Abstract
Interplay between Foxp3+ regulatory T cells (Treg) and dendritic cells (DCs) maintains immunologic tolerance, but the effects of each cell on the other are not well understood. We report that polyclonal CD4+Foxp3+ Treg cells induced ex vivo with transforming growth factor beta (TGFβ) (iTreg) suppress a lupus-like chronic graft-versus-host disease by preventing the expansion of immunogenic DCs and inducing protective DCs that generate additional recipient CD4+Foxp3+ cells. The protective effects of the transferred iTreg cells required both interleukin (IL)-10 and TGFβ, but the tolerogenic effects of the iTreg on DCs, and the immunosuppressive effects of these DCs were exclusively TGFβ-dependent. The iTreg were unable to tolerize Tgfbr2-deficient DCs. These results support the essential role of DCs in ‘infectious tolerance’ and emphasize the central role of TGFβ in protective iTreg/DC interactions in vivo.
Keywords: regulatory T cells, dendritic cells, TGFβ, graft-versus-host disease
Introduction
Foxp3+ regulatory T cells (Treg) consisting of heterogeneous thymus-derived natural cells (nTreg) and those cells induced in the periphery (iTreg) are essential in maintaining immune tolerance and preventing autoimmune diseases (Andersson et al., 2008; Horwitz et al., 2008; Lan et al., 2012). Abnormalities in numbers and/or function of Foxp3+ Treg have been reported in many autoimmune diseases. Treg are short-lived cells with a rapid turnover. Continuous antigen stimulation provided by tolerogenic dendritic cells (DCs) is required for Treg persistence.
DCs are specialized antigen-presenting cells (APCs) that initiate and regulate immune responses against foreign as well as self-antigens (Steinman et al., 2003). Different subsets of DCs direct immune responses depending upon their maturation state. While mature DCs promote adaptive immune responses against microbial invaders, immature and certain semi-mature DCs are tolerogenic (Horwitz et al., 2008). Several cytokines such as transforming growth factor beta (TGFβ), interleukin (IL)-10, IL-27 and other agents such as vitamin D3 and indoleamine-2,3-dioxygenase (IDO) promote the tolerogenic phenotype of DCs (Awasthi et al., 2007; Pallotta et al., 2011). Tolerogenic DCs can control autoimmunity directly by producing anti-inflammatory cytokines such as IL-10 or TGFβ, or indirectly by an IDO-dependent mechanism (Coombes et al., 2007; Kaplan et al., 2007; Favre et al., 2010).
Treg have the ability to induce conventional T cells to become additional tolerant Foxp3+ cells through a mechanism called ‘infectious tolerance’. This phenomenon was first described by Waldmann's group, which was investigating transplantation tolerance (Qin et al., 1993). Subsequent work confirmed that interplay between Treg and DCs involving TGFβ is required for the generation of new CD4+Foxp3+ Treg cells (Cobbold et al., 2009).
We have investigated the protective role of CD4+Foxp3+ Treg in the prevention and treatment of established autoimmune diseases. We have reported that unlike endogenous CD4+CD25+Foxp3+ nTreg which can convert to pathogenic Th17 cells in an inflammatory environment, nTreg treated ex vivo with retinoic acid, and iTreg induced ex vivo with IL-2, TGFβ and retinoic acid are resistant to conversion, and others recently confirmed this finding (Zheng et al., 2008; O'Connor et al., 2010; Zhou et al., 2010a; Lu et al., 2011; Kong et al., 2012). Here, we report that in a chronic graft-versus-host disease (cGVHD) with a lupus-like syndrome, transferred iTreg block the expansion of immunogenic DCs and instead induce tolerogenic DCs that generate more iTreg. These effects were TGFβ-dependent and required TGFBR2 receptor and intact TGFβ signaling in DCs. While IL-10 also contributed to the direct protective effects of iTreg, this cytokine was not required for the necessary iTreg/DC interaction, or for the protective effects of induced tolerogenic DCs.
Results
Phenotypic characteristics of polyclonally differentiated CD4+Foxp3+ cells generated ex vivo with IL-2 and TGFβ
As reported previously, TGFβ is a crucial cytokine that can induce differentiation of iTreg from conventional naïve CD4+CD25− cells (Zheng et al., 2002). Foxp3, an important transcription factor regulating the development and function of Treg (Fontenot et al., 2003), was induced in the CD4+ and CD25+ cell population after TGFβ priming in DBA/2 (D2) WT (Figure 1A, top panel) or C57BL/6 Foxp3gfp knock-in mice (Figure 1A, lower panel). Additionally, these Foxp3+ cells also expressed other Treg-related molecular markers such as CD103, CD39, PD1, CTLA-4, and GITR (Supplementary Figure S1A). These cells expressed some levels of membrane-bound TGFβ and secreted active TGFβ and IL-10 (Supplementary Figure S1A and B). Interestingly, these cells did not express Helios (Supplementary Figure S1A), suggesting that the iTreg might be a different linage compared with nTreg since the latter express high levels of Helios (Thornton et al., 2010). Unlike nTreg, iTreg produced low levels of IL-2 (Supplementary Figure S1A), and this difference may explain the different stabilities of both Treg in the presence of IL-6 since IL-2 can restrain Th17 cell differentiation. As these cells were produced by polyclonal stimulation and displayed suppressive activity, we refer to them as polyclonally differentiated iTreg or simply iTreg.
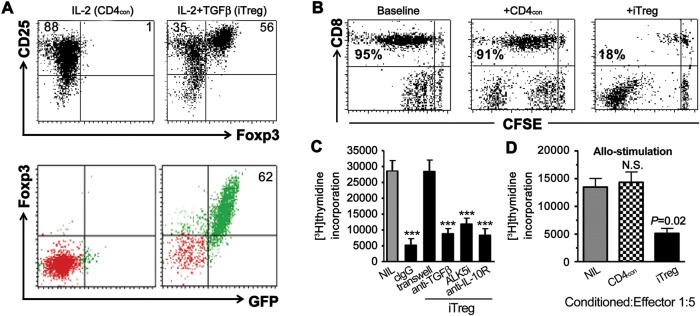
Figure 1.
Characteristics of polyclonally iTreg cells. (A) Naïve CD4+ T cells from DBA/2 or C57BL/6 Foxp3gfp knock-in mice were stimulated with anti-CD3/28 beads and rmIL-2 with (iTreg) or without TGFβ (CD4con) for 3–4 days. CD25 and Foxp3 (or GFP) expression was determined by flow cytometry. Numbers in the panel represents for the frequency of the quadrants. (B) iTreg or CD4con cells generated as before were cultured with CFSE-labeled CD25+-depleted T cells (1:5 ratio) in the presence of anti-CD3 and irradiated APC for 3 days. The proliferation (CFSE dilution) of responder T cells was analyzed by flow cytometry. Cells frequency showed in panel was gated on CD8+ cells. Data were representative of three independent experiments. (C) iTreg cells generated with IL-2 and TGFβ were cultured with CD25+-depleted T cells (1:5 ratio) in the presence of anti-CD3 and syngeneic APC for 3 days. Anti-TGFβ, ALK5i, anti-IL-10R, and isotype IgG (all 10 μg/ml) were added to the culture system separately. Transwell was also used to determine the cell-contact effect. [3H]thymidine was added to cultures for the last 18 h and incorporation was measured. Values were mean ± SEM of three independent experiments. ***P <0.001, iTreg vs. baseline. (D) iTreg or CD4con cells were added to CD25+-depleted T cells in the presence of allogeneic APC for 3 days. [3H]thymidine was used to measure the incorporation by proliferating T cells. Values were mean ± SEM of three independent experiments. P =0.02, iTreg vs. baseline.
iTreg suppress in vitro anti-CD3- and alloantigen-triggered T cell responses by cell contact-dependent mechanism
Similar to nTreg, CD4+ cells primed with TGFβ but not CD4+ control cells (treated without TGFβ, CD4con) suppressed anti-CD3 stimulated T cell proliferation including CD4+ and CD8+ cells. We have documented this result using both CFSE-labeling (Figure 1B) and [3H]thymidine incorporation assays (Figure 1C). Placement of iTreg in a transwell assay plate containing a semi-permeable membrane that separated iTreg from responder T cells abolished the suppressive activity of the iTreg (Figure 1C). Furthermore, the addition of blocking antibodies against TGFβ or IL-10R, or ALK5 (TGFBR1) inhibitor did not significantly diminish the suppressive activity of these cells (Figure 1C), suggesting that cell contact is needed for iTreg suppressive activity in vitro.
It is less known whether polyclonally differentiated iTreg also suppress the antigen-specific immune response. To address this issue, we performed a T cell proliferation assay using responder T cells isolated from D2 mice stimulated with γ-irradiated non-T cells isolated from C57BL/6 mice (allogeneic stimulation). The CD4con or iTreg were added to some cultures (1:5 ratio). After 3-day co-culture, the iTreg but not CD4con cells significantly suppressed alloantigen-stimulated T cell proliferation (Figure 1D), suggesting that iTreg exert both antigen-specific and antigen-non-specific suppressive effect against T cell immune responses.
Polyclonally differentiated iTreg suppress cGVHD with a lupus-like syndrome through TGFβ- and IL-10-dependent signaling pathways
The employed cGVHD is achieved by an adoptive transfer of parental D2 splenocytes, T or CD4+ cells into DBA/2 × C57BL/6 F1 (D2B6F1) mice (Zheng et al., 2004b), and is characterized by a typical lupus-like syndrome including elevated anti-dsDNA antibodies, proteinuria, and lupus nephritis. The disease is initiated by the activation of the donor CD4+ cells (H2d) upon the encounter of the B6 (H2b) antigen. Our previous study has revealed that antigen-specific iTreg suppressed cGVHD syndrome (Zheng et al., 2004b). In the present study, we set out to determine whether polyclonally differentiated iTreg also suppress the alloantigen-mediated cGVHD.
As D2 CD4+ T cells are pathogenic and iTreg originated from D2 cells, we first determined whether the latter exerts a similar pathogenic effect. As shown in Supplementary Figure S2A, adoptive transfer of 12 × 106 D2 CD4+ T cells to D2B6F1 mice resulted in elevated anti-dsDNA antibody titers compared with mice that received no cells. The rapid increase in anti-dsDNA antibodies was observed at 2 weeks and was sustained until at least 12 weeks after cell transfer. The transfer of a similar number of CD4con cells had a similar effect on anti-dsDNA antibody production in D2B6F1 mice. In sharp contrast, injection of 12 × 106 of iTreg did not elicit anti-dsDNA antibody production, which remained at the same level as in naïve D2B6F1 mice. D2B6F1 mice developed marked proteinuria 12 weeks following CD4+ D2 or CD4con cells transfer, but not following iTreg transfer (Supplementary Figure S2B). Collectively, these findings indicate that TGFβ priming alters the phenotype and behavior of CD4+ D2 cells.
We further determined whether iTreg can suppress immune responses in cGVHD mice. To assess this possibility, we transferred 12 × 106 D2 CD4+ cells, or plus either 5 × 106 iTreg or CD4con cells. The number of transferred iTreg was based on our earlier study with antigen-specific Treg cells required for optimal protection from cGVHD symptoms (Zheng et al., 2004b). As shown in Figure 2A, co-transfer of CD4+ D2 cells with iTreg but not with CD4con markedly suppressed the production of anti-dsDNA antibodies. Transfer of CD4+ D2 cells alone resulted in the death of all mice by 20 weeks post-transfer (Figure 2B). Co-transfer of CD4con cells with D2 cells neither prolonged nor shortened the survival of mice. Co-transfer of iTreg with D2 CD4+ T cells significantly prolonged the survival of cGVHD mice (Figure 2B). Collectively, these results show that iTreg exhibit suppressive function in both antigen-specific and antigen-non-specific fashions in vitro and in vivo.
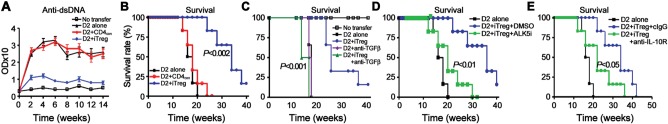
Figure 2.
The suppressive effect of iTreg in cGVHD model with a lupus-like syndrome is almost completely dependent upon TGFβ and partially upon IL-10. 12 × 106 fresh D2 CD4+ cells or together with 5 × 106 iTreg or 5 × 106 CD4con were transferred into D2B6F1 mice (A and B), anti-TGFβ antibody or control IgG was co-administered in some groups (C). (A) Anti-dsDNA levels were examined by ELISA. (B and C) Mice survival was monitored. (D and E) ALK5 inhibitor (ALK5i) or DMSO (vehicle), anti-IL-10R or control IgG was administrated and survival was monitored. Five mice in each group were included in each experiment and data were combined from at least two independent experiments.
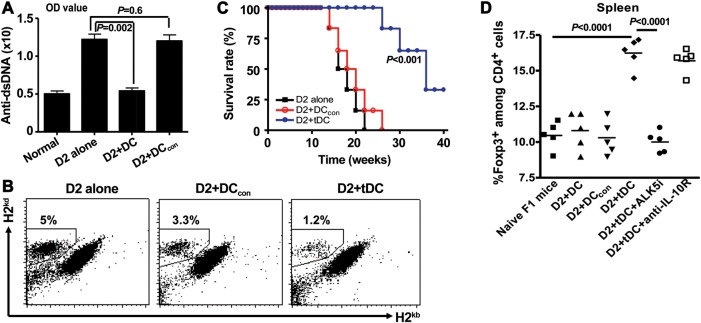
The mechanism(s) whereby Treg suppress immune responses remains incompletely defined. Both nTreg and iTreg express membrane-bound TGFβ and secrete active TGFβ and/or IL-10—features important for their suppressive function as well as Th17 cell conversion (Xu et al., 2007; Zheng et al., 2008). To determine whether these two cytokines are also involved in the suppressive mechanisms of iTreg in cGVHD in vivo, we used neutralizing antibodies and receptor inhibitors. While co-transfer of iTreg with CD4+ D2 cells significantly suppressed IgG upregulation (Supplementary Figure S2C) and prolonged D2B6F1 mice survival (Figure 2C), anti-TGFβ antibody not only completely abolished this protective effect of iTreg, but actually led to a further increase in IgG levels. Further studies are needed to determine whether iTreg have converted to a T helper phenotype following anti-TGFβ treatment. Anti-TGFβ antibody also completely reversed the protective effect of iTreg on mouse survival, with a trend toward accelerated death of cGVHD mice. This result could not be explained by the effect of antibody on the primary disease progression since such doses of anti-TGFβ treatment alone did not alter the levels of IgG or survival in cGVHD mice (Figure 2C and Supplementary Figure S2C).
To further determine whether the TGFβ signaling pathway is crucial for the iTreg-mediated suppression in cGVHD, we also blocked the TGFβ receptor I (ALK5) activity using ALK5 inhibitor (ALK5i) in iTreg-co-transferred D2B6F1 mice. Similar to anti-TGFβ antibody, injection of ALK5i almost completely abolished the protective effect of iTreg on mouse survival (Figure 2D). DMSO (vehicle) alone did not alter the disease course. Moreover, blockade of IL-10 signaling with anti-IL-10R antibody also significantly altered the survival of lupus mice transferred with iTreg, albeit to a lesser degree than anti-TGFβ antibody or ALK5i (Figure 2E). Together, these results suggest that iTreg can suppress lupus-like autoimmune disease primarily through TGFβ and partially via IL-10 signal pathways.
Polyclonally differentiated iTreg induced the formation of tolerogenic DCs through TGFβ signaling pathway in DCs
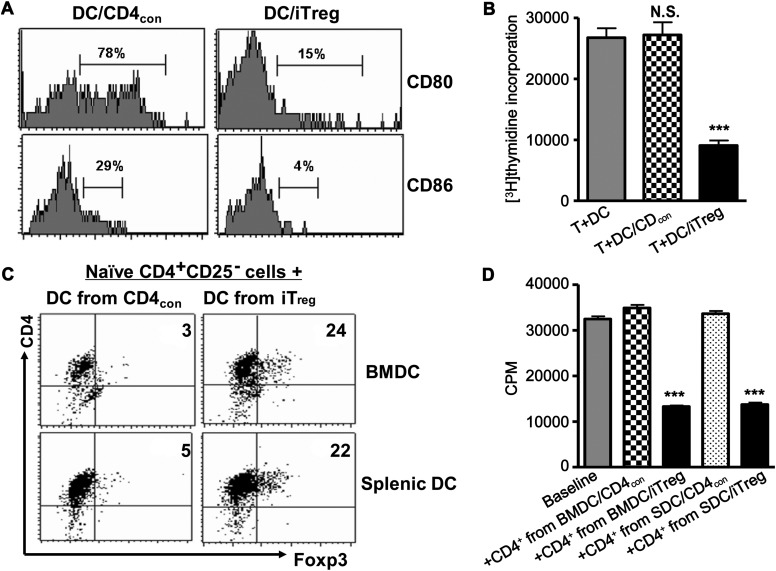
Our previous report demonstrated that adoptively transferred iTreg had a limited lifespan in the recipient mice but sustained long-term protective effect in the prevention of allograft rejection (Zheng et al., 2006). As iTreg can educate naïve T cells to become a new generation of Foxp3+ Treg in vitro (Zheng et al., 2004a) and DCs may be involved in this effect (Andersson et al., 2008; Horwitz et al., 2008), we have tested the effect of iTreg on DC maturation and function. When bone marrow-derived (BMDC) or splenic CD11c+ DCs were co-cultured with CD4con or CD4+ iTreg derived from congenic CD45.1+ C57BL/6 mice, iTreg but not CD4con cells markedly suppressed the up-regulation of CD80 and CD86 expression by DCs (Figure 3A). These DCs produced low levels of IL-12 and IL-23 (data not shown) and displayed decreased antigen-presenting function (Figure 3B). When DCs that had been co-cultured with iTreg were added to allogenic T cells, proliferation of these T cells was significantly reduced compared with T cells stimulated with freshly isolated DCs or DCs that had been co-cultured with CD4con cells (Figure 3B). When naïve CD4+CD25− cells from CD45.2+ C57BL/6 mice were co-cultured with DCs that had been previously conditioned by CD45.1+ iTreg in the absence of exogenous TGFβ for 3 days, about 25% of the naïve CD4+CD25− cells became CD25+Foxp3+ (Figure 3C). This was not observed for DCs conditioned by CD4con cells. These newly induced iTreg were gated on CD45.2, thereby excluding the possibility that the Foxp3+ cells were carried over with the initial iTreg pool. Furthermore, using a T cell suppression assay, we demonstrated that these newly generated CD4+CD25+Foxp3+ cells developed suppressive capacity. Both splenic DCs and BMDC displayed a similar ability to develop into ‘tolerogenic DCs (tDCs)’ (Figure 3D).
Figure 3.
iTreg induce the formation of tolerogenic DCs in vitro. CD4con cells or iTreg generated from CD45.1+ B6 mice and CD11c+ cells isolated from B6 mice were co-cultured (5:1 ratio) in the presence of GM-CSF, IL-4 and anti-CD3 for 3 days. (A) CD80 and CD86 expression on CD11c+ cells was analyzed by flow cytometry. Data are representative of three separate experiments. (B) When cells were harvested, CD4+ cells were removed by magnetic beads and the remaining CD11c+ cells were added to CD25+-depleted T cells (1: 5 ratio) for additional 3 days. [3H]thymidine was added to cultures for the last 18 h and incorporation by proliferating T cells was measured. Values were mean ± SEM of three independent experiments. ***P <0.001, DC primed with iTreg vs. DC primed with CD4con cells. (C) CD11c+ cells isolated from either bone marrow (BMDC) or spleen (SDC) and primed with iTreg or CD4con cells were added to naïve CD4+CD25− cells isolated from CD45.2+ B6 mice (1:5 ratio) in the presence of IL-2 (40 units/ml) for 3 days and Foxp3 expression was measured on gated CD4+CD45.2+cells using flow cytometry. Data are representative of three separate experiments. (D) CD45.2+CD4+ cells primed with DCs were isolated and added to CD25+-depleted T cells in the presence of anti-CD3 and APC for an additional 3 days. [3H]thymidine was added to the cultures for the last 18 h and incorporation by proliferating T cells was measured. Values were mean ± SEM of three independent experiments. ***P <0.001, DCs primed with iTreg vs. DCs primed with CD4con cells.
Since both TGFβ and IL-10 are involved in the suppressive activity of iTreg in vivo and have the functional capacity to induce tDCs (Pallotta et al., 2011), we determined the role of these cytokines in the iTreg-induced formation of tDCs. As shown in Figure 4A and B, co-culture of DCs with iTreg but not CD4con cells suppressed CD80 and CD86 expression. This effect was completely abrogated by the addition of ALK5i, but not by anti-IL-10R antibody. DCs that had been primed with iTreg in the presence of ALK5i but not with anti-IL-10R antibody mostly restored their antigen-presenting capacity (Supplementary Figure S3) and lost the ability to induce naïve CD4+CD25− cells to acquire Foxp3 expression (data not shown). iTreg, therefore, induce the formation of tDCs mainly through TGFβ rather than via the IL-10 signaling pathway.
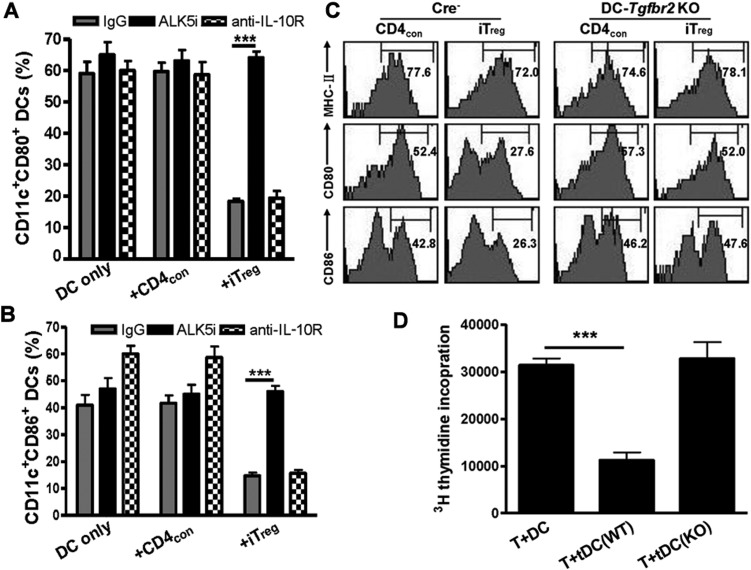
Figure 4.
TGFβ but not IL-10 signaling is required for the formation of tolerogenic DCs induced by iTreg. CD11c+ cells cultured alone or in combination with CD4con or iTreg (1:5 ratio) were stimulated with GM-CSF, IL-4 and anti-CD3 for 3 days. In some cultures, anti-IL-10R, control IgG or ALK5i was added to cultures. (A and B) CD80 and CD86 expression was determined by flow cytometry. Values were mean ± SEM. ***P <0.001, iTreg primed DCs treated with ALK5i vs. with control. (C) Splenic CD11c+ cells isolated from Cre− and DC-Tgfbr2 KO mice were co-cultured with CD4con or iTreg cells (1:5 ratio) in the presence of GM-CSF, IL-4 and anti-CD3 for 3 days. MHC-II, CD80 and CD86 expression on CD11c+ cells was analyzed by flow cytometry. Data are representative of five independent experiments. (D) Splenic CD11c+ cells in C57BL/6 WT (Cre−) or DC-Tgfbr2 KO mice were co-cultured with iTreg for 3 days as above. CD11c+ cells were then sorted and added to CD25+-depleted T cells from D2 mice and T cell proliferation was measured by 3[H]-incorporation. Values are mean ± SEM of three separate experiments. ***P <0.001, DCs primed with WT iTreg vs. DC primed with Tgfbr2 KO iTreg.
We further verified the role of TGFβ signaling pathway on the induction of tDCs using mice with a conditional knockout of Tgfbr2 gene in CD11c+ DCs. The DC-Tgfbr2 KO mice were generated in Kiela's laboratory (see Materials and methods) and their phenotypic features have been described in detail elsewhere (unpublished data). Although these mice eventually succumb to multi-organ autoimmune inflammation and die by 14 weeks of age, in asymptomatic mice between 6 and 8 weeks of age, the CD80 expression by CD11c+ cells was similar between Cre− (wild type) and Cre+ (DC-Tgfbr2 KO) littermates. CD86 expression on CD11c+ cells was almost undetectable in both Cre− and DC-Tgfbr2 KO mice (Supplementary Figure S4). When splenic CD11c+ DCs isolated from Cre− or DC-Tgfbr2 KO mice were stimulated with GM-CSF, TNF-α and IL-2, both CD80 and CD86 expression was similarly up-regulated. Interestingly, the addition of iTreg but not CD4con, significantly suppressed CD80 and CD86 upregulation in DCs derived from Cre− mice but not in DCs from DC-Tgfbr2 KO mice (Figure 4C). Although iTreg did not alter the MHC-II expression on DCs isolated from Cre− mice, the functional ability of these DCs to trigger allogeneic immune responses was decreased. However, DCs from DC-Tgfbr2 KO mice developed potent antigen-stimulating abilities, even after priming with iTreg (Figure 4D). These results indicate that the TGFβ signaling pathway in DCs is crucial for the formation of tDCs induced by iTreg.
Polyclonally differentiated iTreg suppress the expansion and maturation of DCs in a lupus-like mouse model
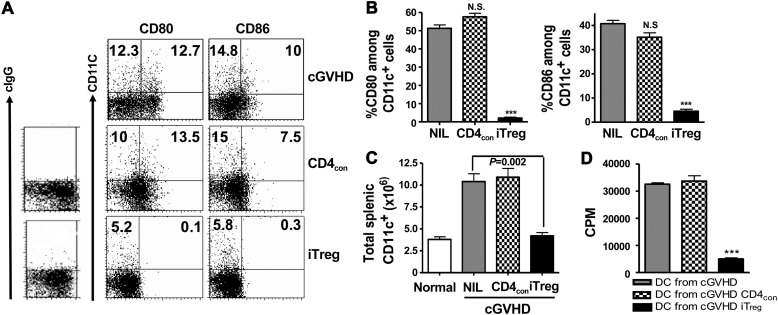
To verify our in vitro findings in an in vivo model of lupus-like syndrome, we employed the T cell transfer cGVHD model. We demonstrated that in D2B6F1 mice 3 weeks after CD4+ D2 cell transfer, CD11c+ cells expressed substantial amounts of CD80 and CD86. Interestingly, co-transfer of pathogenic D2 cells with iTreg, but not with CD4con cells, prevented the upregulation of CD80 and CD86 on CD11c+ cells (Figure 5A and B) as well as B cells (data not shown).
Figure 5.
Adoptive transfer of iTreg to lupus mice suppresses the expansion of DCs and decreases their B7 expression. 12 × 106 fresh D2 CD4+ cells alone or together with 5 × 106 CD4con cells or iTreg were transferred into D2B6F1 mice. (A and B) CD80 and CD86 expression in each group of mice was examined 3 weeks later. ***P <0.001, iTreg vs. CD4con cells. (C) Total splenic CD11c+ cells were counted. Data are representative or values are mean ± SEM of five mice in each experiment and combined from two independent experiments. (D) CD11c+ cells were sorted from each group of D2B6F1 mice as above and added to cultures containing CD25+-depleted T cells isolated from BALB/c mice for 3 days. T cell proliferation was determined as in above. Values are mean ± SEM of three separate experiments. ***P <0.001, DCs primed with iTreg vs. DC primed with CD4con.
DCs play an important role in the pathogenesis of SLE (Monrad and Kaplan, 2007). Consistently, D2B6F1 mice displayed 3-fold expansion of total splenic CD11c+ cells 3 weeks after D2 cell transfer. Co-transfer of CD4con with D2 cells did not alter the total numbers of splenic CD11c+ cells in F1 mice. However, co-transfer of iTreg with D2 cells almost completely suppressed the expansion of CD11c+ cells in F1 mice (Figure 5C). To determine their functional activity, splenic CD11c+ cells were further sorted and added to BALB/c naïve T cells for a 3-day in vitro co-culture. CD11c+ cells sorted from either cGVHD or CD4con cell-transferred cGVHD mice induced strong alloresponses. In contrast, CD11c+ cells sorted from iTreg-transferred cGVHD mice had greatly reduced ability to stimulate allo-T cell proliferation (Figure 5D).
tDCs suppress cGVHD through TGFβ- but not IL-10-dependent signaling pathway
To further analyze the functional characteristics of tDCs in lupus mice treated with iTreg, we isolated CD11c+ DCs from D2B6F1 3 weeks after co-transfer of D2 cells with CD4con or iTreg. Sorted DCs were then co-transferred with fresh D2 cells into naïve D2B6F1 mice. As shown in Figure 6A, compared with D2B6F1 mice which received D2 T cells alone, infusion of CD11c+ cells isolated from lupus mice treated with iTreg (tDCs) significantly prevented the anti-dsDNA antibody serum titers 3 weeks post transfer. These DCs also suppressed the donor T cell engraftments in recipient spleens (Figure 6B). The degree of donor cell engraftment correlates with the disease severity in this lupus model (Zheng et al., 2004b). Importantly, transfer of tDCs also markedly prolonged the survival of lupus mice (Figure 6C). In contrast, transfer of the same number of DCs sorted from lupus mice or from mice treated with CD4con did not suppress anti-dsDNA antibody production and donor engraftment expansion, and didn't prolong the survival. (Figure 6A–C). tDCs suppressed donor engraftment through TGFβ but not the IL-10 signal pathway (Supplementary Figure S5). Because tDCs can suppress immune responses either directly or indirectly, we also examined the Foxp3+ cell frequency in lupus mice after tDC treatment. We observed that transfer of DCs sorted from iTreg-treated lupus mice markedly increased the frequency of Foxp3+ cells when compared with controls. Moreover, the expansion of Foxp3+ cells was dependent on TGFβ rather than IL-10 signaling pathway since only administration of ALK5i could abolish the Foxp3+ cell increase (Figure 6C) following tDCs treatment.
Figure 6.
DCs isolated from lupus mice treated with iTreg but not CD4con cells suppress disease development. 12 × 106 fresh D2 CD4+ cells alone or together with 5 × 106 CD4con or iTreg were transferred into D2B6F1 mice. Three weeks later, spleens were removed, CD11c+ cells were sorted, and 5 × 105 DCs and 12 × 106 fresh D2 CD4+ cells were co-transferred into D2B6F1 mice. (A) Anti-dsDNA antibody level in sera was examined 2 weeks later. (B) Donor engraftments (H-2d+H-2b− cell population) in the spleens were determined 2 weeks following cell transfer. (C) Survival was monitored. (D) ALK5 inhibitor (ALK5i) or DMSO (vehicle), anti-IL-10R or control IgG was administrated. Foxp3+ cell frequency in spleen of D2B6F1 mice at one month following cell transfer was determined by flow cytometry. DCcon mean DCs from cGVHD mice transferred with CD4con cells; tDCs mean DCs from cGVHD mice transferred with iTreg.
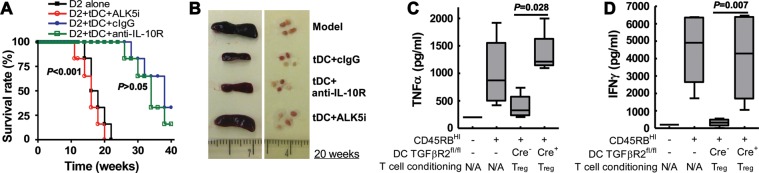
We next attempted to explore the underlying mechanism of how tDCs suppress lupus. As shown in Figure 7, co-transfer of tDCs sorted from lupus mice that received iTreg markedly prolonged survival (Figure 7A) and suppressed splenomegaly and lymph node enlargement (Figure 7B). Administration of ALK5i with tDCs not only completely blocked the suppressive activity of tDCs in cGVHD, but also slightly reduced the survival time in these mice. Anti-IL-10R antibody administration did not significantly decrease survival in the tDCs infusion group (Figure 7A). tDCs also suppressed renal IgG deposition and ALK5i but not anti-IL-10R antibody reversed those protective effects of tDCs (Supplementary Figure S6). These observations suggest that iTreg transfer induces the formation of tDCs in the context of ongoing inflammation in vivo and that these tDCs suppress T cell-mediated immune responses through TGFβ signaling rather than via the IL-10 signaling pathway.
Figure 7.
Tolerogenic DCs suppress lupus through TGFβ but not IL-10-dependent mechanism. 5 × 105 CD11c+ cells were sorted from lupus mice treated with iTreg (tDCs) or CD4con (DCcon) and were co-transferred with 12 × 106 fresh D2 CD4+ cells into D2B6F1 mice. ALK5i or DMSO (vehicle), anti-IL-10R or control IgG was administrated in separate groups. (A) Survival was monitored for 20 weeks. (B) The size of spleens and lymph nodes at 20 weeks following cell transfer were assessed. (C and D) iTreg were co-cultured with DCs from Cre− or DC-Tgfbr2 KO mice for 3 days. These DCs (2 × 105) were then co-transferred with naïve CD4+CD45Rbhigh cells (5 × 105) into Rag1−/−mice. Mice were sacrificed 4 weeks after cell transfer. MLN cell suspensions were cultured with anti-CD3/CD28 antibodies and supernatants analyzed for TNFα and IFNγ with xMAP multiplex assay. All experiments were repeated at least twice with similar results.
To further confirm these findings, we tested the ability of in vitro generated tDCs to control immune activation of naïve CD4+ T cells in a lymphopenic host. iTreg were induced in vitro with TGFβ as above from naïve CD4+ in Foxp3gfp knock-in mice. CD11c+ splenic DCs isolated from Cre− or DC-Tgfbr2 KO mice were then co-cultured with flow-sorted GFP+ iTreg or CD4con cells for 3 days. CD11c+ cells were then re-sorted from the co-culture and co-transferred with naïve CD4+CD45Rbhi cells into Rag1−/− mice. Typically, Rag mice transferred with naïve T cells in an analogous way develop colitis within 6–8 weeks. Due to the anticipated short half-life of the transferred DCs, we limited the evaluation of colitis to 4 weeks post-transfer and to more objective markers of immune activation such as the cytokine production in mesenteric lymph nodes (ELISA) and colonic cytokine expression (real-time RT–PCR). While both interferon (IFN)γ and TNFα protein (Figure 7C and D) and mRNA (Supplementary Figure S7) were significantly elevated 4 weeks after CD4+CD45Rbhi cell transfer, co-transfer of Cre− DCs primed with iTreg but not CD4con attenuated both IFNγ and TNFα production. However, co-transfer of Tgfbr2 KO DCs primed with iTreg or CD4con did not suppress IFNγ and TNFα production, further supporting the notion that iTreg induce the formation of tDCs via a TGFβ-dependent mechanism.
Discussion
It has been well documented that both nTreg and iTreg cells suppress the development of autoimmune diseases. However, the mechanisms whereby Treg subsets suppress immune response remain incompletely understood. Although cell contact is required for the suppression of immune response by nTreg in vitro (Piccirillo et al., 2002), immunosuppressive factors such as TGFβ and/or IL-10 appear to be critically involved in the suppression of immune responses and disease progress by nTreg in vivo (Maloy et al., 2003; Fahlen et al., 2005). In this study, we have demonstrated that TGFβ signaling is indispensable while IL-10 plays a less prominent role in the suppression of lupus, although these soluble factors did not contribute to the suppressive activity of iTreg in vitro. Although the direct link between the active TGFβ secretion and membrane-bound TGFβ expression by iTreg is unidentified, it is possible that iTreg that did not express membrane-bound TGFβ also produce active TGFβ (Tran et al., 2009). It is possible that cell contact may play a dominant role in vitro due to the limited cell mobility in confined spaces. However, it is evident that cytokines produced by Treg have a systemic role in the suppression of immune responses in vivo.
In general, antigen-specific Treg are believed to have a more potent suppressive ability than non-specific Treg (Tang and Bluestone, 2008). Another advantage of antigen-specific Treg is that they can selectively suppress immune responses without compromising other beneficial immune responses, and therefore are especially suitable for providing protection from organ transplant rejection. Nonetheless, in some autoimmune diseases such as lupus, the specific antigens are ill-defined and polyclonal Treg may be suitable in such a situation. In the current study, we demonstrate that polyclonal iTreg suppressed anti-CD3 and alloantigen-stimulated T cell responses and alloantigen-mediated cGVHD, implying that the manipulation of polyclonal iTreg may be of therapeutic value for the systemic autoimmune diseases that lack the identification of specific antigens. An earlier study has demonstrated that the suppressive function of nTreg is antigen-non-specific when they have been activated (Thornton and Shevach, 2000). It is also possible that polyclonal iTreg can be selectively expanded following specific antigen stimulation (Godebu et al., 2008). It should be noted that 5million of iTreg result in an optimally protective effect in cGVHD and Foxp3 GFP knock-in mouse in DBA/2 strain is not available; we have used iTreg that consisted of the mixed populations of 60%–75% Foxp3+ cells and 25%–40% Foxp3− cells. To this end, we demonstrated that the suppressive effect of iTreg mixed populations on cGVHD is comparable to the effect of purified Foxp3+ iTreg in colitis. After TGFβ treatment, these activated CD4+Foxp3− cells do not develop a pathogenic effect, implying an important clinical relevance since current methods are unable to sort the purified Foxp3+ Treg population in humans.
We have documented therapeutically important interactions between polyclonal iTreg and DCs in mice with a lupus-like cGVHD disease. Although these Treg are short-lived, a single injection doubled the survival of these mice. The transferred iTreg not only inhibited co-stimulatory molecule expression and prevented the expansion of immunogenic DCs, but also induced them to become tolerogenic. The secondary transfer of these tDCs into new mice with this fatal syndrome had protective effects equivalent to the initially transferred iTreg. Moreover, both in vitro and in vivo these tDCs converted CD4+ cells into additional Foxp3+ iTreg. Although the protective effects of the transferred iTreg in this model was both TGFβ- and IL-10-dependent, the tolerogenic effects of the iTreg on DCs and the subsequent therapeutic effects of tDCs on lupus were clearly TGFβ-, but not IL-10-dependent. It is likely that the transferred Foxp3+ iTreg predominately produced active TGFβ over IL-10 (Zheng et al., 2002).
Qin et al. (1993) previously reported that CD4+ cells from mice rendered tolerant to MHC mismatched skin grafts could prevent other T cells in these mice from rejecting the grafts and could transfer this suppressive capacity to other T cells by ‘infectious tolerance’. Until recently, it was unclear whether this transfer was a direct effect or mediated by an intermediate APC. In vitro, several groups have reported that Treg have a direct effect which is dependent upon IL-10 (Dieckmann et al., 2002), TGFβ (Jonuleit et al., 2002; Andersson et al., 2008)_ENREF_23, and most recently IL-35 (Chaturvedi et al., 2011). CD4+Foxp3+ Treg have a vital role in sustaining infectious tolerance (Kendal et al., 2011).
It has become apparent that in vivo DCs have an essential intermediate role in enabling Treg to convert other T cells to similar suppressor cells. Initially, CD4+Foxp3+ nTreg were reported to prevent immature DCs from becoming immunogenic by direct contact and inhibiting their expression of B7 (Tang et al., 2006; Onishi et al., 2008). Cobbold et al. (2009) then described a tolerogenic interaction between Treg and DCs in transplant tolerance in vivo. Subsequently, using transplant models, this group has accumulated considerable evidence that Treg effect on DCs leads to a local microenvironment depleted of tryptophan and other essential amino acids with enriched adenosine. This environment has tolerogenic properties that in synergy with TGFβ results in generation of new CD4+Foxp3+ iTreg (Cobbold et al., 2010). In our autoimmune model where Foxp3+ iTreg had the ability to modulate DCs to become tolerogenic and to secondarily induce other Foxp3+ T cells, TGFβ signaling in DCs was similarly critical.
Our findings that adoptive transfer of tDCs also suppressed lupus development are novel. Whether tDCs protection was a direct effect of DCs or due to their ability to induce or expand other CD4+Foxp3+ Treg in vivo cannot be distinguished. It has been known that tDCs produce TGFβ, IL-10, IL-27, retinoic acid, IDO, and vitamin D3 (Wakkach et al., 2003). These factors either suppress immune responses directly or indirectly via the induction of Treg subsets. It is likely that these factors produced by tDCs can enhance the stability of iTreg adoptively transferred in the disease since retinoic acid promotes iTreg and restrains the Th17 cell differentiation. We now observe that TGFβ, but not IL-10, plays an important role in the suppression of lupus-like disease following adoptive transfer of tDCs and the TGFβ signal in DCs is crucial for the formation and function of tDCs. Laouar et al. (2008) have previously reported that TGFβ signaling in DCs is a prerequisite for the control of autoimmune encephalomyelitis. The role for IL-10 in this model was not investigated. IL-10 has broad inhibitory effects on T cell activation, and has been recently reported to enhance the long-term viability of CD4+Foxp3+ Treg (Murai et al., 2009). Although we cannot exclude the direct action of tDCs, we believe that these DCs have at least indirectly caused the suppression of lupus through the increased frequency of Foxp3+ Treg. It is likely that these tDCs secrete TGFβ to educate the recipient's naïve T cells to become a new generation of Foxp3+ Treg in the presence of self or foreign antigens through ‘infectious tolerance’. These new Treg can continue to maintain immune tolerance and control the disease development.
It is important to emphasize that the effects of Treg on DCs in this study were conducted with iTreg differentiated ex vivo with TGFβ. Others have reported that iTreg were unstable and lacked suppressive activity on acute GVHD (Floess et al., 2007; Koenecke et al., 2009). However, in our experimental settings and the cGVHD model of lupus-like disease, these iTreg had evidently protective effects. In our earlier studies comparing both mouse and human nTreg and iTreg, the in vivo protective activity of these subsets was equivalent when transferred at the onset of disease (Zheng et al., 2004b; Zhou et al., 2010b). We have also reported that only iTreg induced ex vivo are resistant to conversion to Th17 cells following treatment with IL-6 in mice, or IL-1 and IL-6 in humans (Zheng et al., 2008; Lu et al., 2010). We have recently observed that only transferred iTreg shifted the balance of Th17 cells in draining lymph nodes from Th17-predominant to Treg-predominant in established CIA and controlled mast cells (Kong et al., 2012; Su et al., 2012). This effect is likely the result of a Treg-induced shift from immunogenic to tolerogenic DC phenotype/function. Thus, the potential therapeutic effect of Treg in chronic inflammatory, immune-mediated disease is likely to be determined by their effects on APCs in vivo.
Materials and methods
Mice
Female DBA/2 (D2, H-2d), C57BL/6 CD45.1 (B6, H-2b), C57BL/6 CD45.2, BALB/c, Rag-1 KO mice, and (DBA/2 × C57BL/6)F1 (D2B6F1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6 Foxp3 knock-in mice were generously provided by Dr Talil Chatilla (UCLA). To create DC-specific Tgfbr2 conditional knockout (CKO) mice, B6.129S6-Tgfbr2tm1Hlm mice (Tgfbr2fl/fl), carrying homozygous loxP site insertion flanking exon 2 of Tgfbr2 gene were crossed with CD11c-Cre transgenic mice (B6.Cg-Tg(Itgax-cre)1-1Reiz/J) (Caton et al., 2007). Cre−Tgfbr2fl/fl littermates were used as controls for Cre+Tgfbr2fl/fl (referred to as Cre− and DC-Tgfbr2 KO in the text, respectively). All animals were treated according to National Institutes of Health guidelines for the use of experimental animal with the approval of the University of Southern California and the University of Arizona Committees for the Use and Care of Animals (IACUC #11481 and #07-126, respectively).
The generation of CD4+ induced regulatory T cells (iTreg) ex vivo
Naïve CD4+CD62L+CD25−CD44low T cells were isolated from spleen cells of DBA2, C57BL/6 or C57BL/6 Foxp3gfp knock-in mice using naïve CD4+ T cell isolation kit (Miltenyi Biotec). Cells were cultured in 48-well plates and stimulated with anti-CD3/CD28 coated beads (1 bead per 5 cells, Invitrogen) in the presence of IL-2 (40 U/ml; R&D) with (iTreg) or without (CD4con) TGFβ (2 ng/ml; R&D) for 4 days. RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 10 mM HEPES (Invitrogen), and 10% heat-inactivated FCS (HyClone Laboratories) was used for all cultures. Foxp3 expression was determined by flow cytometry. The suppressive activity of these cells against T cell proliferation was examined with a standard in vitro suppressive assay as previously reported (Zheng et al., 2007). 5 × 106 cells were transferred to each D2B6F1 mice.
Co-culture of iTreg or CD4con with DCs
CD11c+ cells were isolated from bone marrow or spleens by flow sorting using FACS AriaII (BD Bioscience) and cultured with GM-CSF (500 U/ml) and IL-4 (200 U/ml) for 3 days. In some wells, CD4con or iTreg were added to DCs (5:1 CD4 to DC ratio) and co-cultures were activated with anti-CD3 (0.5 μg/ml; BD Pharmingen) for 3 days. CD80, CD86, MHC-II (all from Biolegend) expression on CD11c+ cells were analyzed by flow cytometry.
Induction and assessment of cGVHD with a lupus-like syndrome
Chronic GVHD with a lupus-like syndrome was induced in D2B6F1 mice by injecting 12 × 106 D2 CD4+ cells through the tail vein as described previously (Shustov et al., 1998). Other groups received this number of D2 cells plus 5 × 106 CD4con, iTreg or 5 × 105 DCs isolated from lupus-like syndrome mice transferred with CD4con or iTreg. To determine the suppressive mechanisms of Treg and tolerogenic DCs in vivo, anti-TGFβ1 (2G.7; R&D) (0.5 mg/kg body weight) or isotype-matched IgG1 antibody, anti-IL-10R (0.25 mg/kg body weight) or isotype-matched IgG1 antibody, or ALK5 inhibitor (ALK5i; LY-364947, 0.5 mg/mouse; Sigma) were administered i.p. weekly for a total of six injections. In most experiments, there were five mice per group, experiments have been repeated at least two times and data represented the accumulated results from all experiments. Before transfer and weekly thereafter, blood was collected and serum IgG and anti-dsDNA autoantibodies were measured by ELISA (Du Clos et al., 1986). All samples tested for anti-dsDNA antibodies were processed at the same time. Serum was diluted 1/400 or 1/800 for anti-dsDNA and 1/40000 for IgG measurement. Proteinuria was assessed using Albustix reagent strips (Bayer). Mice were sacrificed at time points indicated in the different experiments after transfer of parental T cells for assessment of lymphoid hyperplasia and immune complex glomerulonephritis. The total numbers and phenotypes of the spleen cells were determined from single-cell suspensions. The cells were stained with FITC-anti-H-2b, PE-anti-H-2d (BD PharMingen) and single-positive anti-H-2d cells considered to be parental D2 cells. Mouse survival was monitored every 3 days.
Co-transfer of tDCs and naïve T cells into Rag1−/− mice
CD4+CD45Rbhigh cells (0.5 × 106) flow sorted from splenocytes in naïve C57BL/6 mice (95%–100% purity) were intravenously injected into Rag1−/− mice (C57BL/6). Separate groups also received 2 × 105 DCs isolated from Cre− and DC-Tgfbr2 KO mice that were previously primed with iTreg or CD4con cells. Mice were sacrificed 2 weeks after T/DC cell transfer. Colon was removed for RNA isolation, and mesenteric lymph node cell suspensions were cultured in the plates with anti-CD3/CD28 activation beads. Cytokine expression was measured by real-time PCR (TaqMan primer/probe sets from Applied Biosystems) and with an xMAP multiplex assay (Millipore).
Proliferation assay
iTreg generated as above or nTreg expanded as described previously (Zhou et al., 2010b) were added to fresh naïve T cells (1:4 Treg/T cell ratio) and were stimulated with anti-CD3 mAb (0.025 µg/ml) and irradiated APC (30 Gy, 1:1 ratio) for 3 days. In other experiments, T responder cells were stimulated with allogeneic APC or DC. 3H was added to cultures for the last 16–18 h and T cell proliferation ([3H]thymidine incorporation) was measured by using a scintillation counter.
Histology
For histological examination, mice were anesthetized after the final disease index was assessed. Kidneys from each mouse were removed and preserved in 10% buffered formalin or frozen in OCT medium. The specimens were processed, blocked, sectioned, and stained with H&E. Cryostat sections of frozen kidney tissue were examined for deposits of IgG using a standard procedure (Hellmark et al., 1997). Sections were incubated with fluorescence-labeled goat F(ab')2 IgG antiserum to mouse IgG. The sections were read blindly by the same investigator, grading the intensity of fluorescence from 0 to 4+.
Statistical analysis
Results were grouped and calculated by using GraphPad Prism 4.0 software (GraphPad Software) and presented as mean ± SEM. Student's t-test was used to assess statistical significance between two groups, and one-way ANOVA was used to assess statistical significance among multiple groups. P < 0.05 was considered as a statistically significant difference.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported by ACR Within Our Reach Fund, Arthritis Foundation and Wright Foundation (all to S.G.Z.), NSFC (30772150 and 81001307 to Z.L.), International Collaborative Projects of Shanghai Municipal Science and Technology Commission (11410702000 to H.F. and S.G.Z.), National Institute of Health Grant (AR059103 and AI084359 to S.G.Z. and DK067286 to P.R.K.) and Dorrance Foundation Fellowship in Pediatric GI Research (to R.R.).
Conflict of interest: none declared.
Supplementary Material
References
- Andersson J., Tran D.Q., Pesu M., et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J. Exp. Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A., Carrier Y., Peron J.P., et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Caton M.L., Smith-Raska M.R., Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V., Collison L.W., Guy C.S., et al. Cutting edge: Human regulatory T cells require IL-35 to mediate suppression and infectious tolerance. J. Immunol. 2011;186:6661–6666. doi: 10.4049/jimmunol.1100315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cobbold S.P., Adams E., Farquhar C.A., et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl Acad. Sci. USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S.P., Adams E., Nolan K.F., et al. Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol. Rev. 2010;236:203–218. doi: 10.1111/j.1600-065X.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D., Bruett C.H., Ploettner H., et al. Human CD4+CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J. Exp. Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Clos T.W., Rubin R.L., Tan E.M. Monoclonal antibody for DNA measurement in biological fluids. J. Immunol. Methods. 1986;88:185–192. doi: 10.1016/0022-1759(86)90005-0. [DOI] [PubMed] [Google Scholar]
- Fahlen L., Read S., Gorelik L., et al. T cells that cannot respond to TGF-beta escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D., Mold J., Hunt P.W., et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floess S., Freyer J., Siewert C., et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Godebu E., Summers-Torres D., Lin M.M., et al. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J. Immunol. 2008;181:1798–1805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmark T., Niles J.L., Collins A.B., et al. Comparison of anti-GBM antibodies in sera with or without ANCA. J. Am. Soc. Nephrol. 1997;8:376–385. doi: 10.1681/ASN.V83376. [DOI] [PubMed] [Google Scholar]
- Horwitz D.A., Zheng S.G., Gray J.D. Natural and TGF-beta-induced Foxp3+CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Schmitt E., Kakirman H., et al. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.H., Li M.O., Jenison M.C., et al. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J. Exp. Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A.R., Chen Y., Regateiro F.S., et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J. Exp. Med. 2011;208:2043–2053. doi: 10.1084/jem.20110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenecke C., Czeloth N., Bubke A., et al. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur. J. Immunol. 2009;39:3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- Kong N., Lan Q., Chen M., et al. Antigen-specific TGF-β-induced regulatory T cells but not natural Tregs ameliorate autoimmune arthritis by shifting the balance of Th17 toward Treg cells. Arthritis Rheum. 2012;64:2548–2558. doi: 10.1002/art.34513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q., Fan H., Quesniaux V., et al. Induced Foxp3+ regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J. Mol. Cell Biol. 2012;4:22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y., Town T., Jeng D., et al. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA. 2008;105:10865–10870. doi: 10.1073/pnas.0805058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Zhou X., Wang J., et al. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS One. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Ma J., Li Z., et al. All-trans retinoic acid promotes TGF-beta-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS One. 2011;6:e24590. doi: 10.1371/journal.pone.0024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy K.J., Salaun L., Cahill R., et al. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monrad S., Kaplan M.J. Dendritic cells and the immunopathogenesis of systemic lupus erythematosus. Immunol. Res. 2007;37:135–145. doi: 10.1007/BF02685895. [DOI] [PubMed] [Google Scholar]
- Murai M., Turovskaya O., Kim G., et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor R.A., Leech M.D., Suffner J., et al. Myelin-reactive, TGF-beta-induced regulatory T cells can be programmed to develop Th1-like effector function but remain less proinflammatory than myelin-reactive Th1 effectors and can suppress pathogenic T cell clonal expansion in vivo. J. Immunol. 2010;185:7235–7243. doi: 10.4049/jimmunol.1001551. [DOI] [PubMed] [Google Scholar]
- Onishi Y., Fehervari Z., Yamaguchi T., et al. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl Acad. Sci. USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta M.T., Orabona C., Volpi C., et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat. Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- Piccirillo C.A., Letterio J.J., Thornton A.M., et al. CD4+CD25+ regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Cobbold S.P., Pope H., et al. ‘Infectious’ transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- Shustov A., Nguyen P., Finkelman F, et al. Differential expression of Fas and Fas ligand in acute and chronic graft-versus-host disease: up-regulation of Fas and Fas ligand requires CD8+ T cell activation and IFN-gamma production. J. Immunol. 1998;161:2848–2855. [PubMed] [Google Scholar]
- Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Su W., Fan H., Chen M., et al. Induced CD4+ forkhead box protein-positive T cells inhibit mast cell function and established contact hypersensitivity through TGF-β1. J. Allergy Clin. Immunol. 2012;130:444–452.e7. doi: 10.1016/j.jaci.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Tang Q., Bluestone J.A. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Adams J.Y., Tooley A.J., et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Korty P.E., Tran D.Q., et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D.Q., Andersson J., Hardwick D., et al. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakkach A., Fournier N., Brun V., et al. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Xu L., Kitani A., Fuss I., et al. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Gray J.D., Ohtsuka K., et al. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J. Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J.H., Gray J.D., et al. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J. Immunol. 2004a;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J.H., Koss M.N., et al. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J. Immunol. 2004b;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Meng L., Wang J.H., et al. Transfer of regulatory T cells generated ex vivo modifies graft rejection through induction of tolerogenic CD4+CD25+ cells in the recipient. Int. Immunol. 2006;18:279–289. doi: 10.1093/intimm/dxh368. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J., Wang P., et al. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- Zheng S.G., Wang J., Horwitz D.A. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- Zhou X., Kong N., Wang J., et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J. Immunol. 2010a;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Wang J., Shi W., et al. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J. Mol. Cell Biol. 2010b;2:164–169. doi: 10.1093/jmcb/mjq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.