Abstract
Nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are multidisciplinary liver diseases that often accompany type 2 diabetes or metabolic syndrome, which are characterized by insulin resistance. Therefore, effective treatment of type 2 diabetes and metabolic syndrome should target not only the cardiometabolic abnormalities, but also the associated liver disorders. In the last decade, it has been shown that metformin, thiazolidinediones, vitamin E, ezetimibe, n-3 polyunsaturated fatty acids, renin-angiotensin system (RAS) blockers, and antiobesity drugs may improve hepatic pathophysiological disorders as well as clinical parameters. Accordingly, insulin sensitizers, antioxidative agents, Niemann-Pick C1-like 1 (NPC1L1) inhibitors, RAS blockers, and drugs that target the central nervous system may represent candidate pharmacotherapies for NAFLD and possibly NASH. However, the efficacy, safety, and tolerability of long-term treatment (potentially for many years) with these drugs have not been fully established. Furthermore, clinical trials have not comprehensively examined the efficacy of lipid-lowering drugs (i.e., statins, fibrates, and NPC1L1 inhibitors) for the treatment of NAFLD. Although clinical evidence for RAS blockers and incretin-based agents (GLP-1 analogs and dipeptidyl peptidase-4 inhibitors) is also lacking, these agents are promising in terms of their insulin-sensitizing and anti-inflammatory effects without causing weight gain.
1. Introduction
Over the past two decades, the prevalence of metabolic abnormalities such as type 2 diabetes and metabolic syndrome (MetS) has been increasing worldwide together with the escalating obesity pandemic [1–3]. Abdominal obesity, in particular, substantially increases the risk of developing type 2 diabetes, MetS, and fatty liver. According to the American Association for the Study of Liver Diseases (AASLD), fatty liver in the absence of a chronic increase in alcohol intake (i.e., alcohol intake is <20 g ethanol/day) is referred to as nonalcoholic fatty liver disease (NAFLD) [4]. According to the AASLD's practice guidelines for NAFLD [5], NAFLD is histologically subdivided into nonalcoholic fatty liver (NAFL) and a more severe condition, nonalcoholic steatohepatitis (NASH), which sometimes advances over several decades to life-threatening hepatic cirrhosis and hepatocellular carcinoma. The prevalence of NAFLD, as detected by ultrasound, is up to 30–46% in developed countries and nearly 10% in developing nations, making NAFLD the most common liver disorder worldwide [5, 6].
Lifestyle interventions such as diet and moderate exercise, which lead to weight loss, are fundamental for the treatment of NAFLD. Paradoxically, NAFLD has also been reported in nonobese people [7–9]. In India, individuals with a normal BMI (18.5–24.9 kg/m2) have a 2-fold higher risk of developing NAFLD compared with those with a BMI of <18.5 kg/m2 [10]. Therefore, NAFLD is expected to become a major burden in Asian countries where the prevalence of obesity is less than that in Western countries [10, 11]. Notably, NAFLD appears to be an early predictor of metabolic disorders, particularly among normal-weight individuals [7]. This is because NAFLD may be more tightly associated with insulin resistance and with markers of oxidative stress and endothelial dysfunction than with the Adult Treatment Panel III criteria for MetS in nonobese, nondiabetic subjects [8]. Therefore, although obese people are predisposed to develop NAFLD, normal weight and overweight people may, through the development of insulin resistance, also show the pathogenic characteristics of NAFLD.
The clinical relevance of NAFLD is still poorly understood because some investigators [12–15], but not all [16, 17], have shown that NAFLD is associated with higher overall mortality and cardiovascular disease. Since NAFLD is closely associated with obesity, diabetes, and MetS, it is unknown whether the relationship between NAFLD and all-cause mortality and cardiovascular death, if any, is independent of cardiometabolic risk factors (Figure 1) such as MetS and type 2 diabetes.
Figure 1.
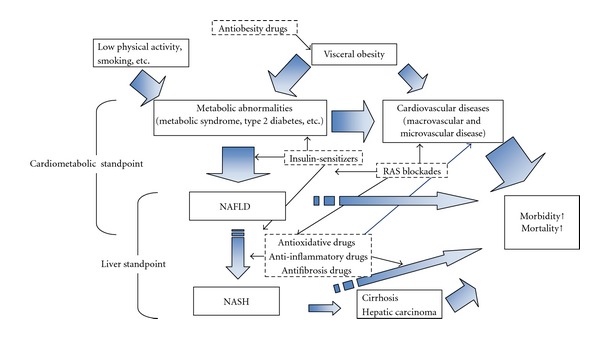
Therapeutic options and their main effects on NAFLD and NASH.
Taken together, NAFLD and NASH are multidisciplinary liver diseases that require interventions targeting the cardiometabolic and liver disorders for the effective treatment of patients with these diseases. Therefore, it is likely that mild NAFLD will require predominantly cardiometabolic pharmacotherapies, whereas moderate to severe NAFLD and NASH will require pharmacotherapies targeting the hepatic disorders. However, since many of the candidate drugs are likely to have broad therapeutic effects targeting multiple aspects of these diseases, distinct classifications are unavailable.
2. Liver-Specific Pathogenic Characteristics of NAFLD and NASH
Ectopic fat deposition in organs other than fat tissue, such as the liver and skeletal muscle, reflects severe energy overaccumulation or disturbed fat distribution. However, hepatocytes can, under physiological conditions, store small amounts of triglyceride in a transient manner [18].
Low physical activity as a result of a sedentary state, other unfavorable lifestyle behaviors (e.g., diet and habitual smoking), and sympathetic overdrive as a result of physical/mental stress may lead to insulin resistance independently of obesity. In turn, insulin resistance suppresses the influx of glucose and free fatty acids (FFAs) into adipose tissue, increasing FFA influx into the liver.
The pathogenic characteristics described above are often observed in metabolically obese young women with a normal body weight [19, 20]. Stefan et al. [21] proposed that reduced ectopic fat in the liver may be more important than reduced visceral fat for the discrimination of benign obesity, in other words metabolically normal obesity with good insulin sensitivity.
Ectopic fat deposition in the liver may be an initial feature of peripheral insulin resistance, particularly in adipose tissue, which predominantly accumulates surplus energy as fat. For many years, it was thought that simple steatosis (i.e., fatty liver) remains benign throughout life. However, studies published in the last two decades have shown that such conditions, without appropriate treatment or intervention, provoke other events, including oxidative stress, inflammation, and fibrosis. These events lead to the degeneration and impaired functioning of liver tissue and ultimately result in NASH. Unfortunately, similar to other cardiometabolic diseases, most people with asymptomatic NAFLD remain untreated until the results of blood test or imaging studies indicate the presence of NAFLD. Serum hepatic enzymes, particularly alanine aminotransferase (ALT), are often elevated beyond the normal range [22–24]. However, they may remain within the normal ranges [23, 25] and may be overlooked until abdominal ultrasound, computed tomography, or magnetic resonance imaging are performed. In clinical practice, people with hypertrophic abdominal fat cells often develop NAFLD at a later date. This phenomenon may be caused by direct influx, via the portal vein, of long-chain FFAs, glycerol, and proinflammatory cytokines (e.g., tumor necrosis factor [TNF], interleukin [IL]-6, and IL-1) from the visceral and upper body fat depots, which exhibit insulin resistance and activation of hormone-sensitive lipase and adipose tissue triacylglycerol lipase [26–28].
FFAs derived from the lipolysis of visceral and subcutaneous fat account for approximately 60% of the total hepatic FFA content [29]. Besides increased FFA influx, hyperinsulinemia increases hepatic lipogenesis by activating sterol regulatory element binding protein 1c (SREBP-1c), a key regulator of lipogenic gene expression. Similarly, hyperglycemia caused by inadequate insulin action results in the activation of carbohydrate response element binding protein, which activates L-type pyruvate kinase and lipogenic genes in hepatocytes [30].
Nevertheless, it seems unexpected that hepatic lipogenesis is not attenuated by decreased insulin sensitivity or insulin resistance. Recent studies have suggested that SREBP-1c and lipogenesis are activated secondary to enhanced endoplasmic reticulum (ER) stress. ER stress activates the cleavage of SREBP-1c independently of insulin, which may explain the paradoxical stimulation of lipogenesis in insulin-resistant liver [31]. Consistently, reducing ER stress in obese animals decreases SREBP-1c activation and lipogenesis, and improves hepatic steatosis and insulin sensitivity [32]. Moreover, ER stress in response to increased hepatic lipid content attenuates triglyceride secretion by limiting apolipoprotein B secretion [33], thus increasing hepatic triglyceride accumulation. Alternatively, reduced mitochondrial β-oxidation, following inhibition of carnitine palmitoyl transferase-1 (CPT-1), may contribute to enhanced fatty acid synthesis [34].
Several animal studies have also demonstrated a relationship between decreased microsomal triglyceride transfer protein (MTP) expression and steatosis [35]. For example, patients with NASH were reported to show reduced very low-density lipoprotein (VLDL) synthesis and impaired hepatic MTP mRNA expression, which was likely due to single nucleotide polymorphisms of the MTP gene [36].
3. Pharmacotherapeutic Candidates for Multidisciplinary Treatment of NAFLD and NASH
In terms of preventing liver damage, the priority for pharmacotherapy of NAFLD is to prevent transformation of NAFLD into NASH and to improve the pathophysiology [5]. Therefore, in theory, antioxidative, antiinflammatory, and antifibrotic agents may constitute first-line treatments for NAFLD. At the same time, treatments should also be initiated to target the cardiometabolic abnormalities and obesity. The potential pharmacotherapies for NAFLD and NASH are listed in Table 1. The order of the drugs is mostly based on the recommendation for use [4–6].
Table 1.
List of drugs and agents as candidates for the treatment of NAFLD.
| Insulin sensitizers | |
| Metformin (Biguanide) | |
| Thiazolidines (Pioglitazone, Rosiglitazone) | |
| Antioxidants | |
| Vitamin E | |
| Vitamin C | |
| Polyphenols (Resveratrol, etc.) | |
| Hepatocyte-protective agents | |
| Ursodeoxycholic acid | |
| n-3 polyunsaturated fatty acids (EPA and DHA) | |
| Antidyslipidemia | |
| Statins | |
| Fibrates | |
| NPC1L1 inhibitors (ezetimibe) | |
| RAS blockers | |
| Angiotensin II receptor blockers | |
| Angiotensin-converting enzyme inhibitors | |
| Antialdosterone (spironolactone and eplerenone) | |
| Renin inhibitor (aliskiren) | |
| Incretin-related agents | |
| GLP-1 agonists/analogs (exenatide and liraglutide) | |
| DPP-4 inhibitors (sitagliptin and vildagliptin) | |
| Antiobesity drugs | |
| Orlistat | |
| Sibutramine | |
| Mazindol |
3.1. Metformin
Metformin (biguanide) is an insulin-sensitizer that is considered to be the first-line treatment of type 2 diabetes because it has relatively few side effects and is inexpensive. Besides reducing hepatic glucose output, metformin also activates AMP kinase, which inhibits the production of glucose, cholesterol, and triglycerides, and stimulates fatty acid oxidation [37].
Nevertheless, the outcomes of clinical trials in NAFLD are conflicting [38, 39]. Metformin was more effective than dietary treatment alone in normalizing several metabolic parameters [40]. Metformin was also better than a prescriptive diet or vitamin E intake for the treatment of NAFLD in patients receiving nutritional counseling [41]. In a clinical study, serum ALT levels were consistently lower in patients treated with metformin compared with those treated with placebo [42]. The effects of metformin in that study were considered to be mediated by weight loss [42].
However, another study showed that metformin only transiently improved hepatic parameters [43]. Moreover, 6 months of treatment with metformin was no better than placebo in terms of improving liver histology in patients with NAFLD [44]. Further well-designed studies are needed to elucidate the significance of metformin treatment for NAFLD.
3.2. Thiazolidinediones
A number of clinical trials, including randomized controlled trials, have shown that thiazolidinediones (peroxisome proliferator activated receptor [PPAR]-γ agonists) improve liver enzyme levels and liver histology in patients with NAFLD and NASH [39, 45]. PPARγ, which belongs to the nuclear hormone receptor family, is predominantly expressed in adipose tissues and plays a key role in adipogenesis and glucose homeostasis [46]. Since PPARγ is also expressed in cardiovascular tissues, such as vascular endothelial cells, smooth muscle cells, macrophages, and cardiomyocytes, altered PPARγ activity may be involved in the etiology of cardiovascular diseases, particularly atherosclerosis [47].
It has been shown that treatment with pioglitazone for 1-2 years improves the overall pathogenic characteristics of NAFLD and NASH [48–50], suggesting that PPARγ agonists may improve the pathophysiology of the liver as well as clinical factors in patients with NAFLD and NASH. PPARγ regulates several key activities, including adipocyte differentiation and fibroblast differentiation into mature adipocyte types [51, 52]. Therefore, the improvements in NAFLD may be due to improvements in the visceral tissue and upper subcutaneous fat, as thiazolidinediones redirect fat accumulation from the liver or muscle into adipose tissues and confine it in adipose tissue at the expense of adipose cell expansion [53], which unfortunately leads to weight gain.
Thiazolidinediones also activate AMP-activated protein kinase and inhibit lipolysis, at least in part by inhibiting the translocation of hormone-sensitive lipase to lipid droplets [54]. Mayerson et al. [55] showed that treatment with rosiglitazone significantly reduced hepatic triglyceride content and suppresses adipocyte lipolysis. In this way, rosiglitazone increased intramyocellular fat storage as triglycerides, which was accompanied by improved muscle insulin sensitivity. This suggests that intramyocellular fat alone does not necessarily reflect insulin resistance.
However, this apparent improvement in insulin resistance often results in adverse outcomes, such as an increase in the number of adipose cells through enhanced differentiation, eventually leading to weight gain and systematic edema [39, 50]. These events increase the heart's workload and aggravate latent heart failure. Consequently, long-term treatment with thiazolidinediones may be poorly tolerated in some people with diabetes because of their adverse effects, although pioglitazone has fewer side effects than rosiglitazone [56, 57] (troglitazone was excluded from the market because of hepatic toxicity). Some adverse effects of thiazolidinediones, particularly weight gain and edema, could be prevented when administered in low dosages. Indeed, even low-dose pioglitazone (15 mg/day) improved liver enzymes without overt adverse effects in a study of 12 patients with biopsy-confirmed NASH [58].
3.3. Vitamin E as an Antioxidative Agent
Some intervention studies in patients with NAFLD have shown that vitamin E, a lipophilic antioxidant, may improve some of the pathogenic characteristics of NAFLD. Vitamin E exerts its antioxidative effects by reducing lipid peroxidation, preventing free radical reactions, and stabilizing cellular phospholipid membranes [59]. Vitamin E also inhibits hepatic transforming growth factor-β1 expression, attenuates cytokine stimulation of stellate cells, and protects against hepatic fibrosis [60], suggesting that vitamin E is more beneficial for NASH rather than NAFLD.
Nevertheless, clinical trials performed to date have yielded conflicting results. Some studies, most of them involving small numbers of patients, showed positive outcomes in terms of the treatment of NAFLD [41, 48, 61, 62]. However, other studies showed negative results or no significant improvement with vitamin E compared with placebo or lifestyle interventions, suggesting that vitamin E alone is insufficient to treat NAFLD [38, 63]. Lavine et al. [38] reported that vitamin E and metformin were not superior to placebo in terms of sustained reductions in ALT levels in children with NAFLD. It was also reported that simple lifestyle interventions (i.e., diet and physical exercise) in children with NAFLD can significantly improve liver function, glucose metabolism, and lipid levels beyond those achieved with antioxidant therapy (600 IU/day vitamin E plus 500 mg/day vitamin C) [63]. Several studies have shown that high-dose vitamin E supplementation may be harmful in some patients because of unexpected adverse outcomes, such as increased cardiovascular disease mortality [64, 65], although other studies found no such risk [66, 67].
Among nondiabetic patients with biopsy-confirmed NASH, current recommendations advocate the administration of 800 IU/day vitamin E [5], as this dose may reduce serum ALT levels and improve steatosis, inflammation, and hepatocyte ballooning.
3.4. Ursodeoxycholic Acid (UDCA)
UDCA is a cytoprotective antiinflammatory agent that is widely used to treat liver diseases, as well as gallstones. Long-term clinical studies have revealed that UDCA safely and effectively improves hepatic enzyme levels, serum fibrosis markers, and selected metabolic parameters [68]. Oral administration of taurine-conjugated UDCA decreased hepatic steatosis in ob/ob mice by cooperatively regulating multiple metabolic pathways, including reduced expression of genes that regulate de novo lipogenesis [69]. Overall, however, there were no significant differences between UDCA and placebo in several clinical trials [70].
3.5. Long-Chain n-3 Polyunsaturated Fatty Acids (PUFAs)
n-3 PUFAs are mainly consumed in the form of marine oils from fatty fish or other seafood. Fish oil contains both docosahexaenoic acid (DHA, C22:6 n-3) and eicosapentaenoic acid (EPA, C20:5 n-3). n-3 PUFA consumption lowers plasma triglycerides and blood pressure and may reduce inflammation and improve vascular function [71]. These effects occur because n-3 PUFAs are natural ligands for several nuclear receptors and transcription factors that regulate gene expression in various tissues [72]. Considering these properties of n-3 PUFAs, several clinical trials have investigated the potential effects of fish oil or n-3 PUFA consumption on the outcomes of cardiovascular disease. Meta-analyses of these studies have shown that fish oil and n-3 PUFA consumption, but not α-linolenic acid, reduce the risk of cardiovascular events and the risk of coronary heart disease-related mortality [71, 73].
Regarding NAFLD, dietary supplementation with long-chain n-3 PUFAs appears to safely reduce nutritional hepatic steatosis in adults [74]. Araya et al. [75] reported that the levels of n-3 PUFAs were decreased while the n-6/n-3 fatty acid ratio was increased in NAFLD patients compared with controls, probably because of defective desaturation of PUFA by inadequate intake of their precursors and increased peroxidation of PUFA. Petit et al. [76] reported that increased erythrocyte n-3 and n-6 PUFA levels are significantly associated with a lower prevalence of steatosis in patients with type 2 diabetes. In addition, the dietary records of middle-aged healthy Japanese men revealed that dietary EPA and EPA + DHA may help to prevent NAFLD [77]. Furthermore, Sato et al. [78] reported that the antiobesity effects of EPA in high-fat/high-sucrose-induced obese mice were associated with the suppression of hepatic lipogenesis and steatosis.
Enjoji and Nakamuta [79] proposed that excess cholesterol intake appears to be one of the main factors associated with NAFLD, particularly in nonobese subjects, because excess cholesterol consumption stimulates the liver X receptor-α–SREBP-1c pathway and enhances fatty acid synthesis. Indeed, it was reported that low-dose (2.0% of total energy) fish oil diets improve hepatic lipid accumulation in mice fed a high-cholesterol diet [80]. However, studies examining the effects of EPA/fish oil on dietary cholesterol-induced NAFLD in humans are still lacking.
3.6. Statins
Statins (3-hydroxy-3-methyglutaryl-coenzyme A reductase inhibitors) are used worldwide to treat lipid disorders, particularly elevated low-density lipoprotein-cholesterol (LDL-C) and substantially reduce cardiovascular events and mortality. Statins also have pleiotropic effects, including antiinflammatory actions [81], as they greatly reduce the levels of proinflammatory cytokines, such as C-reactive protein (CRP) and TNF.
Although statins are putatively associated with some adverse events, including elevated hepatic enzymes and liver dysfunction, an elevated serum ALT level at baseline attributable to NAFLD is unlikely to increase the risk of statin-associated elevations in ALT [82]. Similarly, an elevated baseline serum ALT was not associated with an increased risk of hepatotoxicity in patients treated with lovastatin [83].
In clinical studies, simvastatin and atorvastatin [84–86] were associated with a reduction in hepatic steatosis and may inhibit the progression to NASH. Four years of treatment with atorvastatin (20 mg) combined with vitamins C and E reduced hepatic steatosis by 71% in people with NAFLD at baseline [87]. Taken together with a previous report showing that excess cholesterol consumption may accelerate NAFLD [79], statins may be promising candidates for the treatment of NAFLD. Additionally, some statins improved surrogate markers of hepatic steatosis, such as serum glyceraldehyde-derived advanced glycation end-products [88].
On the other hand, considering that statins are associated with worsening of glucose metabolism [89, 90] and that multifactorial medications, such as antihypertensive and antidiabetic drugs, were used in most of the previous studies of statin therapy [91], well-designed, randomized, placebo-controlled studies are needed to determination the suitability of statins as monotherapy or combination therapy for NAFLD.
3.7. Fibrates
PPARα is highly expressed in the liver and is involved in fatty acid oxidation [92]. Fibrates, PPARα agonists, increase FFA oxidation in the liver, alter TG synthesis, and reduce hepatic synthesis of VLDL [93, 94], and theoretically improve the pathogenic characteristics of MetS and NAFLD. Unlike the striking outcomes of numerous clinical trials using statins, the results of several large studies of fibrates were inconsistent and had varying outcomes, including the incidence of cardiovascular mortality and events [95–99]. In all of these studies, treatment with a fibrate was associated with a large, although nonsignificant, reduction in cardiovascular events in people with type 2 diabetes or components of the metabolic syndrome.
To date, however, few clinical studies, except for a small pilot study [100, 101], have examined the effects of fibrates on the pathophysiology of NAFLD. Fibrates are expected to ameliorate the pathogenic characteristics of NAFLD because they reduce the levels of inflammatory biomarkers, such as CRP and IL-6, and may improve insulin resistance [102–104], via mechanisms that differ from those of statins. Fenofibrate is commonly used in clinical practice because it is generally well tolerated when used as monotherapy and as combination therapy in a wide range of individuals [103, 105].
No clinical studies have examined the effects or safety of a statin in combination with a fibrate compared with monotherapy for treating NAFLD. However, some benefits of combination therapy on cardiovascular and microvascular outcomes were observed in specific subgroups of patients, such as patients with low HDL levels or hypertriglyceridemia [106].
3.8. Niemann-Pick C1-Like 1 (NPC1L1) Inhibitors
NPC1L1 plays a key role in intestinal cholesterol absorption. Ezetimibe, an NPC1L1 inhibitor, was reported to reduce circulating LDL-C levels and improve clinical outcomes in patients at increased risk for cardiovascular events when administered alone or in combination with a statin [107]. Unlike in rodents in which NPC1L1 is mainly expressed in the intestine, NPC1L1 is highly expressed in the liver in humans [108, 109]. This suggests that hepatic NPC1L1 may facilitate hepatic cholesterol accumulation and that ezetimibe may be a potential candidate for NAFLD, especially NAFLD induced by a high-cholesterol diet [79]. In clinical trials, albeit on a small scale, ezetimibe improved biochemical parameters and hepatic enzyme levels, as well as the histological abnormalities of NAFLD [110–112].
3.9. Renin-Angiotensin System (RAS) Blockade
The RAS plays key roles in the regulation of blood pressure and fluid balance, as well as in the pathogenesis of insulin resistance and NAFLD [113, 114]. In addition, inhibition of the RAS may improve the intracellular insulin signaling pathway, offering better control of adipose tissue proliferation and adipokine production [115]. The two main classes of RAS blockers, angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs), are efficient drugs that significantly reduce cardiovascular events and mortality [116–118]. As expected, ARBs and ACEIs improve insulin resistance and possibly lipid profiles, suggesting that these agents may be suitable treatments for NAFLD and NASH. Of interest, telmisartan was reported to be a partial agonist of PPARγ [119, 120], a property that does not appear to be shared by other ARBs [121]. Nevertheless, despite many animal studies showing the beneficial effects of ARBs and ACEIs, clinical studies for NAFLD are still lacking.
Beneficial effects of spironolactone and eplerenone, aldosterone antagonists, were recently shown in a mouse model of NAFLD [121–123]. Additionally, spironolactone in combination with vitamin E was reported to improve insulin resistance in patients with NAFLD [124]. However, these effects of aldosterone antagonists have only been shown in mouse and small-scale clinical studies.
Aliskiren, a direct renin inhibitor used to treat hypertension, provides an organ-protective effect by attenuating oxidative stress and improving insulin resistance in mice models [125–127]. Therefore, although aliskiren is a promising drug in terms of improving insulin resistance and oxidative stress, again, human studies are lacking.
3.10. Incretin Modulators
Treatment with incretin modulators, glucagon-like peptide-1 (GLP-1) analogs, and dipeptidyl peptidase-4 inhibitors reduces weight gain, minimizes hypoglycemia, decreases inflammation, and is cardioprotective in preclinical studies [128, 129]. The most common adverse events are mild gastrointestinal tract symptoms such as nausea, vomiting, and diarrhea, the incidence and severity of which generally decreased during continued therapy [129].
Regarding the beneficial effects of incretin modulators, weight loss (or weight neutrality) and reduced inflammation appear to be particularly promising for the treatment of NAFLD because these effects facilitate improvements in insulin resistance and metabolic abnormalities. Ding et al. [130] reported that administration of exendin-4, a GLP-1 receptor agonist, induces the regression of hepatic steatosis in ob/ob mice by improving insulin sensitivity. GLP-1 appears to protect hepatocytes from fatty acid-related death by suppressing dysfunctional ER stress responses [131]. In addition, GLP-1 suppresses hepatic lipogenesis by activating the AMP kinase pathway and reduces hepatic fat accumulation and nutrient-induced hepatic proinflammatory responses [132]. Tushuizen et al. [133] reported that 44 weeks of exenatide therapy decreased liver fat measured by liver spectroscopy from 15.8% to 4.3% and improved hepatic enzymes in a patient with type 2 diabetes. Taken together, there are promising results in animal models and limited human reports, but clinical studies examining the effects of incretin-based agents on hepatic steatosis have not been performed.
3.11. Antiobesity Drugs
Theoretically, improving NAFLD via weight loss is an ideal approach in obese or overweight people because other complications are simultaneously ameliorated. Of several commonly used antiobesity medications [134], orlistat and sibutramine are available for long-term prescription [135, 136]. Orlistat inhibits dietary triglyceride hydrolysis in the gut, occasionally with mild gastrointestinal symptoms, resulting in a substantial decrease in fat absorption. Zelber-Sagi et al. [137] showed that orlistat improves serum ALT levels and steatosis determined by ultrasound in patients with NAFLD, beyond its weight-lowering effects. Harrison et al. [138] reported that subjects who lost ≥5% of their body weight over 9 months experienced improvements in insulin resistance and steatosis, while subjects who lost ≥9% of their body weight also experienced improvements in hepatic histology.
Sibutramine, a combined norepinephrine and serotonin reuptake inhibitor, reduces food intake and body weight. Both orlistat and sibutramine had beneficial effects on body weight, lipid profiles, glucose metabolism, and inflammatory markers in many trials [135, 137]. However, there is still insufficient safety data regarding the long-term outcomes of antiobesity therapy. Indeed, sibutramine was reported to increase blood pressure and heart rate, which may limit its use in clinical practice [135].
Mazindol, a tetracyclic chemical, has been approved in several countries, including Japan. However, long-term treatment with mazindol is not currently permitted, although treatment with mazindol for a few weeks to several months is allowed in severely obese individuals (e.g., BMI ≥ 35 kg/m2 in Japan). The combination of mazindol and diet therapy is effective in treating severe obesity [139]. Mazindol exerts antiobesity effects by inhibiting the appetite and activating thermogenesis, as well as having antidiabetic effects [140]. However, clinical studies of mazindol have not been conducted in patients with NAFLD, partly because of the restriction for short-term administration.
Rimonabant, a selective cannabinoid-1 (CB1) receptor blocker, was shown to reduce body weight and improve cardiovascular risk factors in obese patients by regulating the energy balance and body composition [141]. Furthermore, administration of 20 mg rimonabant daily in combination with a hypocaloric diet for 1 year, significantly decreased body weight and waist circumference [142]. Based on several trials showing its weight loss benefits, rimonabant entered the European market for the treatment of obesity [143]. However, the drug was withdrawn worldwide in 2008-2009 because of the emergence of significant side effects, particularly psychiatric disorders (e.g., depression and anxiety) [143, 144]. The CB1 receptor is expressed not only in the central nervous system but also in the gastrointestinal tract, adipose tissue, and cardiovascular system [145]. Consistently, animal studies have shown that CB1 receptor antagonists improve glucose homeostasis, insulin levels, fatty liver, and plasma lipid profiles by blocking the CB1 receptor in peripheral tissues, including the liver and visceral fat [146, 147]. Despite these promising effects, the clinical application of CB1 receptor antagonists will be restricted until their critical adverse effects on the central nervous system can be overcome.
3.12. Resveratrol
Polyphenols, ubiquitous dietary components that mainly include flavonoids and tannins, are considered dietary supplements rather than drugs. Several polyphenols obtained from plants may be promising candidate treatments for NAFLD and NASH because they are effective scavengers of reactive oxygen and reactive nitrogen species [148].
Of several polyphenols described to date, resveratrol, a component of several grape species, appears to be particularly relevant in the context of liver disease [149]. For example, resveratrol has antiinflammatory effects mediated through a decrease in proinflammatory cytokines, including TNF. In a study using an animal model of steatosis, resveratrol significantly reduced hepatic steatosis and ALT levels [150]. Resveratrol appeared to reduce liver oxidative stress by increasing the expression of CPT-1a and acyl-coenzyme A oxidase. Another study revealed that resveratrol can also protect the liver from NAFLD by reducing FFA availability [151]. Resveratrol also decreased the severity of NAFLD in rats, at least in part, through its antioxidant effects and by inhibiting TNF [152]. Resveratrol was also reported to protect the liver from NAFLD by enhancing AMP-activated protein kinase phosphorylation [153].
3.13. Alcohol Intake
Habitual alcohol intake, even light consumption, interferes with the development and progression of critical diseases. Intriguingly, light to moderate alcohol consumption is often associated with a low prevalence of fatty liver. Clinical studies have suggested that light or moderate (<10–20 g ethanol/day) alcohol intake protects against NAFLD [154]. Light alcohol consumption (20 g ethanol on 1–3 days/week or 40–140 g ethanol/week) and moderate alcohol consumption (140–280 g ethanol/week) were independently associated with a low prevalence of fatty liver [155, 156], whereas moderate to heavy drinking (>60 g ethanol/week) was associated with the progression of hepatic steatosis and fibrosis [157, 158].
The effects of moderate alcohol consumption on liver enzymes may increase with increasing BMI [159]. In addition, habitual alcohol consumption generally impairs fatty acid oxidation and stimulates lipogenesis [160, 161]. However, the specific mechanisms by which alcohol (alone or in combination with obesity/metabolic abnormalities) causes liver injury are poorly understood. In clinical studies, the causality between light to moderate alcohol consumption and reduced prevalence of fatty liver or NAFLD remains unknown. Thus, patients with NAFLD should not consume heavy amounts of alcohol [5, 162]. Additionally, no recommendation can be made regarding even light to moderate alcohol consumption [5].
4. Conclusion
Since hepatic dysfunction is usually closely associated with systemic disorders, there are no specific medications that ameliorate only the pathogenic characteristics of fatty liver without affecting other organs and tissues. In other words, drugs used, or expected to be used, in the treatment of type 2 diabetes, MetS (i.e., hypertension and dyslipidemia), hypercholesterolemia, and obesity may be candidate drugs for the treatment of NAFLD and NASH. These agents can be more effective than monotherapy, although further evidence from animal and cellular studies, as well as large clinical trials, is needed to examine this possibility.
Conflict of Interests
There was no personal or financial support, or author involvement with organization(s) with any financial interest in this paper. The author has no conflict of interests to declare.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman P. Health risks associated with overweight and obesity. Obesity Reviews. 2007;8(supplement 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Dixon JB. The effect of obesity on health outcomes. Molecular and Cellular Endocrinology. 2010;316(2):104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 6.Smith BW, Adams LA. Non-alcoholic fatty liver disease. Critical Reviews in Clinical Laboratory Sciences. 2011;48:97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Kim HJ, Lee KE, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Archives of Internal Medicine. 2004;164(19):2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 8.Musso G, Gambino R, Bo S, et al. Should nonalcoholic fatty liver disease be included in the definition of metabolic syndrome? A cross-sectional comparison with Adult Treatment Panel III criteria in nonobese nondiabetic subjects. Diabetes Care. 2008;31(3):562–568. doi: 10.2337/dc07-1526. [DOI] [PubMed] [Google Scholar]
- 9.Yap JYK, O’Connor C, Mager DR, Taylor G, Roberts EA. Diagnostic challenges of nonalcoholic fatty liver disease (NAFLD) in children of normal weight. Clinics and Research in Hepatology and Gastroenterology. 2011;35:500–505. doi: 10.1016/j.clinre.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Das K, Das K, Mukherjee PS, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51(5):1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Huang MH, Yang JC, et al. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of Taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. Journal of Clinical Gastroenterology. 2006;40(8):745–752. doi: 10.1097/00004836-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54(12):3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 13.Adams LA, Lymp JF, Sauver JS, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30(8):2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 15.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. Journal of Hepatology. 2008;49(4):608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Ruhl CE, Everhart JE. Non-alcoholic fatty liver disease (NAFLD) and mortality. Journal of Hepatology. 2009;51(3):p. 593. doi: 10.1016/j.jhep.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343 doi: 10.1136/bmj.d6891.d6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Burn P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nature Reviews Drug Discovery. 2004;3(8):695–710. doi: 10.1038/nrd1469. [DOI] [PubMed] [Google Scholar]
- 19.Conus F, Allison DB, Rabasa-Lhoret R, et al. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. Journal of Clinical Endocrinology and Metabolism. 2004;89(10):5013–5020. doi: 10.1210/jc.2004-0265. [DOI] [PubMed] [Google Scholar]
- 20.Dvorak RV, DeNino WF, Ades PA, Poehlman ET. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes. 1999;48(11):2210–2214. doi: 10.2337/diabetes.48.11.2210. [DOI] [PubMed] [Google Scholar]
- 21.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Archives of Internal Medicine. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 22.Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes/Metabolism Research and Reviews. 2006;22(6):437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 23.Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clinical Chemistry. 2007;53(4):686–692. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]
- 24.Ghouri N, Preiss D, Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: a narrative review and clinical perspective of prospective data. Hepatology. 2010;52(3):1156–1161. doi: 10.1002/hep.23789. [DOI] [PubMed] [Google Scholar]
- 25.Tolman KG, Dalpiaz AS. Treatment of non-alcoholic fatty liver disease. Therapeutics and Clinical Risk Management. 2007;3(6):1153–1163. [PMC free article] [PubMed] [Google Scholar]
- 26.Capeau J. Insulin resistance and steatosis in humans. Diabetes & Metabolism. 2008;34(6):649–657. doi: 10.1016/S1262-3636(08)74600-7. [DOI] [PubMed] [Google Scholar]
- 27.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clinical Biochemistry. 2009;42(13-14):1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Gustafson B. Adipose tissue, inflammation and atherosclerosis. Journal of Atherosclerosis and Thrombosis. 2010;17(4):332–341. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro H, Tehilla M, Attal-Singer J, Bruck R, Luzzatti R, Singer P. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clinical Nutrition. 2011;30(1):6–19. doi: 10.1016/j.clnu.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes, Obesity and Metabolism. 2010;12(supplement 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 32.Kammoun HL, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. Journal of Clinical Investigation. 2009;119(5):1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. Journal of Clinical Investigation. 2008;118(1):316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. Journal of Clinical Investigation. 2004;114(2):147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussain MM, Bakillah A. New approaches to target microsomal triglyceride transfer protein. Current Opinion in Lipidology. 2008;19(6):572–578. doi: 10.1097/MOL.0b013e328312707c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujita K, Imajo K, Shinohara Y, et al. Novel findings for the development of drug therapy for various liver diseases: liver microsomal triglyceride transfer protein activator may be a possible therapeutic agent in non-alcoholic steatohepatitis. Journal of Pharmacological Sciences. 2011;115(3):270–273. doi: 10.1254/jphs.10r14fm. [DOI] [PubMed] [Google Scholar]
- 37.Schimmack G, DeFronzo RA, Musi N. AMP-activated protein kinase: role in metabolism and therapeutic implications. Diabetes, Obesity and Metabolism. 2006;8(6):591–602. doi: 10.1111/j.1463-1326.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 38.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin e or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents the tonic randomized controlled trial. Journal of the American Medical Association. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 40.Garinis GA, Fruci B, Mazza A, et al. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. International Journal of Obesity. 2010;34(8):1255–1264. doi: 10.1038/ijo.2010.40. [DOI] [PubMed] [Google Scholar]
- 41.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. American Journal of Gastroenterology. 2005;100(5):1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 42.Krakoff J, Clark JM, Crandall JP, et al. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity. 2010;18(9):1762–1767. doi: 10.1038/oby.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair S, Diehl AM, Wiseman M, Farr GH, Jr., Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Alimentary Pharmacology and Therapeutics. 2004;20(1):23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 44.Haukeland JW, Konopski Z, Eggesbø HB, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scandinavian Journal of Gastroenterology. 2009;44(7):853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 45.Spiegelman BM. Peroxisome proliferator-activated receptor gamma: a key regulator of adipogenesis and systemic insulin sensitivity. European Journal of Medical Research. 1997;2(11):457–464. [PubMed] [Google Scholar]
- 46.Spiegelman BM. PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47(4):507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 47.Wang N, Yin R, Liu Y, Mao G, Xi F. Role of peroxisome proliferator-activated receptor-γ in atherosclerosis: an update. Circulation Journal. 2011;75(3):528–535. doi: 10.1253/circj.cj-11-0060. [DOI] [PubMed] [Google Scholar]
- 48.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England Journal of Medicine. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shadid S, Jensen MD. Effect of pioglitazone on biochemical indices of non-alcoholic fatty liver disease in upper body obesity. Clinical Gastroenterology and Hepatology. 2003;1(5):384–387. doi: 10.1053/s1542-3565(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 50.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 51.Anghel SI, Wahli W. Fat poetry: a kingdom for PPARγ . Cell Research. 2007;17(6):486–511. doi: 10.1038/cr.2007.48. [DOI] [PubMed] [Google Scholar]
- 52.Mandrup S, Lane MD. Regulating adipogenesis. Journal of Biological Chemistry. 1997;272(9):5367–5370. doi: 10.1074/jbc.272.9.5367. [DOI] [PubMed] [Google Scholar]
- 53.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. The Lancet. 2010;375(9733):2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourron O, Daval M, Hainault I, et al. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia. 2010;53(4):768–778. doi: 10.1007/s00125-009-1639-6. [DOI] [PubMed] [Google Scholar]
- 55.Mayerson AB, Hundal RS, Dufour S, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51(3):797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang WHW. Do thiazolidinediones cause heart failure? A critical review. Cleveland Clinic Journal of Medicine. 2006;73(4):390–397. doi: 10.3949/ccjm.73.4.390. [DOI] [PubMed] [Google Scholar]
- 57.Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ. 2009;339:p. b4731. doi: 10.1136/bmj.b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoneda M, Endo H, Nozaki Y, et al. Life style-related diseases of the digestive system: gene expression in nonalcoholic steatohepatitis patients and treatment strategies. Journal of Pharmacological Sciences. 2007;105(2):151–156. doi: 10.1254/jphs.fm0070063. [DOI] [PubMed] [Google Scholar]
- 59.Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD: antioxidants and cytoprotective agents. Journal of Clinical Gastroenterology. 2006;40(supplement 1):S51–S60. doi: 10.1097/01.mcg.0000168648.79034.67. [DOI] [PubMed] [Google Scholar]
- 60.Parola M, Muraca R, Dianzani I, et al. Vitamin E dietary supplementation inhibits transforming growth factor β1 gene expression in the rat liver. FEBS Letters. 1992;308(3):267–270. doi: 10.1016/0014-5793(92)81290-3. [DOI] [PubMed] [Google Scholar]
- 61.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. Journal of Pediatrics. 2000;136(6):734–738. [PubMed] [Google Scholar]
- 62.Ersöz G, Günşar F, Karasu Z, Akay S, Batur Y, Akarca US. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turkish Journal of Gastroenterology. 2005;16(3):124–128. [PubMed] [Google Scholar]
- 63.Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics. 2006;24(11-12):1553–1561. doi: 10.1111/j.1365-2036.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 64.Adinolfi LE, Restivo L. Does vitamin e cure nonalcoholic steatohepatitis? Expert Review of Gastroenterology and Hepatology. 2011;5(2):147–150. doi: 10.1586/egh.11.27. [DOI] [PubMed] [Google Scholar]
- 65.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Annals of Internal Medicine. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 66.Dietrich M, Jacques PF, Pencina MJ, et al. Vitamin E supplement use and the incidence of cardiovascular disease and all-cause mortality in the Framingham Heart Study: does the underlying health status play a role? Atherosclerosis. 2009;205(2):549–553. doi: 10.1016/j.atherosclerosis.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerss J, Köpcke W. The questionable association of vitamin E supplementation and mortality—inconsistent results of different meta-analytic approaches. Cellular and Molecular Biology. 2009;55(supplement 1):1111–1120. [PubMed] [Google Scholar]
- 68.Ratziu V, De Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. Journal of Hepatology. 2011;54(5):1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 69.Yang JS, Kim JT, Jeon J, et al. Changes in hepatic gene expression upon oral administration of taurine-conjugated ursodeoxycholic acid in ob/ob mice. PLoS ONE. 2010;5(11) doi: 10.1371/journal.pone.0013858.e13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duvnjak M, Tomasic V, Gomercic M, Smircic Duvnjak L, Barsic N, Lerotic I. Therapy of nonalcoholic fatty liver disease: current status. Journal of Physiology and Pharmacology. 2009;60(supplement 7):57–66. [PubMed] [Google Scholar]
- 71.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. American College of Cardiology Foundation. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 72.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Current Opinion in Lipidology. 2008;19(3):242–247. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang C, Harris WS, Chung M, et al. n-3 Fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. American Journal of Clinical Nutrition. 2006;84(1):5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 74.Shapiro H, Tehilla M, Attal-Singer J, Bruck R, Luzzatti R, Singer P. The therapeutic potential of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clinical Nutrition. 2011;30(1):6–19. doi: 10.1016/j.clnu.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clinical Science. 2004;106(6):635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 76.Petit JM, Guiu B, Duvillard L, et al. Increased erythrocytes n-3 and n-6 polyunsaturated fatty acids is significantly associated with a lower prevalence of steatosis in patients with type 2 diabetes. Clinical Nutrition. 2012;31:520–525. doi: 10.1016/j.clnu.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 77.Oya J, Nakagami T, Sasaki S, et al. Intake of n-3 polyunsaturated fatty acids and non-alcoholic fatty liver disease: a cross-sectional study in Japanese men and women. European Journal of Clinical Nutrition. 2010;64(10):1179–1185. doi: 10.1038/ejcn.2010.139. [DOI] [PubMed] [Google Scholar]
- 78.Sato A, Kawano H, Notsu T, et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 2010;59(10):2495–2504. doi: 10.2337/db09-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Enjoji M, Nakamuta M. Is the control of dietary cholesterol intake sufficiently effective to ameliorate nonalcoholic fatty liver disease? World Journal of Gastroenterology. 2010;16(7):800–803. doi: 10.3748/wjg.v16.i7.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirako S, Kim HJ, Shimizu S, Chiba H, Matsumoto A. Low-dose fish oil consumption prevents hepatic lipid accumulation in high cholesterol diet fed mice. Journal of Agricultural and Food Chemistry. 2011;59(supplement 1):13353–13359. doi: 10.1021/jf203761t. [DOI] [PubMed] [Google Scholar]
- 81.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23):III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 82.Chalasani N, Aljadhey H, Kesterson J, Murray MD, Hall SD. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126(5):1287–1292. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 83.Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. American Journal of the Medical Sciences. 2005;329(2):62–65. doi: 10.1097/00000441-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 84.Hatzitolios A, Savopoulos C, Lazaraki G, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian Journal of Gastroenterology. 2004;23(4):131–134. [PubMed] [Google Scholar]
- 85.Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. Journal of Hepatology. 2007;47(1):135–141. doi: 10.1016/j.jhep.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 86.Kiyici M, Gulten M, Gurel S, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Canadian Journal of Gastroenterology. 2003;17(12):713–718. doi: 10.1155/2003/857869. [DOI] [PubMed] [Google Scholar]
- 87.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the st francis heart study randomized clinical trial. American Journal of Gastroenterology. 2011;106(1):71–77. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 88.Kimura Y, Hyogo H, Yamagishi SI, et al. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: clinical usefulness of AGEs as a biomarker for the attenuation of NASH. Journal of Gastroenterology. 2010;45(7):750–757. doi: 10.1007/s00535-010-0203-y. [DOI] [PubMed] [Google Scholar]
- 89.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. The Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 90.Koh KK, Quon MJ, Han SH, Lee Y, Kim SJ, Shin EK. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. Journal of the American College of Cardiology. 2010;55(12):1209–1216. doi: 10.1016/j.jacc.2009.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liberopoulos EN, Athyros VG, Elisaf MS, Mikhailidis DP. Statins for non-alcoholic fatty liver disease: a new indication? Alimentary Pharmacology and Therapeutics. 2006;24(4):698–699. doi: 10.1111/j.1365-2036.2006.03021.x. [DOI] [PubMed] [Google Scholar]
- 92.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 93.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–2093. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 94.Barter PJ, Rye KA. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(1):39–46. doi: 10.1161/ATVBAHA.107.148817. [DOI] [PubMed] [Google Scholar]
- 95.Frick MH, Elo O, Haapa K. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. The New England Journal of Medicine. 1987;317(20):1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 96.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. The New England Journal of Medicine. 1999;341(6):410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 97.Oliver M. A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate. Report from the committee of principal investigators. British Heart Journal. 1978;40(10):1069–1118. doi: 10.1136/hrt.40.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Behar S, Brunner D, Kaplinsky E, Mandelzweig L, Benderly M. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the bezafibrate infarction prevention (BIP) study. Circulation. 2000;102(1):21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 99.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. The Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 100.Laurin J, Lindor KD, Crippin JS, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23(6):1464–1467. doi: 10.1002/hep.510230624. [DOI] [PubMed] [Google Scholar]
- 101.Fernández-Miranda C, Pérez-Carreras M, Colina F, López-Alonso G, Vargas C, Solís-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Digestive and Liver Disease. 2008;40(3):200–205. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Okopień B, Krysiak R, Herman ZS. Effects of short-term fenofibrate treatment on circulating markers of inflammation and hemostasis in patients with impaired glucose tolerance. Journal of Clinical Endocrinology and Metabolism. 2006;91(5):1770–1778. doi: 10.1210/jc.2005-1615. [DOI] [PubMed] [Google Scholar]
- 103.Koh KK, Quon MJ, Lim S, et al. Effects of fenofibrate therapy on circulating adipocytokines in patients with primary hypertriglyceridemia. Atherosclerosis. 2011;214(1):144–147. doi: 10.1016/j.atherosclerosis.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 104.Jeong S, Yoon M. Fenofibrate inhibits adipocyte hypertrophy and insulin resistance by activating adipose PPARα in high fat diet-induced obese mice. Experimental and Molecular Medicine. 2009;41(6):397–405. doi: 10.3858/emm.2009.41.6.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jacobson TA. Myopathy with statin-fibrate combination therapy: clinical considerations. Nature Reviews Endocrinology. 2009;5(9):507–518. doi: 10.1038/nrendo.2009.151. [DOI] [PubMed] [Google Scholar]
- 106.Rosenblit PD. Do persons with diabetes benefit from combination statin and fibrate therapy? Current Cardiology Reports. 2012;14(1):112–124. doi: 10.1007/s11886-011-0237-7. [DOI] [PubMed] [Google Scholar]
- 107.Khanderia U, Regal RE, Rubenfire M, Boyden T. The ezetimibe controversy: implications for clinical practice. Therapeutic Advances in Cardiovascular Disease. 2011;5(4):199–208. doi: 10.1177/1753944711410099. [DOI] [PubMed] [Google Scholar]
- 108.Altmann SW, Davis HR, Jr., Zhu LJ, et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 109.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. Journal of Biological Chemistry. 2005;280(13):12710–12720. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 110.Ábel T, Fehér J, Dinya E, Eldin MG, Kovács A. Safety and efficacy of combined ezetimibe/simvastatin treatment and simvastatin monotherapy in patients with non-alcoholic fatty liver disease. Medical Science Monitor. 2009;15(12):MS6–MS11. [PubMed] [Google Scholar]
- 111.Enjoji M, Machida K, Kohjima M, et al. NPC1L1 inhibitor ezetimibe is a reliable therapeutic agent for non-obese patients with nonalcoholic fatty liver disease. Lipids in Health and Disease. 2010;9, article 29 doi: 10.1186/1476-511X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park H, Shima T, Yamaguchi K, et al. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. Journal of Gastroenterology. 2011;46(1):101–107. doi: 10.1007/s00535-010-0291-8. [DOI] [PubMed] [Google Scholar]
- 113.Polyzos SA, Kountouras J, Zavos C, Tsiaousi E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes, Obesity and Metabolism. 2010;12(5):365–383. doi: 10.1111/j.1463-1326.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 114.Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends in Endocrinology and Metabolism. 2005;16(3):120–126. doi: 10.1016/j.tem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Georgescu EF. Angiotensin receptor blockers in the treatment of NASH/NAFLD: could they be a first-class option? Advances in Therapy. 2008;25(11):1141–1174. doi: 10.1007/s12325-008-0110-2. [DOI] [PubMed] [Google Scholar]
- 116.Weber M. Achieving blood pressure goals: should angiotensin II receptor blockers become first-line treatment in hypertension? Journal of Hypertension. 2009;27(5):S9–S14. doi: 10.1097/01.hjh.0000357903.93951.73. [DOI] [PubMed] [Google Scholar]
- 117.Bommer WJ. Use of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy to reduce cardiovascular events in high-risk patients: part 2. Preventive Cardiology. 2008;11(4):215–222. doi: 10.1111/j.1751-7141.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 118.Parhofer KG, Münzel F, Krekler M. Effect of the angiotensin receptor blocker irbesartan on metabolic parameters in clinical practice: the DO-IT prospective observational study. Cardiovascular Diabetology. 2007;6, article 36 doi: 10.1186/1475-2840-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kyvelou SMG, Vyssoulis GP, Karpanou EA, et al. Effects of antihypertensive treatment with angiotensin II receptor blockers on lipid profile: an open multi-drug comparison trial. Hellenic Journal of Cardiology. 2006;47(1):21–28. [PubMed] [Google Scholar]
- 120.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-γ activity. Circulation. 2004;109(17):2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 121.Kurtz TW. Treating the metabolic syndrome: telmisartan as a peroxisome proliferator-activated receptor-gamma activator. Acta Diabetologica. 2005;42(supplement 1):S9–S16. doi: 10.1007/s00592-005-0176-0. [DOI] [PubMed] [Google Scholar]
- 122.Wada T, Kenmochi H, Miyashita Y, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151(5):2040–2049. doi: 10.1210/en.2009-0869. [DOI] [PubMed] [Google Scholar]
- 123.Schäfer A, Vogt C, Fraccarollo D, et al. Eplerenone improves vascular function and reduces platelet activation in diabetic rats. Journal of Physiology and Pharmacology. 2010;61(1):45–52. [PubMed] [Google Scholar]
- 124.Polyzos SA, Kountouras J, Zafeiriadou E, et al. Effect of spironolactone and vitamin E on serum metabolic parameters and insulin resistance in patients with nonalcoholic fatty liver disease. Journal of the Renin-Angiotensin-Aldosterone System. 2011;12:498–503. doi: 10.1177/1470320311402110. [DOI] [PubMed] [Google Scholar]
- 125.Dong YF, Liu L, Kataoka K, et al. Aliskiren prevents cardiovascular complications and pancreatic injury in a mouse model of obesity and type 2 diabetes. Diabetologia. 2010;53(1):180–191. doi: 10.1007/s00125-009-1575-5. [DOI] [PubMed] [Google Scholar]
- 126.Kang YS, Lee MH, Song HK, et al. Aliskiren improves insulin resistance and ameliorates diabetic vascular complications in db/db mice. Nephrology Dialysis Transplantation. 2011;26(4):1194–1204. doi: 10.1093/ndt/gfq579. [DOI] [PubMed] [Google Scholar]
- 127.Chou CL, Lai YH, Lin TY, Lee TJ, Fang TC. Aliskiren prevents and ameliorates metabolic syndrome in fructose-fed rats. Archives of Medical Science. 2011;7(5):882–888. doi: 10.5114/aoms.2011.25566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies—review of the scientific evidence. Journal of Clinical Endocrinology and Metabolism. 2011;96(7):2027–2031. doi: 10.1210/jc.2011-0599. [DOI] [PubMed] [Google Scholar]
- 129.Campbell RK. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clinical Therapeutics. 2011;33(5):511–527. doi: 10.1016/j.clinthera.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 130.Ding X, Saxena NK, Lin S, Gupta N, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43(1):173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025269.e25269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ben-Shlomo S, Zvibel I, Shnell M, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. Journal of Hepatology. 2011;54(6):1214–1223. doi: 10.1016/j.jhep.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 133.Tushuizen ME, Bunck MC, Pouwels PJ, van Waesberghe JHT, Diamant M, Heine RJ. Incretin mimetics as a novel therapeutic option for hepatic steatosis. Liver International. 2006;26(8):1015–1017. doi: 10.1111/j.1478-3231.2006.01315.x. [DOI] [PubMed] [Google Scholar]
- 134.Starfford RS, Radlery DC. National trends in antiobesity medication use. Archives of Internal Medicine. 2003;163:1046–1050. doi: 10.1001/archinte.163.9.1046. [DOI] [PubMed] [Google Scholar]
- 135.Tziomalos K, Krassas GE, Tzotzas T. The use of sibutramine in the management of obesity and related disorders: an update. Vascular Health and Risk Management. 2009;5:441–452. doi: 10.2147/vhrm.s4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bray GA. Lifestyle and pharmacological approaches to weight loss: efficacy and safety. Journal of Clinical Endocrinology and Metabolism. 2008;93(11):s81–s88. doi: 10.1210/jc.2008-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clinical Gastroenterology and Hepatology. 2006;4(5):639–644. doi: 10.1016/j.cgh.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 138.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology. 2009;49(1):80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 139.Yoshida T, Sakane N, Umekawa T, Yoshioka K, Kondo M, Wakabayashi Y. Usefulness of mazindol in combined diet therapy consisting of a low-calorie diet and Optifast in severely obese women. International Journal of Clinical Pharmacology Research. 1994;14(4):125–132. [PubMed] [Google Scholar]
- 140.Yoshida T, Umekawa T, Wakabayashi Y, Yoshimoto K, Sakane N, Kondo M. Anti-obesity and anti-diabetic effects of mazindol in yellow KK mice: its activating effect on brown adipose tissue thermogenesis. Clinical and Experimental Pharmacology and Physiology. 1996;23(6-7):476–482. doi: 10.1111/j.1440-1681.1996.tb02764.x. [DOI] [PubMed] [Google Scholar]
- 141.Després JP, Golay A, Sjöström L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. The New England Journal of Medicine. 2005;353(20):2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 142.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. The Lancet. 2005;365(9468):1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 143.Sam AH, Salem V, Ghatei MA. Rimonabant: from RIO to Ban. Journal of Obesity. 2011;2011 doi: 10.1155/2011/432607.432607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patel PN, Pathak R. Rimonabant: a novel selective cannabinoid-1 receptor antagonist for of treatment obesity. American Journal of Health-System Pharmacy. 2007;64(5):481–489. doi: 10.2146/060258. [DOI] [PubMed] [Google Scholar]
- 145.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 146.Tam J, Vemuri VK, Liu J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity . Journal of Clinical Investigation. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jourdan T, Djaouti L, Demizieux L, Gresti J, Vergès B, Degrace P. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59(4):926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Soory M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: potential for therapeutic targeting. Infectious Disorders. 2009;9(4):400–414. doi: 10.2174/187152609788922537. [DOI] [PubMed] [Google Scholar]
- 149.Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Della Ragione F. Resveratrol: from basic science to the clinic. Cell Cycle. 2007;6(20):2495–2510. doi: 10.4161/cc.6.20.4815. [DOI] [PubMed] [Google Scholar]
- 150.De La Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Molecular Nutrition and Food Research. 2005;49(5):405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 151.Gómez-Zorita S. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. British Journal of Nutrition. 2012;107:202–210. doi: 10.1017/S0007114511002753. [DOI] [PubMed] [Google Scholar]
- 152.Bujanda L, Hijona E, Larzabal M, et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterology. 2008;8, article 40 doi: 10.1186/1471-230X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacologica Sinica. 2008;29(6):698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 154.Cotrim HP, Freitas LA, Alves E, Almeida A, May DS, Caldwell S. Effects of light-to-moderate alcohol consumption on steatosis and steatohepatitis in severely obese patients. European Journal of Gastroenterology and Hepatology. 2009;21(9):969–972. doi: 10.1097/MEG.0b013e328328f3ec. [DOI] [PubMed] [Google Scholar]
- 155.Gunji T, Matsuhashi N, Sato H, et al. Light and moderate alcohol consumption significantly reduces the prevalence of fatty liver in the japanese male population. American Journal of Gastroenterology. 2009;104(9):2189–2195. doi: 10.1038/ajg.2009.361. [DOI] [PubMed] [Google Scholar]
- 156.Moriya A, Iwasaki Y, Ohguchi S, et al. Alcohol consumption appears to protect against non-alcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics. 2011;33(3):378–388. doi: 10.1111/j.1365-2036.2010.04520.x. [DOI] [PubMed] [Google Scholar]
- 157.Wang XF, Yue M. Relationship between alcohol consumption and clinical manifestation of patients with fatty liver: a single-center study. Hepatobiliary and Pancreatic Diseases International. 2011;10(3):276–279. doi: 10.1016/s1499-3872(11)60046-5. [DOI] [PubMed] [Google Scholar]
- 158.Ekstedt M, Franzén LE, Holmqvist M, et al. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scandinavian Journal of Gastroenterology. 2009;44(3):366–374. doi: 10.1080/00365520802555991. [DOI] [PubMed] [Google Scholar]
- 159.Alatalo PI, Koivisto HM, Hietala JP, Puukka KS, Bloigu R, Niemelä OJ. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. American Journal of Clinical Nutrition. 2008;88(4):1097–1103. doi: 10.1093/ajcn/88.4.1097. [DOI] [PubMed] [Google Scholar]
- 160.Crabb DW. Alcohol deranges hepatic lipid metabolism via altered transcriptional regulation. Transactions of the American Clinical and Climatological Association. 2004;115:273–287. [PMC free article] [PubMed] [Google Scholar]
- 161.Donohue TM., Jr. Alcohol-induced steatosis in liver cells. World Journal of Gastroenterology. 2007;13(37):4974–4978. doi: 10.3748/wjg.v13.i37.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sozio MS, Chalasani N, Liangpunsakul S. What advice should be given to patients with NAFLD about the consumption of alcohol? Nature Clinical Practice Gastroenterology and Hepatology. 2009;6(1):18–19. doi: 10.1038/ncpgasthep1314. [DOI] [PubMed] [Google Scholar]


