Abstract
Background and Aims
Plants use a diverse range of visual and olfactory cues to advertize to pollinators. Australian Chiloglottis orchids employ one to three related chemical variants, all 2,5-dialkylcyclohexane-1,3-diones or ‘chiloglottones’ to sexually attract their specific male pollinators. Here an investigation was made of the physiological aspects of chiloglottone synthesis and storage that have not previously been examined.
Methods
The location of chiloglottone production was determined and developmental and diurnal changes by GC-MS analysis of floral tissue extracts was monitored in two distantly related Chiloglottis species. Light treatment experiments were also performed using depleted flowers to evaluate if sunlight is required for chiloglottone production; which specific wavelengths of light are required was also determined.
Key Results
Chiloglottone production only occurs in specific floral tissues (the labellum calli and sepals) of open flowers. Upon flower opening chiloglottone production is rapid and levels remain more or less stable both day and night, and over the 2- to 3-week lifetime of the flower. Furthermore, it was determined that chiloglottone production requires continuous sunlight, and determined the optimal wavelengths of sunlight in the UV-B range (with peak of 300 nm).
Conclusions
UV-B light is required for the synthesis of chiloglottones – the semiochemicals used by Chiloglottis orchids to sexually lure their male pollinators. This discovery appears to be the first case to our knowledge where plant floral odour production depends on UV-B radiation at normal levels of sunlight. In the future, identification of the genes and enzymes involved, will allow us to understand better the role of UV-B light in the biosynthesis of chiloglottones.
Keywords: Chiloglottis trapeziformis; C. seminuda; UV-B, sexual deception; floral odour; pollination; 2,5-dialkylcyclohexane-1,3-diones; secondary metabolism; specialized metabolites
INTRODUCTION
As a first step in achieving pollination, plants often use a diverse range of visual and olfactory cues to advertize to pollinators (Raguso, 2004). Subsequently, food rewards such as nectar are typically provided to secure the service of pollination (Whitehead et al., 2012). Floral scents are often critical for attracting insect pollinators. The often-complex chemical bouquet of odour compounds act as filters to attract pollinators, while repelling unwanted flower visitors such as herbivores (Pichersky and Gershenzon, 2002; Raguso, 2008; Junker and Blüthgen, 2010).
Much recent progress has been made towards understanding both the chemical composition of floral scents (Knudsen et al., 2006; Raguso, 2008), and the biochemical and molecular basis of floral odours (Dudareva and Pichersky, 2000; Pichersky et al., 2006; Piechulla and Effmert, 2010). Scent formation is most often regulated by transcriptional control of biosynthetic gene expression at the site of emission, which in many cases is tissue specific (Piechulla and Effmert, 2010). For example, in snapdragon, Antirrhinum majus, methyl benzoate, a key component of the floral scent, is produced and emitted exclusively from epidermal cells of the petals. What is more, emission of this compound is highest during the day with the oscillation controlled by a circadian clock and correlating with maximum emission during diurnal bee visitation (Pichersky and Gershenzon, 2002).
Plants may use floral scents to exploit the innate olfactory preference of the pollinator (Schiestl et al., 2003), alternatively they may exploit the ability of pollinators to associate specific floral scents with nectar rewards (Riffell, 2011). One group of plants that routinely exploit the innate preference of their pollinators are sexually deceptive orchids. These orchids lure their specific male insect pollinators to their flower by emitting ‘semiochemical’ volatiles that mimic the female-released sex pheromone (Schiestl et al., 1999, 2003; Mant et al., 2005; Stokl et al., 2007; Franke et al., 2009). Pollination occurs during either a pre-copulatory routine, or attempted copulation with the flower – so called pseudocopulation (Peakall, 1990; Schiestl, 2005). Although a combination of olfactory, visual and tactile mimicry may be essential to achieve pollination (Schiestl, 2005; Gaskett, 2011), long-range attraction and the control of pollinator specificity is typically achieved by floral odours (Vereecken and Schiestl, 2009; Peakall et al., 2010; Ayasse et al., 2011). Visual and tactile mimicry may optimize the position of the pollinator for successful pollen removal and deposition, but usually play a limited role in pollinator attraction and specificity.
Sexual deception is employed by several hundred orchid species, with multiple independent evolutionary events on four continents: Australia, Europe, Africa and South America (Paulus and Gack, 1990; Peakall, 1990; Steiner et al., 1994, Singer, 2002; Singer et al., 2004; Schiestl, 2005; Gaskett, 2011). Long thought to be restricted to the Orchidaceae (Peakall, 1990), this pollination strategy has recently been discovered in a South African daisy (Asteraceae) (Ellis and Johnson, 2010). Thus pollination by sexual deception may be more widespread among plants than presently reported.
Within Australia, >150 species of terrestrial orchid sexually exploit male wasps from the parasitic Australasian subfamily Thynninae (Thynnidae) as pollinators (Peakall, 1990; Peakall and Beattie, 1996; Phillips et al., 2009). The orchid genus Chiloglottis, with some 30 species, is the largest exclusively sexually deceptive genus in Australia. Field experiments using artificially presented flowers have shown pollination in this genus is highly specific with an average of 1·1 pollinator species per orchid (Peakall et al., 2010). The specific interaction between Chiloglottis orchids and their wasp pollinators is known to involve one, two or three compounds from a pool of six related chemical variants representing a new class of natural products, all 2,5-dialkylcyclohexane-1,3-diones or ‘chiloglottones’ (Schiestl et al., 2003; Franke et al., 2009; Peakall et al., 2010).
Bioassays with synthetic chiloglottones indicate two mechanisms for controlling the extreme orchid-pollinator specificity in Chiloglottis: (1) a single specific compound is required for pollinator attraction; (2) a blend of two or more compounds in a particular ratio trigger specific attraction. Co-flowering, sympatric Chiloglottis species are always characterized by quantitative or qualitative semiochemical differences. By contrast, some allopatric Chiloglottis orchids are known to use the same semiochemical but attract different, non-overlapping pollinator species (Peakall et al., 2010). When the semiochemicals involved are mapped onto a phylogeny of Chiloglottis orchids it is evident that orchid speciation is always associated with pollinator switching and usually is underpinned by chemical change (Peakall et al., 2010).
Given the critical importance of chiloglottones in pollinator attraction, pollinator specificity and speciation our overarching goal in this study was to understand better the biology of chiloglottone production. Furthermore, in the course of the study we made an unexpected observation. We discovered that plants brought into a growth chamber with artificial light in the visible range (predominantly 550–650 nm), but completely lacking light in the UV range (<400 nm), failed to produce chiloglottone. This observation suggested that chiloglottone production might require UV light.
In this context, we address six specific questions: (1) Which floral tissues produce chiloglottone? (2) Are there changes in chiloglottone production during floral development? (3) Are there diurnal changes in chiloglottone production? (4) Does chiloglottone production require sunlight? (5) Does chiloglottone production require continuous sunlight? (6) Under what wavelengths of artificial light does chiloglottone production occur?
MATERIALS AND METHODS
Study species
Chiloglottis orchids are small terrestrial herbs that grow as clonal colonies in moist locations in the forests and swamps of eastern Australia. Plants consist of just two opposite leaves, usually borne prostrate on the substrate. While colonies may consist of 100s to 1000s of plants, only a few plants produce a single dull-coloured flower in a given year (Peakall et al., 1997, 2002).
Phylogenetic evidence indicates that Chiloglottis consists of three clades (Mant et al., 2002; Peakall et al., 2010). These clades broadly correspond to a recent split of Chiloglottis into three genera (Jones, 2006). However, these taxonomic changes are not universally accepted and for consistency with previous publications we refer here to Chiloglottis in the broad sense. An overlay of orchid floral chemistry onto the phylogeny has revealed polyphyly of the active chiloglottone compounds. For example, 2-ethyl-5-propylcyclohexane-1,3-dione (chiloglottone 1) is found both singularly and in combination in multiple taxa across the three clades and in related genera (Peakall et al., 2010).
For the purpose of this study we focused our investigations on two taxa in different clades that both employ chiloglottone 1 to attract their respective specific male wasp pollinators: the spring (September–October) flowering Chiloglottis trapeziformis which is pollinated by Neozeleboria cryptoides; and the autumn flowering (April–May) C. seminuda, which is pollinated by an undescribed species in the same genus [N. sp. (proxima2)] (Peakall et al., 2010; Griffiths et al., 2011).
Figure 1 shows a labelled diagram of the flower parts of the two study species and the chemical structure of chiloglottone 1 (for additional chemical details, see Franke et al., 2009; Peakall et al., 2010). The key floral difference between species is the labellum morphology. In C. trapeziformis, densely clustered calli (callus) are attached to the labellum by a stalk. In C. seminuda the calli are more extensively developed, extending across the posterior two-thirds of the labellum (Fig. 1). To human eyes, the insectiform callus structure of C. seminuda approximates the shape and size of the female thynnine wasp (R. Peakall, pers. obs.). Irrespective of the morphological differences between the species, the pollinators of both grip the callus structure as they attempt to mate with the anterior tip of the labellum. Thus the callus likely represents a visual and tactile mimic of the female that functions to position and orient the pollinator appropriately for pollen deposition and removal. More importantly the callus is a source of chiloglottone 1, the long-range attractant to the flower.
Fig. 1.
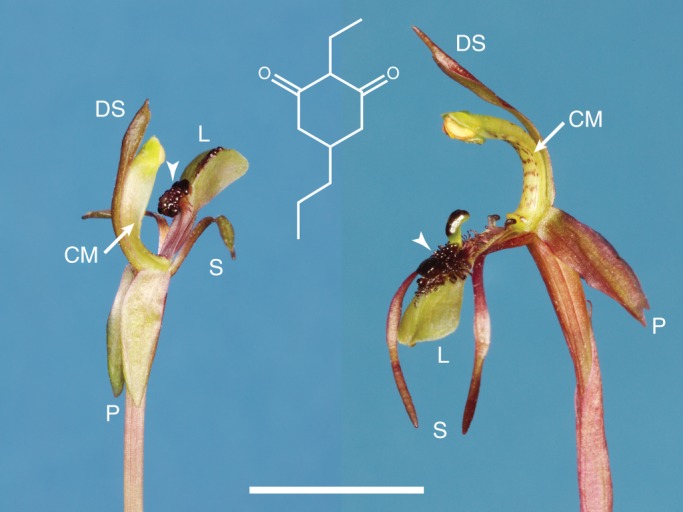
The floral structure of Chiloglottis trapeziformis (left) and C. seminuda (right), and the chemical structure of chiloglottone 1 (2-ethyl-5- propylcyclohexane-1,3-dione), the semiochemical used by both orchids to sexually attract their pollinator. Key to flower parts: CM, column; DS, dorsal sepal; L, labellum; P, petal; S, lateral sepal. The arrowhead points to the callus structure. Scale bar = 10 mm.
Both in the wild and cultivation, very few plants flower in a given season; therefore in order to obtain sufficient floral material we sourced whole plants and picked flowers from wild colonies of the orchids. Chiloglottis trapeziformis was sourced predominantly from a large 10 × 10 m colony growing naturally within the Australian National Botanic Gardens, in Canberra (ACT) during its flowering season in September. Chiloglottis seminuda was sourced from wild populations growing near Mt Werong in the Blue Mountains (NSW) in March.
Chemical analysis
For chemical analysis the flower part of interest was dissected from the flower and washed for 3 min in 100 µL of HPLC grade dichloromethane containing the commercially available 2-methyl-1,3-cyclohexanedione (Aldrich CAS 4341-24-6) at 5 ng μL−1, as an internal standard. Note that because chiloglottones are emitted in such low amounts, neither headspace nor SPME (solid phase micro extraction) techniques can detect them (R. Peakall, pers. obs.).
Gas chromatographic analysis with mass spectrometry (GC-MS) of the floral tissue extracts was performed on an Agilent Technologies 6890N gas chromatograph coupled with a 5973 mass selective detector (Agilent Technologies, USA) equipped with an SGE BP21 column (30 m × 0·25 mm × 0·25 µm) connected directly to the mass spectrometry detector. For each sample, 4 µL of extract was injected splitless into the inlet at 250 °C, the column was held at 40 °C for 1 min, then programmed at 10 °C per min to 230 °C and held for 15 min. Helium served as the carrier gas at a flow of 2 mL min−1.
For some tissue types and experiments only trace amounts of the target chiloglottone 1 were detected. In these cases, we employed a selective ion monitoring (SIM) method designed specifically to target chiloglottones. Our SIM method reduces the detection threshold several orders of magnitude, enabling sensitive measurement of a chiloglottone 1 when in small amounts. Quantitation based on corrected percentage areas was performed relative to the internal standard, using the Agilent Technologies Chemstation software, with appropriate adjustments when SIM rather than standard runs were employed.
Distribution of chiloglottone 1 in flower parts
Each floral tissue type listed in Table 1 was dissected from mature flowers for subsequent floral volatile extraction. Due to the morphological differences between the two species (Fig. 1), there was a minor difference in how we treated the dissection of the labellum. In C. trapeziformis, the stalked callus was removed as a whole from the labellum lamina. In C. seminuda we divided the labellum into the portion containing the calli (called callus in Table 1) and the portion lacking calli. The remaining flower parts were analogous for both species.
Table 1.
Levels of chiloglottone 1 in different floral tissues of Chiloglottis trapeziformis and C. seminuda
| C. trapeziformis | C. seminuda | |
|---|---|---|
| Callus | 301 ± 77 ng | 802 ± 277 ng |
| Labellum | ND | ND |
| 2 × Sepals | ND | 1115 ± 359 ng |
| 2 × Lateral petals | ND | ND |
| Dorsal sepal | ND | ND |
| Column (stigma + anther) | ND | ND |
ND = Not detected.
Mean ± s.e. are shown. Outcomes of ANOVA: for C. trapeziformis F5,10 = 12·2, P < 0·0005; for C. seminuda F5,44 = 7·75, P < 0·0001.
Developmental and diurnal changes in chiloglottone 1 production
By monitoring the flowering colony of C. trapeziformis in the botanic gardens over time, we were able to classify plants into four recognizable phases: young buds, mature buds, freshly opened flowers (1–2 d open, characterized by all green flower parts), and mature flowers (>3 d, characterized by reddish green flower parts). Representative flowers at each stage were sampled for chemical analysis of chiloglottone 1 levels within the stalked callus.
Using the same colony, we also assessed whether there are diurnal changes in chiloglottone 1 production by sampling mature flowers growing under natural sunlight at four time points over 24 h: 1000 h, 1500 h, 1700 h and 1000 h the next day. We also included an additional treatment in which flowers were covered in aluminium foil to ensure complete darkness overnight from 1700 h to 1000 h the next day. It was not possible to conduct these field experiments for C. seminuda.
Light treatments experiments
Preliminary experiments revealed that chiloglottone 1 levels were depleted in both picked and potted flowers of both C. trapeziformis and C. seminuda when they were maintained in the growth chamber with artificial lighting lacking UV light (<400 nm) for 2–3 d. Therefore, we were readily able to obtain plants exhibiting very low starting levels of chiloglottone 1 for the following light experiments.
Does chiloglottone 1 production require sunlight?
To test if chiloglottone production requires sunlight, depleted flowers were exposed to continuous sunlight for 0 (controls), 15, 60 or 120 min, prior to floral odour extraction and analysis.
Does chiloglottone 1 production require continuous sunlight?
Some light-mediated biological processes require light for initiation (i.e. ‘induction’) but not maintenance of the process. To test if chiloglottone production requires continuous light, a set of depleted flowers were exposed to a flash of sunlight for 2 min before being placed back in the growth chamber for 0, 15 or 120 min. In parallel, another set of depleted plants was exposed to continuous sunlight for 15 or 120 min.
Under what wavelengths of artificial light does chiloglottone 1 production occur?
For this experiment we used an LZC-5 Luzchem photoreactor (Luzchem Research Inc., Ontario, Canada) with sidewall irradiation and heat-extracting fan to provide specific UV light within a narrow band width as follows: UV-C (mostly 254 nm); UV-B (mainly 280–315 nm) with a peak at 300 nm; UV-A (315–400 nm) with a peak at 368 and 420 nm (see Supplementary Data Table S1 for additional information on light sources used). Depleted flowers were irradiated for 120 min, prior to floral odour extraction and analysis.
Statistical analysis
At least two replicates, each with a minimum of three flowers per treatment or control group were used in each experiment. The outcomes of experiments were analysed by single-factor ANOVA with comparisons among means assessed by the Tukey–Kramer HSD test. Error bars in the figures represent standard errors of the mean based on a pooled estimate of error variance, with labelled columns not connected by the same letter of the same case deemed significantly different at P < 0·05. All statistical analyses were performed in JMP® 9 (SAS Institute 2010).
RESULTS
Distribution of chiloglottone 1 in flower parts
In C. trapeziformis, chiloglottone 1 is present only in the callus, which is attached to the labellum lamina by a short stalk. In C. seminuda, on the other hand, chiloglottone 1 was found in both the posterior two-thirds of the labellum containing the insectiform callus, which cannot be separated from the labellum lamina, as well as the lateral sepals (Table 1 and Fig. 1)
Changes in chiloglottone 1 production
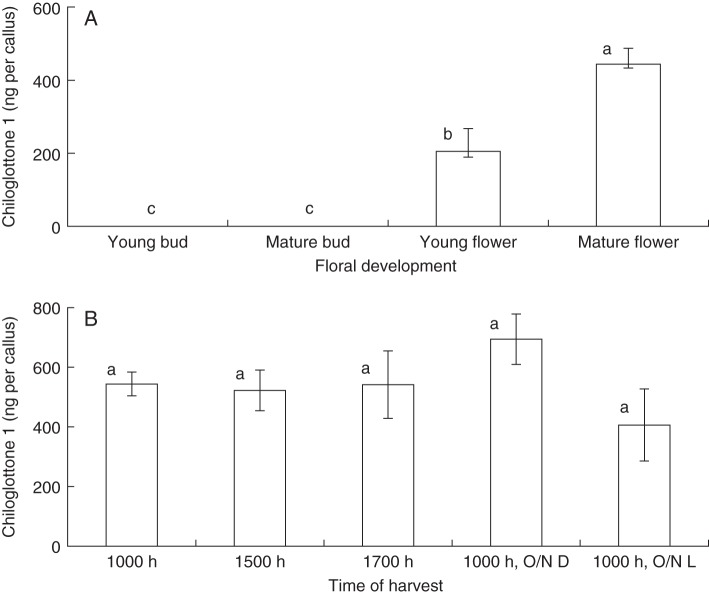
Chiloglottone 1 was detected in both freshly opened and mature flowers of C. trapeziformis, but not in buds (Fig. 2A). Average chiloglottone 1 levels were highest in mature flowers (≥4 d old), although we have detected fluctuation in chiloglottone 1 levels over the 2- to 3-week flowering time of unpollinated flowers (full data not shown). No significant diurnal changes were detected across a 24-h period, with chiloglottone 1 levels similar across the three daytime sampling intervals and both the overnight treatments (Fig. 2B).
Fig. 2.
The levels of chiloglottone 1 in buds and flowers of C. trapeziformis (A) at different developmental stages and (B) at different time points over a period of 24 h. For the treatment “O/N D” flowers were covered with aluminium foil at 1700 h to ensure complete darkness overnight until 1000 h the following day; for the treatment “O/N L”, flowers were exposed overnight to the evening and morning light until 1000 h the following day. Error bars are standard errors of the mean based on pooled estimate of error variance. Labelled columns not connected by the same letter are deemed significantly different at P < 0·05, based on a Tukey–Kramer HSD test. Outcomes of ANOVA: (A) F3,18 = 25·7, P < 0·0001; (B) F4,16 = 1·4, P = 0·2696.
Light requirements for chiloglottone 1 production
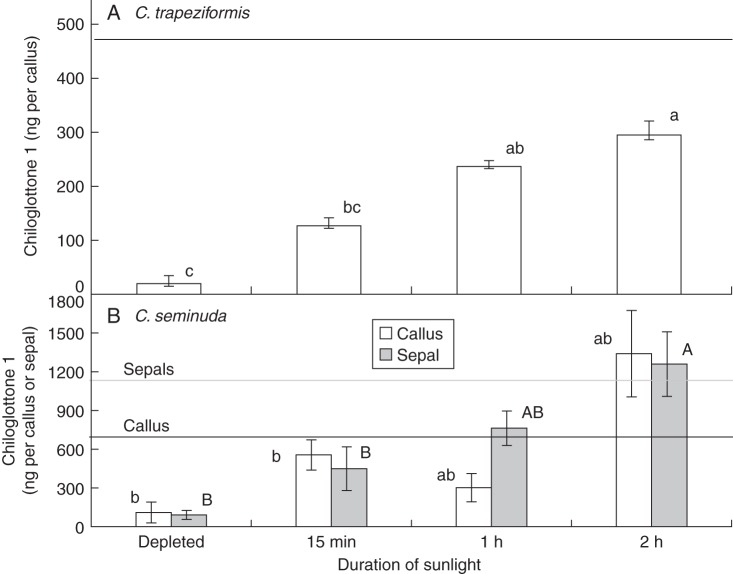
For both C. trapeziformis and C. seminuda, plants collected from the field and held in a growth chamber without UV light for a minimum of 2–3 d contained very little chiloglottone 1 (Fig. 3). However, upon exposure to sunlight, chiloglottone 1 levels rapidly increased in a time-dependent manner in both species. In C. trapeziformis, significantly higher chiloglottone 1 levels were detected after just 1 h, while by 2 h levels approached the average observed in flowers sampled from the field (Fig. 3A). In C. seminuda the sunlight-dependent response was observed in both lateral sepals and callus with significantly higher levels of chiloglottone 1 detected within 2 h, at which point the levels were typical of field plants (Fig. 3B).
Fig. 3.
The time-dependent increase in chiloglottone 1 levels following exposure to sunlight in previously depleted flowers of C. trapeziformis and C. seminuda. Horizontal lines represent the average chiloglottone 1 levels detected under continuous exposure to sun (460 ng per callus, n = 29 for C. trapeziformis; 706 ng per callus and 1070 ng per sepal, n = 14 for C. seminuda). Error bars are standard error of the mean based on pooled estimate of error variance. For ease of comparison, chiloglottone 1 levels in C. seminuda are shown for both callus and sepals in the same graph (B); however, statistical comparisons can only be made within the respective tissue types. Labelled columns not connected by the same letter of the same case are deemed significantly different at P < 0·05, based on a Tukey–Kramer HSD test. Outcomes of ANOVA: for C. trapeziformis (A) F3,26 = 19·2, P < 0·0001; for C. seminuda (B) callus F3,23 = 4·8, P < 0·009 and sepals F3,22 = 6·2, P < 0·003.
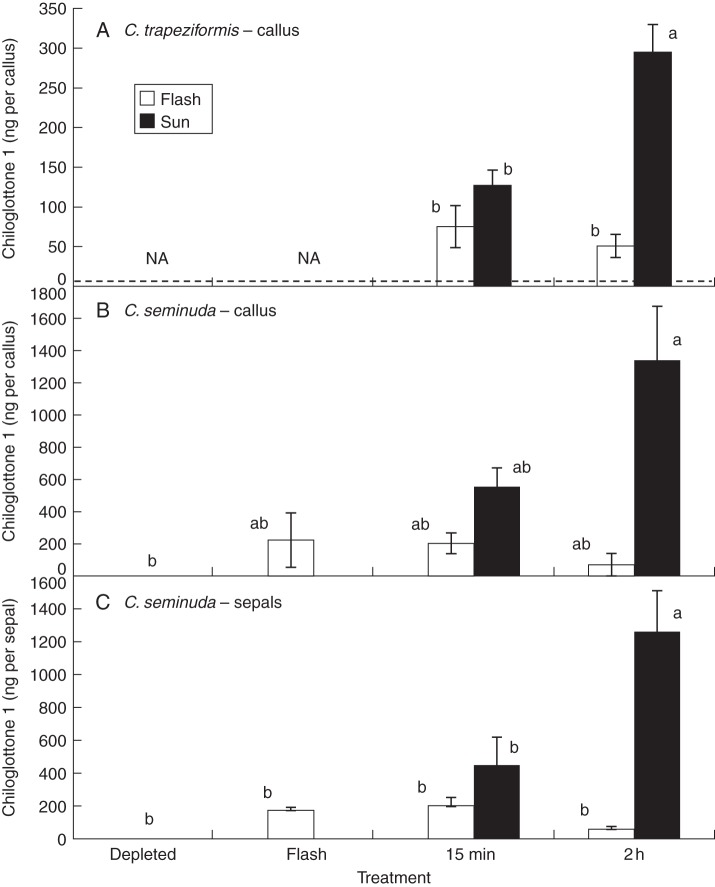
Further experiments were conducted to determine whether continuous sunlight is required for chiloglottone 1 production. In C. trapeziformis, chiloglottone 1 was detected in depleted flowers 15 min after exposure to sunlight for a 2-min interval (Fig. 4). However, in these flowers chiloglottone 1 levels were <40 % of the levels founds in flowers that were continuously exposed to 15 min of sunlight. Furthermore, levels of chiloglottone 1 decreased, rather than increased, over 2 h in the growth chamber, compared with the sunlight treatment (Fig. 4A). A similar result was found in C. seminuda with detectable levels of chiloglottone 1 found in both callus and sepals of previously depleted flowers following a 2-min flash of sunlight. Chiloglottone 1 levels declined in flowers returned to the growth chamber over the next 2 h, while chiloglottone 1 production continued to increase under continuous sunlight (Fig. 4B, C)
Fig. 4.
Comparison of chiloglottone 1 levels after a 2-min flash of sunlight compared with continuous sunlight in previously depleted flowers of C. trapeziformis and C. seminuda. NA = not applicable because the depleted treatment was not formally included in the C. trapeziformis experiment. Instead the dashed line shows the average chiloglottone 1 level in depleted plants (4·4 ng per callus, n = 27 for C. trapeziformis). Error bars are standard error of the mean based on pooled estimate of error variance. Labelled columns not connected by the same letter are deemed significantly different at P < 0·05, based on a Tukey–Kramer HSD test. Outcomes of ANOVA: for C. trapeziformis F3,18 = 9·5, P < 0·0006; for C. seminuda callus F5,27 = 4·9, P < 0·002; for C. seminuda sepals F5,31 = 10·1, P < 0·0001.
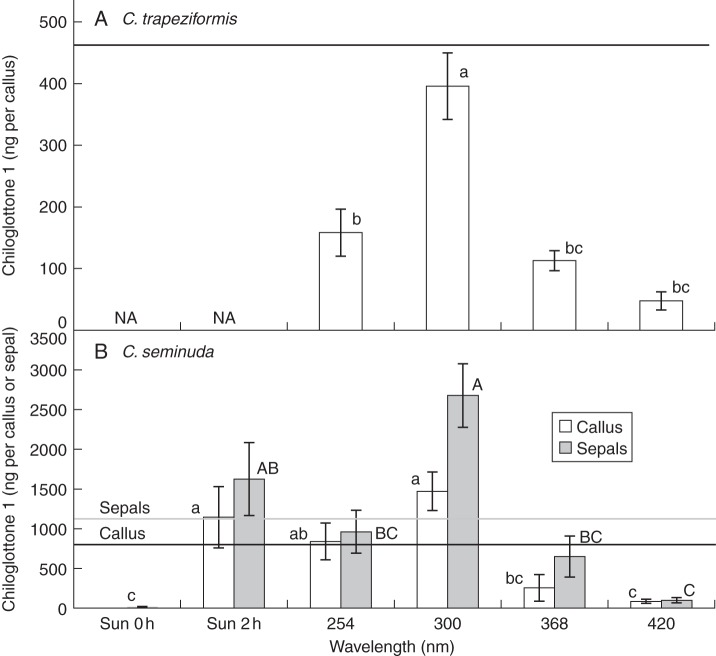
Experiments exposing Chiloglottis flowers to specific UV wavelengths revealed significant differences in chiloglottone 1 levels between different light treatments with radiation in the UV-B range (300 nm) the most efficient in stimulating chiloglottone 1 biosynthesis (Fig. 5). For C. trapeziformis a 2-h UV-B exposure was sufficient for chiloglottone 1 levels to reach the average levels found in flowers under field conditions. In C. seminuda chiloglottone 1 levels in the sepals exceeded by >2-fold the natural levels of chiloglottone 1 under the UV-B treatment, while chiloglottone 1 levels in callus reached control levels in both the UV-C (254 nm) and UV-B (300 nm) treatments within 2 h. In both species, some chiloglottone 1 production was detected at UV-A (368 nm) and 420 nm, but the levels were less than for the lower wavelengths of light (Fig. 5).
Fig. 5.
Comparison of chiloglottone 1 levels after radiation for 2 h at different specific wavelengths of light. Solid lines represent the average chiloglottone 1 levels detected under continuous exposure to sun in callus and sepals across all experiments (see Fig. 3 caption for values). Error bars are standard error of the mean based on pooled estimate of error variance. For ease of comparison, chiloglottone 1 levels in C. seminuda are shown for both callus and sepals in the same graph (B); however, statistical comparisons can only be made within the respective tissue types. Labelled columns not connected by the same letter of the same case are deemed significantly different at P < 0·05, based on a Tukey–Kramer HSD test. Outcomes of ANOVA: for C. trapeziformis callus F4,108 = 19·4, P < 0·0001; for C. seminuda callus F5,34 = 9·6, P < 0·0001; for C. seminuda sepals F5,34 = 17·5, P < 0·0001.
DISCUSSION
Much is now known about how Chiloglottis orchids achieve pollination by sexual deception (Bower, 1996; Peakall et al., 1997; Bower, 2006; Bower and Brown, 2009), including a sound knowledge of the chemical structures of various chiloglottones involved in pollinator attraction (Schiestl et al., 2003; Poldy et al., 2008, 2009, 2012; Franke et al., 2009; Peakall et al., 2010). However, the physiological aspects of chiloglottone synthesis and storage have not yet been extensively examined. For example, it has not yet been reported where chiloglottone production occurs in the flower; how chiloglottone production varies with development stage; or whether there is diurnal variation in chiloglottone production. Here we have obtained data relating to these questions. In addition, we discovered that chiloglottone 1 production requires continuous sunlight, and we narrowed down the relevant wavelengths of sunlight to the UV-B range.
Tissue-specific locations of chiloglottone 1 production
In previous studies of Chiloglottis, chiloglottone extractions have been performed on whole labella (e.g. Peakall et al., 2010). Here by floral dissection in C. trapeziformis we determined that the production of chiloglottone 1 occurs only in the callus tissue of the labellum. By contrast, in C. seminuda both the lateral sepals and calli on the labellum produce chiloglottone 1 (Table 1). This pattern of chiloglottone production in both sepals and labellum appears to be characteristic of Chiloglottis taxa belonging to the ‘reflexa’ clade (for phylogeny, see Peakall et al., 2010). In the other two Chiloglottis clades, the production of chiloglottones appears to be restricted to the labellum callus (R. Peakall, pers. obs.).
Tissue-specific production of the semiochemicals involved in pollinator attraction appears to be a general characteristic of Australian sexually deceptive orchids. In Drakaea, the sister genus to Chiloglottis, the novel pyrazines involved are produced only in the labellum (Bohman et al., 2012a, b). Floral dissections reveal that both the labellum and sepals attract the wasp pollinator of Caladenia tentaculata (Peakall and Beattie, 1996), while only sepals are attractive to the wasp pollinator of Caladenia pectinata (R. D. Phillips and R. Peakall, pers. obs.). Thus, depending on the species, semiochemical production can occur in labella only, sepals and labella, or sepals only.
Changes in chiloglottone 1 production
The buds of the two Chiloglottis species examined here contained no detectable chiloglottone 1. This result is similar to the observation in many other flowering plants, where flowers begin to synthesize and emit scent only after anthesis (e.g. Pichersky et al., 1994). However, upon flower opening the levels of chiloglottone 1 in young Chiloglottis flowers was already at least 50 % of that observed in older flowers, indicating that scent synthesis ramps up quickly as the flowers open, again exhibiting a similar pattern to what is observed in many dicot flowers (Dudareva and Pichersky, 2000; Piechulla and Effmert, 2010)
Although Chiloglottis orchids are pollinated by specific diurnal wasps that only fly during warm and sunny conditions (usually from mid-morning to mid-afternoon), we found no significant differences in chiloglottone 1 levels for samples taken across the day and night (Fig. 2). However, we only measured the internal concentration of this metabolite, not odour emission, which as noted earlier is so low it cannot be detected by standard headspace extraction techniques. In other species such as the nocturnal hawkmoth-pollinated Petunia, a rhythmic emission of a diverse blend of floral volatiles is well known, with emission levels for many compounds peaking in the night. However, storage levels did not fluctuate to the same extent as the emission levels (Orlova et al., 2006). Thus, while chiloglottone 1 storage levels are stable in Chiloglottis, we cannot rule out changes in emission between day and night.
Continuous UV light is required for the production of chiloglottone 1
Plants in general rely on radiation in the visible and UV range as cues to activate many of their physiological and metabolic processes, including the synthesis of specialized (secondary) metabolites such as anthocyanin pigments (Heijde and Ulm, 2012; Van Buskirk et al., 2012). In many plants with scented flowers, scent emission is restricted to a segment of the 24-h period, e.g. the day or night (or even a shorter period, such as dusk) (Pichersky et al., 2006; Piechulla and Effmert, 2010). However, in all examples reported to date, while the patterns of emission often indicate the involvement of a circadian rhythm mechanism (which is itself entrained by light) (e.g. Orlova et al., 2006), we are not aware of any reports on the direct influence of UV radiation on floral scent production.
Our UV light experiments in Chiloglottis were prompted by our initial observation that plants in growth chambers that were provided with a spectrum of visible, but not UV light, do not produce chiloglottone 1. Furthermore, plants collected from the field and maintained in the growth chamber, become depleted of chiloglottone 1 over a 2- to 3-d period. Using these depleted plants to advantage, we have demonstrated in this study that even a short exposure to normal levels of sunlight results in rapid chiloglottone 1 production, with exposure over just 2 h generating chiloglottone 1 amounts similar to that found in wild plants. Furthermore, since plants exposed to sunlight for 2 min then placed back in the growth chamber showed initial accumulation, but a lower rather than higher level after 2 h (Fig. 4), we conclude that constant irradiation is required for continued chiloglottone 1 production.
In our final experiments using specific ranges of the UV spectrum to irradiate the plants, we demonstrated that UV-B (300 nm) was the most effective, although some chiloglottone 1 was produced when other UV wavelengths were used (Fig. 5). In the case of C. seminuda, chiloglottone 1 levels in the sepals were >2-fold the normal levels in wild plants, perhaps reflecting the efficiency of the narrow sepals for maximally capturing the optimal UV light. For both species, chiloglottone 1 quantities in callus tissue reached levels equivalent to wild plants at the optimal UV-B range upon 2 h of exposure.
Possible mechanism for the UV response
The mechanism by which UV-B radiation triggers and sustains chiloglottone 1 synthesis is presently unknown but could involve one or more processes ranging from gene transcription to post-transcriptional and/or post-translational regulation. It could also involve direct biochemical involvement of radiation in the (so far unknown) enzymatic activities that lead to chiloglottone biosynthesis. The observation that chiloglottone 1 can be detected in depleted C. seminuda flowers following just 2 min of natural levels of sunlight (Fig. 4), and more substantial amounts within 15 min of sunlight in both species (Fig. 3), suggests that the enzymes and substrates directly involved in biosynthesis may already be present in the tissue prior to the treatment. It is also possible that UV radiation is required for the reaction itself, although we are not aware of any biochemical example in which UV radiation directly stimulates (or is consumed) in enzymatic reactions analogous to the involvement of blue light in the reaction catalysed by photolyase (Park et al., 1995).
Despite the short response time, we cannot rule out the involvement of regulatory mechanisms in the maintenance of chiloglottone biosynthesis. In many well-studied processes, absorption of red, blue and UV radiation by specific receptors leads, through signal transduction pathways, to de novo transcription of genes encoding the proteins involved in a specific pathway (Van Buskirk et al., 2012). For example, it has been amply demonstrated that a short pulse of light in the red wavelength region (620–750 nm) is sufficient to initiate transcription, and this mechanism is mediated by the red light receptor phytochrome (Franklin and Quail, 2010). Recently a ubiquitous plant UV-B-specific photoreceptor, UVR8, has been identified and shown to induce a number of downstream signal pathways by the induction of transcription factors (Brown et al., 2005; Rizzini et al., 2011; Heijde and Ulm, 2012). It is possible that genes responsive to UVR8 activation might give rise to proteins that constitute or maintain the chiloglottone biosynthetic pathway. Alternatively, absorption of UV radiation could induce metabolic changes in the cell that lead to post-translational modification of the relevant enzymes. Several primary metabolism enzymes in photosynthetic tissue are activated following exposure to visible light via cellular changes in redox-sensitive cysteines (Li et al., 1994).
The future identification of the genes and enzymes involved, will allow us to better understand the role of UV-B light in the biosynthesis of chiloglottones. Interestingly, we have shown here that chiloglottone 1 production occurs in specific floral tissues with strong red pigmentation (Fig. 1). This may suggest a previously unsuspected link between anthocyanin and chiloglottone biosynthesis, despite present hypotheses predicting chiloglottone biosynthesis via fatty acid pathways (Schiestl et al., 2003; Franke et al., 2009).
To date, most of the research on plant responses to UV radiation has been concerned with the damaging effects of excessive radiation [see reviews by Frohnmeyer and Staiger (2003) and Zhang and Bjorn (2009)]. It is well established that excessive UV-B radiation induces a complex cascade of plant secondary metabolism including the production of phenolics and antioxidants that offer protective properties within the cell, as well as anthocyanins and flavonoids that may act as sunscreens (Dolzhenko et al., 2010; Schreiner et al., 2012). There is also much interest in how we might exploit these plant stress responses in food and medicinal plants for enhancing human health (Zhang and Bjorn, 2009).
Although largely overlooked until now, there is increasing awareness that UV-B light at low, ecologically relevant levels, may also function to regulate ‘normal’ plant secondary metabolism (Ioannidis et al., 2002; Zhang and Bjorn, 2009). Our discovery that UV-B radiation at normal levels of sunlight is required for chiloglottone 1 production appears to be the first case, to our knowledge where plant floral odour production is UV-B dependent. What is more, in this case the UV-B dependent chiloglottone production is critical for the reproduction of Chiloglottis orchids since, as in the case of C. trapeziformis and C. seminuda in this study, chiloglottone 1 is exclusively responsible for both long-range attraction and the control of pollinator specificity. We predict that future research will uncover additional new cases where UV-B regulation of plant secondary metabolites plays critical roles in every-day plant–animal interactions, not just those associated with plant stress.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank the Australian National Botanic Gardens for allowing us access to plant material from the gardens, Col Bower for sharing details of orchid locations in the wild, and Prof. Chris Easton (RSC, ANU) for access to the Luzchem photoreactor. This work was supported by the Australian Research Council (DP0451374 to R.P., DP1094453 to R.P., R.A.B. and E.P.).
LITERATURE CITED
- Ayasse M, Stokl J, Francke W. Chemical ecology and pollinator-driven speciation in sexually deceptive orchids. Phytochemistry. 2011;72:1667–1677. doi: 10.1016/j.phytochem.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Bohman B, Jeffares L, Flematti G, et al. Discovery of tetrasubstituted pyrazines as semiochemicals in a sexually deceptive orchid. Journal of Natural Products. 2012;75:1589–1594. doi: 10.1021/np300388y. [DOI] [PubMed] [Google Scholar]
- Bohman B, Jeffares L, Flematti G, et al. The discovery of 2-hydroxymethyl-3-(3-methylbutyl)-5-methylpyrazine: a semiochemical in orchid pollination. Organic Letters. 2012;14:2576–2578. doi: 10.1021/ol300864u. [DOI] [PubMed] [Google Scholar]
- Bower CC. Demonstration of pollinator-mediated reproductive isolation in sexually deceptive species of Chiloglottis (Orchidaceae: Caladeniinae) Australian Journal of Botany. 1996;44:15–33. [Google Scholar]
- Bower CC. Specific pollinators reveal a cryptic taxon in the bird orchi, Chiloglottis valida sensu lato (Orchidaceae) in south-eastern Australiad. Australian Journal of Botany. 2006;54:53–64. [Google Scholar]
- Bower CC, Brown GR. Pollinator specificity, cryptic species and geographical patterns in pollinator responses to sexually deceptive orchids in the genus Chiloglottis: the Chiloglottis gunnii complex. Australian Journal of Botany. 2009;57:37–55. [Google Scholar]
- Brown BA, Cloix C, Jiang GH, et al. A UV-B-specific signaling component orchestrates plant UV protection. Proceedings of the National Academy of Sciences of the USA. 2005;102:18225–18230. doi: 10.1073/pnas.0507187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzhenko Y, Bertea CM, Occhipinti A, Bossi S, Maffei ME. UV-B modulates the interplay between terpenoids and flavonoids in peppermint (Mentha×piperita L.) Journal of Photochemistry and Photobiology B: Biology. 2010;100:67–75. doi: 10.1016/j.jphotobiol.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Biochemical and molecular genetic aspects of floral scents. Plant Physiology. 2000;122:627–633. doi: 10.1104/pp.122.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis AG, Johnson SD. Floral mimicry enhances pollen export: the evolution of pollination by sexual deceit outside of the Orchidaceae. American Naturalist. 2010;176:E143–E151. doi: 10.1086/656487. [DOI] [PubMed] [Google Scholar]
- Franke S, Ibarra F, Schulz CM, et al. The discovery of 2,5-dialkylcyclohexan-1,3-diones as a new class of natural products. Proceedings of the National Academy of Sciences of the USA. 2009;106:8877–8882. doi: 10.1073/pnas.0900646106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Quail PH. Phytochrome function in Arabidopsis development. Journal of Experimental Botany. 2010;61:11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer H, Staiger D. Ultraviolet-B radiation-mediated responses in plant: balancing damage and protection. Plant Physiology. 2003;133:1420–1428. doi: 10.1104/pp.103.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskett AC. Orchid pollination by sexual deception: pollinator perspectives. Biological Reviews. 2011;86:33–75. doi: 10.1111/j.1469-185X.2010.00134.x. [DOI] [PubMed] [Google Scholar]
- Griffiths KE, Trueman JWH, Brown GR, Peakall R. Molecular genetic analysis and ecological evidence reveals multiple cryptic species among thynnine wasps pollinators of sexually deceptive orchids. Molecular Phylogenetics and Evolution. 2011;59:195–205. doi: 10.1016/j.ympev.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Heijde M, Ulm R. UV-B photoreceptor-mediated signalling in plants. Trends in Plant Science. 2012;17:230–237. doi: 10.1016/j.tplants.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Ioannidis D, Bonner L, Johnson CB. UV-B is required for normal development of oil glands in Ocimum basilicum L. (sweet basil) Annals of Botany. 2002;90:453–460. doi: 10.1093/aob/mcf212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL. A complete guide to native orchids of Australia including the Island Territories. Sydney: Reed New Holland; 2006. [Google Scholar]
- Junker RR, Blüthgen N. Floral scents repel facultative flower visitors, but attract obligate ones. Annals of Botany. 2010;105:777–782. doi: 10.1093/aob/mcq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Botanical Review. 2006;72:1–120. [Google Scholar]
- Li D, Stevens FJ, Schiffer M, Anderson LE. Mechanism of light-modulation: identification of potential redox-sensitive cysteines distal to catalytic site in light-activated chloroplast enzymes. Biophysical Journal. 1994;67:29–35. doi: 10.1016/S0006-3495(94)80484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant JG, Schiestl FP, Peakall R, Weston PH. A phylogenetic study of pollinator conservatism among sexually deceptive orchids. Evolution. 2002;56:888–898. doi: 10.1111/j.0014-3820.2002.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Mant J, Brandli C, Vereecken NJ, Schulz CM, Francke W, Schiestl FP. Cuticular hydrocarbons as sex pheromone of the bee Colletes cunicularius and the key to its mimicry by the sexually deceptive orchid, Ophrys exaltata. Journal of Chemical Ecology. 2005;31:1765–1787. doi: 10.1007/s10886-005-5926-5. [DOI] [PubMed] [Google Scholar]
- Orlova I, Marshall-Colón A, Schnepp J, et al. Reduction of benzenoid synthesis in Petunia flowers reveals multiple pathways to benzoic acid and enhancement in auxin transport. The Plant Cell Online. 2006;18:3458–3475. doi: 10.1105/tpc.106.046227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Kim ST, Sancar A, Deisenhofer J. Crystal structure of DNA photolyase from E. coli. Science. 1995;268:1866–1872. doi: 10.1126/science.7604260. [DOI] [PubMed] [Google Scholar]
- Paulus HF, Gack C. Pollinators as prepollinating isolation factors: evolution and speciation in Ophrys (Orchidaceae) Israel Journal of Botany. 1990;39:43–79. [Google Scholar]
- Peakall R. Responses of male Zaspilothynnus trilobatus Turner wasps to females and the sexually deceptive orchid it pollinates. Functional Ecology. 1990;4:159–167. [Google Scholar]
- Peakall R, Beattie AJ. Ecological and genetic consequences of pollination by sexual deception in the orchid Caladenia tentactulata. Evolution. 1996;50:2207–2220. doi: 10.1111/j.1558-5646.1996.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Bower CC, Logan AE, Nicol HI. Confirmation of the hybrid origin of Chiloglottis × pescottiana (Orchidaceae: Diurideae). 1. Genetic and morphometric evidence. Australian Journal of Botany. 1997;45:839–855. [Google Scholar]
- Peakall R, Jones L, Bower CC, Mackey BG. Bioclimatic assessment of the geographic and climatic limits to hybridisation in a sexually deceptive orchid system. Australian Journal of Botany. 2002;50:21–30. [Google Scholar]
- Peakall R, Ebert D, Poldy J, Barrow R, Francke W, Bower C, Schiestl F. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: implications for pollinator-driven speciation. New Phytologist. 2010;188:437–450. doi: 10.1111/j.1469-8137.2010.03308.x. [DOI] [PubMed] [Google Scholar]
- Phillips RD, Faast R, Bower CC, Brown GR, Peakall R. Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae) Australian Journal of Botany. 2009;57:287–306. [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Raguso RA, Lewinsohn E, Croteau R. Flower scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpenes emission and linalool synthase activity. Plant Physiology. 1994;106:1533–1540. doi: 10.1104/pp.106.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Noel JP, Dudareva N. Biosynthesis of plant volatiles: Nature's diversity and ingenuity. Science. 2006;311:808–811. doi: 10.1126/science.1118510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla B, Effmert U. Biosynthesis and regulation of flower scent. In: Pua E-C, Davey MR, editors. Plant developmental biology: biotechnological perspectives. Vol. 2. Berlin: Springer-Verlag: 2010. [Google Scholar]
- Poldy J, Peakall R, Barrow RA. Pheromone analogs from Neozeleboria wasps and the orchids that seduce them: a versatile synthesis of 2,5-dialklated 1,3-cyclohexanediones. Tetrahedron Letters. 2008;49:2446–2449. [Google Scholar]
- Poldy J, Peakall R, Barrow R. Synthesis of chiloglottones – semiochemicals from sexually deceptive orchids and their pollinators. Organic and Biomolecular Chemistry. 2009;7:4296–4300. doi: 10.1039/b912233h. [DOI] [PubMed] [Google Scholar]
- Poldy J, Peakall R, Barrow RA. Identification of the first alkenyl chiloglottone congener. European Journal of Organic Chemistry. 2012;2012:5818–5827. [Google Scholar]
- Raguso RA. Flowers as sensory billboards: progress towards an integrated understanding of floral advertisement. Current Opinion in Plant Biology. 2004;7:434–440. doi: 10.1016/j.pbi.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Raguso RA. Wake up and smell the roses: the ecology and evolution of floral scent. Annual Review of Ecology, Evolution, and Systematics. 2008;39:549–569. [Google Scholar]
- Riffell JA. The neuroecology of a pollinator's buffet: olfactory preferences and learning in insect pollinators. Integrative and Comparative Biology. 2011;51:781–793. doi: 10.1093/icb/icr094. [DOI] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science. 2011;332:103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- Schiestl FP. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften. 2005;92:255–264. doi: 10.1007/s00114-005-0636-y. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, et al. Orchid pollination by sexual swindle. Nature. 1999;399:421–422. [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, et al. The chemistry of sexual deception in an orchid–wasp pollination system. Science. 2003;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- Schreiner M, Mewis S, Huyskens-Keil S, et al. UV-B-Induced secondary plant metabolites – potential benefits for plant and human health. Critical Reviews in Plant Sciences. 2012;31:229–240. [Google Scholar]
- Singer RB. The pollination mechanism in Trigonidium obtusum Lindl (Orchidaceae: Maxillariinae): sexual mimicry and trap-flowers. Annals of Botany. 2002;89:157–163. doi: 10.1093/aob/mcf021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Flach A, Koehler S, Marsaioli AJ, Amaral MDE. Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae : Maxillariinae) Annals of Botany. 2004;93:755–762. doi: 10.1093/aob/mch091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner KE, Whitehead VB, Johnson SD. Floral and pollinator divergence in 2 sexually deceptive South African orchids. American Journal of Botany. 1994;81:185–194. [Google Scholar]
- Stokl J, Twele R, Erdmann DH, Francke W, Ayasse M. Comparison of the flower scent of the sexually deceptive orchid Ophrys iricolor and the female sex pheromone of its pollinator Andrena morio. Chemoecology. 2007;17:231–233. [Google Scholar]
- Van Buskirk EK, Decker PV, Chen M. Photobodies in light signalling. Plant Physiology. 2012;158:52–60. doi: 10.1104/pp.111.186411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecken NJ, Schiestl FP. On the roles of colour and scent in a specialized floral mimicry system. Annals of Botany. 2009;104:1077–1084. doi: 10.1093/aob/mcp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead MR, Phillips RD, Peakall R. Pollination: the price of attraction. Current Biology. 2012;22:R680–682. doi: 10.1016/j.cub.2012.06.072. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Bjorn LO. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia. 2009;80:207–218. doi: 10.1016/j.fitote.2009.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.