Abstract
Pulmonary eosinophilia comprises a heterogeneous group of diseases that are defined by eosinophilia in pulmonary infiltrates or in tissue. Drugs can cause almost all histopathologic patterns of interstitial pneumonias, such as cellular and fibrotic nonspecific interstitial pneumonia, pulmonary infiltrates and eosinophilia, organizing pneumonia, lymphocytic interstitial pneumonia, desquamative interstitial pneumonia, a pulmonary granulomatosis-like reaction, and a usual interstitial pneumonia-like pattern. We present a very rare case of chronic eosinophilic pneumonia due to radiographic contrast infusion diagnosed with video-assisted thoracoscopy. The patient after 1 year is still under corticosteroid treatment with the disease stabilized.
Keywords: interstitial lung disease, radiographic contrast, orphan disease
An ever-increasing number of drugs can induce a variety of interstitial lung diseases (ILD), including most forms of interstitial pneumonias, alveolar involvement, and vasculitis. Drug-induced ILD (DI-ILD) accounts for 3% of all causes of ILD.1,2 Drugs can cause almost all histopathologic patterns of interstitial pneumonias, such as cellular and fibrotic nonspecific interstitial pneumonia, pulmonary infiltrates and eosinophilia (PIE), organizing pneumonia, lymphocytic interstitial pneumonia, desquamative interstitial pneumonia, a pulmonary granulomatosis-like reaction, and a usual interstitial pneumonia-like pattern. PIE or pulmonary eosinophilia is a heterogeneous group of disorders associated with eosinophilia within the lung parenchyma and/or blood eosinophilia. More than 100 medications have been associated with PIE, such as antibiotics, nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, β blockers, and amiodarone. The radiographic contrast medium is not a common cause of PIE.3–7 There is only one published case report that describes eosinophilic pneumonia associated with reaction to radiographic contrast medium. We are reporting a case of chronic interstitial pneumonitis with eosinophilia that occurred 3 weeks after the intravenous administration of iodinated contrast medium during a coronary angiography and rapidly developed into pulmonary fibrosis.
Case report
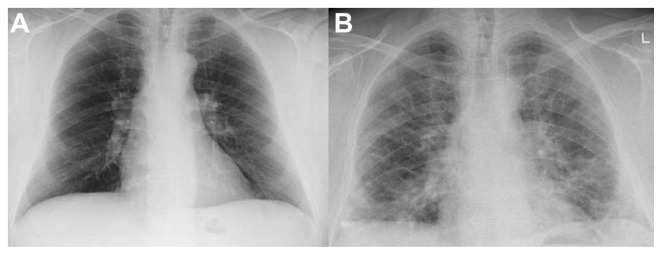
A 51-year-old patient was admitted to the hospital with retrosternal pain in December 2010. In his medical history, the patient mentioned hypothyroidism and has been on L-thyroxin 150 mg/day for 13 years. The patient’s medical history also revealed hypertension and mild heart failure, he had been receiving angiotensin-converting-enzyme (ACE) inhibitors (ramipril 5 mg/day) and β blockers (bisoprolol 5 mg/day) as treatment for the past 2 years. He occasionally received a nonsteroidal anti-inflammatory drug (NSAID) for musculoskeletal pain. In order to complete the diagnostic work up, the patient underwent a coronary angiography on January 19, 2011. During the examination, just after radiographic contrast media administration, (he received 60 mL iopromide intravenous [iv]), he presented symptoms of an allergic reaction, including dyspnea, tachypnea, tachycardia, and hypoxia. In addition, his lung X-ray showed a bilateral reticular thickening, an image that agreed with a possible allergic alveolitis. The patient was treated with corticosteroids (40 mg prednisolon iv). His clinical situation promptly improved, but the X-ray findings persisted (the patient had a normal X-ray image before the examination) (Figure 1A and B). He had no prior history of allergic bronchial asthma or other allergies, and this was the first time that he received a contrast media agent.
Figure 1.
Posteroanterior chest radiograph. (A) Normal posteroanterior chest radiograph. The examination was obtained one year before EPF started. (B) Two months later the signs and findings of the EPF were more prominent. Reticular and nodular opacities and areas of consolidation are seen.
Abbreviation: EPF, eosinophilic pulmonary fibrosis.
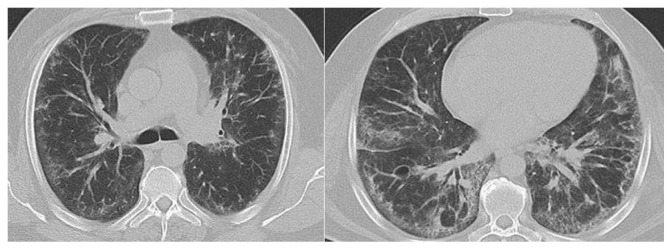
Throughout the first 15 days, the patient’s X-ray findings remained abnormal and he presented with hypoxia and desaturation at room temperature (pO2 47–49 mmHg, pCO2 37–39 mmHg [FiO2 21%]). He additionally demonstrated decreased ability for exercise (6-minute walking test [6MWT] 360 m). The echocardiogram did not show significant pathologic findings (left ventricular ejection fraction 65%, interventricular septal thickness 14 mm). However, the body plethysmography was compatible with a restrictive disorder and severe diffusion disorder (TLCO 34%, KCO 61%, TLC 3.81 [55%], FEV1 1.8 L [51%], VC max 2.2l [49%]). The high resolution computed tomography (HRCT) of the lungs revealed an eosinophilic pulmonary fibrosis pattern of airspace disease and patchy peripheral ground-glass opacities (Figure 2). The cardiopulmonary exercise test confirmed a limitation of pulmonary function (Wmax 95 W [53 W, ramp 12 W/minute]; VO2 max 1614 mL/minute [63% predicted] = 17.7 mL/minute/kg; Wmax 95 W = 43% predicted; O2 pulse demonstrated a plateau at 11.4 mL/beat [67%], VE/VO2 or VE/VCO2 under maximal workload [without any further reserve]).
Figure 2.
Axial, high resolution CT images of the lungs in a patient with eosinophilic pulmonary fibrosis demonstrate peripheral distribution of airspace disease and patchy peripheral ground-glass opacities.
Abbreviation: CT, computed tomography.
Finally, we performed a right heart catheterization and we confirmed the history of a true diastolic dysfunction of the left ventricular (wedge pressure 18–20, pulmonary artery pressure systolic/diastolic/mean 42/20/29; cardiac output 5.9 mL/minute). The laboratory tests that were performed in order to exclude the connective tissue disorders (IgG, IgM, IgA, ANA, p- and c-ANCA, C3, C4, and ENA) throughout his hospitalization were negative. Possible infections from viruses, microbes, Chlamydia, or tuberculosis (polymerase chain reaction [PCR] negative) were also excluded.
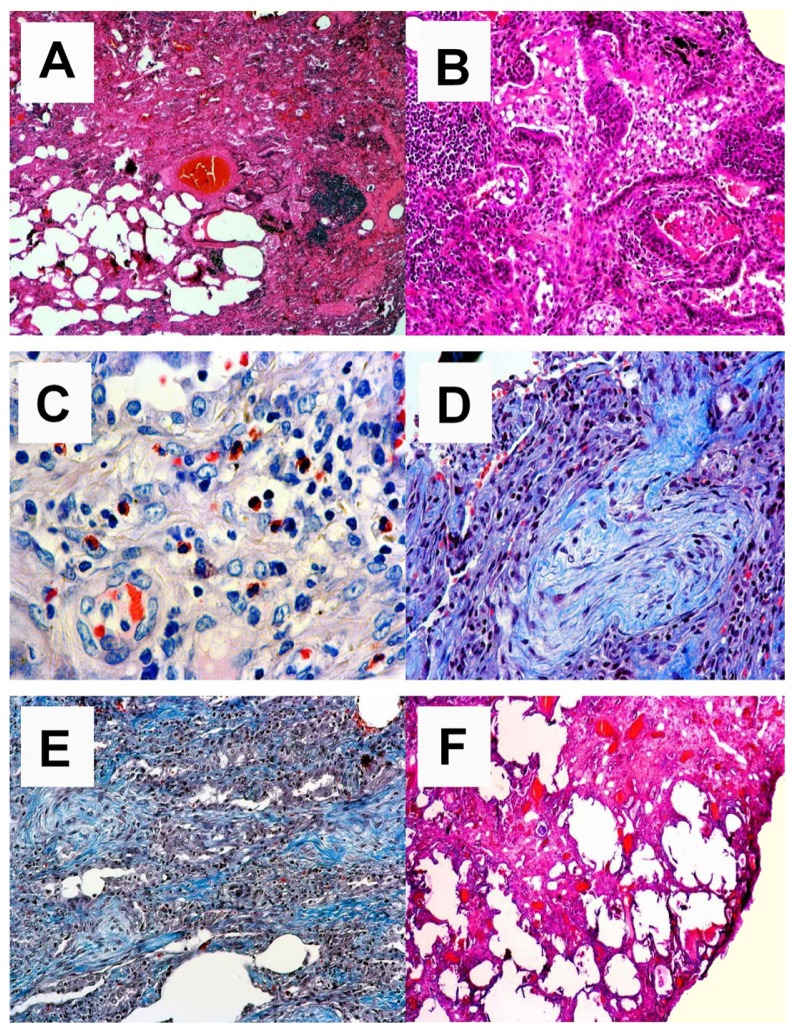
Three weeks later after all the above tests had already been performed, the patient underwent a video-assisted thoracoscopy so that we could reach a final diagnosis. The biopsy revealed a very severe fibrotic disorder throughout most of the lung (end-stage lung) (Figure 3). Histomorphologically, the lesion did not resemble some of the typical interstitial lung patterns (usual interstitial pneumonia, non-specific interstitial pneumonia, desquamative interstitial pneumonia, bronchiolitis obliterans organizing pneumonia, acute interstitial pneumonia, respiratory bronchiolitis -interstitial lung disease). At that time there was a lymphocyte-stimulating test performed with all other possible causative drugs (β blockers, angiotensin-converting-enzyme-inhibitor), but the results were negative showing no clear immunologic trigger mechanism from the chronically taken drugs.
Figure 3.
Overview of the destructive inflammatory process. (A) Acute and chronic inflammatory changes with some scarring (upper right side) are shown. HE, magnification 25×. (B) Acute alveolitis; florid inflammatory changes with highly activated pneumocytes and overspill of acute inflammatory cells into the alveoli. HE, magnification 100×. (C) Interstitial eosinophilia; in areas of longstanding inflammation one can observe a high frequency of eosinophils. They are located in the interstitium without angiocentricity and do not show overspill into the alveoli. These eosinophilic infiltrates are the major clue to a drug-induced reaction. Giemsa, magnification 200×. (D) Bronchiolitis obliterans organizing pneumonia; within the alveoli one can observe fibrobastic proliferations following acute alveolitis shown in B. CAB, magnification 100×. (E) Scarring; in more advanced stage disease one finds an interstitial accumulation of newly synthesized collagen fibers (blue color) without accompanying inflammatory infiltrate. CAB, magnification 100×. (F) End stage; interstitial scar. Note there is no honeycombing. HE, magnification 40×.
Abbreviations: HE, hematoxylin eosin; CAB, Chromotrop-Anilinblue trichrome staining method.
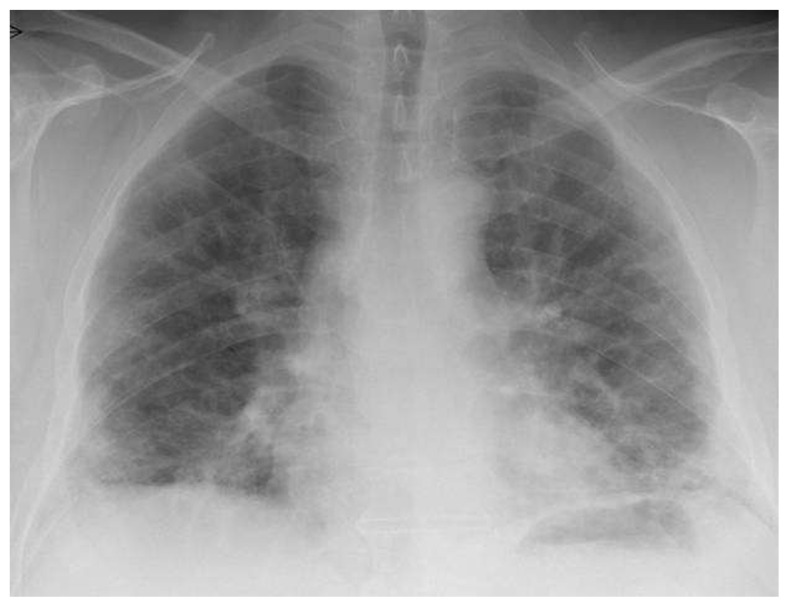
This was a chronic interstitial pneumonitis with eosinophils and fibrosis with a honeycombing image. This process was acute and progressing very rapidly and was directly dependent on the patient’s radiographic contrast media exposure. The patient was treated with corticosteroids, receiving a gradually reducing dose (from 60 mg to 25 mg). One year later, in a scheduled check-up visit, his clinical condition was improved (6MWT, lung function parameters [TLC 55% predicted, FEV1 51% predicted]) (Figure 4). Throughout the patient’s evaluation period, no peripheral blood eosinophilia was observed.
Figure 4.
Posteroanterior chest radiograph.
Note: One year later, posteroanterior chest radiograph demonstrates prominent areas of ground-glass opacity and consolidation.
Discussion
Pulmonary eosinophilia comprises a heterogeneous group of diseases that are defined by eosinophilia in pulmonary infiltrates (identified through bronchoalveolar lavage) or in tissue (identified through open lung or transbronchial biopsy). Although the inflammatory infiltrate consists of macrophages, lymphocytes, neutrophils, and eosinophils and although lung injury results from all these cells rather than from the eosinophils alone, eosinophilia is a significant marker for the diagnosis and treatment.8 A modern classification categorizes the eosinophilic lung diseases into those of unknown cause (simple pulmonary eosinophilia [SPE], acute eosinophilic pneumonia [AEP], chronic eosinophilic pneumonia [CEP], idiopathic hypereosinophilic syndrome [HIS]) and those of known cause (allergic bronchopulmonary aspergillosis [ABPA], bronchocentric granulomatosis [BG], parasitic infection, drug-induced reaction, fungal and mycobacterial infection, pulmonary diseases caused by radiation or toxins). Another group contains eosinophilic vasculitis (allergic angiitis, granulomatosis [Churg–Strauss syndrome]).9,10 PIE can also be associated with connective tissue diseases and neoplasms.
The eosinophil cell is a polymorph nuclear leucocyte containing several eosinophil-specific proteins in cytoplasmic granules. An eosinophil can serve as an end-stage effector cell but can also have specialized roles in the host defense mechanism. However, an eosinophil cell may infiltrate the lung tissue and can sometimes harm the host by releasing specific proteins that are potentially cytotoxic. Thus, it impairs gas exchange and causes several histopathologic lesions and many symptoms, such as dyspnea, fever, and coughing.11,12
Drug exposure, a major cause of PIE, was first described by Liebow and Carrington in 1969.13 Some of the most frequently mentioned drugs,5 are the following: some antibiotics (nitrofurantoin, ampicillin, penicillin, clarithromycin, and sulfonamides), nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, β blockers, amiodarone, bleomycin, carbamazepine, phenytoin, and hydrochlorothiazide. Toxins are suspected of causing eosinophilic pneumonia together with cigarette smoke and illicit drugs. Iodinated contrast material and blood transfusions have also been reported as possible causes.3–7 Patients with drug-induced PIE can present with a variety of pathologic conditions ranging from a mild SPE-like syndrome to a fulminant AEP-like syndrome, which is why desquamative interstitial pulmonary interstitial eosinophilia (DI-PIE) is indistinguishable from idiopathic eosinophilic pneumonia (simple, acute, or chronic) by clinical, radiographic, and histopathologic criteria.8–10
The diagnosis of DI-PIE is normally made by a meticulous exclusion of all other possible causes. The most valuable clinical information is derived from the patient’s history. Exposure to certain parasites (in endemic regions) or toxic products (a history of smoking), a history of asthma and atopy (which may raise suspicion for Churg–Strauss syndrome, ABPA, or BG), connective tissue diseases, and neoplasia must be carefully excluded. The diagnosis is also supported by a definite temporal association between exposure to the agent and the development of respiratory signs and symptoms.9 There should be no evidence of ILD prior to treatment with the suspected drug; thus, review of earlier chest films is required. In general, drug-induced pulmonary toxicity occurs during, rather than after, treatment with the drug, more often via the oral or parenteral route; ILD rarely occurs after an overdose of the drug. More commonly, however, DI-ILD occurs with normal doses and develops unexpectedly as an idiosyncratic reaction in a few patients.11 This makes early detection and prevention of drug-induced diseases difficult. The condition usually resolves with removal from the agent and recurs with rechallenge. Difficulties arise when signs and symptoms develop after the drug is discontinued or when drug withdrawal does not translate into improvement, as in patients with pulmonary fibrosis.10,11
BAL can be useful in the evaluation of patients with eosinophilic lung disease. The detection of an increased number of eosinophils in the bronchoalveolar lavage fluid may provide the first (or sometimes the only) indication of a PIE. Peripheral eosinophilia and elevated IgE levels are usually present but cannot be considered as a specific marker because a large variety of pulmonary diseases may be associated with them. Chest X-rays in DI-PIE demonstrate nonspecific findings, including pleural effusion, reticulonodular densities, consolidation, and hilar adenopathy. Chest computed tomography (CT) demonstrates a more characteristic pattern and distribution of parenchymal opacities, findings that can often be helpful for the diagnosis. However, there is still a considerable overlap of CT findings among the various eosinophilic lung diseases as some drugs can produce more than one pattern of histopathologic involvement in the same patient.3–7 Lung biopsy (transbronchial or open) is not necessary for the diagnosis of pulmonary eosinophilia but is performed to rule out the hypotheses of infection and neoplasia as well as to make the differential diagnosis with other interstitial diseases or to confirm the diagnosis (tissue eosinophilia).
The mechanism of drug-associated PIE remains unclear.11 One possible explanation is related to antigen (derived from the offending drug) presentation by alveolar macrophages, which are responsible for the activation of T helper 2 (Th2) lymphocytes. Activated Th2 lymphocytes release interleukin-5, which promotes eosinophil production and migration to the lung and degranulation.
The key treatment for DI-PIE syndrome is early recognition and discontinuation of possible causative medication. When drug withdrawal does not translate into measurable improvement in patients with extensive involvement, corticosteroid therapy is required; generally this quickly reverses all manifestations of PIE.
Drug-induced pulmonary fibrosis (DI-PF) is a rare but serious complication. Treatment with most cytotoxic drugs (chemotherapeutic agents), amiodarone, gold, sulfasalazine, or methotrexate is mainly suspected. Because many points about pulmonary fibrosis pathogenesis remain unclear, the diagnosis of drug-induced fibrosis is often difficult unless there is a definite temporal association between exposure to the drug and onset of the disease.10,11 When chemotherapeutic drugs are used, pulmonary fibrosis can develop during or many years after termination of treatment. Amiodarone-induced fibrosis can develop just after an episode of nonresolving classic amiodarone pulmonary toxicity, especially if corticosteroids are not given or given late. In addition, amiodarone-induced fibrosis may have a more rapid progression as compared to idiopathic pulmonary fibrosis. In any case, drug administration must be promptly interrupted, although this is rarely followed by improvement. The response to corticosteroid drugs is often limited. Lung transplantation is an option for few patients.3–7,11
In our case report, the patient presented with dyspnea and signs of allergic alveolitis just after radiographic contrast medium administration. The symptoms appeared to be in remission after treatment with corticosteroids (40 mg prednisolon). Three weeks later a diagnosis of chronic eosinophilic pneumonia and pulmonary fibrosis was made. CEP is a severe disorder of insidious onset. It can be secondary to known causes (drugs, parasites, irradiation) or more often idiopathic. The symptoms (dry cough, dyspnea, and systemic manifestations) persist for 2 to 4 weeks. The erythrocyte sedimentation rate is usually elevated. Histologic examination typically shows accumulation of eosinophils and lymphocytes in the alveoli and interstitium, with interstitial fibrosis. The radiological profile reveals peripheral pulmonary infiltrates. The CT scan demonstrates nonsegmental areas of airspace consolidation with peripheral predominance, and very rarely fibrotic opacities may be found. There are few reported cases in which CEP resulted in significant pulmonary fibrosis, with honeycombing and digital clubbing. Persistent stimulation from activated eosinophils could be related to progression to lung fibrosis since the eosinophil granule proteins play an important role during the process of endomyocardial fibrosis in hypereosinophilic syndrome. The use of corticosteroids usually improves the symptoms in 24–48 hours. Unfortunately, a high recurrence rate follows discontinuation or dose reduction, which is why there is no consensus regarding treatment duration.
The radiographic contrast media that the patient received was an iodinated benzyne ring, specifically a nonionic monomer (iopromide), which is included with the new agents of radiographic contrast media. These new molecules have a lower osmolality in order to reduce the risk of adverse reactions.3 The most common adverse reactions related to radiographic contrast media are hypersensitivity, anaphylaxis, and nephrotoxicity; however, based on a case report from 1991, radiographic contrast media is also suspected of causing eosinophilic pneumonia.5 In addition, the patient had received β blockers (bisoprolol) and angiotensin-converting enzyme inhibitors (ramipril) for the past 2 years; β blockers can cause eosinophilic pneumonia and pulmonary fibrosis, and angiotensin-converting enzyme inhibitors are also responsible for eosinophilic pneumonia.5
Based on the above information, it is clear that this acute and extremely rapidly progressive pulmonary disorder was drug-associated. β Blockers and ACE inhibitors could be blamed for this serious interstitial lung disease; however, the procedure took place immediately after the dispensation of iodinated contrast medium. This last fact could possibly have been the cause that triggered the immunologic mechanisms and set in motion multiple reactions, resulting eventually in the destruction of the lung. This is a hypothesis that needs further research to confirm, but it presents a possible explanation.
Acknowledgment
The authors thank Dr Martin C Freund, Radiology Department, University of Innsbruck, Austria, for his expert evaluation on the radiology figures.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yoshida K, Shijubo N, Koba H, et al. Chronic eosinophilic pneumonia progressing to lung fibrosis. Eur Respir J. 1994;7(8):1541–1544. doi: 10.1183/09031936.94.07081541. [DOI] [PubMed] [Google Scholar]
- 2.Jederlinic PJ, Sicilian L, Gaensler EA. Chronic eosinophilic pneumonia: a report of 19 cases and a review of the literature. Medicine. 1988;67:154–162. doi: 10.1097/00005792-198805000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Jennings CA, Deveikis J, Azumi N, Yeager H., Jr Eosinophilic pneumonia associated with reaction to radiographic contrast medium. South Med J. 1991;84(1):92–95. doi: 10.1097/00007611-199101000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Drug-induced interstitial pneumonia. Prescrire Int. 2008;17(94):61–63. [No authors listed] [PubMed] [Google Scholar]
- 5.Camus P, Fanton A, Bonniaud P, Camus C, Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71(4):301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 6.Tseng OL, Kelsall JT, Wilcox PG. Piperacillin-associated pulmonary infiltrates with eosinophilia: a case report. Can Respir J. 2010;17(2):e24–e26. doi: 10.1155/2010/670153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon J, Schwarz M. Drug-, toxin-, and radiation therapy-induced eosinophilic pneumonia. Semin Respir Crit Care Med. 2006;27(2):192–197. doi: 10.1055/s-2006-939522. [DOI] [PubMed] [Google Scholar]
- 8.Eosinophilic diseases of the lungs. Ter Arkh. 2012;84(3):67–73. Russian. [no authors listed] [PubMed] [Google Scholar]
- 9.Katz U, Shoenfeld Y. Pulmonary eosinophilia. J Bras Pneumol. 2009;35(6):561–573. doi: 10.1590/s1806-37132009000600010. [DOI] [PubMed] [Google Scholar]
- 10.Campos LE, Pereira LF. Pulmonary eosinophilia. J Bras Pneumol. 2009;35(6):561–573. doi: 10.1590/s1806-37132009000600010. [DOI] [PubMed] [Google Scholar]
- 11.Jeong YJ, Kim KI, Seo IJ, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics. 2007;27(3):617–637. doi: 10.1148/rg.273065051. discussion 637–639. [DOI] [PubMed] [Google Scholar]
- 12.Miller BA, Gray A, Leblanc TW, Sexton DJ, Martin AR, Slama TG. Acute eosinophilic pneumonia secondary to daptomycin: a report of three cases. Clin Infect Dis. 2010;50(11):e63–e68. doi: 10.1086/652656. [DOI] [PubMed] [Google Scholar]
- 13.Liebow AA, Carrington DB. The interstitial pneumonias. In: Simon M, Potchen EJ, LeMay M, editors. Frontiers of Pulmonary Radiology. New York: Grune & Stratton; 1969. pp. 102–141. [Google Scholar]