Abstract
Purpose: To evaluate multiple applicator implant configurations of interstitial ultrasound devices for large volume ablation of liver tumors.
Methods: A 3D bioacoustic-thermal model using the finite element method was implemented to assess multiple applicator implant configurations for thermal ablation with interstitial ultrasound energy. Interstitial applicators consist of linear arrays of up to four 10 mm-long tubular ultrasound transducers, each under separate and dynamic power control, enclosed within a water-cooled delivery catheter (2.4 mm OD). The authors considered parallel implants with two and three applicators (clustered configuration), spaced 2–3 cm apart, to simulate open surgical placement. In addition, the authors considered two applicator implants with applicators converging and diverging at angles of ∼20°, 30°, and 45° to simulate percutaneous placement. Heating experiments (10–15 min) were performed and compared against simulations employing the same experimental parameters. To estimate the performance of parallel, multiple applicator configurations in an in vivo setting, simulations were performed taking into account a range of blood perfusion levels (0, 5, 12, and 15 kg m−3 s−1) that may occur in tumors of varying vascularity. The impact of tailoring the power supplied to individual transducer elements along the length of applicators is explored for applicators inserted in non-parallel (converging and diverging) configurations. Thermal dose (t43 > 240 min) and temperature thresholds (T > 52 °C) were used to define the ablation zones, with dynamic changes to tissue acoustic and thermal properties incorporated within the model.
Results: Experiments in ex vivo bovine liver yielded ablation zones ranging between 4.0–5.6 cm × 3.2–4.9 cm, in cross section. Ablation zone dimensions predicted by simulations with similar parameters to the experiments were in close agreement (within 5 mm). Simulations of in vivo heating showed that 15 min heating and interapplicator spacing less than 3 cm are required to obtain contiguous, complete ablation zones. The ability to create complete ablation zone profiles for nonparallel implants was illustrated by tailoring applied power levels along the length of applicators.
Conclusions: Parallel implants consisting of three interstitial ultrasound applicators in a triangular configuration yield complete ablation zones measuring up to 6.2 cm × 5.7 cm after 15 min heating. At larger interapplicator spacing, the level of blood perfusion in the tumor may yield indentations along the periphery of the ablation zone. Tailoring applied power along the length of the applicator can accommodate for nonparallel implants, without compromising safety.
Keywords: liver ablation, thermal ablation, interstitial ultrasound, high intensity ultrasound, multiple applicator ablation, computer model
INTRODUCTION
Thermal ablation is a well established therapy for the treatment of unresectable hepatocellular carcinoma and select secondary hepatic tumors.1, 2, 3 Ablation is also used for treatment of cancer and benign disease in bone, kidney, lung, prostate and uterine fibroids. Radio frequency (RF) currents, cryoablation, microwaves (MW), and interstitial laser fibers are the most commonly used energy modalities for minimally invasive thermal ablation of liver tumors, and high intensity focused ultrasound has emerged as a noninvasive technique for tissue ablation.4, 5, 6, 7 Thermochemical ablation and irreversible electroporation have recently emerged as other modalities for tissue ablation.8, 9, 10, 11 Irrespective of energy modality, the goal of thermal ablation treatment of liver tumors is to raise target (tumor and a 5–10 mm margin encompassing the tumor) temperatures to lethal levels, while ensuring thermal protection of surrounding critical structures. If a single ablation does not adequately cover the target, sequential overlapping ablations must be performed until the entire target is treated adequately.12
Catheter-based ultrasound devices are under development for thermal therapy of cancer and benign tissue in the prostate, uterine fibroids, liver, bone, and other organs. These devices consist of independently powered tubular, planar, or curvilinear transducers arranged in a linear array. Devices suitable for percutaneous ablation (13–14 g) have been developed and successfully evaluated in vivo for application in highly perfused organs such as the liver,13, 14, 15 prostate,16, 17 brain18 and uterine fibroids.19 Compared to other needle-based ablation devices, minimally invasive catheter based ultrasound devices have the unique ability to control the spatial energy deposition profile along the length of the applicator,14, 20 as well as across the angular expanse.16, 17 Ultrasound applicators for thermal therapy also offer the potential to be used as an imaging device to determine proper applicator placement as well as to monitor treatment progression.13, 21
A randomized clinical trial comparing percutaneous thermal ablation to surgical resection for tumors < 5 cm in diameter, reported no differences in overall and disease-free survival between the two treatments.1 However, for larger tumors >5 cm in diameter, studies have reported lower complete ablation rates, 24% and 62% in tumors measuring 5–9.5 cm and 5–7 cm.8 Similar results have been reported with early MW ablations systems. Suggested reasons for recurrence due to inadequate ablation include limitations of the heat-sink effect associated with large blood vessels, imprecise placement of applicators during sequential ablations resulting in nonoverlapping ablation zones, limitations of the heating technology, and poor visualization of the treatment zone on preoperative and postoperative imaging.22 It is also noted that the overall and disease free survival rates for patients with large tumors (>5 cm), are significantly lower than those with smaller tumors (<5 cm), possibly due to the more aggressive tumor biology.23
Several strategies for increasing ablation zone sizes with RF and MW ablation systems have been explored, including: modification of tissue physical properties (e.g. saline infusion),24 modification of blood flow,25, 26 power delivery strategies incorporating feedback control,27 and simultaneous multiple-applicator ablation.28, 29, 30 Compared to sequential ablations, simultaneous multiple applicator ablations offer the potential for synergistic heating between applicators, leading to faster ablation of targets, however, there is a small risk of skin burns as has been observed in some clinical studies.31, 32, 33 A recent study by Sindram et al., reported that the occurrence of skin burns is a function of the array insertion depth and the minimum interapplicator spacing, in the case of nonparallel implants,34 and could be avoided by placing constraints on insertion depth and applicator spacing.
Investigators have explored applicator designs and power delivery strategies for increasing sizes of ablation zones created by catheter-based ultrasound devices. Some of the techniques that have been considered are: applicator cooling (catheter cooled vs. internally cooled),15, 17, 35 transducer sizes and shapes,36, 37, 38 multifrequency transducers39 and power modulation schemes.40 While some studies have explored the feasibility of multiple applicator ablation with catheter-based ultrasound devices, there have been few systematic studies of multiple applicator configurations for treatment of large volume targets in the liver. Diederich et al.41 explored the possibility of creating conformal ablation zones in the prostate using interstitial ultrasound applicators in conjunction with a transurethral heating applicator. Nau et al.17, 42 demonstrated the feasibility of creating conformal ablation zones in muscle and prostate targets in vivo, using directional interstitial applicators. This work builds upon a preliminary investigation43 to extensively analyze the performance of parallel and nonparallel configurations of interstitial ultrasound applicators used for liver ablation.
The objective of this study was to use a previously validated theoretical model and ex vivo experiments to define performance of interstitial ultrasound devices in multiple applicator configurations suitable for creating large volume ablation zones in the liver. We used finite element method (FEM) computer models of liver ablation with multiapplicator implants in parallel, diverging, and converging configurations which bracket common surgical approaches. Specific tests included the feasibility of creating conformal ablation zones with nonparallel implants by tailoring applied power levels along the length of the applicator. Heating experiments were performed in ex vivo bovine liver using a subset of configurations as informed by computational models. Critical temperature and thermal dose thresholds, and visible coagulation were used to assess the ablation volumes under different configurations, and to compare experiments to theoretical models.
METHODS
Minimally invasive ultrasound applicators for liver ablation
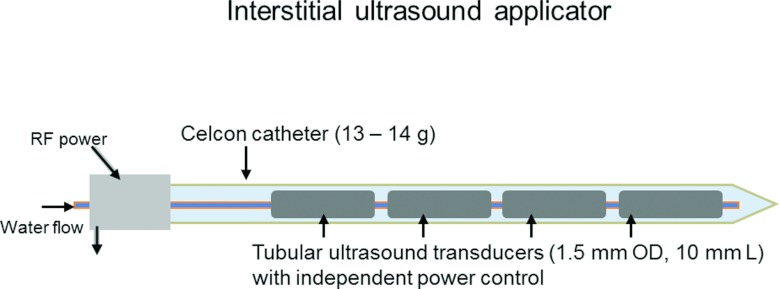
Minimally invasive, catheter-based ultrasound devices for thermal ablation have been described in detail elsewhere,17, 44, 46 and are only described briefly in this section. Catheter-based ultrasound applicators considered in this study consist of linear arrays of 1.5 mm (OD) ultrasound transducers, each 10 mm long (Fig. 1). Transducers are mounted on a polyimide tube, and enclosed in a Celon catheter (13 g, 2.4 mm OD). Cooling water is circulated through and over the transducers to protect them from thermal effects, couple the ultrasound energy, and to increase thermal penetration in to the target volume. Transducers are independently powered, enabling tailored heating along the length of the applicator.
Figure 1.
Interstitial ultrasound applicator consisting of a linear array of independently powered ultrasound applicators enclosed in a Celcon catheter (13–14 g). Energy deposition along length of the applicator can be controlled by tailoring power applied to each individual transducer.
Applicator configurations
We considered two types of configurations: (1) Configurations with interstitial applicators inserted parallel to each other and (2) configurations with interstitial applicators inserted in a nonparallel fashion. Parallel implants consisted of two and three applicators spaced 2 or 3 cm apart (see Fig. 2). Nonparallel implants consisted of two applicators in converging or diverging configurations inserted at angles of 20°, 30°, and 45° to a target volume within the liver (see Fig. 3). For converging implants, the spacing between applicator tips was set to 1 cm at a depth of 5 cm, while considering insertion angles of 20°, 30°(nominal), and 45° to the liver surface. For diverging implants, we considered interapplicator tip spacing of 2 cm, 3 cm (nominal), and 4 cm at a depth of 5 cm. Table 1 lists all the applicator configurations considered in this study. We performed heating experiments in ex vivo liver tissue with applicators in configurations 1-5 (only 30° case for configurations 4 and 5). Computer simulations of ex vivo heating (nonperfused liver) were performed and compared against experiments. Computer simulations of in vivo heating were then performed for promising implant configurations.
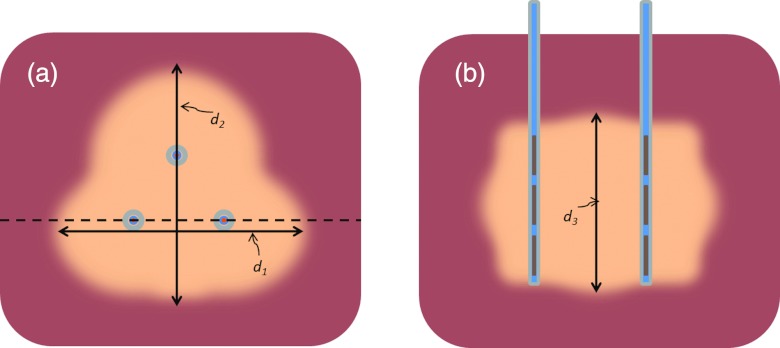
Figure 2.
Illustration of measurements of ablation zones from theoretical models and ex vivo experiments. (a) Top view of slice in a plane perpendicular to applicator insertion showing diameters of ablation zone, d1 and d2. Dashed line indicates slice along length of applicator (b), used to measured length of the ablation zone, d3.
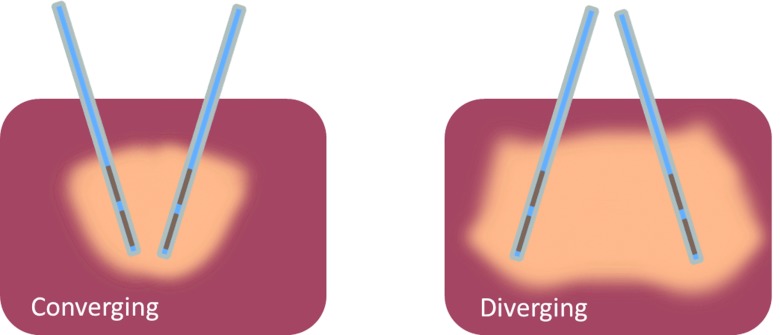
Figure 3.
Sketch of applicators inserted into liver in nonparallel (converging and diverging) configuration.
Table 1.
Multiple applicator configurations for liver ablation considered in this study.
| Configuration number | Number of applicators | Applicator spacing (cm) | Notes |
|---|---|---|---|
| 1 | 2 | 2 | Parallel |
| 2 | 3 | 2 | Parallel |
| 3 | 3 | 3 | Parallel |
| 4 | 2 | 3 | Converging (20°, 30°, 45°) |
| 5 | 2 | 3 | Diverging (20°,30°, 45°) |
Ex vivo experiments
While experiments in appropriate in vivo large animal models offer the most comprehensive evaluation, heating experiments in ex vivo liver tissue are a commonly used developmental step for benchmarking ablation technology. We performed ablations in ex vivo liver tissue using applicators in configurations 1–5. Fresh bovine livers were obtained from a local abattoir and transported to our laboratory immediately after excision. Bovine livers were sliced into blocks approximately 8 cm × 8 cm × 6 cm in size and placed in an isotonic saline solution maintained at 37 °C. Ablations were performed on liver samples within 24 h of being brought into the laboratory. Those samples not used immediately were placed in sealed containers containing isotonic saline and preserved on ice. Custom acrylic templates were built to insert applicators into liver at appropriate distances/angles for the various configurations considered in this study. Initial estimates of optimal transducer operating frequencies were obtained by determining the frequency of maximum real impedance using a network analyzer. Acoustic force-balance measurements were performed to determine the electro-acoustic efficiency, or acoustic output energy relative to applied electrical power. Applicators were inserted into the liver tissue up to a minimum depth of 3.5 cm. Independent four-channel RF amplifiers (Advanced Surgical Systems, Tucson, AZ, USA) were used to power individual transducers at their optimal frequencies. All ex vivo ablations were performed with ∼15 W/cm2 applied to each transducer for 10 min. This power level was selected based on previous experience with interstitial ultrasound devices. After each application of power, the sample under test was left in the isotonic saline bath for a minimum of five minutes to allow for thermal dose accrual. Samples were then sliced orthogonally across the applicator implant within the central heating plane to take measurements and photographs of the ablation zone (as indicated by the extents of visible tissue discoloration). Figure 2 illustrates the dimensions of the ablation zone reported in this study.
Bioacoustic-thermal model
A 3D bioacoustic-thermal model using the FEM was implemented to determine the transient temperature profile during heating with interstitial ultrasound applicators. Heat transfer in liver tissue was modeled using the Pennes bioheat equation [Eq. 1].
| (1) |
where ρ [kg m−3] is density, c [J kg−1 K−1] is specific heat capacity, k [W m−1 K−1] is thermal conductivity, Qs [W m−3] is the acoustic power deposition term, [kg m−3 s−1]is blood mass perfusion rate, cbl [J kg−1 K−1] is specific heat capacity of blood, and Tbl [°C] is the temperature of inflowing arterial blood. The acoustic power deposition due to each individual transducer was calculated using Eq. 2.
| (2) |
where α [Np m−1 MHz−1] is acoustic absorption coefficient, Is [W m−2] is acoustic intensity on the transducer surface, r [m] is the radial distance from the transducer center to a point in the computational domain, r0 [m] is radius of the transducer, μ is acoustic attenuation coefficient, r′ [m] is the radial distance from the transducer surface (r′ = r − r0). The cumulative power deposition at each point in the computational domain is the sum of acoustic energy due to individual ultrasound transducers. While the Pennes equation has limitations, particularly in accounting for heat transfer due to large blood vessels, it remains a reasonable approximation for modeling heat transfer in tissue and is widely used for evaluating performance of ablation technology.47, 48, 49 Recent studies have validated the temperature and thermal damage profile computed using Pennes model with experiments in perfused ex vivo liver, as well as in vivo animal models.49, 50, 51 The tissue acoustic and thermal properties used in the model for Eqs. 1, 2 are listed in Table 2.44
Table 2.
Tissue physical properties used in computational models of hepatic thermal ablation.
| Symbol | Tissue property | Value |
|---|---|---|
| ρ | Density | 1050 kg m−3 |
| c | Specific heat capacity | 3639 J kg−1 K−1 |
| k | Thermal conductivity | 0.51 W m−1 K−1 |
| Blood perfusion rate | {5,12,15} → 0 kg m−3 s−11 | |
| cbl | Specific heat capacity of blood | 3720 kg m−3 s−1 |
| L | Latent heat of tissue water vaporization | 1.53 × 106 J kg−1 K−1 |
| Tbl | Temperature of inflowing arterial blood | 37 °C |
| α | Ultrasound absorption coefficient | 4.5 → 9 Np m−1 MHz−12 |
| μ | Ultrasound attenuation coefficient | 4.5 → 9 Np m−1 MHz−12 |
We considered nominal blood perfusion rates of 5 kg m−3 s−1, 12 kg m−3 s−1, and 15 kg m−3s−1. Blood perfusion was reduced from a nominal value to 0 after a thermal dose of t43 = 300 min was accrued.
The FEM model was implemented in COMSOL Multiphysics v3.5a (COMSOL Inc., Burlington, MA) and all postprocessing was performed in MATLAB (The Mathworks, Inc., Natick, MA). Initial tissue temperature was set to 37 °C. A Dirichlet boundary condition on the edge of the modeled tissue (14 cm × 14 cm × 10 cm) was employed setting the boundary to a fixed temperature of 37 °C. A convective heat transfer boundary condition was applied on the inner catheter wall of each applicator to simulate water cooling, given by Eq. 3.
| (3) |
where h = 1000 W m−1 K−1 is the convective heat transfer coefficient and T∞ = 25 °C is temperature of the cooling water.52 An irregular FEM mesh consisting of Lagrangian elements was used to discretize the solution space. A submillimeter mesh resolution (maximum edge length ∼0.5 mm) was employed at each applicator surface, with progressively increasing mesh element size away from the applicator. Maximum element edge length was restricted to 3 mm within the entire computational domain. A nonlinear implicit solver with variable time steps was used to solve Eq. 1 for 10–15 min ablations.
Tissue damage due to thermal injury was determined using the Sapareto-Dewey thermal dose of equivalent minutes at 43 °C model, as specified in Eq. 4.
| (4) |
Thermal dose calculations were performed at each time step and used to update values of tissue physical properties. The transient FEM solver was allowed to run for a period of 3 min after power to all transducers was turned off, in order to account for accumulated thermal dose due to elevated temperatures. Previous studies indicate a threshold of t43 ≥ 240 min corresponds to coagulative necrosis in soft tissues, and is used as an indicator of treatment endpoint for in vivo simulations and clinical treatments.53, 54, 55, 56 Due to the sharp thermal dose gradients at the edge of the ablation zone, a conservative threshold of t43 ≥ 600 min was used as an indicator of visible thermal coagulation for ex vivo experiments and simulations, similar to a previously validated model of catheter-based ultrasound ablation.43
Tissue acoustic and thermal properties were dynamically adjusted during the course of ablations as a function of accrued thermal dose and tissue temperature exposure.57 In particular, the acoustic attenuation and absorption coefficients of liver were adjusted linearly with the logarithm of accrued thermal dose, until they attained a value twice that of the nominal value.42 For simulations of in vivo heating, microvascular blood perfusion was held constant at its nominal value for tissue with accrued thermal dose t43 ≥ 300 min, and set to zero for all tissue with accrued thermal dose t43 > 300 min and T > 50 °C, an approximation to simulate the effect of microvascular stasis after sustained heating.44 Theoretical models employing these changes in acoustic and thermal properties of liver tissue have been previously shown to closely predict experimentally measured temperature profiles and ablation zone boundaries.44, 58, 59
When modeling heating of ex vivo liver tissue, we applied the same power/time settings as used in experiments (15 W/cm2 applied power per transducer for 10–15 min, 0 kg m−3 s−1 blood perfusion). For in vivo simulations, we considered a range of microvascular blood perfusion levels ranging from 5 kg m−3 s−1 to 15 kg m−3 s−1. While normal liver tissue has a relatively high blood perfusion rate (∼15 kg m−3 s−1), solid tumors may have lower blood perfusion.60, 61 In the in vivo setting, where there is a substantial heat sink associated with blood flow, larger applied power levels were specified for heating durations of 10–15 min. The selected power levels were chosen to be the highest power levels which yielded a maximal tissue temperature below 100 °C for 15 min ablations. This yielded power levels up to 25 W/cm2. It is noted that such power levels have been applied to similar applicators in prior in vivo studies.17 Above 100 °C, tissue water vaporizes and subsequent bubble formation may lead to increased ultrasound attenuation (reduced penetration) and unpredictable heating.62 Furthermore, in the in vivo setting we considered applicators consisting of 3 and 4 tubular ultrasound transducers in a linear array, similar in configurations as applied for hyperthermia with HDR brachytherapy.63 We also included some simulations to approximate application of elevated power levels, where tissue temperatures in excess of 100 °C are expected. In these simulations, tissue specific heat capacity was modified to account for the substantial heat sink associated with tissue water vaporization.54, 64 This is achieved by incorporating the latent heat of water vaporization as shown in Eq. 5:
| (5) |
where C(T) [J kg−1 K−1] is the tissue specific heat capacity at 37 °C, L [J kg−1 K−1] is the latent heat of tissue water vaporization, and ΔT is the temperature interval over which water vaporization occurs (taken to be 1 °C in this work).
RESULTS
Parallel implants: Ex vivo simulations and heating experiments
Table 3 lists dimensions (mean and standard deviation) of the extents of the observed ablation zone (as indicated by visibly discolored tissue) measured in orthogonal cross sections after 10 min ablations in ex vivo bovine liver for implant configurations 1–3. Table 3 also lists extents of the ablation zone as predicted by the t43 ≥ 600 min isodose margin, computed using theoretical models of heating in ex vivo tissue, employing the same parameters used in the experiments. Maximal diameters (in the plane perpendicular to applicator insertion) of the ablation zone ranged between 4.0 cm (configuration 1) and 5.6 cm (configuration 2). Experimental measurements were repeatable, with standard deviations ranging between 2 and 5 mm. Dimensions of ablated tissue obtained from the presented ex vivo experimental study and the theoretical models developed here listed in Table 3, were quantitatively compared using a one-sampled student's t-test. Based on a 5% significance, statistical equivalence could be established for measured and simulated values of metrics d2 and d3 for implant configurations 1, 2, and 3, and metric d1 for configuration 2. When compared to experimental measurements, theoretical models overestimated d1 by 16% for configuration 1, and underestimated d1 by 10% for configuration 3, at a statistical significance of 5%. Figure 4 shows images of ex vivo bovine liver samples ablated with a single applicator and multiple applicators in configurations 1–3. Both the experimental and computational results show that, in the absence of blood perfusion, contiguous ablation zones can be achieved using all the parallel applicator configurations considered in this study. We note that the ablated tissue in Fig. 4c (configuration 3) appears to be more desiccated and tightly coagulated than the other configurations, suggesting probable explosive tissue disruption in the central region between the clustered array. This may be expected since this configuration has the greatest potential for synergistic heating (greater temperatures and thermal dose exposure) applicators with three applicators spaced 2 cm apart (compared to two applicators in configuration 1, and three applicators spaced 3 cm apart in configuration 2).
Table 3.
Dimensions of ablation zone in ex vivo liver for parallel implants (configurations 1–3). Experimental data are the mean ± standard deviation of n experiments. Simulation data correspond to the t43 ≥ 600 min isodose boundary calculated by the bioacoustics-thermal model, which did not include the effects of perfusion.
| Experiment |
Simulation |
||||||
|---|---|---|---|---|---|---|---|
| Configuration | n | d1 (cm) | d2 (cm) | d3 (cm) | d1 (cm) | d2 (cm) | d3 (cm) |
| 1 | 6 | 4.0 ± 0.3 | 3.2 ± 0.4 | 2.9 ± 0.2 | 4.5 | 3.0 | 2.9 |
| 2 | 9 | 4.6 ± 0.5 | 4.2 ± 0.5 | 3.3 ± 0.5 | 4.7 | 4.6 | 3.2 |
| 3 | 8 | 5.6 ± 0.5 | 4.9 ± 0.5 | 2.8 ± 0.3 | 5.2 | 4.9 | 2.8 |
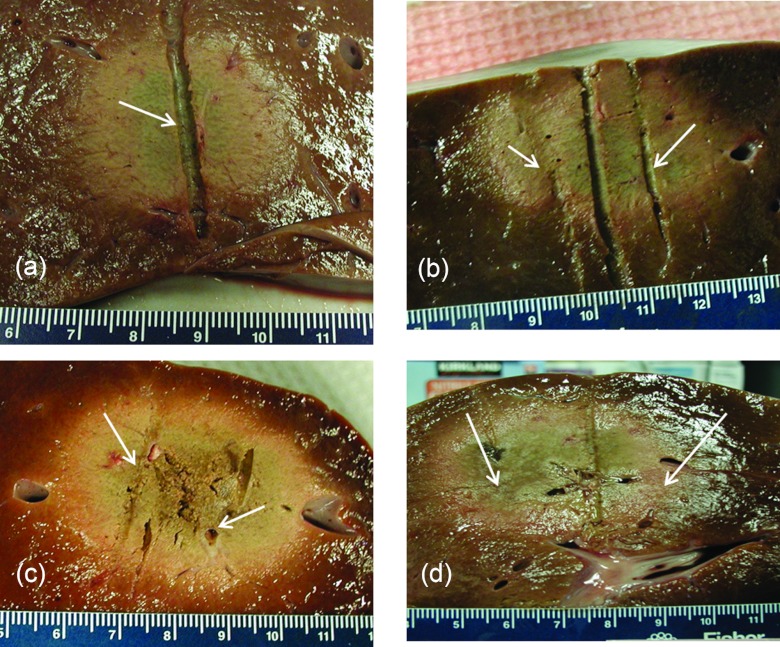
Figure 4.
Ablation zones in ex vivo liver tissue after 10 min ablations with (a) a single applicator, (b) two applicators spaced 2 cm apart (configuration 1), (c) three applicators spaced 2 cm apart (configuration 2) and (d) three applicators spaced 3 cm apart (configuration 3). Arrows indicate the position of ultrasound applicators. The indentation through the center of figures B and D correspond to place holders used to mark the midpoint between applicators.
Parallel implants: In vivo simulations
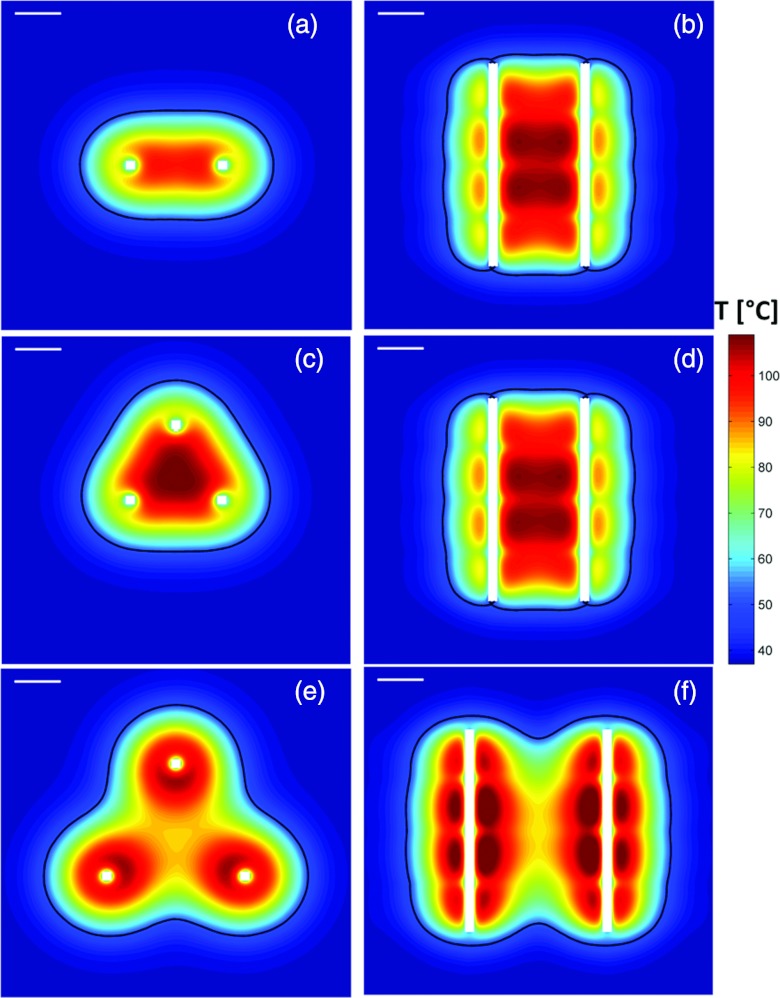
Table 4 lists dimensions of the extents of the ablation zone, as assessed by the t43 > 240 min isodose boundary, after 10 and 15 min ablations using configurations 1–3. For each configuration, results are listed for applicators consisting of linear arrays of 3 and 4 transducers. Furthermore, we modeled heating with unrestricted applied power levels for configurations 1–3, with dimensions of the ablation zone for these simulations listed in Table 5. Figure 5 shows sample temperature profiles in orthogonal planes for the parallel implant configurations under evaluation.
Table 4.
Dimensions and volumes of the in vivo ablation zones, estimated from t43≥ 240 min isodose boundary calculated by computer models (T restricted to ∼ 100 °C), after 10 and 15 min ablations with applicators in configurations 1–3. (NC = noncontiguous).
|
mbl = 5 kg m−3 s−1 |
mbl = 12 kg m−3 s−1 |
mbl = 15 kg m−3 s−1 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Configuration | d1 (cm) | d2 (cm) | d3 (cm) | V (cm3) | d1 (cm) | d2 (cm) | d3 (cm) | V (cm3) | d1 (cm) | d2 (cm) | d3 (cm) | V (cm3) |
| Three transducers (each 1.2 mm OD × 10 mm L) per catheter | |||||||||||||
| 10 min | |||||||||||||
| 1 | 4.5 | 2.8 | 4.0 | 33.8 | 3.7 | 1.6 | 3.4 | 16.3 | NC | NC | NC | 6.1 | |
| 2 | 4.0 | 3.8 | 3.7 | 34.7 | 4.1 | 3.9 | 3.9 | 37.6 | 3.6 | 3.3 | 3.6 | 24.8 | |
| 3 | 5.6 | 4.8 | 4.0 | 62.2 | 5.6 | 4.6 | 4.1 | 57.3 | NC | NC | 4.0 | 42.5 | |
| 15 min | |||||||||||||
| 1 | 5.0 | 3.5 | 4.4 | 47.3 | 4.2 | 2.5 | 3.8 | 27.4 | 3.7 | 1.5 | 3.4 | 15.1 | |
| 2 | 4.5 | 4.4 | 4.3 | 50.4 | 4.6 | 4.5 | 4.4 | 53.1 | 4.2 | 4.0 | 4.1 | 40.6 | |
| 3 | 6.1 | 5.6 | 4.5 | 87.6 | 6.0 | 5.4 | 4.4 | 80.7 | 5.7 | 4.9 | 4.1 | 65.3 | |
| Four transducers (each 1.2 mm OD × 10 mm L) per catheter | |||||||||||||
| 10 min | |||||||||||||
| 1 | 4.5 | 2.9 | 5.2 | 46.7 | 4.2 | 2.3 | 5.0 | 35.6 | 3.8 | 1.6 | 4.8 | 23.8 | |
| 2 | 4.0 | 3.9 | 4.9 | 48.0 | 4.1 | 3.9 | 5.1 | 51.3 | 3.6 | 3.3 | 4.7 | 34.4 | |
| 3 | 5.7 | 4.9 | 5.2 | 85.8 | 5.6 | 4.6 | 5.3 | 79.0 | NC | NC | 5.1 | 58.9 | |
| 15 min | |||||||||||||
| 1 | 5.0 | 3.6 | 5.6 | 65.2 | 4.6 | 3.1 | 5.3 | 51.2 | 4.3 | 2.6 | 5.1 | 39.2 | |
| 2 | 4.6 | 4.5 | 5.5 | 68.9 | 4.6 | 4.5 | 5.6 | 71.8 | 4.2 | 4.1 | 5.2 | 55.3 | |
| 3 | 6.2 | 5.7 | 5.5 | 118.4 | 6.1 | 5.5 | 5.5 | 110.0 | 5.8 | 5.0 | 5.4 | 90.0 | |
Table 5.
Dimensions and volumes of the in vivo ablation zones, estimated from t43 ≥ 240 min isodose boundary calculated by computer models with no restriction on power levels (i.e., T > 100 °C), after 10 and 15 min ablations with applicators (three 10 mm long transducers each) in configurations 1–3.
|
mbl = 5 kg m−3 s−1 |
mbl = 12 kg m−3 s−1 |
mbl = 15 kg m−3 s−1 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Configuration | d1 (cm) | d2 (cm) | d3 (cm) | V (cm3) | d1 (cm) | d2 (cm) | d3 (cm) | V (cm3) | d1 (cm) | d2 (cm) | d3 (cm) | V (cm3) |
| 10 min | |||||||||||||
| 1 | 5.6 | 4.1 | 4.9 | 68.9 | 5.0 | 3.3 | 4.5 | 48.6 | 4.8 | 3.1 | 4.3 | 43.0 | |
| 2 | 5.8 | 5.6 | 5.2 | 97.9 | 5.2 | 5.0 | 4.7 | 72.5 | 5.0 | 4.8 | 4.6 | 65.5 | |
| 3 | 6.6 | 6.1 | 4.8 | 110.1 | 6.0 | 5.2 | 4.4 | 75.7 | 5.7 | 4.8 | 4.3 | 64.5 | |
| 15 min | |||||||||||||
| 1 | 6.0 | 4.6 | 5.2 | 87.1 | 5.3 | 3.8 | 4.7 | 59.9 | 5.1 | 3.6 | 4.6 | 53.1 | |
| 2 | 6.2 | 6.2 | 5.5 | 121.0 | 5.5 | 5.4 | 5.0 | 87.2 | 5.3 | 5.2 | 4.9 | 78.4 | |
| 3 | 7.1 | 6.7 | 5.1 | 141.0 | 6.3 | 5.8 | 4.6 | 97.2 | 6.1 | 5.4 | 4.5 | 84.6 | |
Figure 5.
Temperature profiles in axial (left column) and sagittal (right) planes after 15 min ablations with applicators in configurations 1 (a,b), 2 (c,d) and 3 (e,f). The contours indicating extents of the ablation zone correspond to the t43 ≥ 240 min isodose boundary. Temperature profiles shown here are calculated with a nominal perfusion rate of 15 kg m−3 s−1. The white line in the top left corner of each panel indicates a scale of 1 cm.
Our simulations indicate that ablation zone volumes ranging between 6.1 cm3 and 90 cm3 can be created with applicators in parallel implant configurations (for mbl = 15 kg m−3 s−1) after 10–15 min heating, while limiting peak tissue temperatures to ∼100 °C. At elevated blood perfusion rates (mbl = 15 kg m−3 s−1), contiguous ablation zones cannot be obtained using only two applicators (configuration 1). Using three applicators spaced 2 cm apart (configuration 2), maximal ablation zone diameters of 4.0 and 4.6 cm could be obtained after 10 and 15 min heating. When inter applicator spacing was increased to 3 cm, maximal ablation zone diameters of 6.0–6.2 cm were obtained for perfusion rates up to 12.5 kg m–3 s–1. Our simulations indicate that, contiguous ablation zones can be created with configuration 2 within 10 min for all the blood perfusion levels considered in this study. At a nominal perfusion rate of 15 kg m−3 s−1, 15 min ablations are required to create contiguous ablation zones using configurations 1 and 3. Figure 6 illustrates (a) the impact of blood perfusion and (b) treatment times on the extents of the ablation zone in a plane perpendicular to applicator insertion.
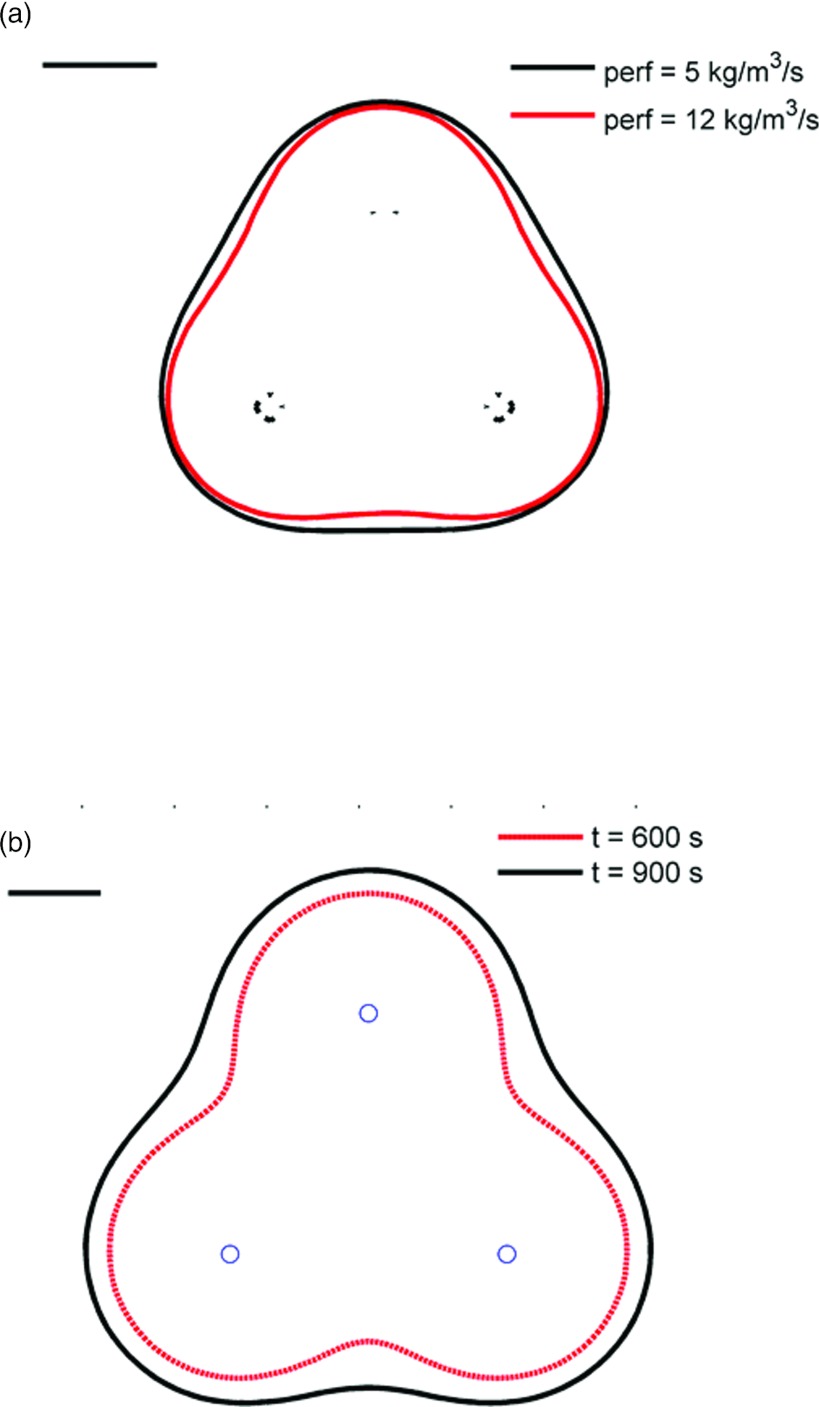
Figure 6.
(a) Extents of the ablation zone after 10 min ablation with applicators in configuration 2, computed using two different nominal blood perfusion rates. At higher blood perfusion rates, there are indentations along the periphery of the ablation zone. (b) Extents of the ablation zone after 10 and 15 min ablation with applicators in configuration 3 at a nominal perfusion rate of 5 kg m−3 s−1. The ablation zone profile after 10 min exhibits scalloping/clefting along the boundary. After 15 min of heating, a contiguous ablation zone is formed. The black line in the top left corner indicates a scale of 1 cm.
Nonparallel implants
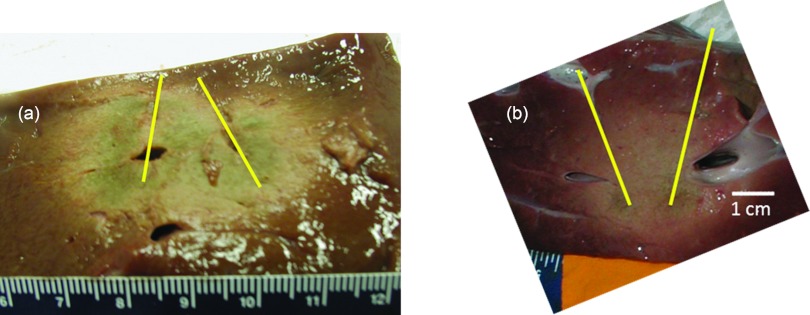
We performed ex vivo ablations with two applicators in converging and diverging configurations (see Fig. 5). In a converging configuration, applied power levels were 12.5 W/cm2 to proximal transducers, and 8.5 W/cm2 to distal transducers. Dimensions of the ablation zone were measured to be 3.2 ± 0.2 cm × 2.4 ± 0.2 cm (n = 3 experiments). In a diverging configuration, applied power levels were 12.5 W/cm2 to proximal transducers and 15 W/cm2 to distal transducers. Dimensions of the ablation zone were measured to be 4.8 ± 0.5 cm × 3.0 ± 0.1 cm (n = 4) experiments. Figure 7 shows sample ablation zones created in ex vivo liver tissue with two applicators inserted diverging and converging configurations.
Figure 7.
Sample ablation zone in ex vivo bovine liver after 10 min ablation with two applicators inserted in (a) diverging configuration and (b) converging configuration. Increased power levels were applied to the tip transducers, and lower power levels were applied to the proximal transducers. Complete, contiguous ablation zones were obtained with minimal clefts or indentations along the periphery.
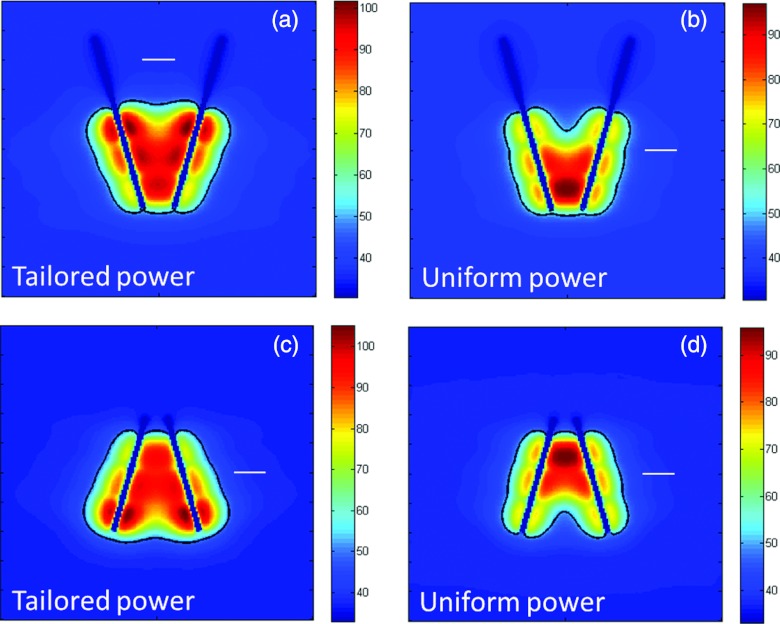
We simulated in vivo ablations with two applicators each containing three independently powered transducers. For converging applicators, power levels applied to transducers decreased from the proximal transducer (17 W/cm2) towards the tip (25 W/cm2), whereas the reverse power delivery scheme was applied for diverging applications. We also included simulations of ablations with uniform power (20 W/cm2) applied to transducers along the length of the applicator. Figure 8 shows the temperature profiles and lethal thermal dose profiles after 10 min ablations with applicators in nonparallel configurations using tailored and uniform power delivery profiles. In a converging configuration, implants with uniform power profiles create a hotspot where applicators are closest to each other. Conversely, in diverging implants, uniform power profiles result in noncontiguous ablation zones (clefts) where the applicators are furthest apart. These undesired heating profiles (clefts and hotspots) are avoided when tailored power profiles can be applied
Figure 8.
Temperature maps and extents of the ablation zone after 10 min ablations with two applicators inserted in converging (a and b) and diverging (c and d) implants. When power supplied to individual transducers is tailored along the length of the applicators (a and c), there are no clefts or indentations in the ablation zone. However, applying uniform powers to all transducers results in hotspots where applicators are closest to each other, and clefts or indentations in the ablation zone where applicators are furthest apart. The white line in each panel corresponds to a 1 cm scale.
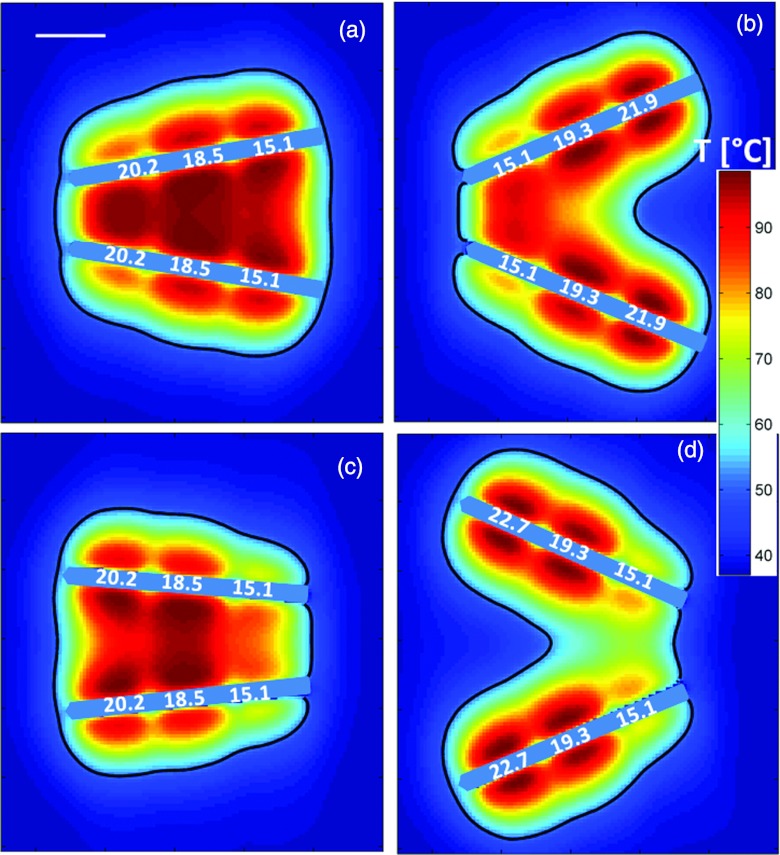
Figures 9a, 9b show temperature profiles and estimated extents of the ablation zone for applicators in converging configurations at angles of 20° and 45°. At 20°, a contiguous coagulation zone can be achieved by increasing applied power levels from the tip (15.1 W/cm2) to the proximal transducer (20.2 W/cm2). At an angle of 45°, however, a contiguous ablation zone cannot be obtained even with application of increased power levels (22.7 W/cm2) to the proximal transducers. Figures 9c, 9d shows temperature profiles and estimated extents of the ablation zone for applicators in a diverging configuration with interapplicator tip spacings of 2 cm and 4 cm. The model predicts a contiguous ablation zone cannot be obtained within 10 min of heating while limiting peak tissue temperatures to 100 °C.
Figure 9.
Temperature maps and extents of the ablation zone after 10 min ablations with two applicators inserted in converging (a and b) and diverging (c and d) implants at varying angles. For converging implants, applicator tips are spaced 1 cm apart, with applicators inserted at angles (a) 30° and (b) 45° to the normal. For diverging implants, applicator tips are spaced (c) 3 cm and (d) 4 cm apart. Applied intensity levels [W/cm2] for each transducer are indicated along the length of the applicators.
DISCUSSION
This study was performed to evaluate interstitial ultrasound in implant configurations suitable for multiple-applicator liver ablation with parallel and nonparallel implants, and explore the feasibility of tailoring ablation zones with longitudinal power control. Heating experiments in ex vivo bovine tissue demonstrated the ability to create contiguous ablation zones with applicators consisting of two transducers. The axial and cross section dimensions of the ablation zone predicted by computer models were in very good agreement with those observed experimentally. The computer model was used to determine the performance of these configurations in an in vivo setting at various blood perfusion rates.
Blood perfusion in highly perfused organs such as the liver poses a substantial heat sink during thermal ablation procedures. Using multiple applicators spaced up to 3 cm apart results in synergistic heating, leading to larger ablation zones. Ex vivo experiments and simulations indicated that complete, contiguous ablation zones could be created within 10 min with interstitial ultrasound applicators in configurations 1–3. When limiting peak tissue temperatures to 100 °C, our simulations indicated that blood perfusion rates were a major limiting factor for the size of ablation zones in vivo. At low and moderate blood perfusion rates (5 and 12 kg m−3 s−1), contiguous ablation zones could be obtained using two applicators (2 cm spacing) or three applicators (spaced up to 3 cm apart) within 10 min of heating. At a blood perfusion rate of 15 kg m−3 s−1, however, 15 min of heating were required to create a contiguous ablation zone using configurations 1 and 3, while restricting maximum tissue temperatures to 100 °C.
Of the three implant configurations considered in this study, configuration 2 created contiguous ablation zones with axial cross section closest to a circle. For all perfusion ranges, the difference between d2 and d1 remained under 2 mm using configuration 2 (an ideal circle would have d2 = d1). On the other hand, differences between d2 and d1 range between 5 and 10 mm for configuration 3, with larger differences for 10 min ablations and higher blood perfusion rates. Larger differences between d2 and d1 indicate scalloping along the periphery of the ablation zone (Fig. 6). These results suggest that interapplicator spacing should be restricted to 2 cm if near-spherical ablation zones are desired.
At a given perfusion level, increasing heating duration from 10 to 15 min results in increase of the maximum ablation zone diameter by up to 4–6 mm. Furthermore, for configurations 1 and 3, 10 min of heating were not sufficient for creating contiguous ablation zones at high blood perfusion rates. Both the experimental and simulation studies indicate that the length of the ablation zone (d3) is restricted to the segment of the applicator containing the tubular ultrasound transducers. At all blood perfusion rates and heating durations, the ablation zones did not extend more than 5 mm beyond the edges of the proximal/distal transducers, consistent with other studies. For configurations 2 and 3, adding an additional transducer along the length of the applicator had little impact on d1 and d2. However, for configuration 1, adding a fourth transducer at higher perfusion rates results in up to 4 mm increases in d1 and d2. This is due to the increased power deposition and resultant increase in growth of the ablation zone by thermal conduction. These results demonstrate ability to control the length of the ablation zone with minimal impact on cross sectional dimensions of the ablation zone using interstitial ultrasound technology.
When power levels were restricted to maintain tissue temperature below 100 °C, simulations of in vivo heating showed that 10–15 min ablations were required to create complete, contiguous ablation zones for three applicators, spaced 2 cm apart, ranging from 4.5 × 4.4 cm to 3.3 × 3.6 cm for blood perfusion rates between 5 and 15 kg m−3 s−1. When higher power levels were used, ablation zones ranged from 5.6 cm × 4.1 cm to 5.1 cm × 3.6 cm for blood perfusion rates between 5 and 15 kg m−3 s−1. Applicators in configuration 3, a clustered array spaced 3 cm apart, yielded noncontiguous ablation zones after 10 min heating. The synergistic heating from three applicators after 10 min was unable to overcome the large heat sink due to blood perfusion. Contiguous ablation zones extending 6.1 cm × 5.6 cm were formed after 15 min of heating for low perfusion cases (mbl = 5 kg m−3 s−1), however, at high perfusion rates (mbl = 15 kg m−3 s−1) the outer boundary of the ablation zone is scalloped in between applicators reducing effective treatment diameter to 5.7 × 4.9 cm. We note that in these simulations, fairly high tissue perfusion values were utilized, however, necrotic tumors may have severely reduced blood perfusion rates. Furthermore, the type of the tumor, location in the liver, and extent of liver cirrhosis may have a dramatic impact on nominal perfusion values within the target.65
Multiple applicator ablation with other energy modalities (RF and MW) are in clinical use for treatment of large tumors, and are briefly compared here to interstitial ultrasound technology. The commercially available Covidien 915 MHz Evident system creates ablation zones up to 4.6 cm × 4.6 cm × 5.1 cm in ex vivo bovine liver after 10 min ablations with three water cooled antennas spaced 2 cm apart.66 The BSD Microtherm-X creates ablation zones 5.2 cm × 5.5 cm × 6 cm, operating synchronously with 45 W applied power for 10 min.67 Similarly, clustered RF applicators have been demonstrated to produce ablation zones of 4.7 cm diameter in ex vivo liver after 15 min, and 4.5–7 cm for treatment of intrahepatic colorectal metastases in humans.68 In a similar setting, the multiapplicator interstitial ultrasound implants were shown to produce comparable ablation zones of 4.9–5.6 cm diameter in the ex vivo experiments, and length of the ablation zone ranging from 3.8 to 5.6 cm depending on the number of ultrasound transducers in each catheter. These data indicate that multiple-applicator interstitial ultrasound devices can create ablation zones which may be slightly smaller than MW systems, and comparable to RF ablation systems, within similar heating times.
One advantage of interstitial ultrasound compared to other ablation modalities is the ability to tailor heating along the length of the applicators, through the use of linear arrays of independently powered ultrasound elements. While parallel insertion can be readily achieved in experimental studies using well-defined templates, this may not always be possible in practice due to anatomical considerations. The inability to tailor the heating pattern along the length of the applicator, may lead to hot spots where applicators are closest to each other, and cold spots (inadequate ablation) where applicators are furthest away (Fig. 8). While tailoring the applied power levels may enable the physician to adjust for nonparallel implants, we note that the ability to obtain contiguous ablation zones is dependent on the maximum spacing between applicators. When spacing between transducers exceeds 3 cm [Figs. 9b, 9d], contiguous ablation zones cannot be obtained within 10 min of heating while maintaining tissue temperatures below 100 °C. This is consistent with the limitations of interstitial ultrasound heating technology as analyzed in the parallel implant portion of this study. Knowledge of the applicator positions obtained from preprocedural and periprocedural imaging, placement of invasive temperature sensors, and predictive computer models may enable tailoring of heating profiles along the length of interstitial ultrasound applicators in nonparallel configurations to conform to patient-specific targets. Based on the positions of implanted applicators as determined by imaging, forward and/or inverse treatment planning tools may be used to determine appropriate power levels for adequate thermal coverage of the target, as has been implemented for interstitial ultrasound hyperthermia procedures.69 CT and ultrasound are the most commonly used imaging modalities for guiding percutaneous tumor ablation.70 For parallel implants, the use of surface templates (as used for currently available RF and MW ablation systems) can augment imaging data to assess applicator positioning. Recent developments in precise placement and electromagnetic tracking of applicators and multimodality image fusion provide the promise of improved applicator localization relative to the tumor, and may enable tailored heating with the use of catheter-based ultrasound technology.71 A detailed study of the feasibility of tailoring heating profiles—from nonparallel, multiple interstitial ultrasound applicators—to patient-specific targets is a subject of ongoing research in our laboratory.
Another advantage of interstitial ultrasound applicators is the relative independence of their energy deposition patterns from applicator insertion depth or proximity of adjacent devices. The wavelengths of ultrasound energy in the 6–8 MHz range are small in soft tissue, thus the energy emitted from each transducer segment is collimated and well-defined by the dimensions of the respective element.14 This is in contrast to the wavelength of MW energy in the 915 MHz–2.45 GHz range, which varies from 4.5 to 2 cm, thereby making it difficult to control or vary heating length of individual devices. Currently available MW antennas, typically have a minimal insertion depth below which the antenna efficiencies and radiation pattern diverge from prescribed values.72 A recent study by Sindram et al.,34 noted the occurrence of skin burns when a cluster of MW applicators was inserted at a moderate angle (10°) and at insertion depths < 5 cm. Since ultrasound energy from interstitial applicators is tightly collimated to the individual transducers along the length of the applicator,16 it offers the potential to avoid skin burns and unpredictable energy deposition patterns, especially when heating tumors closer to the surface of the liver. It should be noted that this collimation and high operating frequency (6–8 MHz) precludes the possibility of using phasing strategies between applicators for focusing energy, which is possible with MW applicators.72
SUMMARY
We evaluated configurations of multiple, interstitial ultrasound applicators for large volume liver ablation using bioacoustic-thermal models and experiments in ex vivo bovine liver.
Simulations of these implant configurations within moderate to highly perfused liver tissue (mbl = 5–15 kg m−3 s−1), indicated that lesion dimensions with 2–3 cm clustered arrays vary from 3.8 × 1.6 cm (15 kg m−3 s−1) to 6.2 × 5.7 cm (5 kg m−3 s−1), with scalloping and variation of diameter more apparent at the higher perfusion and shorter times. When unrestricted power is applied to transducers without consideration of maximum tissue temperatures, ablation zone up to 6.1 × 5.4 cm can be attained in 15 min, even at high perfusion rates (15 kg m−3 s−1). Ex vivo ablations with 2–3 parallel applicators spaced 2–3 cm apart yielded ablation zones of 4.0–5.6 cm × 3.2–4.9 cm in cross section. Simulations of ex vivo heating using the same parameters as the experiments yielded ablation zone dimensions in close agreement to experiment. These are comparable to current values obtained for clustered arrays of RF and MW ablation systems. Further, the ability to tailor heating along the length of interstitial ultrasound applicators allows the possibility to adjust heating profiles for converging or diverging implants as anticipated for percutaneous insertion under image guidance, independent of insertion depth and alignment with adjacent applicators, and may enable conformal heating of patient-specific targets.
ACKNOWLEDGMENT
The authors acknowledge support through NIH (Grant Nos. R01CA122276, 5R44CA134169, 5R44CA112852).
References
- McWilliams J. P., Yamamoto S., Raman S. S., Loh C. T., Lee E. W., Liu D. M., and Kee S. T., “Percutaneous ablation of hepatocellular carcinoma: Current status,” J. Vasc. Interv. Radiol. 21(8), S204–213 (2010). 10.1016/j.jvir.2009.11.025 [DOI] [PubMed] [Google Scholar]
- Flanders V. L. and Gervais D. A., “Ablation of Liver Metastases: Current Status,” J. Vasc. Interv. Radiol. 21(8), S214–S222 (2010). 10.1016/j.jvir.2010.01.046 [DOI] [PubMed] [Google Scholar]
- Forner A., Llovet J. M., and Bruix J., “Hepatocellular carcinoma,” Lancet 379(9822), 1245–1255 (2012). 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- O’Rourke A. P., Haemmerich D., Prakash P., Converse M. C., Mahvi D. M., and Webster J. G., “Current status of liver tumor ablation devices,” Expert Rev. Med. Devices 4(4), 523–537 (2007). 10.1586/17434440.4.4.523 [DOI] [PubMed] [Google Scholar]
- Padma S., Martinie J. B., and Iannitti D. A., “Liver tumor ablation: Percutaneous and open approaches,” J. Surg. Oncol. 100(8), 619–634 (2009). 10.1002/jso.21364 [DOI] [PubMed] [Google Scholar]
- Stafford R. J., Fuentes D., Elliott A. A., Weinberg J. S., and Ahrar K., “Laser-induced thermal therapy for tumor ablation,” Crit. Rev. Biomed. Eng. 38(1), 79–100 (2010). 10.1615/CritRevBiomedEng.v38.i1.70 [DOI] [PubMed] [Google Scholar]
- Fischer K., Gedroyc W., and Jolesz F. A., “Focused ultrasound as a local therapy for liver cancer,” Cancer J. 16(2), 118–124 (2010). 10.1097/PPO.0b013e3181db7c32 [DOI] [PubMed] [Google Scholar]
- Cressman E. N. K., Geeslin M. G., Shenoi M. M., Hennings L. J., Zhang Y., Iaizzo P. A., and Bischof J. C., “Concentration and volume effects in thermochemical ablation in vivo: Results in a porcine model,” Int. J. Hyperthermia 28(2), 113–121 (2012). 10.3109/02656736.2011.644621 [DOI] [PubMed] [Google Scholar]
- Farnam J. L., Smith B. C., Johnson B. R., Estrada R., Edelman T. L., Farah R., and Cressman E. N. K., “Thermochemical ablation in an ex-vivo porcine liver model using acetic acid and sodium hydroxide: Proof of concept,” J. Vasc. Interv. Radiol. 21(10), 1573–1578 (2010). 10.1016/j.jvir.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Charpentier K. P., Wolf F., Noble L., Winn B., Resnick M., and Dupuy D. E., “Irreversible electroporation of the liver and liver hilum in swine,” HPB (Oxford) 13(3), 168–173 (2011). 10.1111/j.1477-2574.2010.00261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball C., Thomson K. R., and Kavnoudias H., “Irreversible electroporation: a new challenge in ‘out of operating theater’ anesthesia,” Anesth. Analg. 110(5), 1305–1309 (2010). 10.1213/ANE.0b013e3181d27b30 [DOI] [PubMed] [Google Scholar]
- Dodd G. D., Frank M. S., Aribandi M., Chopra S., and Chintapalli K. N., “Radiofrequency thermal ablation computer analysis of the size of the thermal injury created by overlapping ablations,” AJR, Am. J. Roentgenol. 177(4), 777–782 (2001). [DOI] [PubMed] [Google Scholar]
- Owen N. R., Bouchoux G., Seket B., Murillo-Rincon A., Merouche S., Birer A., Paquet C., Delabrousse E., Chapelon J. Y., Berriet R., Fleury G., and Lafon C., “In vivo evaluation of a mechanically oscillating dual-mode applicator for ultrasound imaging and thermal ablation,” IEEE Trans. Biomed. Eng. 57(1), 80–92 (2010). 10.1109/TBME.2009.2023994 [DOI] [PubMed] [Google Scholar]
- Deardorff D. L., Diederich C. J., and Nau W. H., “Control of interstitial thermal coagulation: Comparative evaluation of microwave and ultrasound applicators,” Med. Phys. 28(1), 104–117 (2001). 10.1118/1.1334606 [DOI] [PubMed] [Google Scholar]
- Deardorff D. L. and Diederich C. J., “Ultrasound applicators with internal water-cooling for high-powered interstitial thermal therapy,” IEEE Trans. Biomed. Eng. 47(10), 1356–1365 (2000). 10.1109/10.871409 [DOI] [PubMed] [Google Scholar]
- Kinsey A. M., Diederich C. J., Tyreus P. D., Nau W. H., Rieke V., and Pauly K. B., “Multisectored interstitial ultrasound applicators for dynamic angular control of thermal therapy,” Med. Phys. 33(5), 1352–1363 (2006). 10.1118/1.2184443 [DOI] [PubMed] [Google Scholar]
- Nau W. H., Diederich C. J., and Burdette E. C., “Evaluation of multielement catheter-cooled interstitial ultrasound applicators for high-temperature thermal therapy,” Med. Phys. 28(7), 1525–1534 (2001). 10.1118/1.1381550 [DOI] [PubMed] [Google Scholar]
- Kangasniemi M., Diederich C. J., Price R. E., Stafford R. J., Schomer D. F., Olsson L. E., Tyreus P. D., Nau W. H., and Hazle J. D., “Multiplanar MR temperature-sensitive imaging of cerebral thermal treatment using interstitial ultrasound applicators in a canine model,” J. Magn. Reson. Imaging 16(5), 522–531 (2002). 10.1002/jmri.10191 [DOI] [PubMed] [Google Scholar]
- Nau W. H., Diederich C. J., Simko J., Juang T., Jacoby A., and Burdette E. C., “Ultrasound interstitial thermal therapy (USITT) for the treatment of uterine myomas,” in Thermal Treatment of Tissue: Energy Delivery and Assessment IV, Proc. SPIE6440 (2011). [DOI] [PMC free article] [PubMed]
- Lafon C., Melodelima D., Salomir R., and Chapelon J. Y., “Interstitial devices for minimally invasive thermal ablation by high-intensity ultrasound,” Int. J. Hyperthermia 23(2), 153–163 (2007). 10.1080/02656730601173029 [DOI] [PubMed] [Google Scholar]
- Owen N. R., Chapelon J. Y., Bouchoux G., Berriet R., Fleury G., and Lafon C., “Dual-mode transducers for ultrasound imaging and thermal therapy,” Ultrasonics 50(2), 216–220 (2010). 10.1016/j.ultras.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Hong K. and Georgiades C., “Radiofrequency ablation: Mechanism of action and devices,” J. Vasc. Interv. Radiol. 21(8), S179–186 (2010). 10.1016/j.jvir.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Ng K., Vauthey J.-N., Pawlik T., Lauwers G., Regimbeau J.-M., Belghiti J., Ikai I., Yamaoka Y., Curley S., Nagorney D., Ng I., Fan S., and Poon R., “Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database,” Ann. Surg. Oncol. 12(5), 364–373 (2005). 10.1245/ASO.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Livraghi T., Goldberg S. N., Monti F., Bizzini A., Lazzaroni S., Meloni F., Pellicanò S., Solbiati L., and Gazelle G. S., “Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases,” Radiology 202(1), 205–210 (1997). [DOI] [PubMed] [Google Scholar]
- Yamasaki T., Kurokawa F., Shirahashi H., Kusano N., Hironaka K., and Okita K., “Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Comparison with standard percutaneous radiofrequency ablation therapy,” Cancer 95(11), 2353–2360 (2002). 10.1002/cncr.10966 [DOI] [PubMed] [Google Scholar]
- Chang I., Mikityansky I., Wray-Cahen D., Pritchard W. F., Karanian J. W., and Wood B. J., “Effects of perfusion on radiofrequency ablation in swine kidneys,” Radiology 231(2), 500–505 (2004). 10.1148/radiol.2312021248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerich D. and Webster J. G., “Automatic control of finite element models for temperature-controlled radiofrequency ablation,” Biomed. Eng. Online 4, 42 (2005). 10.1186/1475-925X-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeseke P. F., Sampson L. A., Haemmerich D., Brace C. L., Fine J. P., Frey T. M., Winter T. C., and Lee F. T., “Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: In vivo porcine liver results,” Radiology 241(1), 116–124 (2006). 10.1148/radiol.2411051271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace C. L., Laeseke P. F., Sampson L. A., Frey T. M., van der Weide D. W., and Lee F. T., “Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: Results from an in vivo swine liver model,” Radiology 244(1), 151–156 (2007). 10.1148/radiol.2441052054 [DOI] [PubMed] [Google Scholar]
- Haemmerich D. and Lee F. T., “Multiple applicator approaches for radiofrequency and microwave ablation,” Int. J. Hyperthermia 21(2), 93–106 (2005). 10.1080/02656730412331286894 [DOI] [PubMed] [Google Scholar]
- Seki S., Sakaguchi H., Kadoya H., Morikawa H., Habu D., Nishiguchi S., Shiomi S., Kitada T., and Kuroki T., “Laparoscopic microwave coagulation therapy for hepatocellular carcinoma,” Endoscopy 32(8), 591–597 (2000). 10.1055/s-2000-9014 [DOI] [PubMed] [Google Scholar]
- Dong B. W., Liang P., Yu X. L., Zeng X. Q., Wang P. J., Su L., Wang X. D., Xin H., and Li S., “Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study,” AJR, Am. J. Roentgenol. 171(2), 449–454 (1998). [DOI] [PubMed] [Google Scholar]
- Iannitti D. A., Martin R. C. G., Simon C. J., Hope W. W., Newcomb W. L., McMasters K. M., and Dupuy D., “Hepatic tumor ablation with clustered microwave antennae: the US Phase II Trial,” HPB (Oxford) 9(2), 120–124 (2007). 10.1080/13651820701222677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindram D., Haley K., McKillop I. H., Martinie J. B., and Iannitti D. A., “Determination of angle, depth, and distance of antennae as skin burn risks in microwave ablation in a porcine model,” J. Interv. Oncol. 3(1), 31–36 (2010). [Google Scholar]
- Deardorff D. L., Diederich C. J., and Nau W. H., “Air-cooling of direct-coupled ultrasound applicators for interstitial hyperthermia and thermal coagulation,” Med. Phys. 25(12), 2400–2409 (1998). 10.1118/1.598451 [DOI] [PubMed] [Google Scholar]
- Tyreus P. D., Nau W. H., and Diederich C. J., “Effect of applicator diameter on lesion size from high temperature interstitial ultrasound thermal therapy,” Med. Phys. 30(7), 1855–1863 (2003). 10.1118/1.1584125 [DOI] [PubMed] [Google Scholar]
- Lafon C., de L., Theillere Y., Prat F., Chapelon J. Y., and Cathignol D., “Optimizing the shape of ultrasound transducers for interstitial thermal ablation,” Med. Phys. 29(3), 290–297 (2002). 10.1118/1.1445416 [DOI] [PubMed] [Google Scholar]
- Nau W. H., Diederich C. J., and Stauffer P. R., “Directional power deposition from direct-coupled and catheter-cooled interstitial ultrasound applicators,” Int. J. Hyperthermia 16(2), 129–144 (2000). 10.1080/026567300285330 [DOI] [PubMed] [Google Scholar]
- Chopra R., Luginbuhl C., Foster F. S., and Bronskill M. J., “Multifrequency ultrasound transducers for conformal interstitial thermal therapy,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 50(7), 881–889 (2003). 10.1109/TUFFC.2003.1214507 [DOI] [PubMed] [Google Scholar]
- Delabrousse E., Salomir R., Birer A., Paquet C., Mithieux F., Chapelon J., Cotton F., and Lafon C., “Automatic temperature control for MR-guided interstitial ultrasound ablation in liver using a percutaneous applicator: Ex vivo and in vivo initial studies,” Magn. Reson. Med. 63(3), 667–679 (2010). 10.1002/mrm.22328 [DOI] [PubMed] [Google Scholar]
- Diederich C. J., Nau W. H., Burdette E. C., Bustany I. S., Deardorff D. L., and Stauffer P. R., “Combination of transurethral and interstitial ultrasound applicators for high-temperature prostate thermal therapy,” Int. J. Hyperthermia 16(5), 385–403 (2000). 10.1080/026567300416695 [DOI] [PubMed] [Google Scholar]
- Nau W. H., Diederich C. J., Ross A. B., Butts K., Rieke V., Bouley D. M., Gill H., Daniel B., and Sommer G., “MRI-guided interstitial ultrasound thermal therapy of the prostate: A feasibility study in the canine model,” Med. Phys. 32(3), 733–743 (2005). 10.1118/1.1861163 [DOI] [PubMed] [Google Scholar]
- Prakash P., Salgaonkar V. A., Burdette E. C., and Diederich C. J., “Hepatic ablation with multiple interstitial ultrasound applicators: Initial ex vivo and computational studies,” Proc. SPIE 7901 (2011). [Google Scholar]
- Tyreus P. D. and Diederich C. J., “Theoretical model of internally cooled interstitial ultrasound applicators for thermal therapy,” Phys. Med. Biol. 47(7), 1073–1089 (2002). 10.1088/0031-9155/47/7/306 [DOI] [PubMed] [Google Scholar]
- Moros E. G., Dutton A. W., Roemer R. B., Burton M., and Hynynen K., “Experimental evaluation of two simple thermal models using hyperthermia in muscle in vivo,” Int. J. Hyperthermia 9(4), 581–598 (1993). 10.3109/02656739309005054 [DOI] [PubMed] [Google Scholar]
- Diederich C. J., Nau W. H., and Stauffer P. R., “Ultrasound applicators for interstitial thermal coagulation,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control 46(5), 1218–1228 (1999). 10.1109/58.796127 [DOI] [PubMed] [Google Scholar]
- Haemmerich D. and Schutt D. J., “RF ablation at low frequencies for targeted tumor heating: In vitro and computational modeling results,” IEEE Trans. Biomed. Eng. 58(2), 404–410 (2011). 10.1109/TBME.2010.2085081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phasukkit P., Tungjitkusolmun S., and Sangworasil M., “Finite-element analysis and in vitro experiments of placement configurations using triple antennas in microwave hepatic ablation,” IEEE Trans. Biomed. Eng. 56(11), 2564–2572 (2009). 10.1109/TBME.2009.2027128 [DOI] [PubMed] [Google Scholar]
- He X., McGee S., Coad J. E., Schmidlin F., Iaizzo P. A., Swanlund D. J., Kluge S., Rudie E., and Bischof J. C., “Investigation of the thermal and tissue injury behavior in microwave thermal therapy using a porcine kidney model,” Int. J. Hyperthermia 20(6), 567–593 (2004). 10.1080/0265673042000209770 [DOI] [PubMed] [Google Scholar]
- Fuentes D., Cardan R., Stafford R. J., Yung J., Dodd G. D., and Feng Y., “High-fidelity computer models for prospective treatment planning of radiofrequency ablation with in vitro experimental correlation,” J. Vasc. Interv. Radiol. 21(11), 1725–1732 (2010). 10.1016/j.jvir.2010.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K. S., Frericks B. B., Holmer C., Schenk A., Weihusen A., Knappe V., Zurbuchen U., Peitgen H. O., Buhr H. J., and Ritz J. P., “In vivo validation of a therapy planning system for laser-induced thermotherapy (LITT) of liver malignancies,” Int. J. Colorectal Dis. 26(6), 799–808 (2011). 10.1007/s00384-011-1175-y [DOI] [PubMed] [Google Scholar]
- Wootton J. H., Prakash P., Hsu I.-C. J., and Diederich C. J., “Implant strategies for endocervical and interstitial ultrasound hyperthermia adjunct to HDR brachytherapy for the treatment of cervical cancer,” Phys. Med. Biol. 56(13), 3967–3984 (2011). 10.1088/0031-9155/56/13/014 [DOI] [PubMed] [Google Scholar]
- Dewey W. C., “Arrhenius relationships from the molecule and cell to the clinic,” Int. J. Hyperthermia 10(4), 457–483 (1994). 10.3109/02656739409009351 [DOI] [PubMed] [Google Scholar]
- Shafirstein G., Novak P., Moros E. G., Siegel E., Hennings L., Kaufmann Y., Ferguson S., Myhill J., Swaney M., and Spring P., “Conductive interstitial thermal therapy device for surgical margin ablation: in vivo verification of a theoretical model,” Int. J. Hyperthermia 23(6), 477–492 (2007). 10.1080/02656730701591476 [DOI] [PubMed] [Google Scholar]
- Boyes A., Tang K., Yaffe M., Sugar L., Chopra R., and Bronskill M., “Prostate tissue analysis immediately following magnetic resonance imaging guided transurethral ultrasound thermal therapy,” J. Urol. 178(3), 1080–1085 (2007). 10.1016/j.juro.2007.05.011 [DOI] [PubMed] [Google Scholar]
- McDannold N., Hynynen K., and Jolesz F., “MRI monitoring of the thermal ablation of tissue: Effects of long exposure times,” J. Magn. Reson. Imaging 13(3), 421–427 (2001). 10.1002/jmri.1061 [DOI] [PubMed] [Google Scholar]
- Prakash P. and Diederich C. J., “Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: implications for treatment planning, monitoring and control,” Int. J. Hyperthermia 28(1), 69–86 (2012). 10.3109/02656736.2011.630337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier C., Lafon C., and Dillenseger J. L., “3-D modeling of the thermal coagulation necrosis induced by an interstitial ultrasonic transducer,” IEEE Trans. Biomed. Eng. 55(2), 833–837 (2008). 10.1109/TBME.2007.914543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast T. D., Makin I. R., Faidi W., Runk M. M., Barthe P. G., and Slayton M. H., “Bulk ablation of soft tissue with intense ultrasound: Modeling and experiments,” J. Acoust. Soc. Am. 118(4), 2715–24 (2005). 10.1121/1.2011157 [DOI] [PubMed] [Google Scholar]
- Ahmed M., Liu Z., Humphries S., and Goldberg S. N., “Computer modeling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumor ablation,” Int. J. Hyperthermia 24(7), 577–588 (2008). 10.1080/02656730802192661 [DOI] [PubMed] [Google Scholar]
- Liu Z., Ahmed M., Sabir A., Humphries S., and Goldberg S. N., “Computer modeling of the effect of perfusion on heating patterns in radiofrequency tumor ablation,” Int. J. Hyperthermia 23(1), 49–58 (2007). 10.1080/02656730601094415 [DOI] [PubMed] [Google Scholar]
- Makin I. R., Mast T. D., Faidi W., Runk M. M., Barthe P. G., and Slayton M. H., “Miniaturized ultrasound arrays for interstitial ablation and imaging,” Ultrasound Med. Biol. 31(11), 1539–1550 (2005). 10.1016/j.ultrasmedbio.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Diederich C. J., Wootton J., Prakash P., Salgaonkar V., Juang T., Scott S., Chen X., Cunha A., Pouliot J., and Hsu I. C., “Catheter-based ultrasound hyperthermia with HDR brachytherapy for treatment of locally advanced cancer of the prostate and cervix,” Proc. SPIE 7901 (2011). 10.1117/12.876401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Converse M. C., Mahvi D. M., and Webster J. G., “Expanding the bioheat equation to include tissue internal water evaporation during heating,” IEEE Trans. Biomed. Eng. 54(8), 1382–1388 (2007). 10.1109/TBME.2007.890740 [DOI] [PubMed] [Google Scholar]
- Schutt D. J. and Haemmerich D., “Effects of variation in perfusion rates and of perfusion models in computational models of radio frequency tumor ablation,” Med. Phys. 35(8), 3462–3470 (2008). 10.1118/1.2948388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EvidentTM MWA System– Antennas, available at : http://www.valleylab.com/mwablation/antennas-surg.html (2012).
- “MicroThermX® Microwave Ablation System | Microwave Ablation | BSD Medical Corporation,” available at: http://bsdmedical.com/products_microthermx.php (2012).
- Goldberg S. N., Solbiati L., Hahn P. F., Cosman E., Conrad J. E., Fogle R., and Gazelle G. S., “Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases,” Radiology 209(2), 371–379 (1998). [DOI] [PubMed] [Google Scholar]
- Chen X., Diederich C. J., Wootton J. H., Pouliot J., and Hsu I. C., “Optimisation-based thermal treatment planning for catheter-based ultrasound hyperthermia,” Int. J. Hyperthermia 26(1), 39–55 (2010). 10.3109/02656730903341332 [DOI] [PubMed] [Google Scholar]
- Rempp H., Boss A., Helmberger T., and Pereira P., “The current role of minimally invasive therapies in the management of liver tumors,” Abdom. Imaging 36(6), 635–647 (2011). 10.1007/s00261-011-9749-2 [DOI] [PubMed] [Google Scholar]
- Wood B. J., Kruecker J., Abi-Jaoudeh N., Locklin J. K., Levy E., Xu S., Solbiati L., Kapoor A., Amalou H., and Venkatesan A. M., “Navigation systems for ablation,” J. Vasc. Interv. Radiol. 21(8), S257–S263 (2010). 10.1016/j.jvir.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. P., Turner P. F., and Hamilton B., “Interstitial microwave transition from hyperthermia to ablation: historical perspectives and current trends in thermal therapy,” Int. J. Hyperthermia 26(5), 415–433 (2010). 10.3109/02656731003639356 [DOI] [PubMed] [Google Scholar]
- Damianou C. A., Sanghvi N. T., Fry F. J., and Maass-Moreno R., “Dependence of ultrasonic attenuation and absorption in dog soft tissues on temperature and thermal dose,” J. Acoust. Soc. Am. 102(1), 628–34 (1997). 10.1121/1.419737 [DOI] [PubMed] [Google Scholar]