Abstract
Context:
Several studies have reported that antioxidants play an important role in Parkinson's disease (PD). Garcinia indica extract is a natural antioxidant, the present study was undertaken to evaluate the neuroprotective effect of methanolic extract of Garcinia indica (GIM) against 6-hydroxydopamine (6-OHDA) neurotoxicity for striatal dopaminergic neurons in the rat.
Materials and Methods:
Thirty adult Wistar rats were randomly divided into five groups namely control, 6-OHDA model, and GIM (100, 200, and 400 mg/kg body weight suspended in one ml of 0.1% carboxymethyl cellulose). The treatment was started three days before surgery and continued for next 14 days. The surgery was done on third day in all groups for administration of 6-OHDA into the right striatum and right substantia nigra, whereas control group injected with 6-OHDA vehicle. Various behavior and biochemical tests (Apomorphine-induced rotational behavior, Stepping test, Initiation time, Postural balance test, and Disengage time) were used to evaluate the neuroprotective effect of GIM. One-way analysis of variance (ANOVA) followed by Dunnett's test was used to compare inter-group differences. P<0.05 was considered as statistically significant.
Results:
GIM had significant (P<0.05, P<0.01) preventive effect in biochemical tests, i.e., dopamine and its metabolites measurement and in various behavior tests, i.e., apomorphine-induced rotational behavior, stepping test, initiation time, postural balance test, and disengage time as compared to 6-OHDA-treated rats.
Conclusions:
Our results demonstrated that GIM acted as an effective neuroprotective agent for striatal dopaminergic neurons in 6-OHDA lesioned rat model of PD.
KEY WORDS: 6-OHDA, Garcinia indica, Parkinson's disease
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta. Currently, levodopa has been used for PD; however, its long-term use is limited due to adverse effects such as dyskinesia, on-off phenomenon, and psychiatric effects. Thus, new anti-parkinsonism drugs with better safety profile need to be discovered.[1]
Garcinia indica is known as a kokam in Gujarat, India. It is a traditional home remedy in case of flatulence, heat strokes, and infections.[2,3] It is also used in as an appetizer, an anti-inflammatory agent, a liver tonic, and to relieve muscle tremor.[4] Garcinol, a polyisoprenylated benzophenone purified from Garcinia indica fruit rind has showed antioxidant and anti-ulcer properties.[5,6] Apart from hydroxycitric acid and garcinol, kokam contains other compounds with potential antioxidant properties. These include citric acid, malic acid, polyphenols, carbohydrates, anthocyanin pigments, and ascorbic acid.[7]
PD is characterized by tremor, rigidity, bradykinesia, and postural instability. The oxidative stress and inflammation are highly implicated in the degeneration of nigrostriatal neurons in PD.[8,9] Antioxidant and anti-inflammatory activity has been reported for aqueous and ethanolic extract of Garcinia indica fruit.[5,10,11] Methanolic extract of Garcinia species also reported antioxidant and antispasmodic effects.[12,13] Therefore, present study was undertaken to evaluate the effect of methanolic extract of Garcinia indica (GIM) in dopaminergic neuronal loss induced by 6-hydroxydopamine (6-OHDA) in rat model of PD.
Materials and Methods
Animals
Thirty male adult Wistar rats, weighing 200 to 250 g, were maintained under 12: 12-h light: dark cycle with food and water provided ad libitum. The study protocol (IAECNo:09/NIPER/CPCSEA/351) was approved by Institutional Animal Ethics Committee for the care and use of laboratory animals. All efforts were made to reduce the number of animals used and their suffering.
Extract Preparation
G. indica fruits were purchased from a local supplier and were identified by a pharmacognosy expert in the Department of Pharmacy, NIPER-Guwahati. The fruits were finely powdered in automix blender (600 g), which were then extracted with methanol using Soxhlet extractor. The resultant solution was filtered and dried using a rotary evaporator in a water bath at a temperature not exceeding 60°C.
Experimental Design
The animals were randomly allocated to five groups as Vehicle, 6-OHDA, GIM (100, 200, and 400 mg/kg body weight) of six rats each. Vehicle and 6-OHDA groups were received 1 ml of 0.1% carboxymethyl cellulose (CMC) solution in water while GIM groups were treated with the selected doses, suspended in 1 ml of 0.1% CMC in water. All treatments were given orally; daily in morning at 10 am, for 17 days (includes three days pre-treatment before surgery). On the third day, brain surgery was performed, after 60 minutes of each treatment, in all groups for 6-OHDA administration except vehicle-treated group where vehicle is administered. Dose of GIM was selected as per previous study.[10,11]
Operative Procedure
Unilateral striatal lesion was produced by stereotaxic injection of 7 μg 6-OHDA into the right striatum and 7 μg 6-OHDA into the right substantia nigra according to the atlas of Paxinos and Watson.[1,14]
Briefly, the animal was anesthetized with pentobarbital anesthesia (Sigma, 45 mg/kg, i.p.) and placed in stereotaxis instruments. The solution was prepared in a 0.2 mg/ml ascorbate saline and injected into right striatum with a Hamilton syringe at a rate of 1 μl/min. The stereotaxic co-ordinates were 1 mm anterior to bregma, 2 mm lateral from midline, and 4.5 mm below the dura for striatum and 5.8 mm posterior to bregma, 1.6 mm lateral from midline, and 8 mm below the dura for SN part of brain with the incisor bar located 3.3 mm below the interaural line on the non-dominant side.
Quantitation of Rotational Behavior
On 14th day, after right striatum stereotaxic injection of 6-OHDA, animals were subjected to rotational behavior testing. Rats were injected with apomorphine hydrochloride subcutaneously (Sigma, 0.05 mg/kg) and after 15 minutes from injection, contralateral turns were recorded for a 30-minute period. In order to exclude the influence of apomorphine, rotational test was performed at last of the all other behavior test.[1,15]
Stepping Test
This test was used for measurement of akinesia. The rat was held with one hand by the experimenter fixing the hind limbs (slightly raising the torso) and with the other hand fixing the forelimb that was not to be monitored. In this way, the other forepaw had to bear the weight. When the rats were moved with a speed of 90 cm per 5 s in forward and backward along the table, the free forelimb had to step with the movement of the experimenter to keep balance. The steps taken to keep balance were recorded as the adjusting steps. This was done for both the contralateral and ipsilateral forepaw and finally counted together. The numbers of adjusting steps for both directions were counted.[1,16]
Initiation Time
The rats were pre-trained for two days to turn up a wooden ramp (1.1 m) into their home cage. During the test, the rat was held as per the stepping test. Time was measured until the rat initiated movement with the forelimb was not fixed by the experimenter. This duration was defined as the initiation time and 180 s was used as the break-off point. The test was performed once a day for each forelimb on three consecutive days and the mean of the three test sessions was calculated.[1,17]
Postural Test
The rat was held as described for the stepping test and then in a fast movement tilted toward the side of the paw touching the table, which caused a loss of balance. The animal tried to regain balance with an adjusting step that was recorded by a scoring system ranging from 0 to 3: (0) no detectable muscle reaction, the rat falls onto the side; (1) clear forelimb reaction, but the rat cannot move limb under the body toward the center of gravity and thus still falls onto the side; (2) incomplete recovery of balance, i.e., the rat moves its limb under the body but not yet fully into the center of gravity, and thus the forelimb is not aligned vertically to the body; further, the forepaw might not be placed in a plain position on the table and digits might be crossed over each other; (3) complete recovery of balance. The test was repeated six times a day on both sides giving a maximum daily score of 18. Final results were expressed as average of the three-test day's score. Six animals repeated three times every day for three days, then mean calculated from 54 values for six rats in each group.[1,18]
Disengage Time
A blunt wooden probe touched the perioral region beneath the vibrissae of the rat repeatedly at 1 s intervals when the rat was engaged in eating a piece of milk chocolate. The latency of the orienting response, i.e., turning of the head toward the stimulus, was recorded; an immediate response was scored as 1 s. Stimulation was discontinued if the animal did not respond within a period of 180 s. The test was performed once a day on each side over two days and the mean of the two subtests was calculated.[1,19]
All these tests (Rotational test, Stepping test, Initiation time, Postural test, Disengage time) were performed by a blind investigator, not aware of the treatment given to the animals.
Measurement of Dopamine and Its Metabolites
Animals were euthanized day after rotational test to exclude the effect of apomorphine. The striatal samples were separated and weighed immediately after dissection and stored at -70°C until assay. The striatal samples were sonicated in ice cold 0.2M perchloric acid containing 0.05% ethylenediamminetetraacetic acid (EDTA). The homogenates were immediately centrifuged at 10,000 rpm at 4°C for 10 minutes. The supernatants were filtered using 0.45-μm pore filters and were used for determination of dopamine (DA) and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) using high-performance liquid chromatography with electrochemical detector. The mobile phase consisted of a mixture of 0.1M citric acid monohydrate, 0.1M sodium acetate, 7% methanol, 100mM EDTA, and 0.01% sodium octane sulfonic acid. The flow rate of mobile phase was maintained at 1 ml/min and the injection volume was 20 ml.[20]
Statistical Analysis
Data are expressed as mean±standard error of the mean (SEM). Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by Dunnett's test. P<0.05 was considered statistically significant.
Results
Rats subjected to stereotaxic injection of 6-OHDA and received GIM had significant (P<0.05) effect in various behavior tests.
A significant (P < 0.05, P<0.01) protective effect of GIM treated rats were observed in apomorphine-induced rotational behavior. In 6-OHDA group, there was significant (P<0.001) increase in number of rotation observed as compare to control group. The increased dose of GIM (100, 200, and 400 mg/kg) showed dose-dependent decrease in the number of rotations as compare to 6-OHDA group [Figure 1].
Figure 1.
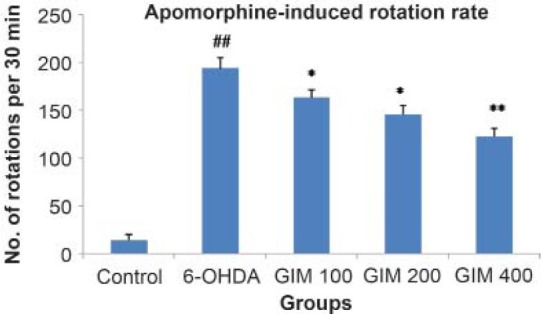
Effect of GIM on amphetamine-induced rotation test in 6-OHDA rat model for Parkinson's Disease
Values are expressed as mean ± SEM, (n = 6 in each group) *P<0.05, **P<0.01 vs 6-OHDA group, ##P<0.001 vs control group, GIM = methanolic extract of Garcinia indica, 6-OHDA = 6-hydroxydopamine,
A significant (P<0.01) decrease in number of steps in forehand direction in 6-OHDA-treated group was observed as compared to control group. GIM treatment significantly increased the number of steps in dose-dependent fashion in forehand direction as compare to 6-OHDA (P<0.05, P<0.01) [Table 1]. 6-OHDA also significantly decreased number of steps in backhand direction as compared to control group (P<0.01). GIM treatment significantly increased the number of steps in dose-dependent fashion in backhand direction as compare to 6-OHDA (P<0.05) [Table 1].
Table 1.
Effect of GIM on behavioral tests in 6-OHDA-induced rat PD model
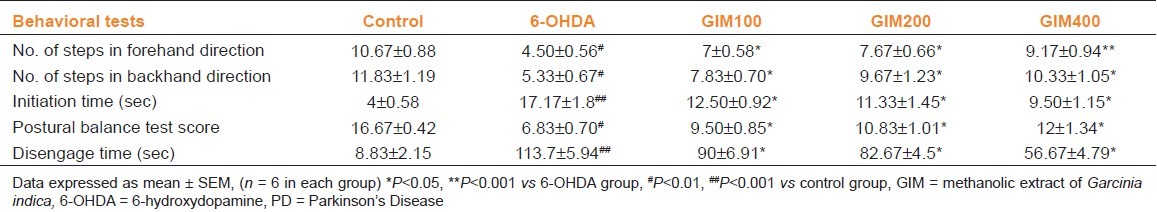
The initiation time in 6-OHDA-treated rats was significantly (P<0.001) increased as compared to control group indicating akinesia. GIM-treated groups significantly (P<0.05) decreased the initiation time as compared to 6-OHDA group [Table 1].
The postural balanced test showed that 6-OHDA significantly (P<0.01) decreased score as compared to control group. The GIM-treated groups showed dose-dependent restoration of the postural balance (P<0.05) [Table 1].
Similarly, 6-OHDA significantly (P<0.001) increased disengage time as compared to control group. GIM treatment significantly (P<0.05) decreased disengage time in dose-dependent manner as compared to 6-OHDA [Table 1].
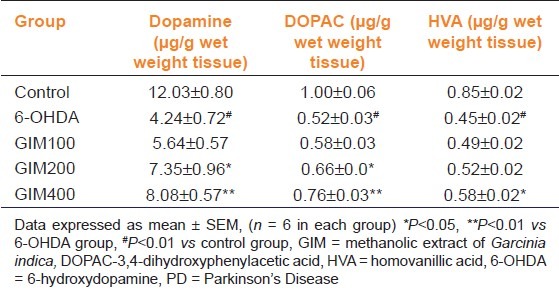
The striatal DA and its metabolite levels in the control and treated groups showed that 6-OHDA administration significantly (P<0.001) decreased DA, DOPAC, and HVA as compared to control group. However, GIM (100 mg/kg) increased DA and its metabolite level, but failed to show changes at significant level. Although GIM (200 mg/kg) significantly (P<0.05) increased DA and DOPAC levels compared to 6-OHDA. Moreover, GIM (400 mg/kg) significantly (P<0.05) increased DA, DOPAC, and HVA levels as compared to 6-OHDA [Table 2]. So, GIM (200, and 400 mg/kg) can be used as an effective dose for protective effect in PD.
Table 2.
Effect of GIM on striatal dopamine, DOPAC, and HVA level in 6-OHDA injured rat PD model
Discussion
The findings of the present study demonstrated that chronic treatment of GIM corrected a hemi-Parkinson's condition in rats caused by intra brain application of the neurotoxin 6-OHDA. Various behavior and biochemical tests were used as an index of striatal dopaminergic function. GIM improved these behavior and biochemical tests in 6-OHDA-induced rat model of PD.
PD is a neurodegenerative movement disorder of unknown etiology. It is characterized by the progressive loss of dopaminergic neurons in the substantia nigra, depletion of DA in the striatum, and abnormal mitochondrial that may be closely associated with pathological and clinical abnormalities.[21] 6-OHDA-induced PD models also produce similar changes.[22]
Our study results showed a dose-dependent effect of GIM treatment in increasing number of steps in forehand and backhand direction, postural balance and decreased apomorphine-induced rotations and initiation time to become active and decreased disengage time in 6-OHDA-induced PD animals. These behavior parameters were used as an index of dopaminergic neuron function. Thus, it can be stated that GIM pretreatment have a neuroprotective effect against 6-OHDA.
A previous study showed that intranigral administration of 6-OHDA appeared to produce widespread loss of dopaminergic terminals in the striatum with a marked reduction in both DA and its metabolites DOPAC and HVA.[23] These results are consistent with our results of the decrease of DA and its metabolite DOPAC and HVA levels in the striatum by 6-OHDA administration. However, GIM dose dependently prevents the 6-OHDA-induced reduction of DA and its metabolite DOPAC and HVA levels in the striatum. These results suggest that GIM pretreatment have a neuroprotective effect against 6-OHDA.
Increasing evidence indicates that both oxidative stress and inflammation may play a fundamental role in the pathogenesis of PD. Oxidative stress is characterized by increase in reactive oxygen species and depletion of glutathione. Lipid mediators for oxidative stress include 4-hydroxynonenal, isoprostanes, isofurans, isoketals, neuroprostanes, and neurofurans are also increased in PD.[24]
Previous study showed that Garcinia seeds significantly increased GSH level, antioxidant enzyme activity and significantly decreased the levels of malondialdehyde, aspartate transaminase, alanine aminotransferase, and urea in brain of Wistar albino rats exposed to gamma-radiation. Thus, it protects against gamma-radiation-induced oxidative stress in brain of exposed rats.[25]
In addition, oxidative stress is also linked to other components of the degenerative process, such as mitochondrial dysfunction, excitotoxicity, nitric oxide toxicity, and inflammation. A neuroinflammation is widely present in PD, characterized by activated microglial cells that generate proinflammatory cytokines, such as TNF-α and IL-1β.[26]
The garcinol, one of the active constitute of Garcinia indica, prevents NO accumulation in lipopolysaccharide (LPS)-treated astrocytes. Garcinol significantly reduce the expression of LPS-induced inflammatory mediators, such as inducible NO synthase (iNOS) and Cyclooxygenase-2 (COX-2).[27] These observed antioxidant and anti-inflammatory properties of Garcinia indica encouraged us to evaluate neuroprotective effect against PD.
In conclusion, this study showed that GIM has a neuroprotective effect against 6-OHDA in various behavioral and biochemical models. However, further work is necessary to find out the exact mechanism of the extract.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Singh S, Ahmed R, Sagar RK, Krishana B. Neuroprotection of the nigrostriatal dopaminergic neurons by melatonin in hemiparkinsonium rat. Indian J Med Res. 2006;124:419–26. [PubMed] [Google Scholar]
- 2.Ambasta SP, editor. The wealth of India, Raw materials. Vol. 4. New Delhi: Publication and Information Directorate, CSIR; 1998. [Google Scholar]
- 3.Dushyantha DK, Girish DN, Suvarna VC, Dushyantha DK. Native lactic acid bacterial isolates of kokum for preparation of fermented beverage. Europ J Biol Sci. 2010;2:21–4. [Google Scholar]
- 4.Charaka Samhita. 5th ed. Varanasi: Chaukhamba Sanskrit Sansthan; 2001. Agnivesha. Prameha Chikitsa. [Google Scholar]
- 5.Yamaguchi F, Ariga T, Yoshimura Y, Nakazawa H. Antioxidative and anti-glycation activity of garcinol from Garciniaindica fruit rind. J Agric Food Chem. 2000;48:180–5. doi: 10.1021/jf990845y. [DOI] [PubMed] [Google Scholar]
- 6.Tamil Selvi A, Joseph GS, Jayaprakasha GK. Inhibition of growth and aflatoxin production in Aspergillus flavus by Garcinia indica extract and its antioxidant activity. Food Microbiol. 2003;20:455–60. [Google Scholar]
- 7.Mishra A, Bapat MM, Tilak JC, Devasagayam T. Antioxidant activity of Garcinia indica (kokam) and its syrup. Curr Sci. 2006;91:90–3. [Google Scholar]
- 8.Miller RL, James-Kracke M, Sun GY, Sun AY. Oxidative and inflammatory pathways in Parkinson's disease. Neurochem Res. 2009;34:55–65. doi: 10.1007/s11064-008-9656-2. [DOI] [PubMed] [Google Scholar]
- 9.Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Khatib NA, Pawase K, Patil PA. Evaluation of anti inflammatory activity of garciniaindica fruit rind extracts in wistar rats. Inter J Res Ayurveda Pharmacy. 2010;1:449–54. [Google Scholar]
- 11.Amol BD, Vinayak DS, Nilofer SN. Antioxidant and hepatoprotective effect of Garcinia indica Linn fruit rind. Pharmacie Globale (IJCP) 2011;6:1–5. [Google Scholar]
- 12.Udia PM, Braide VB, Owu DU. Antispasmodic and spasmolytic effects of methanolic extract from seeds of Garcinia kola on isolated rat small intestine. Niger J Physiol Sci. 2009;24:111–6. doi: 10.4314/njps.v24i2.52912. [DOI] [PubMed] [Google Scholar]
- 13.Okoko T. In vitro antioxidant and free radical scavenging activities of Garcinia kola seeds. Food Chem Toxicol. 2009;47:2620–3. doi: 10.1016/j.fct.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Sydney: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- 15.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–93. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 16.Schallert T, De Ryck M, Whishaw IQ, Ramirez VD, Teitelbaum P. Excessive bracing reactions and their control by atropine and L-DOPA in an animal analog of Parkinsonism. Exp Neurol. 1979;64:33–43. doi: 10.1016/0014-4886(79)90003-7. [DOI] [PubMed] [Google Scholar]
- 17.Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: Differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–75. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler C, Sauer H, Lee CS, Björklund A. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson's disease. J Neurosci. 1996;16:7206–15. doi: 10.1523/JNEUROSCI.16-22-07206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 20.Eghwrudjakpor PO, Miyake H, Kurisaka M, Mori K. Central nervous system bioaminergic responses to mechanical trauma: An experimental study. Surg Neurol. 1991;35:273–9. doi: 10.1016/0090-3019(91)90004-s. [DOI] [PubMed] [Google Scholar]
- 21.Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. Biochim Biophys Acta. 1998;1366:211–23. doi: 10.1016/s0005-2728(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 22.Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–24. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 23.Costa G, Abin-Carriquiry JA, Dajas F. Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantianigra. Brain Res. 2001;888:336–42. doi: 10.1016/s0006-8993(00)03087-0. [DOI] [PubMed] [Google Scholar]
- 24.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 25.Adaramoye OA. Protective effect of kolaviron, a biflavonoid from Garcinia kola seeds, in brain of Wistar albino rats exposed to gamma-radiation. Biol Pharm Bull. 2010;33:260–6. doi: 10.1248/bpb.33.260. [DOI] [PubMed] [Google Scholar]
- 26.Farooqui T, Farooqui AA. Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson's disease. Parkinsons Dis. 2011;2011:247467. doi: 10.4061/2011/247467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao CH, Ho CT, Lin JK. Effects of garcinol on free radical generation and NO production in embryonic rat cortical neurons and astrocytes. Biochem Biophys Res Commun. 2005;329:1306–14. doi: 10.1016/j.bbrc.2005.02.110. [DOI] [PubMed] [Google Scholar]


