Abstract
Objective:
The objective was to investigate the antiurolithiatic and antioxidant activity of ethanolic extract of Hordeum vulgare seeds (EHV) on ethylene glycol-induced urolithiasis in Wistar albino rats.
Materials and Methods:
Urolithiasis was produced in Wistar albino rats by adding 0.75% v/v ethylene glycol (EG) to drinking water for 28 days. The ethanolic extract of Hordeum vulgare seeds (EHV) was assessed for its curative and preventive action in urolithiasis. In preventive treatment, the EHV given from 1st day to 28th day, while in the curative regimen, the EHV was given from 15th day to 28th day. Various renal functional and injury markers such as urine volume, calcium, phosphate, uric acid, magnesium, urea, and oxalate were evaluated using urine, serum, and kidney homogenate. Antioxidant parameters such as lipid peroxidation, superoxide dismutase, and catalase were also determined.
Results:
The EHV treatment (both preventive and curative) increased the urine output significantly compared to the control. The EHV treatment significantly reduced the urinary excretion of the calcium, phosphate, uric acid, magnesium, urea, and oxalate and increased the excretion of citrate compared to EG control. The increased deposition of stone forming constituents in the kidneys of calculogenic rats were significantly lowered by curative and preventive treatment with EHV. It was also observed that the treatment with EHV produced significant decrease in lipid peroxidation, and increased levels of superoxide dismutase and catalase.
Conclusion:
These results suggest the usefulness of ethanolic extract of Hordeum vulgare seeds as an antiurolithiatic and antioxidant agent.
KEY WORDS: Antioxidant, ethylene glycol, Hordeum vulgare, urolithiasis
Introduction
Urinary calculi are the third prevalent disorder of the urinary system. Approximately 80% of these calculi are composed of calcium oxalate and calcium phosphate. Lithiasis is a male-predominant disorder, with a recurrence rate of 70–80% in males and 47–60% in females. Currently, no allopathic medications are available for urolithiasis. Surgery, lithotripsy, and local calculus disruption using a high-power laser are used to treat calculi. However, these procedures are expensive and recurrence is quite common.[1]
As per Ayurveda, the seeds of Hordeum vulgare Linn. are reported to be useful in the treatment of a wide range of aliments including urinary stones.[2] However, no scientific data are available to establish the antiurolithiatic property of the seed extract of H. vulgare Linn. In the present study, an effort has been made to establish the scientific validity of the antiurolithiatic activity of Hordeum vulgare seed extract using ethylene glycol-induced urolithiasis using male Wistar albino rats.
Materials and Methods
Adult male albino rats of Wistar strain (150-200 g) were procured from Zydus Research Centre, Ahmedabad. For acute toxicity studies, albino mice of either gender (25-30 g) were also procured. The animals were acclimatized to standard laboratory conditions (temperature: 23 ± 2°C) and maintained on 12-hour light/dark cycle. They were provided with regular rat chow (VRK Nutritional Solutions, Pune, India) with free access to drinking water ad libitum for the period of 28 days. Institutional Animal Ethics Committee (IAEC) approval (Protocol no.: IICP/PH/02-2010/04 dated 15.03.2010) was obtained and care of the animals was taken as per guidelines of CPCSEA, Ministry of Social Justice and Empowerment, Government of India.
Chemicals
Ethylene glycol was obtained from Qualigen Fine Chemicals, Mumbai, India and cystone (Himalaya Health Care, india) was procured from the local market. All other chemicals and reagents used were of analytical grade.
Preparation of Plant Extract
The dried seeds of Hordeum vulgare Linn. were received from commercial supplier of Anand, Gujarat, India and were identified by Dr. G. C. Jadeja, Professor and Head, Department of Agricultural Botany, B. A. College of Agriculture, Anand Agriculture University, Anand. A voucher specimen (voucher no. IICP/11-JGS/03-HV) was deposited in the herbarium of the Department of Pharmacognosy, Indukaka Ipcowala College of Pharmacy, New Vallabh Vidyanagar, Anand, Gujarat, India.
The air-dried seeds (500 g) were powdered and extracted with ethanol in soxhlet apparatus for 24 hours. The extract was evaporated under reduced pressure to give solid residues, which was stored at 0–4°C for subsequent experiments. The yield of the extract was 4.30% w/w.
Acute Toxicity Studies
Acute toxicity study was performed as per the OECD guideline (no. 420) using albino mice prior to the evaluation of antiurolithiatic activity. The EHV was tested using graded doses (500, 1000, 2000, and 5000 mg/kg) in mice. Furthermore, the general behavior of mice was recorded continuously for 12 hours, and daily for the next 2 weeks for any mortality.[3]
Experimental Design
Ethylene glycol-induced urolithiasis model was used to assess the antiurolithiatic activity in albino Wistar rats.[4] Animals were divided into nine groups containing six animals each. Group I served as control and received regular rat food and drinking water ad libitum. Ethylene glycol (0.75% v/v) in drinking water was fed to groups II–IX for 28 days to induce formation of renal calculi. Group III received standard antiurolithiatic drug, cystone (750 mg/kg b.w.; p.o.).[5] Groups IV, V, and VI served as curative regimen (CR) and received EHV 100 mg/kg, 250 mg/kg, and 500 mg/kg body weight respectively from 15th to 28th day. Groups VII, VIII, and XI received EHV 100 mg/kg, 250 mg/kg, and 500 mg/kg body weight respectively from the 1st day till the 28th day and served as preventive regimen (PR). All extracts and standard were given once daily by oral route.
Analysis of Urine and Serum
At the end of treatment, all animals were kept in individual metabolic cages and 24-hour urine samples were collected and measured on the 28th day. Animals had free access to drinking water during the urine collection period. A drop of concentrated hydrochloric acid was added to the collected urine before being stored at 4°C. The urine was analyzed for calcium, magnesium, phosphate, citrate, oxalate, urea, and uric acid.
On the 29th day, the animals were anesthetized with diethyl ether[6] and blood was collected from the retro-orbital sinus under mild anesthesia. Serum was separated by centrifugation at 15,000 rpm for 20 minutes and analyzed for calcium, magnesium, phosphate, citrate, oxalate, urea, uric acid, BUN, and creatinine.
Kidney Homogenate Analysis
The abdomen was cut open to remove both kidneys from each animal. Isolated kidneys were rinsed in an ice-cold physiological solution, after the extraneous tissues were removed. The right kidney was fixed in 10% neutral buffered formalin, processed in a series of graded alcohol and xylene, embedded in paraffin wax, sectioned at 5 μm and stained with hematoxylin and eosin (H and E) for histopathological examination. The slides were examined under a light microscope to study the architecture of the kidney and calcium oxalate deposits. A sample of 100 mg of the dried kidney was boiled in 10 ml of 1N hydrochloric acid for 30 minutes and homogenized. The homogenate was centrifuged at 2000 rpm for 10 minutes and the supernatant was separated. The calcium, phosphate, uric acid, and oxalate content in the kidney homogenate were determined.
Enzyme Assay
A portion of kidney was taken from all the groups, and a 30% w/v homogenate was prepared in 0.9% buffered KCl (pH 7.4) for the estimation of protein, superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA).
Statistical Analysis
Results were expressed as mean ± SEM. Differences among data were determined using one-way ANOVA followed by Dunnett's multiple comparison tests (Graph pad Prism software for Windows, Version 2.03.1998). P < 0.05 was considered to be statistically significant.
Results
From the acute toxicity study, the LD50 cut-off dose was found to be 5 g/kg body weight for the extract. Hence, the therapeutic dose was taken as 100 mg/kg, 250 mg/kg, and 500 mg/kg body weight for the ethanolic extract.
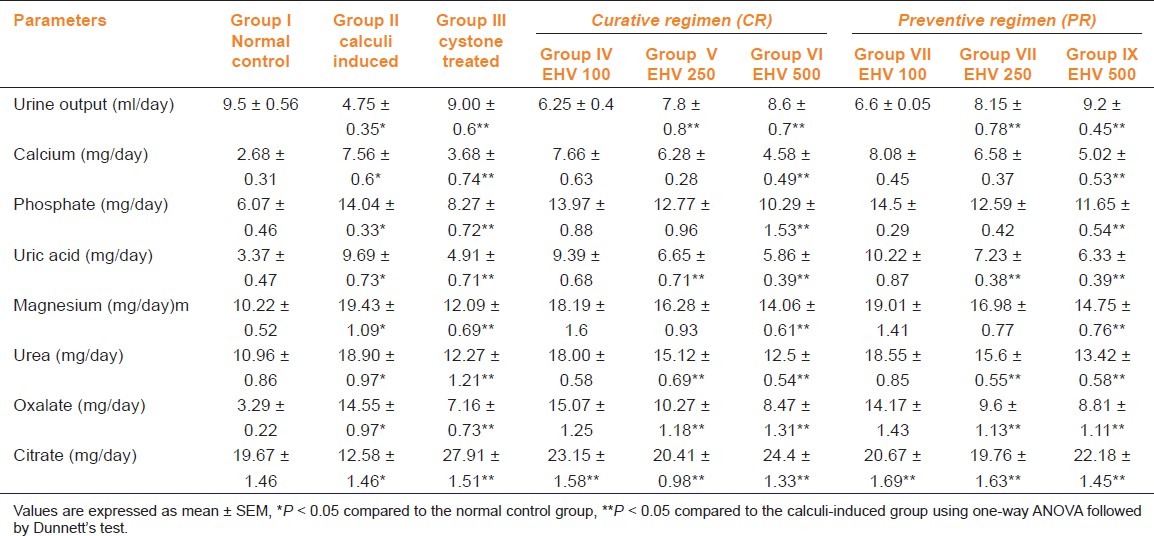
Table 1 depicts the urinary biochemical data that were obtained at the end of the experiment in each group. In the present study, EHV significantly (P < 0.05) increased the urine volume at doses of 250 and 500 mg/kg indicating its diuretic activity. Chronic administration of 0.75% (v/v) ethylene glycol aqueous solution to male wistar rats resulted in hyperoxaluria. There was an increase in urinary calcium, phosphate, uric acid, magnesium, urea, and oxalate in calculi induced animals [Table 1, group II]. However, supplementation with EHV (250 and 500 mg/kg) significantly (P < 0.05) inhibited these changes in urinary calcium, phosphate, uric acid, magnesium, urea, and oxalate excretion dose-dependently in both curative and preventive regimen [Table 1, group IV-IX]. Urinary citrate excretion was decreased after ethylene glycol administration in group II compared to the normal group (group I). However, supplementation with EHV (100, 250, and 500 mg/kg) significantly increased (P < 0.05) this parameter and restores the urinary citrate levels to near-normal value. The results were consistent with cystone-treated animals [Table 1, group III].
Table 1.
Effect of ethanolic extract of seeds of H. vulgare Linn. on urinary parameters of control and experimental animals
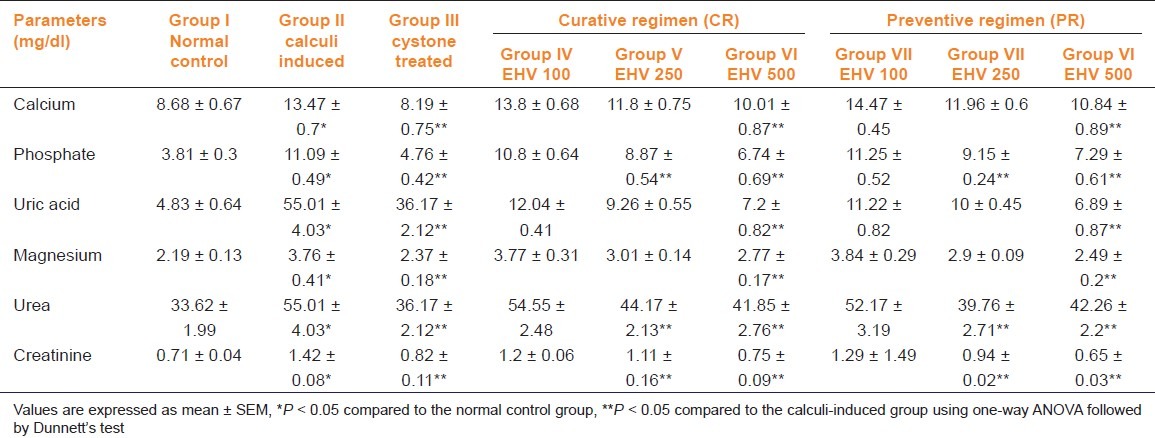
Renal stone induction caused impairment of renal functions of the untreated rats as evident from the markers of glomerular and tubular damage, i.e., elevated serum creatinine, uric acid, and urea. These markers were significantly (P < 0.05) reduced in the animals which were treated with EHV in a dose-dependent manner. The serum calcium, inorganic phosphate, and magnesium were significantly increased (P < 0.05) in calculi-induced animals compared to group I [Table 2, group II] indicating marked renal damage. However, treatment with EHV (250 and 500 mg/kg) significantly (P < 0.05) lowered the elevated serum level of calcium, inorganic phosphate, and magnesium in both curative and preventive regimens [Table 2, groups IV–IX].
Table 2.
Effect of ethanolic extract of seeds of H. vulgare Linn. on serum biochemistry of control and experimental animals
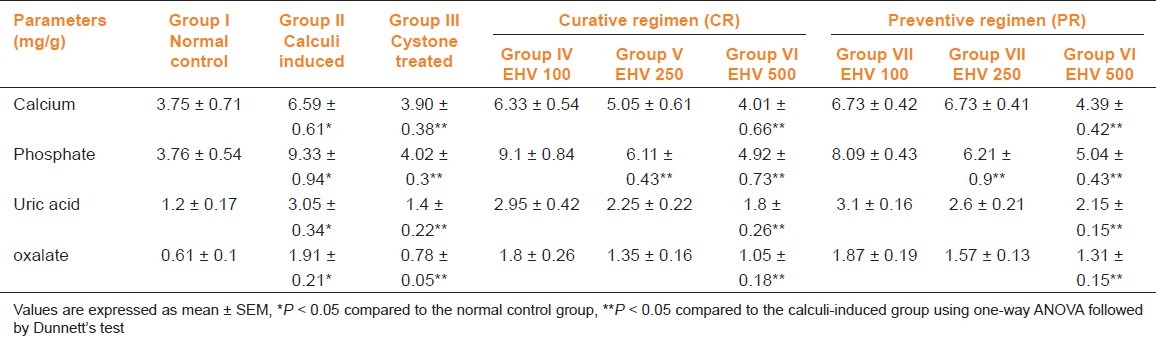
The calcium, phosphate, uric acid, and oxalate levels were significantly elevated in kidney homogenate of the calculi-induced animal group (group II) compared to group I [Table 3]. The EHV (500 mg/kg) and cystone treatment significantly (P < 0.05) diminished the levels of all parameters mentioned above in both regimens. Low dose of EHV (250 mg/kg) failed to exhibit significant reduction in calcium, uric acid, and oxalate level in kidney homogenate except phosphate level.
Table 3.
Effect of ethanolic extract of seeds of H. vulgare Linn. on biochemical parameters of kidney homogenate of control and experimental animals
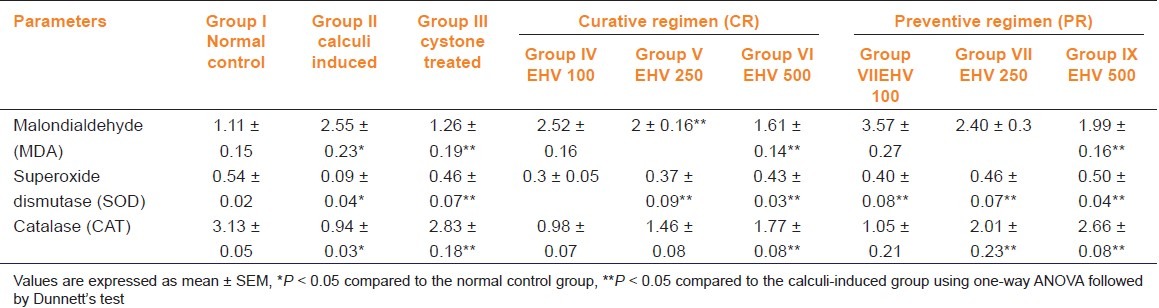
Ethylene glycol treatment significantly (P < 0.05) increased the MDA levels and decreased SOD and CAT levels in calculi-induced animals compared to normal animals [table 4, group II]. The treatment with EHV (250 and 500 mg/kg) produced significant (P < 0.05) reduction in MDA and improved the level of antioxidant enzymes like SOD compared to group II [Table 4, group IV–IX]. The elevated level of CAT was significantly (P < 0.05) maintained by the treatment with EHV 500 in both curative and preventive treatment. [Table 4, groups VI, IX].
Table 4.
Effect of ethanolic extract of seeds of H. vulgare Linn. on markers of oxidation in control and experimental animals
Histopathological analysis revealed no calcium oxalate deposits or other abnormalities in the nephron segment of the vehicle treatment group [Figure 1a]. On the other hand, several calcium oxalate deposits inside the tubules and dilation of the proximal tubules along with interstitial inflammations were observed in the renal tissue of urolithiatic rats [Figure 1b]. The number of calcium oxalate deposits in the tubules of EHV treated rats (groups IV–IX) and cystone treated rats (group III) were less than group II [Figures 1c–e].
Figure 1.
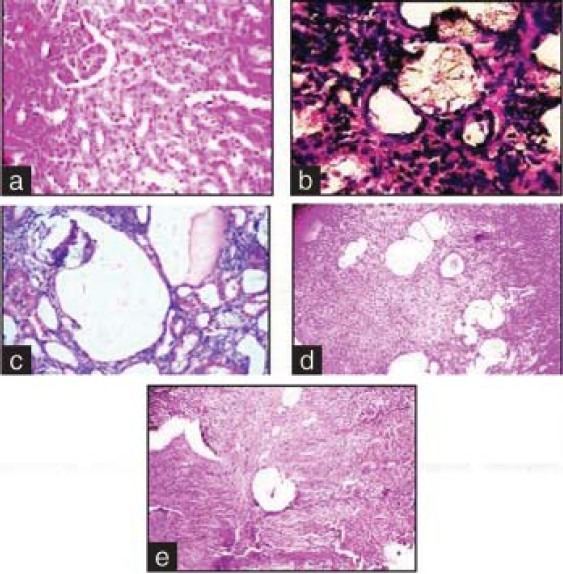
Light microscopic architecture and calcium oxalate deposits in the section of kidney. Sections of (a) normal control, (b) urolithic, (c) cystone treated, (d) curative treatment with EHV at the dose of 500 mg/kg, (e) preventive treatment with EHV at the dose of 500 mg/kg
Discussion
A number of renal pathological diseases, including calcium oxalate kidney stones, have resulted due to the oxalate-induced damage to the renal cells.[7,8] Elevated levels of oxalate is responsible for the toxic effects on the renal epithelial cells via alteration in membrane integrity, generation of reactive oxygen species, and depleted source of antioxidant enzymes.[9,10]
In the present study, male rats were selected to induce renal stone because their urinary system resembles that of humans.[11] Evidence in previous studies indicated that in response to the 28-day period of ethylene glycol (0.75%, v/v) administration, young male albino rats form renal calculi composed mainly of calcium oxalate, minute amounts of calcium phosphate and uric acid stone.[8] Ethylene glycol increases the risk of urolithiasis by increasing urinary levels of stone constituents (calcium, oxalate, phosphate, and uric acid) and facilitating an optimal environment for stone growth like low citrate level. Ethylene glycol increases oxalate production by way of increasing substrate availability which induces the activity of oxalate synthesizing liver enzyme, glycolate oxidase.[12] In view of its medicinal use as an antiurolithic, Hordeum vulgare seed extract was studied to evaluate its potential to prevent calcium oxalate urolithiasis. To our knowledge this is the first study to show the antiurolithiatic effect of EHV in ethylene glycole-induced urolithiasis.
The present study showed an increased in urine output of EHV treated animals which dilutes the concentration of urinary electrolytes. As a result, calcium and phosphorus are flushed out via the urine and there are lesser chances of precipitation, decreased formation as well as the growth of urinary stone. The excretion of oxalate and calcium were progressively increased in calculi-induced animals which is consistent with the previous reports.[13] Most calculi in the urinary system arise from a common component of urine such as calcium oxalate (CaOx) and hypercalciuria, representing up to 80% of analyzed stones.[14] Increased urinary calcium is a factor favoring the nucleation and precipitation of calcium oxalate or apatite (calcium phosphate) from urine and subsequent crystal growth.[15] However, EHV lowered the levels of oxalate as well as calcium excretion, which is beneficial in preventing calculi formation.
Increased urinary inorganic phosphate excretion observed on ethylene glycol administration along with oxalate stress seems to provide an environment appropriate for stone formation by forming calcium phosphate crystals, which eventually induces calcium oxalate deposition.[16] Treatment with EHV restored inorganic phosphate level, thus reducing the risk of stone formation. Magnesium, a potent inhibitor of CaOx crystallization in vitro, binds to oxalate to form a soluble complex, consequently reducing the concentration available for CaOx precipitation.[12,17] Magnesium deficiency accelerates the deposition of renal tubular calcium oxalate in rats. Experiments in animal models have shown protection against CaOx deposition in kidneys by magnesium but clinical studies have not shown any beneficial effects in impeding the formation of CaOx kidney stones.[18] However, treatment with EHV significantly reduced the level of magnesium in urine and serum and significant elevation in ethylene glycol control animals. The reason for this is unclear.
Citrate is an important urolithiasis inhibitor, which forms a soluble complex with calcium and inhibits precipitation and aggregation of calcium oxalate and phosphate. In our study, EHV and cystone treatment led to increase in citrate concentration which might have reduced crystallization of calcium oxalate.[19]
The glomerular filtration rate decreases in urolithiasis due to the obstruction to the outflow of urine by stones in the urinary system and the waste products such as urea and uric acid get accumulated in blood. This indicates marked damage of kidney. The uric acid crystals adsorb glutamic acid and other organic compounds and promote calcium oxalate crystals growth. The results showed a significant increase in uric acid level in serum as well as in urine in the ethylene glycol control group compared to normal control. The uric acid levels decreased after treatment with EHV and cystone, thereby hastening the process of dissolving the preformed stones and prevention of new stone formation in the urinary system.[20,21]
It has been postulated from several in vitro and in vivo studies that high levels of oxalate may have a detrimental effect on renal architecture mediated through intracellular oxidative stress, followed by changes in membrane structure, membrane lipid peroxidation, and cell death.[22] It was observed in the present study that administration of ethylene glycol increased MDA content of kidneys and decreased activity of the antioxidant enzymes in the kidneys. The EHV treatment protected against the changes associated with oxidative stress. Several studies have shown that crystal formation results in cell damage and cell detachment from the basement membrane and the released degradation products further promote nucleation of crystals.[23] Renal epithelial injury promotes crystal retention, as epithelial injury exposes a variety of crystal adhesion molecules on epithelial surfaces.[24,25] Histopathological changes also support the above results. Administration of EHV to ethylene glycol rats prevented super saturation of calcium oxalate and thus decreased their deposition in renal tubules.
In conclusion, the results indicate that administration of seed extract of Hordeum vulgare Linn. reduced and prevented the growth of urinary stones. It also seems that the treatment effect is more effective than its preventive effect. The underlying mechanism could be due to its diuretic effect, antioxidant, nephroprotective property, and lowering the concentration of urinary stone-forming constituents. Further experimental and clinical studies are required to elucidate the chemical constituents of the extract and the mechanism(s) that are responsible for the pharmacological activities.
Footnotes
Source of Support: Nil.
Conflicts of interest: No.
References
- 1.Prasad K, Sujatha D, Bharathi K. Herbal drugs in urolithiasis – a review. Pharmacognosy Res. 2007;1:175–9. [Google Scholar]
- 2.Khare CP. Indian medicinal plants - An illustrated dictionary. Heidelberg: Springer Publishers; 2007. pp. 314–5. [Google Scholar]
- 3.Ghosh MN. Fundamentals of experimental pharmacology. 4th ed. Calcutta: Hilton & Company; 2008. [Google Scholar]
- 4.Anupama S, Handa SS. Hepatoprotective activity of andrographolide from Andrographis paniculata against CCl4. Indian J Med Res. 1990;92:276–83. [PubMed] [Google Scholar]
- 5.Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92:137–40. doi: 10.1046/j.1464-410x.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaur T, Bijarnia RK, Singla SK, Tandon C. In vivo efficacy of Trachyspermum ammi anticalcifying protein in urolithiatic rat model. J Ethnopharmacol. 2009;126:459–62. doi: 10.1016/j.jep.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Scheid CR, Koul HK, Kennington L, Hill WA, Luber-Narod J, Jonassen J, et al. Oxalate-induced damage to renal tubular cells. Scanning Microsc. 1995;9:1097–105. [PubMed] [Google Scholar]
- 8.Kurien TB, Selvam R. Induction of lipid peroxidation in calcium oxalate stone formation. Indian J Exp Biol. 1989;27:450–3. [PubMed] [Google Scholar]
- 9.Miller C, Kennington L, Cooney R, Kohjimoto Y, Cao LC, Honeyman T, et al. Oxalate toxicity in renal epithelial cells: Characteristics of apoptosis and necrosis. Toxicol Appl Pharmacol. 2000;162:132–41. doi: 10.1006/taap.1999.8835. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen CW. Essays in Experimental Biology. Chicago: University of Chicago Press; 1962. Experiments on causation of urinary calculi; pp. 253–69. [Google Scholar]
- 11.Richardson TK, Tolbert NE. Oxidation of glycolic acid to oxalic acid by glycolic acid oxidase. J Biol Chem. 1961;57:816–82. [PubMed] [Google Scholar]
- 12.Robertson WG, Peacock M, Selby PL. A multicenter trial to evaluate three treatments for recurrent idiopathic calcium stone disease: A preliminary report. In: Schwille PO, Smith LH, Roberston WG, Vahlensieck W, editors. Urolithiasis and Related Clinical Research. New York: Plenum Press; 1985. pp. 545–8. [Google Scholar]
- 13.Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 2010;48:1013–8. doi: 10.1016/j.fct.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Lemann J, Worcestor EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis. 1991;27:386–91. doi: 10.1016/s0272-6386(12)80628-7. [DOI] [PubMed] [Google Scholar]
- 15.Roger K, Low MD, Stoller ML. Uric acid nephrolithiasis. Urol Clin North Am. 1997;24:135–48. doi: 10.1016/s0094-0143(05)70359-1. [DOI] [PubMed] [Google Scholar]
- 16.Kohri K, Garside J, Blacklock NJ. The role of magnesium in calcium oxalate urolithiasis. Br J Urol. 1988;61:107–15. doi: 10.1111/j.1464-410x.1988.tb05057.x. [DOI] [PubMed] [Google Scholar]
- 17.Rushton HG, Spector M. Effects of magnesium deficiency on intratubular calcium oxalate formation and crystalluria in hyperoxaluric rats. J Urol. 1982;127:598–604. doi: 10.1016/s0022-5347(17)53920-8. [DOI] [PubMed] [Google Scholar]
- 18.Stitchantrakul W, Kochakaran W, Ruangraksa C, Domrongkitchaiporn S. Urinary risk factors for recurrent calcium stone formation in Thai stone formers. J Med Assoc Thai. 2007;90:688–98. [PubMed] [Google Scholar]
- 19.Ruckmani K, Kavimani S, Anandan R, Jaykar B. Effect of Moringa oleifera Lam.on paracetamol induced hepatotoxicity. Indian J Pharm Sci. 1998;60:33–5. [Google Scholar]
- 20.Tripathi YB, Shukla S, Tripathi P. Medicinal plants of Rasayana roup as antioxidants. International Seminar on Free Radicals Mediated Diseases & Ayurveda, Faculty of Ayurveda, IMS, BHU, Varanasi, Uttar Pradesh, India. 1996:A–33. [Google Scholar]
- 21.Purnima A, Basavaraj C, Koti AH. Vishwanathswamy. Anti-urolithiatic and antioxidant activity of Minusops elengi on ethylene glycol induced urolithiasis in rats. Indian J Pharmacol. 2010;42:380–3. doi: 10.4103/0253-7613.71925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashir S, Gilani AH, Siddiqui AA, Pervez S, Khan SR, Sarfaraz NJ, et al. Berberis vulgaris root bark extract prevents hyperoxaluria induced urolithiasis in rats. Phytother Res. 2010;24:1250–5. doi: 10.1002/ptr.3196. [DOI] [PubMed] [Google Scholar]
- 23.Khan SR, Hackett RL. Retention of calcium oxalate crystals in renal tubules. Scanning Microsc. 1991;5:707–12. [PubMed] [Google Scholar]
- 24.Bijarnia RK, Kaur T, Aggarwal K, Singla SK, Tandon C. Modulatory effects of N-acetylcysteine on hyperoxaluric manifestations in rat kidney. Food Chem Toxicol. 2008;46:2274–8. doi: 10.1016/j.fct.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–57. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]


