Abstract
Development of a commercially successful animal vaccine is not only influenced by various immunological factors, such as type of antigen but also by formulation and delivery aspects. The latter includes the need for a vector or specific delivery system, the choice of route of administration and the nature of the target animal population and their habitat. This review describes the formulation and delivery aspects of various types of antigens such as killed microorganisms, proteins and nucleic acids for the development of efficacious and safe animal vaccines. It also focuses on the challenges associated with the different approaches that might be required for formulating and delivering species specific vaccines, particularly if their intended use is for improved animal management with respect to disease and/or reproductive control.
KEYWORDS: Delivery systems, domestic animals, formulation, immunocontraception, immunological adjuvants, vaccines, wildlife
In the last decade, many articles have reviewed research on the development and status of animal vaccines, especially those developed primarily for use in disease prevention for domestic animals (pets and production livestock).[1–3] This review addresses the challenges associated with formulation and delivery of such vaccines and how this assists researchers who are endeavoring to deliver vaccines for either disease management or for fertility control of wildlife. We define wildlife as animals living as wild populations. Thus, wildlife includes introduced vertebrates, which have become pest species in the natural environmental and/or agricultural landscape as well as overabundant native species that require management for conservation, disease or economic reasons in the same landscapes. Delivery of vaccines to these wild populations remains a major challenge though some progress is being made.
Successful vaccines, whether for animals or humans, induce an effective and sustained immune response, have minimal side effects and can be produced cost-effectively at a large scale. Despite these overt requirements, many of the developmental issues regarding effective delivery of vaccines are specific to the animal situation. The delivery of vaccines to domestic pets and livestock is a straightforward scenario compared to delivery to pest animals and wildlife populations. Domesticated species can generally be individually handled for vaccination, including booster immunizations. Efficient access to wildlife is problematic if the vaccine needs to be delivered as an injection. One of the difficulties for wildlife vaccination is to be able to achieve coverage of an appropriate proportion of the target population, and it may require repeated treatment over time to achieve efficacy. Oral, rather than parenteral, delivery would be ideal, but it also raises the need for the development of species specific delivery systems. These challenges along with regulatory and commercial hurdles in the development and commercialization of animal vaccines have constrained the number of commercial products developed for animals, especially for wildlife.
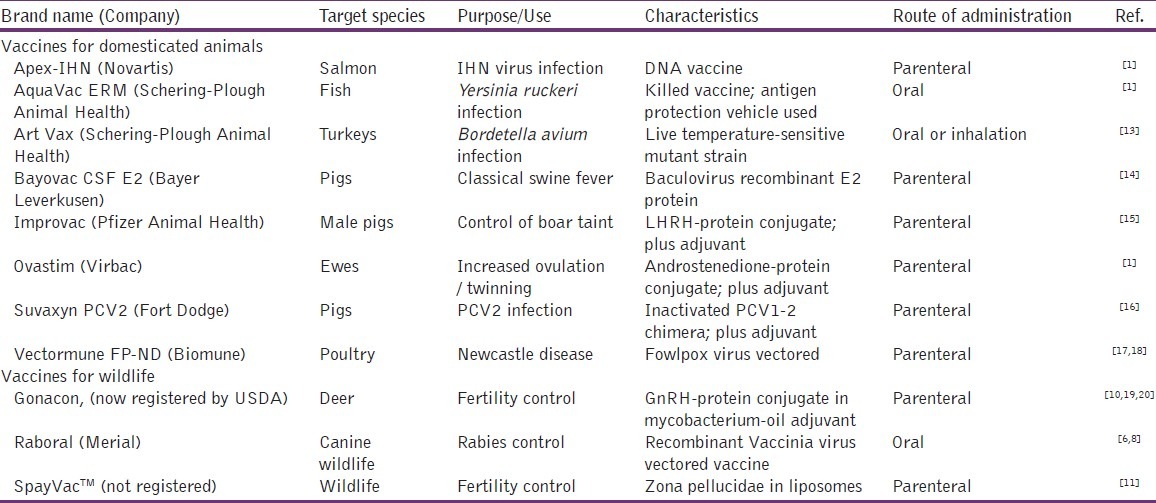
Domestic animal vaccines are used in order to increase food quality and productivity and to prevent and treat diseases. There is also increasing pressure from consumer groups to restrict the use of antibiotics and other drugs in domestic animals, especially in the developed world.[4] Veterinary vaccines offer many benefits over chemical drugs and antibiotics including the absence of residues in foodstuffs, lower frequency of administration, cost-effectiveness and avoidance of the selection of antibiotic-resistant, food-borne bacteria in food animals.[5] On the other hand, an important application for wildlife vaccines is elimination of infections, transmitted directly or indirectly to human beings (zoonotic diseases) or to endangered animal species.[1] One highly successful example of a vaccine for wildlife has been the development of an oral recombinant vaccinia virus, expressing the G-glycoprotein of the rabies virus. It has been widely used in northern Europe since the late 1980s to reduce the incidence of rabies in European red foxes[6,7] and is also currently in broad use for rabies control in raccoons, gray fox and coyote in northern America.[8] In addition, fertility control of wildlife using contraceptive vaccines, which induce an immune response against a specific reproductive antigen (e.g. gonadotrophin releasing hormone, zona pellucidae proteins, sperm proteins), as an alternative method of pest management, has been researched for various invasive animal species in the recent past, and research and development is ongoing.[9–12] Advances in vaccine research have started to provide not only more commercial vaccine products but also novel applications for animal uses [Table 1].
Table 1.
Examples of available animal vaccines and their characteristics
This review describes recent research developments and ongoing challenges in the formulation and delivery of various types of antigens such as killed microorganisms, proteins and nucleic acids for the development of efficacious animal vaccines. It also provides a comparative discussion of specific vaccine delivery requirements, depending on the purpose of the vaccine and its use in different animals (livestock, domestic pets, introduced pest mammals or overabundant native species).
Types of animal vaccines
Formulation issues related to animal vaccines not only vary with the target animal population but also are significantly influenced by the characteristics of selected antigen(s) to be formulated. Vaccines can be categorized as two types, living or non-living, and these raise different issues for formulation and delivery.
Live or live-attenuated vaccines for disease control/protection
Conventionally, many of the vaccines licensed for animal use employ live or live-attenuated microorganisms as antigens. Live vaccines usually induce both cellular (e.g. cytotoxic lymphocytes) and humoral (e.g. systemic antibodies) immunity because of the ability of live microorganisms to infect target cells.[21] These vaccines can be very important for disease management, prevention and even eradication. For example, a live-attenuated vaccine, known as the “Plowright” vaccine, has been used in a vaccination program to eradicate rinderpest virus infection around the world.[22–24]
Serious safety issues, related to live or live-attenuated vaccines such as risk of residual virulence, reversion to pathogenic wild types and potential for unintended consequences if other than target species ingest the vaccines, have resulted in stricter regulatory requirements for live or live-attenuated vaccines. These requirements were highlighted during a vaccination program to control porcine respiratory and reproductive syndrome (PRRS) in Denmark. There are two main types of PRRS virus, European (Lelystad virus strain) and North American, which show 55% to 79% identity at the nucleotide level but show differences in serological cross-reactivity.[25] Following vaccination with the live-attenuated North American PRRS vaccine against the European PRRS virus type in Denmark, the vaccine virus spread within vaccinated as well as non-vaccinated herds, leaving both virus types in the Danish pig population.[26] The other issue with live or live-attenuated vaccines (e.g. against Foot and Mouth Disease, FMD) is that they can confound disease surveillance, based on serological testing of animals – false positives may result in the loss of a country's disease-free status.[27]
Non-living vaccines
Some of the disadvantages associated with live microorganism-based vaccines along with advances in novel applications in vaccinology and biotechnology have paved the way for the development and use of non-living antigens in the vaccines. Whole inactivated or killed vaccines are generally safer than live vaccines, but their inability to infect cells usually results in induction of humoral immune responses without any cell-mediated immunity, making them less effective compared to live or live-attenuated vaccines.[28] Consequently, inactivated or killed antigens usually require the addition of immunological adjuvants and multiple dosing to achieve and sustain a desired level of protective immunity. Additionally, effective oral administration of inactivated microorganisms may require incorporation of a specific protective carrier system. The necessity for immunological adjuvants, carrier systems and multiple dosing highlights the need for specific formulation strategies for these antigens. Indeed, many commercial vaccines, based on killed or inactivated microorganisms, have been successfully developed and are in use for production animals and some wildlife [Table 1].
Developments in biotechnology and molecular biology have enabled the utilization of sub-cellular subunits such as proteins, peptides, carbohydrates and nucleic acids as antigens in vaccines. DNA vaccines represent a growing theme in animal vaccination, and all aspects of DNA veterinary vaccines were recently reviewed by Redding and Weiner.[29] The identification and isolation of sub-cellular components of microorganisms, which play important roles in induction of immunity, prompted their use as safe, non-replicating antigens in vaccines. Acellular antigens, especially proteins, carbohydrates and peptides can be widely used for animal vaccinations due to their non-replicating nature, however, on their own they generally induce poor immunity. The latter can be overcome, at least in part, by conjugation of the small peptides with large immunogenic proteins (such as keyhole limpet hemocyanin), the use of suitable carrier systems and co-formulation of immunological adjuvants with the antigens.[30,31] In addition, most of these antigens tend to be unstable under environmental conditions, such as direct exposure to high temperature, humidity and light[32,33] and when subjected to the acidity and enzymatic activity of the gastrointestinal tract.[34,35] Again, developments in synthetic peptide and polymer chemistry could potentially offer solutions for poor antigen stability.[36,37]
The use of acellular antigens in vaccines has certainly flourished in the last few decades not only because of their safe profile but also because they have enabled novel applications to be investigated. One interesting application has been for reproductive control by immunizing animals against sex hormones, gametes or other key targets in the reproductive tract [Figure 1].[38–42] The most studied reproductive hormone as a vaccine target is luteinizing hormone releasing hormone (LHRH), also known as gonadotrophin-releasing hormone (GnRH).[43–52] GnRH is a small 10 amino acid peptide released from the hypothalamus in all species of mammals and has a central regulatory role in reproductive functions. Immunization against GnRH can result in immunocontraception and control of sexual behavior in both sexes [Table 1] though greater efficacy is achieved in females. Strategies to control fertility using vaccines based on gamete antigens, particularly zona pellucida proteins, have been the most widely researched.[9,12,42,53–55] However, commercial successes for immunocontraceptive vaccines have been relatively few primarily because of poor immunogenicity of small peptide self antigens and difficulties in cost-effective practical delivery of the vaccines to target wildlife species. Another interesting evolution which took place with the use of acellular antigens is development of combined vaccines incorporating two or more antigens, initially used for humans and which later paved the way for numerous combined veterinary vaccines.[56]
Figure 1.
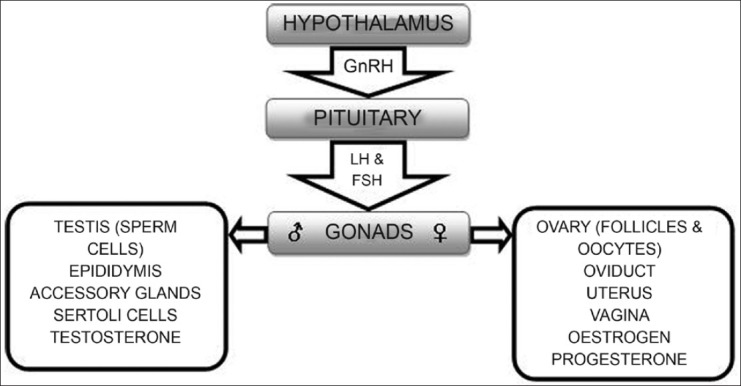
Schematic representation of reproductive targets used for the development of immunocontraceptive vaccines
Formulation of non-living vaccines
Formulation research for antigens began about a century ago when scientists discovered that combining certain reagents, such as saponins, lecithin, aluminum compounds and killed mycobacterium with the antigen for injection could enhance the immune response against the antigen.[57–60] These initial discoveries slowly led to development of immunological adjuvants and delivery systems that boost or alter immune responses to the co-administered antigen. We consider the developments in the formulation of animal vaccines under two categories: (a) use of immunological adjuvants, and (b) use of antigen delivery or carrier systems.
Immunological adjuvants
Immunological adjuvants can provide artificial signals to the immune system to initiate the immune response against a co-administered antigen.[31] In the development of a vaccine, it is important to establish the type of immune response, essential for optimal vaccine efficiency, and then select adjuvant(s) that will help to induce and enhance that type of immune response without unacceptable adverse effects. Different immunological adjuvants can induce one or more type of immune response depending on the route of administration although some of these also induce significant, undesirable side effects [Table 2].
Table 2.
Examples of immunological adjuvants which enhance immune responses against acellular antigens
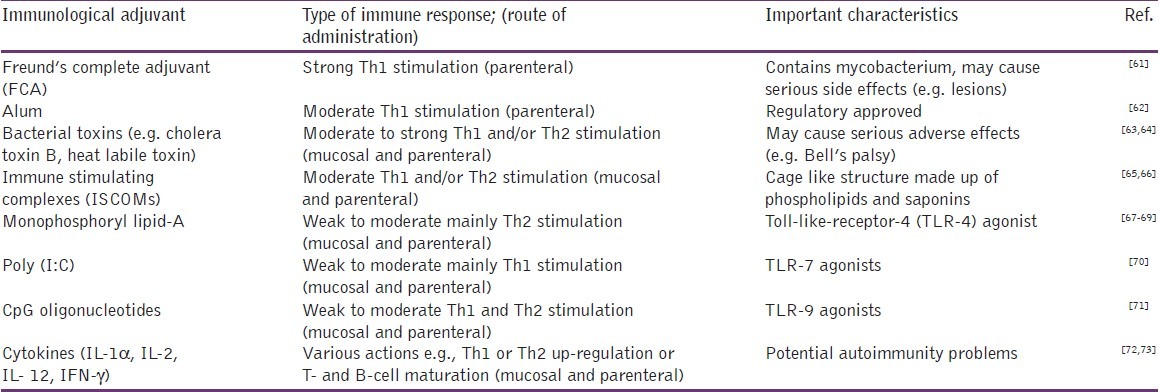
The different properties of the various adjuvants indicate the need for individual formulation strategies for particular vaccines based on (a) intended use, (b) type of immune responses required, (c) route of administration and (d) target animal population. An example highlighting the importance of formulation for an animal reproductive vaccine is the commercial failure of Vaxstrate® vaccine, which comprised a LHRH-ovalbumin conjugate, formulated with an oil emulsion adjuvant system.[74] It was used in Australia in 1990s but withdrawn a few years later due to poor sales as a result of its frequent side effects and poor efficacy in the field.[1] Many of the conventional immunological adjuvants such as Freund's complete adjuvant (FCA), bacterial toxins and non-purified crude agents (e.g. lipid A) usually induce strong stimulant effects but also frequently induce adverse effects upon administration to animals, and any vaccine incorporating these adjuvants is unlikely to be approved by regulatory authorities.[61,75] Notwithstanding these effects, the recently registered and highly efficacious single-shot GnRH vaccine (GonaCon, USDA) uses an adjuvant comprising a Mycobacterium species, M.avium, and mineral oil (AdjuVac),[10] and it induces very limited side effects. However, newer alternative reagents, such as purified and/or receptor-specific adjuvants (e.g. monophosphoryl lipid A, ISCOMs, CpG oligonucleotides), which show moderate immune-stimulating effects, are being investigated. Many of these newer adjuvants are toll-like receptor (TLR) agonists (e.g. CpG oligonucleotides, monophosphoryl lipid A and poly (I:C)) and act by stimulating particular types of TLRs present on antigen presenting cells (APCs) such as dendritic cells situated in different lymphatic tissues.[76] Many of these newer adjuvants could be used for either mucosal or for parenteral routes of administration and are, therefore, an important consideration in formulation of an animal vaccine. Some of these adjuvants have been used successfully in commercial animal vaccines. One example is the Improvac® vaccine, a diethylaminoethyl dextran adjuvanted LHRH-protein conjugate formulation, which is used for the control of boar taint in pigs [Table 1].[15,77]
Antigen delivery/carrier systems
Various carrier systems have been investigated for antigen delivery, including liquid systems (e.g., emulsions), particulate delivery systems (e.g., liposomes, microparticles, nanoparticles, archaeosomes) and viral or bacterial vectors. Particulate delivery systems offer potential advantages such as co-delivery of antigen(s) and adjuvant(s), depot of antigen at the site of injection, presentation of an ordered and repetitive array of B-cell epitopes, stimulation of cell-mediated immune response against acellular antigens, and increased uptake of acellular antigens after mucosal administration.[5,78–80] Some delivery systems such as virosomes, chitosan nanoparticles and archaeosomes also exhibit an in-built immunostimulant property, which could eliminate or reduce the requirement for any additional adjuvant in the vaccine formulation.[81–86]
The use of a live, non-pathogenic virus or bacterium as a vector/carrier (e.g., Canarypox virus, Vaccinia virus, Fowlpox virus and Lactobacilli bacteria)[87–89] offers similar advantages to those for live vaccines, including the possibility of mucosal administration and widespread distribution to animals. However, as with live vaccines, there are regulatory and safety issues associated with replicating vectors, which are genetically modified to express foreign antigens, and prior immunity and/or induction of immunity against the vector itself may reduce efficacy. Despite such disadvantages, vector systems have been successfully used in several commercial animal vaccine products (e.g. Purevax feline rabies vaccine and RecombitekEquine West Nile Virus vaccine).[1,90] This approach was extensively investigated for the species-specific delivery of reproductive control for introduced pest species (wild house mice, European rabbits and European red foxes) in the Australian environment and was termed viral-vectored immunocontraception, VVIC.[91–94] As an example, for the wild house mouse, murine cytomegalovirus was engineered to express mouse zona pellucida 3, and it induced 100% permanent infertility within 3 weeks of an infection. However, poor transmission of the recombinant virus limited its efficacy as a disseminating product for mice. Despite concerted efforts over 10-15 years for mice, rabbits and foxes, technical difficulties precluded the full development of species-specific VVIC vaccines for field release.[95] Nevertheless, the approach still offers an excellent promise as a disseminating or non-disseminating delivery system for immunocontraceptive antigens to pest mammals.
Bacterial ghost systems have been investigated not only as vaccine candidates against their own envelope structures, but also as carrier and adjuvant vehicles for foreign acellular target antigens.[96] Bacterial ghosts are produced by protein E-mediated lysis of Gram negative bacteria (e.g. Escherichia coli, Vibrio cholerae, enterotoxigenic and enterohemorrhagic strains).[97] and show in-built adjuvant properties on both parenteral and mucosal (e.g. inhalational) administration.[98,99] Bacterial ghosts have been assessed for their ability to deliver the zona pellucida proteins of the brush-tailed possum, a pest in New Zealand, and showed some promise.[100]
Some of the newer particulate delivery systems, such as virosomes and virus-like particles (VLPs), have been developed to retain the advantages of live viral systems but not the constraints posed by a replicating microorganism. These systems consist of one or more non-replicating viral components, such as membrane proteins for strong stimulation of immune responses against the incorporated antigen, but circumvent replication and side effects induced by other components of viruses.[2] VLPs are composed of one or several recombinantly-expressed viral proteins, which spontaneously assemble into particulate structures, resembling infectious viruses or, in some cases, sub-viral particles. VLPs have been produced for many different viruses that infect animals, such as Bluetongue virus, Nodavirus and Feline calicivirus.[101–103] They can also be used as a platform for inducing immune responses against selected antigens by incorporating antigenic epitopes by genetic fusion (chimeric VLPs) or by conjugating antigens to VLPs.[104,105]
Virosomes are unilamellar liposomes, carrying viral envelope proteins and can be used to incorporate different types of subunit acellular antigens for delivery by mucosal or parenteral routes.[82] Virosomes have been investigated for vaccination against retroviruses, Sendai virus and Newcastle disease.[106–108] Moreover, discoveries of TLRs on antigen presenting cells (APCs) and their specific agonists have endorsed the development of cell and/or receptor specific particulate delivery systems for vaccines.[109,110] Recently, an intensive emphasis has been given to targeting dendritic cells because of their role in detecting antigens in peripheral tissues, including vaccines at an injection site and then migrating to the T cell areas of lymphoid organs to initiate immunity.[111] These developments in targeted and novel delivery systems have opened new avenues for the development of effective but safe vaccines.
Delivery of animal vaccines
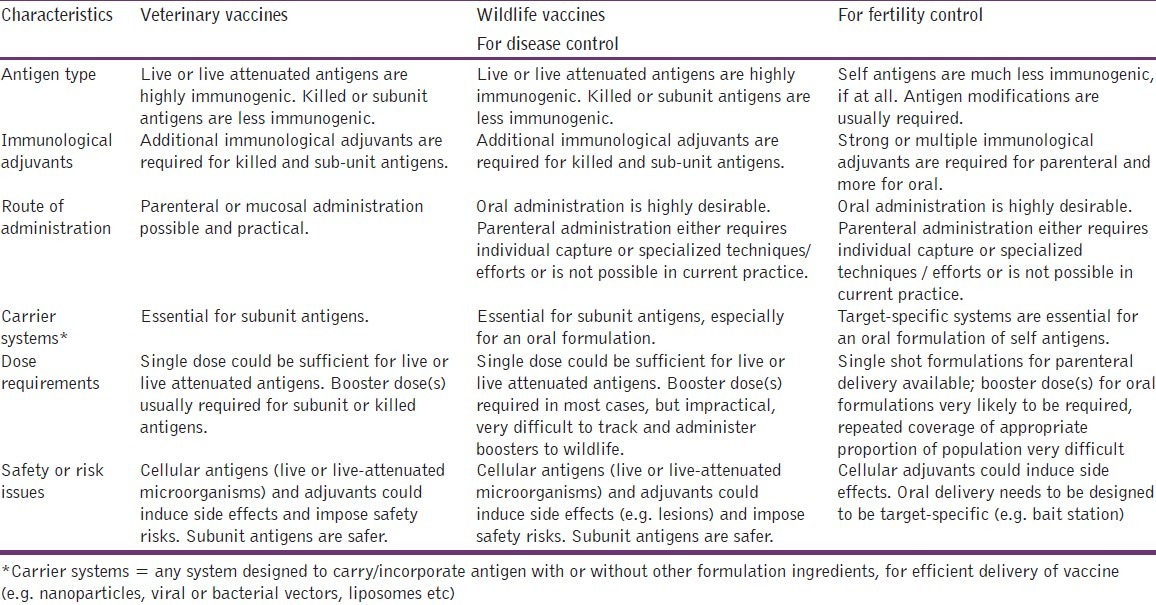
A successful vaccine requires not only careful consideration of its formulation but also an effective mechanism for its delivery to different animals, living in various environments. Delivery devices and delivery systems will vary depending on the target species (domestic, introduced pest or native wildlife), environment and scale of immunization. Table 3 lists desirable formulation and delivery characteristics for veterinary and wildlife vaccines.
Table 3.
Veterinary and wildlife vaccines: Desirable formulation and delivery characteristics
Various specialized parenteral delivery devices for intradermal gene gun delivery, short-distance dart delivery and long-distance ballistic vaccination have been developed and investigated for use in animal vaccination.[92,112–115] Specialized delivery systems such as biodegradable “Biobullets”, made of photopolymerized PEG hydrogels, have also been investigated to make ballistic delivery efficient and feasible.[116] Ballistic delivery devices are usually air-powered rifles, which shoot the vaccine formulation into the muscle of large animal species.[5] In addition, nasal and transdermal routes of vaccination have been used for domestic animal vaccinations; for example Purevax® recombinant canarypox vectored feline leukemia vaccine has been commercialized for transdermal administration, using VET JET™ microneedle technology.[117,118] These delivery systems have provided some success in vaccination of domesticated livestock and pets. For broad-scale, mass vaccination of wildlife, however, it has been accepted that parenteral vaccination generally would not be practical due to high resource requirements; thus if efficacious, oral delivery could be developed; it could be the most suitable and efficient route of vaccination.
Oral animal vaccination and research developments
The oral route of vaccine administration offers various advantages in that animals do not need to be captured for treatment, and distribution and delivery devices (e.g. bait stations) can be designed to provide target specificity. An ideal oral animal vaccine, whether for reproductive or for disease management, must be safe, species-specific, able to induce strong immune responses after one administration and must be formulated into a stable delivery system as a food bait attractive to the target animal species. The advantages of oral vaccination for wildlife have been exploited with some of the live or live virus vectored vaccines, for example, eradication of rabies in many countries using live or live-attenuated oral vaccines delivered in food baits.[119–121] However, this strategy shares the limitations of live or live-attenuated vaccines. On the other hand, poor stability of acellular antigens under environmental and gastrointestinal conditions poses serious formulation challenges in the oral delivery of acellular antigens. For successful oral delivery to wildlife, an acellular antigen should be formulated such that antigens and adjuvants are (a) protected from direct sunlight, high temperature, gastric and intestinal environments; (b) targeted to Peyer's patches in the intestinal tract; (c) effectively presented to antigen presenting cells and an immune response generated is sustained and effective.
VLPs and recombinant live dietary bacteria (e.g. Lactobacillus plantarum and Lactobacillus lactis) have been investigated as oral carriers for acellular antigens. As a vaccine-delivery vehicle, Lactobacilli effectively induces immune responses against acellular antigens by the oral route.[122] Another interesting approach has been the development of membranous or microfold cell (M-cell) targeted delivery systems, using M-cell surface targeting ligands (e.g. lectins, a monoclonal antibody targeting sialyl Lewis A).[123] Recently, many lipid and polymer-based oral food bait systems have been investigated and patented for the delivery of vaccines to wildlife.[124] However, further research is essential to achieve practical application.
The concept of using whole plant or plant fruits or seeds (e.g. rice, tomato) as an oral edible vaccine system has been suggested as a possible solution to various problems associated with vaccine delivery.[125–127] Proteins produced in transgenic plants are capable of invoking immune responses against many pathogens.[128–134] In theory, the use of plants for vaccine delivery to wildlife could overcome some of the challenges in oral delivery. However, the delivery of reproductive self-antigens expressed by transgenic plants is likely to pose a greater challenge for the induction of an efficacious immune response, particularly as their consumption may induce tolerance rather than immunity. Furthermore, apart from the likelihood that digestion and degradation of edible plant vaccines will occur in the gastrointestinal tract, safety and regulatory requirements for transgenic plants, probable contamination of human food supplies and people's attitudes to genetically modified products makes it highly unlikely that any commercial animal vaccine would be available in a transgenic plant form in the near future.
Conclusion
Research developments in biotechnology and molecular biology have provided many breakthroughs in the identification of effective and safe antigens and adjuvants, which could be used for vaccination of animals to prevent spread of diseases or for other applications such as immunocontraception. In spite of these successes, commercial and field applications of animal vaccines are limited due to formulation and delivery constraints. Recent developments in the formulation and delivery of acellular antigens along with investigations of newer immunological adjuvants should enhance progress. However, it will be important to reduce production costs of the newer adjuvants, delivery systems and delivery devices to ensure commercial success. Oral delivery of vaccines to animals, particularly to wildlife and large groups of farm animals, is challenging and consolidated efforts by veterinary, wildlife, biotechnology and pharmaceutical researchers will be required for the foreseeable future.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
References
- 1.Meeusen EN, Walker J, Peters A, Pastoret PP, Jungersen G. Current status of veterinary vaccines. Clin Microbiol Rev. 2007;20:489–510. doi: 10.1128/CMR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brun A, Bárcena J, Blanco E, Borrego B, Dory D, Escribano JM, et al. Current strategies for subunit and genetic viral veterinary vaccine development. Virus Res. 2011;157:1–12. doi: 10.1016/j.virusres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Shams H. Recent developments in veterinary vaccinology. Vet J. 2005;170:289–99. doi: 10.1016/j.tvjl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Shryock TR. The future of anti-infective products in animal health. Nat Rev Microbiol. 2004;2:425–30. doi: 10.1038/nrmicro887. [DOI] [PubMed] [Google Scholar]
- 5.Scheerlinck JP, Greenwood DL. Particulate delivery systems for animal vaccines. Methods. 2006;40:118–24. doi: 10.1016/j.ymeth.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Brochier B, Kieny MP, Costy F, Coppens P, Bauduin B, Lecocq JP, et al. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature. 1991;354:520–2. doi: 10.1038/354520a0. [DOI] [PubMed] [Google Scholar]
- 7.Cross ML, Buddle BM, Aldwell FE. The potential of oral vaccines for disease control in wildlife species. Vet J. 2007;174:472–80. doi: 10.1016/j.tvjl.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Slate D, Rupprecht CE, Rooney JA, Donovan D, Lein DH, Chipman RB. Status of oral rabies vaccination in wild carnivores in the United States. Virus Res. 2005;111:68–76. doi: 10.1016/j.virusres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Hardy CM, Hinds LA, Kerr PJ, Lloyd ML, Redwood AJ, Shellam GR, et al. Biological control of vertebrate pests using virally vectored immunocontraception. J Reprod Immunol. 2006;71:102–11. doi: 10.1016/j.jri.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Fagerstone KA, Miller LA, Eisemann JD, O’Hare JR, Gionfriddo JP. Registration of wildlife contraceptives in the United States of America, with OvoControl and GonaCon immunocontraceptive vaccines as examples. Wildlife Res. 2008;35:586–92. [Google Scholar]
- 11.Fraker MA, Brown RG, Gaunt GE, Kerr JA, Pohajdak B. Long-lasting, single-dose immunocontraception of feral fallow deer in British Columbia. J Wildlife Manage. 2002;66:1141–7. [Google Scholar]
- 12.Kirkpatrick JF, Lyda RO, Frank KM. Contraceptive vaccines for wildlife: A review. Am J Reprod Immunol. 2011;66:40–50. doi: 10.1111/j.1600-0897.2011.01003.x. [DOI] [PubMed] [Google Scholar]
- 13.Jackwood MW, Saif YM. Efficacy of a commercial turkey coryza vaccine (Art-Vax) in turkey poults. Avian Dis. 1985;29:1130–9. [PubMed] [Google Scholar]
- 14.Moormann RJ, Bouma A, Kramps JA, Terpstra C, De Smit HJ. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet Microbiol. 2000;73:209–19. doi: 10.1016/s0378-1135(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 15.Dunshea FR, Colantoni C, Howard K, McCauley I, Jackson P, Long KA, et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J Anim Sci. 2001;79:2524–35. doi: 10.2527/2001.79102524x. [DOI] [PubMed] [Google Scholar]
- 16.Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J Virol. 2004;78:6297–303. doi: 10.1128/JVI.78.12.6297-6303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veits J, Wiesner D, Fuchs W, Hoffmann B, Granzow H, Starick E, et al. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc Natl Acad Sci U S A. 2006;103:8197–202. doi: 10.1073/pnas.0602461103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park MS, Steel J, García-Sastre A, Swayne D, Palese P. Engineered viral vaccine constructs with dual specificity: Avian influenza and Newcastle disease. Proc Natl Acad Sci U S A. 2006;103:8203–8. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller LA, Gionfriddo JP, Fagerstone KA, Rhyan JC, Killian GJ. The single-shot GnRH immunocontraceptive vaccine (GonaCon) in white-tailed deer: Comparison of several GnRH preparations. Am J Reprod Immunol. 2008;60:214–23. doi: 10.1111/j.1600-0897.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 20.Miller LA, Rhyan JC, Drew M. Contraception of bison by GnRH vaccine: A possible means of decreasing transmission of brucellosis in bison. J Wildl Dis. 2004;40:725–30. doi: 10.7589/0090-3558-40.4.725. [DOI] [PubMed] [Google Scholar]
- 21.Alexandersen S. Advantages and disadvantages of using live vaccines risks and control measures. Acta Vet Scand Suppl. 1996;90:89–100. [PubMed] [Google Scholar]
- 22.Baron MD, Banyard AC, Parida S, Barrett T. The Plowright vaccine strain of Rinderpest virus has attenuating mutations in most genes. J Gen Virol. 2005;86:1093–101. doi: 10.1099/vir.0.80751-0. [DOI] [PubMed] [Google Scholar]
- 23.Robertshaw D. Credit to Plowright for rinderpest eradication. Science. 2010;330:1477. doi: 10.1126/science.330.6010.1477-a. [DOI] [PubMed] [Google Scholar]
- 24.Plowright W. The duration of immunity in cattle following inoculation of rinderpest cell culture vaccine. J Hyg (Lond) 1984;92:285–96. doi: 10.1017/s0022172400064524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtaugh MP, Elam MR, Kakach LT. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995;140:1451–60. doi: 10.1007/BF01322671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortensen S, Stryhn H, Søgaard R, Boklund A, Stärk KD, Christensen J, et al. Risk factors for infection of sow herds with porcine reproductive and respiratory syndrome (PRRS) virus. Prev Vet Med. 2002;53:83–101. doi: 10.1016/s0167-5877(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 27.Pluimers FH. Foot-and-Mouth disease control using vaccination: The Dutch experience in 2001. Dev Biol (Basel) 2004;119:41–9. [PubMed] [Google Scholar]
- 28.Roth JA, Henderson LM. New technology for improved vaccine safety and efficacy. Vet Clin North Am Food Anim Pract. 2001;17:585–97. doi: 10.1016/s0749-0720(15)30008-6. vii. [DOI] [PubMed] [Google Scholar]
- 29.Redding L, Weiner DB. DNA vaccines in veterinary use. Expert Rev Vaccines. 2009;8:1251–76. doi: 10.1586/erv.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh M, O’Hagan DT. Recent advances in veterinary vaccine adjuvants. Int J Parasitol. 2003;33:469–78. doi: 10.1016/s0020-7519(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 31.Spickler AR, Roth JA. Adjuvants in veterinary vaccines: Modes of action and adverse effects. J Vet Intern Med. 2003;17:273–81. doi: 10.1111/j.1939-1676.2003.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 32.Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of protein pharmaceuticals: An update. Pharm Res. 2010;27:544–75. doi: 10.1007/s11095-009-0045-6. [DOI] [PubMed] [Google Scholar]
- 33.Manning MC, Patel K, Borchardt RT. Stability of protein pharmaceuticals. Pharm Res. 1989;6:903–18. doi: 10.1023/a:1015929109894. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S, Kulkarni J, Pawar AP. Permeation enhancers in the transmucosal delivery of macromolecules. Pharmazie. 2006;61:495–504. [PubMed] [Google Scholar]
- 35.Soares AF, Carvalho Rde A, Veiga F. Oral administration of peptides and proteins: Nanoparticles and cyclodextrins as biocompatible delivery systems. Nanomedicine (Lond) 2007;2:183–202. doi: 10.2217/17435889.2.2.183. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Aal AB, Batzloff MR, Fujita Y, Barozzi N, Faria A, Simerska P, et al. Structure-activity relationship of a series of synthetic lipopeptide self-adjuvanting group A streptococcal vaccine candidates. J Med Chem. 2008;51:167–72. doi: 10.1021/jm701091d. [DOI] [PubMed] [Google Scholar]
- 37.Horváth A, Olive C, Karpati L, Sun HK, Good MF, Toth I. Toward the development of a synthetic group a streptococcal vaccine of high purity and broad protective coverage. J Med Chem. 2004;47:4100–4. doi: 10.1021/jm040041w. [DOI] [PubMed] [Google Scholar]
- 38.Cowan DP, Hinds LA. Fertility control for wildlife preface. Wildlife Res. 2008;35:iii–iv. [Google Scholar]
- 39.Holland MK, Beagley K, Hardy C, Hinds L, Jones RC. Immunocontraceptive vaccines for the control of wild animal populations: Antigen selection and delivery. In: Gagnon C, editor. Male Gamete: From Basic Science to Clinical Applications. 1st ed. St Louis: Cache River Press; 1999. pp. 493–500. [Google Scholar]
- 40.McLaughlin EA, Aitken RJ. Is there a role for immunocontraception? Mol Cell Endocrinol. 2011;335:78–88. doi: 10.1016/j.mce.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 41.McLeod SR, Saunders G, Twigg LE, Arthur AD, Ramsey D, Hinds LA. Prospects for the future: Is there a role for virally vectored immunocontraception in vertebrate pest management? Wildlife Res. 2007;34:555–66. [Google Scholar]
- 42.Hardy CM, ten Have JF, Mobbs KJ, Hinds LA. Assessment of the immunocontraceptive effect of a zona pellucida 3 peptide antigen in wild mice. Reprod Fertil Dev. 2002;14:151–5. doi: 10.1071/rd01112. [DOI] [PubMed] [Google Scholar]
- 43.Fromme B, Eftekhari P, van Regenmortel M, Hoebeke J, Katz A, Millar R. A novel retro-inverso gonadotropin-releasing hormone (GnRH) immunogen elicits antibodies that neutralize the activity of native GnRH. Endocrinology. 2003;144:3262–9. doi: 10.1210/en.2002-221135. [DOI] [PubMed] [Google Scholar]
- 44.Dalin AM, Andresen O, Malmgren L. Immunization against GnRH in mature mares: Antibody titres, ovarian function, hormonal levels and oestrous behaviour. J Vet Med A. 2002;49:125–31. doi: 10.1046/j.1439-0442.2002.00427.x. [DOI] [PubMed] [Google Scholar]
- 45.Janett F, Caspari K, Thun R. Influence of immunization against GnRH on semen quality and testicular function in the adult boar. Reprod Domest Anim. 2010;45:90. [Google Scholar]
- 46.Burger D, Janett F, Vidament M, Stump R, Fortier G, Imboden I, et al. Immunization against GnRH in adult stallions: Effects on semen characteristics, behaviour and shedding of equine arteritis virus. Anim Reprod Sci. 2006;94:107–11. [Google Scholar]
- 47.Jaros P, Bürgi E, Stärk KD, Claus R, Hennessy D, Thun R. Effect of active immunization against GnRH on androstenone concentration, growth performance and carcass quality in intact male pigs. Livest Prod Sci. 2005;92:31–8. [Google Scholar]
- 48.Cook RB, Popp JD, McAllister TA, Kastelic JP, Harland R. Effects of immunization against GnRH, melengestrol acetate, and a trenbolene acetate/estradiol implant on growth and carcass characteristics of beef heifers. Theriogenology. 2001;55:973–81. doi: 10.1016/s0093-691x(01)00458-7. [DOI] [PubMed] [Google Scholar]
- 49.Thompson DL. Immunization against GnRH in male species (comparative aspects) Anim Reprod Sci. 2000;60:459–69. doi: 10.1016/s0378-4320(00)00116-0. [DOI] [PubMed] [Google Scholar]
- 50.Turzillo AM, Nett TM. Effects of bovine follicular fluid and passive immunization against gonadotropin-releasing hormone (GnRH) on messenger ribonucleic acid for GnRH receptor and gonadotropin subunits in ovariectomized ewes. Biol Reprod. 1997;56:1537–43. doi: 10.1095/biolreprod56.6.1537. [DOI] [PubMed] [Google Scholar]
- 51.Becker SE, Enright WJ, Katz LS. Active Immunization against a Gnrh-ovalbumin conjugate in female white-tailed deer. Zoo Biol. 1999;18:385–96. [Google Scholar]
- 52.Finnerty M, Enright WJ, Prendiville DJ, Roche JF. Immunization of bulls against gonadotropin-releasing-hormone (Gnrh) - growth, testes size, behavior and blood testosterone concentrations. Irish J Agr Res. 1991;30:80–1. [Google Scholar]
- 53.Li H, Piao YS, Zhang ZB, Hardy CM, Hinds LA. Molecular cloning and assessment of the immunocontraceptive potential of the zona pellucida subunit 3 from Brandt's vole (Microtus brandti) Reprod Fertil Dev. 2006;18:331–8. doi: 10.1071/rd05049. [DOI] [PubMed] [Google Scholar]
- 54.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–8. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 55.Fayrer-Hosken R. Controlling animal populations using anti-fertility vaccines. Reprod Domest Anim. 2008;43:179–85. doi: 10.1111/j.1439-0531.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 56.Desmettre P. Veterinary vaccines in the development of vaccination and vaccinology. In: Plotkin SA, editor. History of Vaccine Development. 1st ed. New York: Springer; 2011. pp. 57–65. [Google Scholar]
- 57.Ramon G, Zoeller C. The ‘associated vaccines’ by unions of a toxoid and a microbial vaccine (TAB) or by toxoid mixtures. C R Seances Soc Biol Fil. 1926;94:106–9. [Google Scholar]
- 58.Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes XVII.-XXIV. J Pathol Bacteriol. 1926;29:31–40. [Google Scholar]
- 59.Freund J, Casals J, Hosmer EP. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Exp Biol Med. 1937;37:509–13. [Google Scholar]
- 60.Opie EL, Freund J. An experimental study of protective inoculation with heat killed tubercle bacilli. J Exp Med. 1937;66:761–88. doi: 10.1084/jem.66.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Claassen E, de Leeuw W, de Greeve P, Hendriksen C, Boersma W. Freund's complete adjuvant: An effective but disagreeable formula. Res Immunol. 1992;143:478–83. doi: 10.1016/0923-2494(92)80057-r. discussion 572. [DOI] [PubMed] [Google Scholar]
- 62.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21:23–9. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Wu HY, Russell MW. Comparison of systemic and mucosal priming for mucosal immune responses to a bacterial protein antigen given with or coupled to cholera toxin (CT) B subunit, and effects of pre-existing anti-CT immunity. Vaccine. 1994;12:215–22. doi: 10.1016/0264-410x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 64.Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell's palsy. N Engl J Med. 2004;350:860–1. doi: 10.1056/NEJMp048006. [DOI] [PubMed] [Google Scholar]
- 65.Duewell P, Kisser U, Heckelsmiller K, Hoves S, Stoitzner P, Koernig S, et al. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J Immunol. 2011;187:55–63. doi: 10.4049/jimmunol.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun HX, Xie Y, Ye YP. ISCOMs and ISCOMATRIX. Vaccine. 2009;27:4388–401. doi: 10.1016/j.vaccine.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 67.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–9. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 68.Romero CD, Varma TK, Hobbs JB, Reyes A, Driver B, Sherwood ER. The toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect Immun. 2011;79:3576–87. doi: 10.1128/IAI.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casella CR, Mitchell TC. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–40. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rajagopal D, Paturel C, Morel Y, Uematsu S, Akira S, Diebold SS. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of toll-like receptor 7 agonists. Blood. 2010;115:1949–57. doi: 10.1182/blood-2009-08-238543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta K, Cooper C. A review of the role of CpG oligodeoxynucleotides as toll-like receptor 9 agonists in prophylactic and therapeutic vaccine development in infectious diseases. Drugs R D. 2008;9:137–45. doi: 10.2165/00126839-200809030-00001. [DOI] [PubMed] [Google Scholar]
- 72.Nash AD, Lofthouse SA, Barcham GJ, Jacobs HJ, Ashman K, Meeusen EN, et al. Recombinant cytokines as immunological adjuvants. Immunol Cell Biol. 1993;71:367–79. doi: 10.1038/icb.1993.43. [DOI] [PubMed] [Google Scholar]
- 73.Heath AW, Playfair JH. Cytokines as immunological adjuvants. Vaccine. 1992;10:427–34. doi: 10.1016/0264-410x(92)90389-2. [DOI] [PubMed] [Google Scholar]
- 74.Hoskinson RM, Rigby RD, Mattner PE, Huynh VL, D’Occhio M, Neish A, et al. Vaxstrate: An anti-reproductive vaccine for cattle. Aust J Biotechnol. 1990;4:166–70. [PubMed] [Google Scholar]
- 75.Shah NM, Mangat GK, Balakrishnan C, Buch VI, Joshi VR. Accidental self-injection with Freund's complete adjuvant. J Assoc Physicians India. 2001;49:366–8. [PubMed] [Google Scholar]
- 76.Gnjatic S, Sawhney NB, Bhardwaj N. Toll-like receptor agonists are they good adjuvants? Cancer J. 2010;16:382–91. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fàbrega E, Velarde A, Cros J, Gispert M, Suárez P, Tibau J, et al. Effect of vaccination against gonadotrophin-releasing hormone, using Improvac (R), on growth performance, body composition, behaviour and acute phase proteins. Livest Sci. 2010;132:53–9. [Google Scholar]
- 78.Sharma S, Mukkur TK, Benson HA, Chen Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J Pharm Sci. 2009;98:812–43. doi: 10.1002/jps.21493. [DOI] [PubMed] [Google Scholar]
- 79.Bachmann MF, Rohrer UH, Kündig TM, Bürki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–51. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 80.Neutra MR, Pringault E, Kraehenbuhl JP. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 81.Sharma S, Mukkur TK, Benson HA, Chen Y. Enhanced immune response against pertussis toxoid by IgA-loaded chitosan-dextran sulfate nanoparticles. J Pharm Sci. 2012;101:233–44. doi: 10.1002/jps.22763. [DOI] [PubMed] [Google Scholar]
- 82.Bungener L, Huckriede A, de Mare A, de Vries-Idema J, Wilschut J, Daemen T. Virosome-mediated delivery of protein antigens in vivo: Efficient induction of class I MHC-restricted cytotoxic T lymphocyte activity. Vaccine. 2005;23:1232–41. doi: 10.1016/j.vaccine.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Lambkin R, Oxford JS, Bossuyt S, Mann A, Metcalfe IC, Herzog C, et al. Strong local and systemic protective immunity induced in the ferret model by an intranasal virosome-formulated influenza subunit vaccine. Vaccine. 2004;22:4390–6. doi: 10.1016/j.vaccine.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 84.Kapczynski DR. Development of a virosome vaccine against avian metapneumovirus subtype C for protection in turkeys. Avian Dis. 2004;48:332–43. doi: 10.1637/7115. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez RO, Higa LH, Cutrullis RA, Bilen M, Morelli I, Roncaglia DI, et al. Archaeosomes made of halorubrum tebenquichense total polar lipids: A new source of adjuvancy. BMC Biotechnol. 2009;9:71. doi: 10.1186/1472-6750-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krishnan L, Dennis Sprott G. Archaeosomes as self-adjuvanting delivery systems for cancer vaccines. J Drug Target. 2003;11:515–24. doi: 10.1080/10611860410001670044. [DOI] [PubMed] [Google Scholar]
- 87.Publicover J, Ramsburg E, Rose JK. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J Virol. 2004;78:9317–24. doi: 10.1128/JVI.78.17.9317-9324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Medina E, Guzman CA. Use of live bacterial vaccine vectors for antigen delivery: Potential and limitations. Vaccine. 2001;19:1573–80. doi: 10.1016/s0264-410x(00)00354-6. [DOI] [PubMed] [Google Scholar]
- 89.Mekalanos JJ. Live attenuated vaccine vectors. Int J Technol Assess Health Care. 1994;10:131–42. doi: 10.1017/s0266462300014057. [DOI] [PubMed] [Google Scholar]
- 90.Minke JM, Audonnet JC, Fischer L. Equine viral vaccines: The past, present and future. Vet Res. 2004;35:425–43. doi: 10.1051/vetres:2004019. [DOI] [PubMed] [Google Scholar]
- 91.Redwood AJ, Harvey NL, Lloyd M, Lawson MA, Hardy CM, Shellam GR. Viral vectored immunocontraception: Screening of multiple fertility antigens using murine cytomegalovirus as a vaccine vector. Vaccine. 2007;25:698–708. doi: 10.1016/j.vaccine.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 92.Tyndale-Biscoe CH. Virus-vectored immunocontraception of feral mammals. Reprod Fertil Dev. 1994;6:281–7. doi: 10.1071/rd9940281. [DOI] [PubMed] [Google Scholar]
- 93.van Leeuwen BH, Kerr PJ. Prospects for fertility control in the European rabbit (Oryctolagus cuniculus) using myxoma virus-vectored immunocontraception. Wildlife Res. 2007;34:511–22. [Google Scholar]
- 94.Strive T, Hardy CM, Reubel GH. Prospects for immunocontraception in the European red fox (Vulpes vulpes) Wildlife Res. 2007;34:523–9. [Google Scholar]
- 95.Tyndale-Biscoe H, Hinds LA. Introduction – virally vectored immunocontraception in Australia. Wildlife Res. 2007;34:507–10. [Google Scholar]
- 96.Jalava K, Hensel A, Szostak M, Resch S, Lubitz W. Bacterial ghosts as vaccine candidates for veterinary applications. J Control Release. 2002;85:17–25. doi: 10.1016/s0168-3659(02)00267-5. [DOI] [PubMed] [Google Scholar]
- 97.Mayr UB, Walcher P, Azimpour C, Riedmann E, Haller C, Lubitz W. Bacterial ghosts as antigen delivery vehicles. Adv Drug Deliv Rev. 2005;57:1381–91. doi: 10.1016/j.addr.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 98.Katinger A, Lubitz W, Szostak MP, Stadler M, Klein R, Indra A, et al. Pigs aerogenously immunized with genetically inactivated (ghosts) or irradiated Actinobacillus pleuropneumoniae are protected against a homologous aerosol challenge despite differing in pulmonary cellular and antibody responses. J Biotechnol. 1999;73:251–60. doi: 10.1016/s0168-1656(99)00143-1. [DOI] [PubMed] [Google Scholar]
- 99.Marchart J, Rehagen M, Dropmann G, Szostak MP, Alldinger S, Lechleitner S, et al. Protective immunity against pasteurellosis in cattle, induced by Pasteurella haemolytica ghosts. Vaccine. 2003;21:1415–22. doi: 10.1016/s0264-410x(02)00635-7. [DOI] [PubMed] [Google Scholar]
- 100.Walcher P, Cui X, Arrow JA, Scobie S, Molinia FC, Cowan PE, et al. Bacterial ghosts as a delivery system for zona pellucida-2 fertility control vaccines for brushtail possums (Trichosurus vulpecula) Vaccine. 2008;26:6832–8. doi: 10.1016/j.vaccine.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 101.Di Martino B, Marsilio F, Roy P. Assembly of feline calicivirus-like particle and its immunogenicity. Vet Microbiol. 2007;120:173–8. doi: 10.1016/j.vetmic.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Stewart M, Bhatia Y, Athmaran TN, Noad R, Gastaldi C, Dubois E, et al. Validation of a novel approach for the rapid production of immunogenic virus-like particles for bluetongue virus. Vaccine. 2010;28:3047–54. doi: 10.1016/j.vaccine.2009.10.072. [DOI] [PubMed] [Google Scholar]
- 103.Thiéry R, Cozien J, Cabon J, Lamour F, Baud M, Schneemann A. Induction of a protective immune response against viral nervous necrosis in the European sea bass dicentrarchus labrax by using betanodavirus virus-like particles. J Virol. 2006;80:10201–7. doi: 10.1128/JVI.01098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raja KS, Wang Q, Gonzalez MJ, Manchester M, Johnson JE, Finn MG. Hybrid virus-polymer materials. 1. Synthesis and properties of PEGdecorated cowpea mosaic virus. Biomacromolecules. 2003;4:472–6. doi: 10.1021/bm025740+. [DOI] [PubMed] [Google Scholar]
- 105.Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kündig T, et al. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine. 2002;20:3104–12. doi: 10.1016/s0264-410x(02)00266-9. [DOI] [PubMed] [Google Scholar]
- 106.Kapczynski DR, Tumpey TM. Development of a virosome vaccine for newcastle disease virus. Avian Dis. 2003;47:578–87. doi: 10.1637/6082. [DOI] [PubMed] [Google Scholar]
- 107.Bagai S, Sarkar DP. Targeted delivery of hygromycin B using reconstituted sendai viral envelopes lacking hemagglutinin-neuraminidase. FEBS Lett. 1993;326:183–8. doi: 10.1016/0014-5793(93)81787-z. [DOI] [PubMed] [Google Scholar]
- 108.Singh R, Verma PC, Singh S. Immunogenicity and protective efficacy of virosome based vaccines against newcastle disease. Trop Anim Health Prod. 2010;42:465–71. doi: 10.1007/s11250-009-9444-2. [DOI] [PubMed] [Google Scholar]
- 109.Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32:3094–105. doi: 10.1016/j.biomaterials.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grossmann C, Tenbusch M, Nchinda G, Temchura V, Nabi G, Stone GW, et al. Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic toll-like receptor ligands. BMC Immunol. 2009;10:43. doi: 10.1186/1471-2172-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steinman RM. Dendritic cells in vivo: A key target for a new vaccine science. Immunity. 2008;29:319–24. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 112.Watkins C, Hopkins J, Harkiss G. Reporter gene expression in dendritic cells after gene gun administration of plasmid DNA. Vaccine. 2005;23:4247–56. doi: 10.1016/j.vaccine.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 113.Torres CA, Iwasaki A, Barber BH, Robinson HL. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–32. [PubMed] [Google Scholar]
- 114.Olsen SC, Christie RJ, Grainger DW, Stoffregen WS. Immunologic responses of bison to vaccination with Brucella abortus strain RB51: Comparison of parenteral to ballistic delivery via compressed pellets or photopolymerized hydrogels. Vaccine. 2006;24:1346–53. doi: 10.1016/j.vaccine.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 115.Oliveira SC, Harms JS, Rosinha GM, Rodarte RS, Rech EL, Splitter GA. Biolistic-mediated gene transfer using the bovine herpesvirus-1 glycoprotein D is an effective delivery system to induce neutralizing antibodies in its natural host. J Immunol Methods. 2000;245:109–18. doi: 10.1016/s0022-1759(00)00267-2. [DOI] [PubMed] [Google Scholar]
- 116.Christie RJ, Findley DJ, Dunfee M, Hansen RD, Olsen SC, Grainger DW. Photopolymerized hydrogel carriers for live vaccine ballistic delivery. Vaccine. 2006;24:1462–9. doi: 10.1016/j.vaccine.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 117.Grosenbaugh DA, Leard T, Pardo MC, Motes-Kreimeyer L, Royston M. Comparison of the safety and efficacy of a recombinant feline leukemia virus (FeLV) vaccine delivered transdermally and an inactivated FeLV vaccine delivered subcutaneously. Vet Ther. 2004;5:258–62. [PubMed] [Google Scholar]
- 118.Grosenbaugh DA, Leard T, Pardo MC. Protection from challenge following administration of a canarypox virus-vectored recombinant feline leukemia virus vaccine in cats previously vaccinated with a killed virus vaccine. J Am Vet Med Assoc. 2006;228:726–7. doi: 10.2460/javma.228.5.726. [DOI] [PubMed] [Google Scholar]
- 119.Grimm R. The history of the eradication of rabies in most European countries. Hist Med Vet. 2002;27:295–301. [PubMed] [Google Scholar]
- 120.Brochier B, Pastoret PP. Rabies eradication in Belgium by fox vaccination using vaccinia-rabies recombinant virus. Onderstepoort J Vet Res. 1993;60:469–75. [PubMed] [Google Scholar]
- 121.Flamand A, Coulon P, Lafay F, Kappeler A, Artois M, Aubert M, et al. Eradication of rabies in Europe. Nature. 1992;360:115–6. doi: 10.1038/360115a0. [DOI] [PubMed] [Google Scholar]
- 122.Seegers JF. Lactobacilli as live vaccine delivery vectors: Progress and prospects. Trends Biotechnol. 2002;20:508–15. doi: 10.1016/s0167-7799(02)02075-9. [DOI] [PubMed] [Google Scholar]
- 123.Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010;6:e1001147. doi: 10.1371/journal.ppat.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ballesteros C, de la Lastra JM, de la Fuente J. Recent developments in oral bait vaccines for wildlife. Recent Pat Drug Deliv Formul. 2007;1:230–5. doi: 10.2174/187221107782331610. [DOI] [PubMed] [Google Scholar]
- 125.Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, et al. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci U S A. 2007;104:10986–91. doi: 10.1073/pnas.0703766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salyaev RK, Rekoslavskaya NI, Shchelkunov SN, Stolbikov AS, Hammond RV. Study of the mucosal immune response duration in mice after administration of a candidate edible vaccine based on transgenic tomato plants carrying the TBI-HBS gene. Dokl Biochem Biophys. 2009;428:232–4. doi: 10.1134/s1607672909050020. [DOI] [PubMed] [Google Scholar]
- 127.Loza-Rubio E, Rojas E, Gómez L, Olivera MT, Gómez-Lim MA. Development of an edible rabies vaccine in maize using the Vnukovo strain. Dev Biol (Basel) 2008;131:477–82. [PubMed] [Google Scholar]
- 128.Yuki Y, Tokuhara D, Nochi T, Yasuda H, Mejima M, Kurokawa S, et al. Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine. 2009;27:5982–8. doi: 10.1016/j.vaccine.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 129.Hernández M, Cabrera-Ponce JL, Fragoso G, López-Casillas F, Guevara-García A, Rosas G, et al. A new highly effective anticysticercosis vaccine expressed in transgenic papaya. Vaccine. 2007;25:4252–60. doi: 10.1016/j.vaccine.2007.02.080. [DOI] [PubMed] [Google Scholar]
- 130.Dorokhov YL, Sheveleva AA, Frolova OY, Komarova TV, Zvereva AS, Ivanov PA, et al. Superexpression of tuberculosis antigens in plant leaves. Tuberculosis (Edinb) 2007;87:218–24. doi: 10.1016/j.tube.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 131.Zhang X, Buehner NA, Hutson AM, Estes MK, Mason HS. Tomato is a highly effective vehicle for expression and oral immunization with norwalk virus capsid protein. Plant Biotechnol J. 2006;4:419–32. doi: 10.1111/j.1467-7652.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 132.Streatfield SJ. Oral hepatitis B vaccine candidates produced and delivered in plant material. Immunol Cell Biol. 2005;83:257–62. doi: 10.1111/j.1440-1711.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- 133.Lamphear BJ, Jilka JM, Kesl L, Welter M, Howard JA, Streatfield SJ. A corn-based delivery system for animal vaccines: An oral transmissible gastroenteritis virus vaccine boosts lactogenic immunity in swine. Vaccine. 2004;22:2420–4. doi: 10.1016/j.vaccine.2003.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tregoning J, Maliga P, Dougan G, Nixon PJ. New advances in the production of edible plant vaccines: Chloroplast expression of a tetanus vaccine antigen, TetC. Phytochemistry. 2004;65:989–94. doi: 10.1016/j.phytochem.2004.03.004. [DOI] [PubMed] [Google Scholar]


