Abstract
The purpose of this study was to assess the influence of sport-specific and nonspecific bouts of exercise on athletes' redox state. Blood samples were collected from 14 handball players immediately before and after graded exercise test on the cycle ergometer and handball training. Levels of superoxide anion radical (O2 −), hydrogen peroxide (H2O2), nitrites (NO2 −) as markers of nitric oxide, index of lipid peroxidation (TBARs), glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) activity were determined. Exercise intensity was assessed by a system for heart rate (HR) monitoring. Average athletes' HR was not significantly different between protocols, but protocols differed in total time and time and percentage of time that athletes spent in every HR zone. The laboratory exercise test induced a significant increase of H2O2 and TBARs as well as the decrease of the SOD and CAT activity, while after specific handball training, levels of NO2 − were increased and SOD activity decreased. It seems that unaccustomed short intensive physical activity may induce oxidative stress in trained athletes, while sport-specific activity of longer duration and proper warm-up period may not. Further research should show whether the change of protocol testing and the implementation of various supplementations and manual methods can affect the redox equilibrium.
1. Introduction
Reactive oxygen species (ROS) are constantly being generated in the body in a small extent even at rest, and since they have a potential to react with a variety of chemical species, they have multiple functions in cell signaling and enzymology [1]. In general, the body has adequate antioxidant reserves to cope with the production of ROS under physiological conditions and perhaps during low-moderate intensity exercise [2], but when ROS production is excessive, such as during prolonged intensive physical efforts, an imbalance between oxidants and antioxidants in favor of the oxidants may occur, leading to a disruption of redox signaling and control and/or molecular damage [3].
Exercise provides an excellent model to study the dynamic balance between oxidative challenge and antioxidant defense in the biological system, so the relationship between exercise and oxidative stress has been a topic of intensive scientific research for more than 30 years [4]. Although there is some inconsistency present within the literature, it is clear that both aerobic and anaerobic exercises have the potential to result in increased free radical production, which may or may not result in acute oxidative stress [5–7]. The extent of redox homeostasis disturbance induced by an acute bout of exercise depends on many factors, inter alia, exercise mode, intensity and duration, participant's state of training, gender, age, and nutritional habits [8–10].
There are many studies on exercise-induced oxidative stress performed on sedentary participants, recreational and elite athletes in dominantly aerobic, anaerobic, and even mixed anaerobic-aerobic conditions [11–13], but there are a very limited number of studies that investigated the effects of different types of exercise on the redox state of the same participants [14]. Different types of exercise may induce varying levels of RONS and affect plasma redox state in a specific way. Sports engagement includes upregulation of many cell processes and physiological functions, but those improvements are sport specific. When exposed to unaccustomed physical activities, athletes experience significant stress that may induce, among other undesirable effects, delayed onset of muscle soreness (DOMS), a phenomenon whose etiology is still not clear, but there are some implications that reactive oxygen and nitrogen species (RONS) may be involved in its appearance and existence [15]. So, the main aim of our study was to compare the effects of two types of exercise, different in energy demands, intensity, duration, and specificity, on the redox state of well-trained young handball players.
2. Materials and Methods
2.1. Subjects
The study was performed on 14 young (19.1 ± 1.1 years old) well-trained male handball players. Athletes had at least 5 years of sports experience and were engaged in regular handball (mixed aerobic-anaerobic) training 5 times a week for 90 min. The study was performed during the first part of the competition season, during the game-free week between two competitive rounds of the national league. In order to be included in the analyses, players had to attend more than 90% of the training sessions during the pre-competition period and the current competition season.
All handball players and the head coach were explained study's purposes, risks, and benefits, they were familiarized with study's protocol, and they gave a written informed consent. The study was approved by the Ethical committee of the Faculty of Medical Sciences, University of Kragujevac.
2.2. Study Design
To determine the effects of two different types of exercise on the redox state, handball players were subjected to a laboratory graded exercise test (GXT) on the cycle ergometer (exercise protocol 1) and to the specific handball training (HT) (exercise protocol 2). Those two exercise protocols were chosen because they differ in specificity, intensity, and duration, that is, physiologic, motoric, and psychologic demands. Laboratory graded exercise test represents sport-nonspecific unaccustomed activity for these athletes, while handball training was designed to replicate their usual training session and competitional activities. The study design is illustrated in Figure 8.
Figure 8.
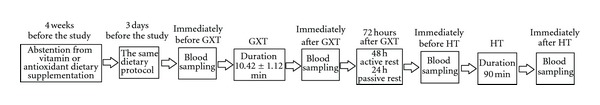
The study design.
Immediately before and after both exercise bouts, blood samples were taken from athletes (except goalkeepers) in order to determine the levels of superoxide anion radical (O2 −), hydrogen peroxide (H2O2), nitrites (NO2 −) as markers of nitric oxide, index of lipid peroxidation (TBARs), glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) activity. The effects of exercise on the redox state were determined independently for each experimental protocol and changes of biochemical parameters, induced by different exercise protocols, were compared.
Four weeks prior to blood sampling, the athletes were instructed to abstain from any vitamin or antioxidant dietary supplementation. None of the participants reported any eating disorders, had no ongoing or previous (last half-year) injuries, were not on any medication known to affect oxidative stress, and were nonsmokers. Exercise protocol 2 (handball training) took place 72 h after exercise protocol 1 (laboratory exercise test). During the first 48 h after exercise protocol 1, athletes were allowed to perform light aerobic exercise as active recovery, while 24 h before both exercise protocols physical activity was forbidden. To exclude the influence of different dietary intakes on nitrite level, all participants were on the same dietary protocol 3 days before the study and during the study.
2.3. Preliminary Measurements
Before the beginning of the study, athletes passed standard sports-medicine examination that included a health questionnaire, electrocardiographic examination, blood pressure, and anthropometrical measurement. Measurement of body composition was performed using an apparatus for bioelectrical impedance analysis In Body 720 (Biospace, Korea) whose validity was previously confirmed [16]. Measurement was performed according to the manufacturer's instructions. Body weight was measured with accuracy within 0.1 kg and body fat with an accuracy of 0.1%. Body height was measured by means of an anthropometer (GPM, Switzerland) and the results of the measurements were accurate within 0.1 cm.
2.4. Exercise Protocols
Athletes' cardiorespiratory fitness was assessed during performance of graded exercise test that was designed as exercise protocol 1 in the first part of the study. Maximal progressive exercise test was performed on a cycle ergometer AX1 (Kettler, Germany). Athletes were familiarized to testing procedure. The saddle height was adjusted for each athlete and athletes were instructed to keep the revolution rate at 60 rpm. The load was set to 2 W/kg and increased by 50 W every 3 min until the end of the exercise test. The test was performed until voluntary exhaustion and athletes stated their subjective feeling of exhaustion by using Borg's CR10 exhaustion scale of at least 8 [17]. During each test, athletes breathed through a two-way mouthpiece (Hans Rudolph, Kansas City, USA). Maximal oxygen consumption (VO2max) and heart rate were followed by an automated cardiopulmonary exercise system (FitMate Pro, COSMED, Italy) whose validity, reliability, and accuracy were previously reported [18]. We considered that VO2max was reached when the oxygen consumption reached its plateau (when an increase in workload cannot induce an increase in oxygen consumption) [19].
One and a half hour handball training consisted of 20 min warm-up (running along the court in pairs while ball passing and shooting (5 min), individual counterattack without defensive player (5 min), stretching (5 min), and shooting from specialized position (5 min)) after which players were assigned to two teams and played a regular handball game (2 × 30 min, 10 min halftime). Intensity of exercise in situational conditions was assessed by Polar Team2 System (Polar Electro Oy, Finland) for heart rate monitoring.
2.5. Analytical Procedures
Blood samples were taken from an antecubital vein into Vacutainer test tube containing sodium citrate anticoagulant. Blood samples were processed and stored immediately. Blood was centrifuged to separate plasma and red blood cells (RBCs). Biochemical parameters were measured spectrophotometrically. The level of superoxide anion radical (O2 −) was measured using nitro blue tetrazolium (NBT) reaction in tris buffer combined with plasma samples and read at 530 nm [20]. The protocol for measurement of hydrogen peroxide (H2O2) was based on oxidation of phenol red in the presence of horseradish peroxidase [21]. Two hundred μL sample with 800 μL phenol red solution (PRS) and 10 μL horseradish peroxidase (POD) were combined (1 : 20). The level of H2O2 was measured at 610 nm. Nitric oxide (NO) decomposes rapidly to form stable metabolite nitrite/nitrate products. Nitrite (NO2 −) was determined as an index of nitric oxide production with Griess reagent [22]. 0.1 mL 3 N perchloric acid (PCA), 0.4 mL 20 mM ethylenediaminetetraacetic acid (EDTA), and 0.2 mL plasma were put on ice for 15 min, then centrifuged for 15 min at 6000 rpm. After pouring off the supernatant, 220 μL K2CO3 was added. Nitrites were measured at 550 nm. Distilled water was used as a blank probe. The degree of lipid peroxidation in plasma was estimated by measuring of thiobarbituric acid reactive substances (TBARs) using 1% thiobarbituric acid (TBA) in 0.05 M NaOH, incubated with plasma at 100°C for 15 min and read at 530 nm. Distilled water was used as a blank probe. TBA extract was obtained by combining 0.8 mL plasma and 0.4 mL trichloroacetic acid (TCA), and then samples were put on ice for 10 minutes and centrifuged for 15 min at 6000 rpm. This method was described previously [23]. Hemoglobin determination, necessary for calculation of activity of endogenous antioxidants, was performed according to the Drabkin method [24]. Superoxide dismutase (SOD) activity was determined by the epinephrine method of Misra and Fridovich [25]. A hundred μL lysate and 1 mL carbonate buffer were mixed, and then 100 μL of epinephrine was added. Detection was performed at 470 nm. Isolated RBCs were washed three times with 3 volumes of ice-cold 0.9 mmol/L NaCl, and hemolysates containing about 50 g Hb/L (prepared according to McCord and Fridovich [26]) were used for the determination of catalase (CAT) activity. CAT activity was determined according to Beutler [27]. Lysates were diluted with distilled water (1 : 7 v/v) and treated with chloroform-ethanol (0.6 : 1 v/v) to remove hemoglobin [28]. Then 50 μL catalase buffer, 100 μL sample, and 1 mL 10 mM H2O2 were mixed. Detection was performed at 360 nm. Distilled water was used as a blank probe. The level of reduced glutathione (GSH) was determined based on GSH oxidation with 5,5-dithiobis-6,2-nitrobenzoic acid, using the Beutler method [29]. The concentration is expressed as nanomoles per milliliter of red blood cells (RBCs).
2.6. Statistical Analysis
The statistical analysis was performed using SPSS 19.0 for Windows. Results are expressed as means ± standard deviation of the mean. Data distribution was checked with the Shapiro-Wilk, test and depending on its results, the appropriate parametric or nonparametric test was used. The differences between the values of means from two related samples (before and after the maximal exercise test, before and after handball training) were assessed by Wilcoxon's test, while the difference between two unrelated samples (characteristics of exercises protocol) was assessed by t-test. Alpha level for significance was set to P < 0.05.
3. Results
Subjects' characteristics are presented in Table 1.
Table 1.
Subject's characteristics.
| Characteristic (X ± SD) |
Handball players (n = 14) |
|---|---|
| Age (years) | 19.1 ± 1.1 |
| Height (cm) | 183.8 ± 6.6 |
| Weight (kg) | 80.6 ± 9.7 |
| Body mass index (kg/m2) | 23.9 ± 2.7 |
| Fat (%) | 12.1 ± 4.8 |
| Muscle (%) | 50.2 ± 2.8 |
| Training experience (years) | 8.5 ± 2.4 |
| Maximal oxygen consumption (mL/kg/min) | 48.7 ± 6.1 |
Characteristics of the two exercise protocols are presented in Table 2. Athletes' average and maximal heart rate did not differ between exercise protocols. But those two exercise protocols significantly differed in total time and time and percentage of time that athletes spent in every heart rate zone. Athletes spent significantly more time, both in minutes and percentages, in submaximal and maximal zones of intensity during handball training.
Table 2.
Characteristics of two exercise protocols: laboratory graded exercise test (GXT) and specific handball training (HT).
| Characteristic (X ± SD) | GXT | HT | t-test |
|---|---|---|---|
| Duration (min) | 10.42 ± 1.12 | 90 | P = 0.000 |
| Heart rate (beat/min) | 157.89 ± 7.68 | 152.07 ± 14.76 | P = 0.078 |
| Max heart rate (beat/min) | 185.78 ± 8.44 | 186.57 ± 11.40 | P = 0.948 |
| Time in HR zone (min) | |||
| <120 | 0 | 5.22 ± 5.63 | P = 0.000 |
| 120–140 | 1.45 ± 1.57 | 10.26 ± 10.08 | P = 0.000 |
| 140–160 | 4.40 ± 1.50 | 19.48 ± 6.93 | P = 0.000 |
| 160–180 | 3.05 ± 1.90 | 36.02 ± 11.07 | P = 0.000 |
| >180 | 1.5 ± 1.0 | 14.37 ± 14.68 | P = 0.001 |
| % of time in HR zone | |||
| <120 | 0 | 5.80 ± 6.25 | P = 0.000 |
| 120–140 | 12.91 ± 12.54 | 11.40 ± 11.21 | P = 0.877 |
| 140–160 | 42.08 ± 13.24 | 21.65 ± 7.70 | P = 0.000 |
| 160–180 | 30.15 ± 13.08 | 40.02 ± 12.3 | P = 0.033 |
| >180 | 14.84 ± 10.14 | 15.97 ± 16.31 | P = 1.000 |
| Workload (Watt) | 294.28 ± 39.25 | / | / |
The effects of exercise protocols on levels of pro/antioxidants in subjects' blood are shown in Figures 1, 2, 3, 4, 5, 6, and 7. Laboratory exercise test that lasted 10.42 ± 1.12 min and in which levels of athlete's heart rate corresponded to a submaximal and maximal intensity zone for less than 5 min induced significant increase of H2O2 and TBARs as well as the decrease of SOD and CAT activity, while after 90 min of specific handball training, during which players spent about 50 min in submaximal and maximal zone of intensity, only levels of NO2 − were significantly increased and SOD activity decreased.
Figure 1.
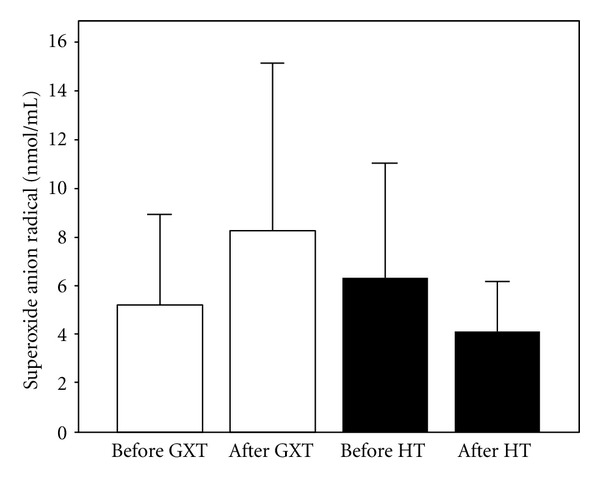
Values of superoxide anion radical (X ± SE) in athletes before and after exercise protocol 1 (laboratory graded exercise test—GXT) and exercise protocol 2 (specific handball training—HT).
Figure 2.
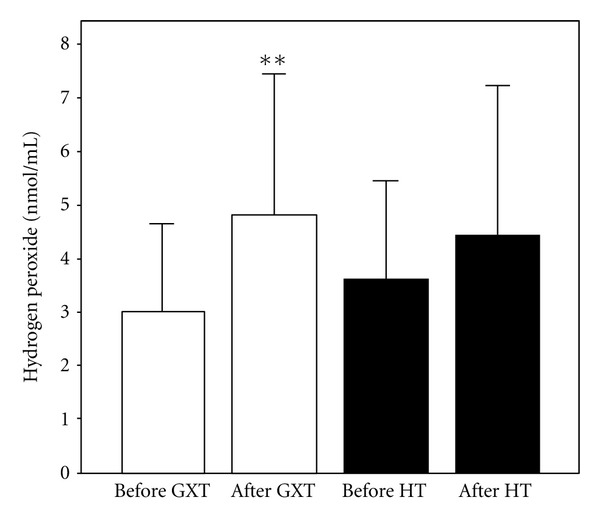
Values of hydrogen peroxide (X ± SE) in athletes before and after exercise protocol 1 (laboratory graded exercise test—GXT) and exercise protocol 2 (specific handball training—HT); **P < 0.01, Wilcoxon's test.
Figure 3.
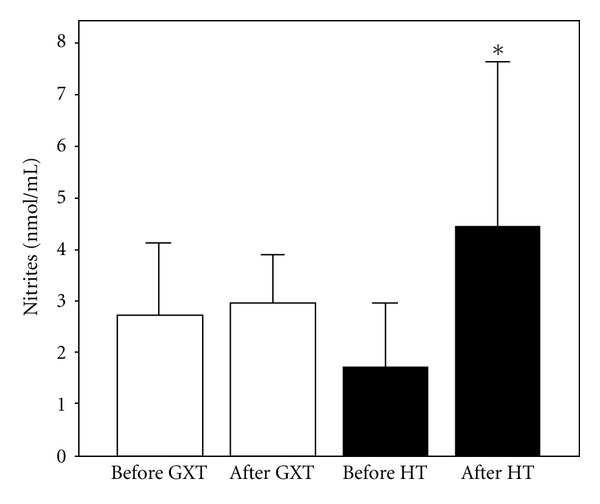
Values of nitrites (X ± SE) in athletes before and after exercise protocol 1 (laboratory graded exercise test—GXT) and exercise protocol 2 (specific handball training—HT); *P < 0.05, Wilcoxon's test.
Figure 4.
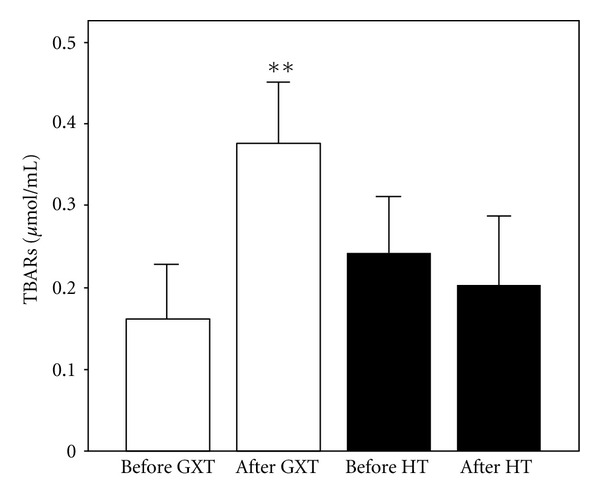
Values of index of lipid peroxidation (X ± SE) in athletes before and after exercise protocol 1 (laboratory graded exercise test—GXT) and exercise protocol 2 (specific handball training—HT); **P < 0.01, Wilcoxon's test.
Figure 5.
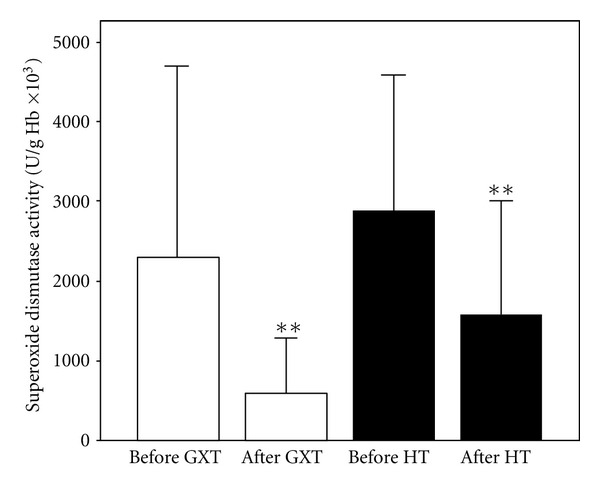
Superoxide dismutase activity (X ± SE) in athletes before and after exercise protocol 1 (laboratory graded exercise test—GXT) and exercise protocol 2 (specific handball training—HT); **P < 0.01, Wilcoxon's test.
Figure 6.
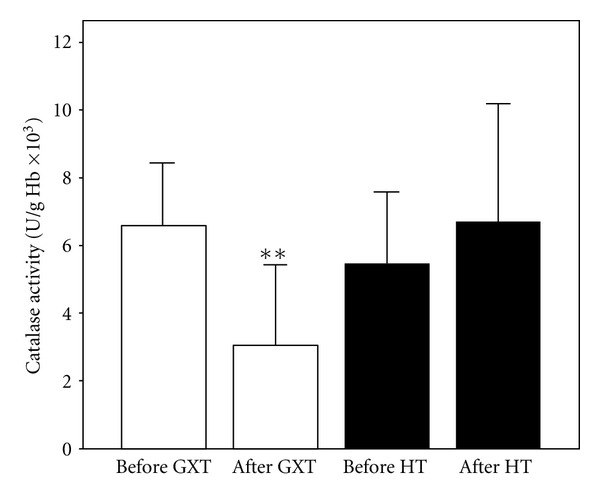
Catalase activity (X ± SE) in athletes before and after exercise protocol 1 (laboratory graded exercise test—GXT) and exercise protocol 2 (specific handball training—HT); **P < 0.01, Wilcoxon's test.
Figure 7.
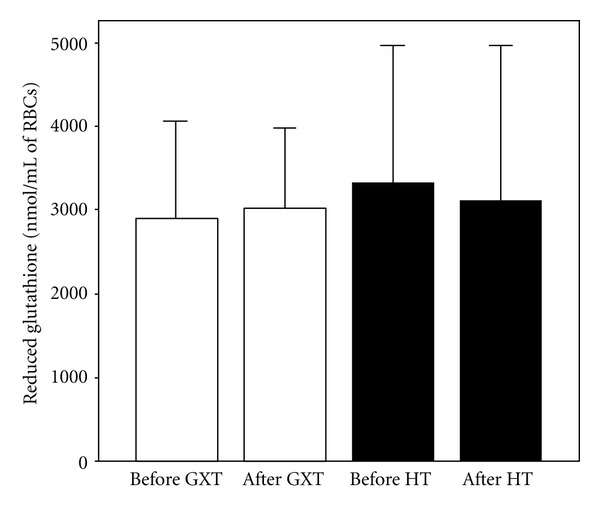
Values of glutathione (X ± SE) in athletes before and after exercise protocol 1 (laboratory graded exercise test—GXT) and exercise protocol 2 (specific handball training—HT).
4. Discussion
The aim of the present study was to compare the effects of two exercise protocols different in energy demands, intensity, duration, and specificity on redox state of well-trained young handball players. The results of the study showed that maximal laboratory graded exercise test performed on the cycle ergometer was followed by significant disturbance of the athlete's redox state. The changes in investigated redox parameters after GXT included significant increase of prooxidants (H2O2 and TBARs) and the decrease of endogenous antioxidants (SOD and CAT), which clearly suggests that athletes experienced oxidative stress after this type of exercise. On the other hand, HT induced changes of a smaller number of biochemical parameters: only changes in levels of nitrites (markers of nitric oxide production) and SOD activity were observed. The fact that SOD activity was decreased after handball training but levels of ROS (O2 −, H2O2, and TBARs) and other endogenous antioxidants (CAT and GSH) were not changed significantly suggests that the first line of antioxidant defense was enough to prevent the exercise-induced oxidative stress. The rise in NO (NO2 −) levels after HT may be the consequence of increased blood flow, that is, the effect of relatively long exposure of blood vessels to shear stress [30, 31].
From the previously published literature on the relationship between exercise and oxidative stress, it is clear that an exercise of sufficient volume, intensity, and duration can lead to an increase in ROS production, which may lead to the oxidation of several biological molecules (lipids, proteins, and nucleic acids) [4–6]. But how much physical effort is enough to induce significant changes in athlete's redox state? Exercise protocols in our study were designed to differ in volume, intensity, and duration. As presented in the Results Section, they significantly differed in total time and time and percentage of time that athletes spent in every heart rate zone, especially in submaximal and maximal zones of intensity. It should be expected that handball training would represent greater challenge for maintaining desirable redox state because all its load parameters were much greater than the ones during GXT, but our results showed the opposite. Laboratory exercise test that lasted 10.42 ± 1.12 minutes and in which levels of athlete's heart rate corresponded to a submaximal and maximal intensity zone for less than 5 minutes induced significant oxidative stress, while after 90 minutes of specific handball training, during which players spent about 50 minutes in submaximal and maximal zone of intensity, the redox state was much less affected. The answer to this paradox may lie in specificity of the exercise activities. Sport training is a process of continuous exposure of an athlete to various kinds of stress, in order to initiate adaptations, that is, structural and functional changes that enable the improvement of an athlete's sports performance, but those adaptations are sport specific and in most cases not transferable. When exposed to an unaccustomed exercise protocol, athletes experienced significantly greater undesirable changes of redox state than when exposed to exercise protocol that was characterized by accustomed physiologic, motoric, and psychologic demands. Another explanation of observed differences in redox responses to those two exercise protocols may lie in the duration of exercise, since there is evidence that prolonged physical activity induces less oxidative stress than acute short exercise (4, 6). This, in part, may be due to differences in the warm-up period between the protocols and the time available to achieve stable redox state. When exposed to maximal GXT protocol, athletes experienced significantly greater undesirable changes of redox state probably due to rapid load, with ischemic reaction occurring in the early stages of loading and the subsequent development of the metabolic defects at the level of the muscle. Ischemic response induced inflammatory response that induced disorder of redox balance during the exercise test. For this reason, it is necessary to consider possible modifications of the protocol to test athletes in terms of extending the warm-up phase and the application of manual techniques in the sense of massage to alleviate the initial ischemic reaction. In addition, it would be necessary to introduce a specific diet and supplementation immediately before the exercise test, especially vitamin E, alpha lipoic acid, coenzyme Q10, glutathione, and N-acetyl cysteine.
5. Conclusions
Our results point out to the conclusion that an unaccustomed physical activity may induce oxidative stress in well-trained athletes, while sport-specific activity may not. These results indicate the importance of the introduction of antioxidant supplementation at a time when athletes are exposed to activities that do not match the specific physical training loads. Although these findings suggest that this type of load should be avoided in regenerative stages of training cycle. Further research should show whether the change of protocol testing and implementation of various supplementation and manual methods can affect the redox state equilibrium.
The limitation of our study is that we did not follow the changes of redox parameters during recovery, but these results are the starting point for our further research in that area. Monitoring of changes in the redox state after these two exercise protocols could give the answer to the question about the role of ROS in exercise-induced muscle fatigue and the possible delayed onset of muscle soreness.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgment
This work was supported by Grant no. 175043 from the Ministry of Science and Technical Development of the Republic of Serbia.
References
- 1.Jacob C, Winyard PG. Introduction. In: Jacob C, Winyard PG, editors. Redox Signaling and Regulation in Biology and Medicine. Weinheim, Germany: Wiley-VCH; 2009. pp. 1–12. [Google Scholar]
- 2.Ji LL. Oxidative stress during exercise: implication of antioxidant nutrients. Free Radical Biology and Medicine. 1995;18(6):1079–1086. doi: 10.1016/0891-5849(94)00212-3. [DOI] [PubMed] [Google Scholar]
- 3.Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radical Biology and Medicine. 2011;51(5):942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dynamic Medicine. 2009;8(1, article 1) doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomer RJ, Goldfarb AH. Anaerobic exercise and oxidative stress: a review. Canadian Journal of Applied Physiology. 2004;29(3):245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 6.Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Medicine. 2006;36(4):327–358. doi: 10.2165/00007256-200636040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Vollaard NBJ, Shearman JP, Cooper CE. Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Medicine. 2005;35(12):1045–1062. doi: 10.2165/00007256-200535120-00004. [DOI] [PubMed] [Google Scholar]
- 8.Djordjevic D, Cubrilo D, Zivkovic V, Barudzic N, Vuletic M, Jakovljevic V. Pre-exercise superoxide dismutase activity affects the pro/antioxidant response to acute exercise. Serbian Journal of Experimental and Clinical Research. 2010;11(4):135–139. [Google Scholar]
- 9.Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxidative Medicine and Cellular Longevity. 2012;2012:12 pages. doi: 10.1155/2012/756132.756132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong TK, Lin H, Lippi G, Nie J, Tian Y. Serum oxidant and antioxidant status in adolescents undergoing professional endurance sports training. Oxidative Medicine and Cellular Longevity. 2012;2012:7 pages. doi: 10.1155/2012/741239.741239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djordjevic D, Jakovljevic V, Cubrilo D, et al. Coordination between nitric oxide and superoxide anion radical during progressive exercise in elite soccer players. Open Biochemistry Journal. 2010;4:100–106. doi: 10.2174/1874091X01004010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djordjevic D, Cubrilo D, Barudzic N, et al. Comparison of blood pro/antioxidant levels before and after acute exercise in athletes and nonathletes. General Physiology and Biophysics. 2012;31(2):211–219. doi: 10.4149/gpb_2012_025. [DOI] [PubMed] [Google Scholar]
- 13.Cubrilo D, Djordjevic D, Zivkovic V, et al. Oxidative stress and nitrite dynamics under maximal load in elite athletes: relation to sport type. Molecular and Cellular Biochemistry. 2011;355(1-2):273–279. doi: 10.1007/s11010-011-0864-8. [DOI] [PubMed] [Google Scholar]
- 14.Bloomer RJ, Goldfarb AH, Wideman L, McKenzie MJ, Consitt LA. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. Journal of Strength and Conditioning Research. 2005;19(2):276–285. doi: 10.1519/14823.1. [DOI] [PubMed] [Google Scholar]
- 15.Close GL, Ashton T, McArdle A, MacLaren DPM. The emerging role of free radicals in delayed onset muscle soreness and contraction-induced muscle injury. Comparative Biochemistry and Physiology. 2005;142(3):257–266. doi: 10.1016/j.cbpa.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Lim JS, Hwang JS, Lee JA, et al. Cross-calibration of multi-frequency bioelectrical impedance analysis with eight-point tactile electrodes and dual-energy X-ray absorptiometry for assessment of body composition in healthy children aged 6–18 years. Pediatrics International. 2009;51(2):263–268. doi: 10.1111/j.1442-200X.2008.02698.x. [DOI] [PubMed] [Google Scholar]
- 17.Borg GAV. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 18.Howley ET, Bassett DR, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Medicine and Science in Sports and Exercise. 1995;27(9):1292–1301. [PubMed] [Google Scholar]
- 19.Nieman DC, Austin MD, Benezra L, et al. Validation of cosmed’s FitMate™ in measuring oxygen consumption and estimating resting metabolic rate. Research in Sports Medicine. 2006;14(2):89–96. doi: 10.1080/15438620600651512. [DOI] [PubMed] [Google Scholar]
- 20.Auclair C, Voisin E. Nitroblue tetrazolium reduction. In: Auclair C, Voisin E, editors. Handbook of Methods for Oxygen Radical Research. Boca Raton, Fla, USA: CRC Press; 1985. pp. 123–132. [Google Scholar]
- 21.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. Journal of Immunological Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 22.Green LC, Wagner DA, Glogowski J. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Drabkin D, Austin H. Spectrophotometric studies II. Preparations from washed blood cells: nitric oxide, hemoglobin and sulfhemoglobin. Journal of Biological Chemistry. 1935;(112):51–65. [Google Scholar]
- 25.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 26.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) Journal of Biological Chemistry. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 27.Beutler E. Catalasered Cell Metabolism, a Manual of Biochemical Methods. In: Beutler E, editor. New York, NY, USA: Grune and Stratton; 1982. pp. 105–106. [Google Scholar]
- 28.Tsuchihashi M. Zur Kernntnis der blutkatalase. Biochemische Zeitschrift. 1923;140:65–72. [Google Scholar]
- 29.Beutler E. Reduced glutathione (GSH) In: Beutler E, editor. Red Cell Metabolism, a Manual of Biochemical Methods. New York, NY, USA: Grune and Stratton; 1975. pp. 112–114. [Google Scholar]
- 30.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. Journal of Physiology. 2004;561(1):1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiorana A, O’Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Medicine. 2003;33(14):1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]


