Abstract
Background and Aims
This study aims to determine the role that both direct effects of fire and subsequent daily temperature fluctuations play in the seed bank dynamics of obligate seeders from the Mediterranean Basin. The short yet high soil temperatures experienced due to passage of fire are conflated with the lower, but longer, temperatures experienced by daily fluctuations which occur after removing vegetation. These germination cues are able to break seed dormancy, but it is difficult to assess their specific level of influence because they occur consecutively after summer fires, just before the flush of germination in the wet season (autumn).
Methods
By applying experimental fires, seed treatments were imposed that combined fire exposure/non-fire exposure with exposure to microhabitats under a gradient of disturbance (i.e. gaps opened by fire, mechanical brushing and intact vegetation). The seeds used were representative of the main families of obligate seeders (Ulex parviflorus, Cistus albidus and Rosmarinus officinalis). Specifically, an assessment was made of (1) the proportion of seeds killed by fire, (2) seedling emergence under field conditions and (3) seeds which remained ungerminated in soil.
Key Results
For the three species studied, the factors that most influenced seedling emergence and seeds remaining ungerminated were microhabitats with higher temperature fluctuations after fire (gaps opened by fire and brushing treatments). The direct effect of fire decreased the seedling emergence of U. parviflorus and reduced the proportion of seeds of R. officinalis remaining ungerminated.
Conclusions
The relevance of depleting vegetation (and subsequent daily temperature fluctuation in summer) suggests that studies focusing on lower temperature thresholds for breaking seed dormancy are required. This fact also supports the hypothesis that the seeding capacity in Mediterranean Basin obligate seeders may have evolved as a response to a wide range of disturbances, and not exclusively to fire.
Key words: Adaptation, Cistus albidus, exaptation, fire-adaptative trait, fire heat, post-fire germination, Rosmarinus officinalis, seed bank dynamics, seedling emergence, temperature fluctuation, Ulex parviflorus
INTRODUCTION
Fire is one of the most recurrent disturbances in Mediterranean Basin (MB) ecosystems (Di Castri et al., 1981) and, consequently, plant species should present strategies that enable them to persist. Species often resprout from the ground biomass that survives the passage of fire (resprouters), recruit new individuals from a fire-resistant seed bank (seeders) or combine both strategies (facultative species, sensu Keeley, 1986). Traditionally, the appearance of the different strategies in MB ecosystems has been associated with their lineage age (Herrera, 1992; Verdú, 2000). Normally, resprouters are taxa that evolved during the Tertiary period, before the Mediterranean climate appeared (Pausas et al., 2006). In contrast, seeding capacity evolved later during the Quaternary period and concomitantly with the Mediterranean climate and frequent fires (Pausas and Verdú, 2005; Pausas et al., 2006; Saura-Mas and Lloret, 2007). In fact, it is postulated that, in addition to climate and soil, fire has been an important selective pressure in the evolution of the post-fire seeding trait (high germination and establishment ability immediately after fire; Keeley et al., 2012).
In MB ecosystems, shrub species with an ‘obligate seeder’ strategy are limited to three families: Cistaceae, Fabaceae and Lamiaceae (Pausas and Verdú, 2005). Nevertheless, these families are a major component of fire-prone shrublands and they successfully regenerate from soil seed banks (Quintana et al., 2004; Santana et al., 2012). Cistaceae and Fabaceae seeds possess a hard coat that can allow them to persist in the soil seed bank over an extended period of time. In addition, this coat establishes a physical dormancy that heat can break and, consequently, trigger germination (Thanos et al., 1992; Baeza and Vallejo, 2006; Paula and Pausas, 2008). Lamiaceae species are not hard coated, but some exhibit enhanced germination when seeds are exposed to heat and/or smoke (Moreira et al., 2010). This directly fire-stimulated germination may enhance plant fitness by different mechanisms and may, thus, be under positive selection (Keeley et al., 2012). For example, seedlings can rapidly access more resources and grow more rapidly, time to maturity is shortened and the amount of seeds stored before the next fire can increase (Bond and van Wilgen, 1996; Ladd et al., 2005; Verdú and Traveset, 2005).
Fire also leads to indirect effects that may strengthen the flush of germination and seedling establishment. The low red/far-red ratio experienced under canopies inhibits the germination of many species (Gorsky et al., 1978; Rees, 1997), including Fabaceae and Cistaceae species (Roy and Sonié, 1992; Baeza and Roy, 2008). Therefore, microhabitat modification by canopy consumption may change the light spectrum and promote germination. Indirectly, the incidence of solar radiation promotes a shift in the range of daily soil temperatures by sustaining high temperatures over significant periods of time (Bradstock and Auld, 1995; Baeza and Roy, 2008). Recent studies have indicated that these fluctuations may also act as germination cues for species with physical dormancy (Baeza and Roy, 2008; Santana et al., 2010b, 2012; Ooi et al., 2012). Specifically, thresholds for breaking physical dormancy would be exceeded in summer, when daily soil temperatures reach the highest values and fluctuate more widely. In line with this, it has been suggested that, alternatively to fire heat, the seasonal climatic patterns of the Mediterranean climate (i.e. dry summer periods with high temperatures followed by wet, mild autumns) could also have participated in the selection of hard seed dormancy (Pausas et al., 2006; Baeza and Roy, 2008). Seasonal dryness is the most limiting factor for seedling establishment in Mediterranean species (Baskin and Baskin, 1998). Thus, dormancy breakage by summer temperatures could avoid germination and seedling establishment failures before and during dry periods (Baeza and Roy, 2008).
One point that has been the object of little study is the specific influence that direct (heat shock produced by fire passage) and indirect (microhabitat modification and subsequent daily soil temperature fluctuations) effects of fire have on the breakage of seed dormancy. The short yet high soil temperatures experienced due to passage of fire are conflated with the lower, but longer, temperatures experienced by the daily fluctuations. These two germination cues occur consecutively after summer fires, just before the flush of germination in the wet season (i.e. autumn; Quintana et al., 2004; Santana et al., 2012). Thus, it is difficult to assess their specific influence independently by direct field observations. Many laboratory experiments have attempted to identify the optimal and lethal temperature thresholds for a wide range of species (see Paula and Pausas, 2008, and references therein); however, these experiments have mostly focused on fire temperatures and have neglected daily soil temperature fluctuations. Furthermore, laboratory experiments obviate the possible complex interactions between the direct effects of fire and the subsequent microhabitat modification. To answer the question of which factor is more important in determining seed bank dynamics (i.e. direct effects of fire vs. microhabitat modification) may throw light on which selective pressure has predominated in shaping the post-disturbance seeding trait.
This study first aims to determine the temperature patterns that can potentially affect seed bank dynamics during three experimental fires and subsequent daily fluctuations in summer. Its second aim is to determine the role that both direct effects of fire and microhabitat modification play in the seed bank dynamics of obligate seeders from the MB. Specifically, we assessed (1) the proportion of seeds killed by fire, (2) seedling emergence under field conditions and (3) the seeds which remained ungerminated in soil. For this purpose, we placed containers with a controlled content of seeds in conditions combining fire exposure/non-fire exposure with exposure to microhabitats under a gradient of disturbance (i.e. gaps opened by fire, mechanical brushing and intact vegetation). The seeds used were representative of the species of the three families of obligate seeders: Ulex parviflorus (Fabaceae), Cistus albidus (Cistaceae) and Rosmarinus officinalis (Lamiaceae).
MATERIALS AND METHODS
Study area and site selection
The study was carried out inland in the Valencian Community (SE Spain) at three sites: Onil (38°39′N-0°39′W), Pardines (38°40′N-0°39′W) and Ayora (39°07′N-0°57′W). In all cases, study sites were shrublands with a history of recurrent fires (two fires in the last 25 years; Santana et al., 2010a). At the onset of this study, vegetation consisted of shrublands (approx. 1–1·5 m in height) dominated by the obligate seeders Cistus albidus, Rosmarinus officinalis and Ulex parviflorus. Resprouting shrubs, such as Quercus coccifera and Juniperus oxycedrus, were scarce, and the grass Brachypodium retusum was the main herbaceous species. Altitude ranges between 900 and 1050 m a.s.l., and climate is typically Mediterranean. Mean annual rainfall ranges between 466 (Onil) and 537 mm (Ayora). There is a pronounced summer drought from June to August, with no more than 65 mm of rain at any site. The mean annual temperature is approx. 14 °C, and the mean maximum temperature for the hottest month (July) is 30 °C. To minimize environmental variability between sites, all the sites were northwardly oriented, located on marls and their soils were Regosols (FAO, 1988).
Monitoring of soil temperature during experimental fires
We selected one plot of approx. 30 m × 20 m at each site, where we set up an experimental fire. The areas to be burned were previously delimited by a 5 m wide fire break in which vegetation was eliminated through mechanical brushing. All three sites were burned in June 2006, and there was a 1 week interval between each experimental fire. Fires were ignited as a line encompassing the entire upwind flank of the experimental plot (head-fires). As a safety measure, fire fighters and forest rangers were present for each experimental fire. In these ecosystems dominated by obligate seeders, the maximum density of seeds stored in the seed bank was found in the first centimetre of the soil profile (2–5 times greater than in deeper layers; Ferrandis et al., 1999; Baeza, 2001; Traba et al., 2004; Clemente et al., 2007). Following this premise, we measured the soil temperature at a 1 cm depth. For this purpose, 15 insulated chrome–alumel thermocouples (K-type) were distributed throughout each plot. Thermocouples were equally distributed following a stratified design under main species patches, i.e. B. retusum, C. albidus, R. officinalis and U. parviflorus (Santana et al., 2011). Thermocouples were protected from fire by a stainless steel sheath and were connected to a data logger (CR1000; Campbell Scientific, North Logan, UT, USA). Records were taken from 20 min before the experimental fire was initiated until 2 h after the fire had been extinguished. Soil temperature was recorded every 10 s. For more details of fire performance and behaviour, see Santana et al. (2011). It is worth noting that fire temperatures in experimental fires are expected to be lower and of shorter duration than in natural fires, where environmental conditions are more severe.
Monitoring of daily soil temperature throughout summer
After the experimental fires, we discerned three different microhabitats under a gradient of disturbance for assessing summer soil temperatures: (1) the canopy gaps deriving from the complete consumption of vegetation by fire (gap hereafter); (2) mechanical brushing located in the 5 m wide fire break contiguous to the experimental fires (here, all woody vegetation was eliminated through mechanical brushing to be left lying on the soil surface; brushing hereafter); and (3) intact vegetation contiguous to the experimental burning plots (vegetation hereafter). Treatments represented a gradient in soil cover, from 0 % for gaps to 100 % in vegetation. Brushing had intermediate values (approx. 60 %).
Soil temperatures were recorded in all three microhabitats over a 2 month period in summer 2006 (from July 21 to September 21). They were recorded every hour with a temperature probe (Hobo® Event, Onset Computer Corporation, Bourne, MA, USA). Similarly to fire temperatures, soil temperatures were also measured at a 1 cm depth. A narrow hole (3 cm wide, 2 cm deep approximately) was excavated and sensors were inserted horizontally at the desired depth. Then, soil was restored as closely as possible to its original state. If there was any litter present, it was removed and replaced after setting up the sensors.
Monitoring of seed bank dynamics
To explore the role that both direct effects of fire and microhabitat modification have on seed bank dynamics, we employed a set of containers with a controlled content of seeds. These containers were distributed within an experimental design which combined the fire exposure/non-fire exposure with a later exposure to different microhabitats. Containers were rectangular and handmade, with a 0·5 mm stainless steel mesh that was open at the top (11 × 7 × 7 cm; L × W × H). The mesh was big enough to allow water permeability, but avoided loss of seeds. We used seeds of the dominant species: C. albidus (Cistus hereafter), R. officinalis (Rosmarinus hereafter) and U. parviflorus (Ulex hereafter). Cistus and Ulex are obligate seeders with hard-coated seeds broken by heat, for example they undergo enhanced germination if exposed to dry heat (80–120 °C for 5–10 min; Baeza and Vallejo, 2006; Moreira et al., 2010). Rosmarinus is a soft-seeded species in which germination is stimulated by high temperatures (80–120 °C, 5–10 min) and smoke (liquid smoke solution; Moreira et al., 2010). The seeds of Rosmarinus and Ulex were collected by hand early in summer 2006 from at least 20 plants at the Pardines site. We collected seeds when fruits ripened, but just before release from the parent plant. For Cistus, seeds were collected directly from plant capsules in late August 2005. All the seeds were stored under laboratory conditions (dry; <22 °C) until they were processed for treatment applications in June 2006. In order to check the initial degree of physical dormancy in hard-coated species (Ulex and Cistus), we submerged 100 seeds of each species in distilled water for 24 h. Imbibed seeds gave us an estimation of non-dormant seeds (8 % for Cistus and 1 % for Ulex). Containers were first filled with soil with similar site characteristics, but which was extracted from a semi-arid zone (>35 km away from the experimental sites) where the studied species were absent. We placed 30 seeds of each species at a 1 cm depth. Then, the container was filled completely and buried in each microhabitat (see below for more details). After burying the containers, the microhabitat was restored as closely as possible to its original state.
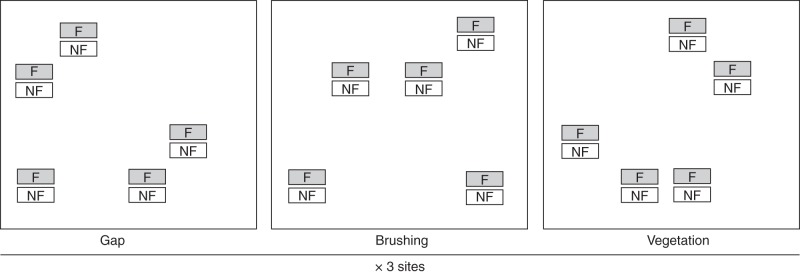
In order to arrange the containers exposed directly to fire effects, 20 containers were buried within each burned plot the day before the experimental fires. Since fire intensity was likely to increase as the flame front moved upslope, containers were distributed as uniformly as possible within a 10 × 10 m square in the centre of the plots. The day after the fire, these containers were extracted and divided randomly into sets of five. Then, they were placed in the three different microhabitats: gap, brushing and vegetation. To do this, a 5 × 5 m plot was laid out within each microhabitat, and five points within this plot were randomly chosen from a 1 × 1 m grid (Fig. 1). The remaining five containers were taken to the laboratory to check the proportion of seeds killed by fire. Additionally, we arranged a second set of 20 containers that had not been exposed to the direct effect of fire. They were placed the day after the fire together with the other containers at each point and microhabitat (Fig. 1). In summary, the experimental design allowed us to provide six treatments that experienced summer conditions in different microhabitats, and which were exposed or not exposed to fire. Specifically: (1) seeds exposed only to the gap environment after fire; (2) seeds exposed to the effects of fire plus the gap environment; (3) seeds exposed to brushing; (4) seeds exposed to fire plus brushing; (5) seeds exposed to vegetation; and (6) seeds exposed to fire plus vegetation (Fig. 1).
Fig. 1.
Arrangement of the containers within the experimental design (F = containers exposed to fire, NF = containers not exposed to fire).
With these containers, we determined different seed bank dynamics parameters. First, we assessed the proportion of seeds killed by fire by means of containers extracted immediately after fire. Secondly, we assessed seedling emergence under field conditions over the next 2 years (approximately every 10 d in the first year and every month in the second year). Once seedlings had been identified, they were removed, and the depth of emergence was carefully checked. Thirdly, we assessed the proportion of seeds which remained ungerminated in soil. In summer 2008, 2 years after beginning treatments, all the containers were extracted and taken to the laboratory. There, the soil from each container was removed from the container, disaggregated and sieved (0·5 mm mesh). This allowed us to recover the seeds that remained ungerminated directly. The viability of the recovered seeds was checked by cutting and soaking them in tetrazolium solution (1 % concentration) for 24 h. Only those embryos which stained completely red or pink were considered viable. As we carefully checked the depth of seedling emergence and the seeds clearly seen on the soil surface were discarded, we assumed that there were no losses or inputs of seeds as regards the initial pool.
Statistical analysis
The proportion of seeds killed by fire was analysed by means of t-tests. The seeds of those containers from the same site were pooled and then compared with three similar sets from the initial pool of seeds.
The roles that direct effects of fire and microhabitat modification play in seedling emergence and ungerminated seeds were analysed, in a first step, using Generalized Linear Models (GLMs). In model construction, we considered fire exposure (fire and no fire) and microhabitat (gap, brushing and vegetation) as fixed effects. The interaction between microhabitats and fire exposure was also considered in the GLM. Starting from the full model, the minimal adequate GLM was obtained by sequential removal of non-significant model terms (analysis of deviance, F-tests, P > 0·005). Since we knew the initial number of seeds, the data showed a binary response (i.e. a seed either emerged or did not, or remained or did not). Thus, we assumed a binomial distribution with a logit-link function. The logits in the baseline were defined by vegetation in microhabitats and the non-fire exposure. Because random effects are not included in GLM, in a second step, we used Generalized Linear Mixed Models (GLMMs) to include these effects (i.e. sites and points where containers were laid out) in the previously selected models (Bolker et al., 2009).
The GLMs and GLMMs were performed using the MASS package in the R software environment (2·14·2; R Development Core Team, Vienna, Austria, http://r-project.org/). We used the glm and glmmPQL (the penalized quasi-likelihood approach) functions to fit the data (Bolker et al., 2009).
RESULTS
Soil temperatures during and after summer fires
During the experimental fires, the maximum temperatures recorded at a 1 cm depth ranged from 81 to 99 °C (Table 1). The time at which the temperature was >40 °C (temperature residence time) varied, ranging between 0·4 and 1·1 h for the different fires. Temperatures >50 °C lasted a relatively short time and did not exceed 0·1 h in any case (Table 1).
Table 1.
Maximum temperature, mean maxima and temperature residence times observed for the different experimental fires
| Site | Maximum observed (°C) | Mean maxima (°C, mean ± s.d.) | Residence time (h) |
|
|---|---|---|---|---|
| >40 °C (mean ± s.d.) | >50 °C (mean ± s.d.) | |||
| Onil | 99·1 | 42·7 ± 17·1 | 0·4 ± 1·1 | 0·1 ± 0·6 |
| Pardines | 80·7 | 45·6 ± 14·5 | 1·1 ± 1·5 | 0·1 ± 0·1 |
| Ayora | 94·8 | 47·8 ± 15·1 | 0·2 ± 0·4 | 0·1 ± 0·1 |
Temperatures were recorded by means of thermocouples (n = 15) at a 1 cm depth of the soil profile.
Soil temperatures during the subsequent summer were variable, depending on microhabitats. Gap experienced the highest soil temperature values, with mean daily maxima ranging from 49 to 53 °C at all three sites. A maximum of 68 °C was observed at the Pardines site (Table 2) and the residence times at temperatures >40 °C fell within the 5·8–2·1 h range per day. These residence times decreased at higher temperatures to 0·3–0·6 h d−1 at temperatures of >60 °C (Table 2). Vegetation, in contrast, presented the lowest temperature values; the mean daily maximum was approx. 22 °C and the absolute maxima observed did not exceed 29 °C in any case (Table 2). Brushing had intermediate values; the mean daily maximum ranged from 32 to 39 °C, and the maximum temperature recorded was 56 °C (Table 2). The residence times >40 °C were low and did not exceed 2 h d−1 (Table 2).
Table 2.
Maximum temperature, mean daily maxima and temperature residence times observed for different microhabitats in summer 2006
| Residence time (h) |
||||||
|---|---|---|---|---|---|---|
| Site | Microhabitat | Maximum observed (°C) | Mean daily maxima (°C, mean ± s.d.) | >40 °C (mean ± s.d.) | >50 °C (mean ± s.d.) | >60 °C (mean ± s.d.) |
| Onil | Gap | 65·8 | 49·0 ± 9·9 | 4·9 ± 3·1 | 2·1 ± 2·3 | 0·3 ± 0·8 |
| Brushing | 41·1 | 31·7 ± 5·9 | 0·1 ± 0·3 | 0 | 0 | |
| Vegetation | 28·7 | 22·7 ± 3·1 | 0 | 0 | 0 | |
| Pardines | Gap | 68·3 | 52·9 ± 11·0 | 5·8 ± 3·3 | 2·9 ± 2·5 | 0·6 ± 1·3 |
| Brushing | 53·3 | 38·7 ± 8·3 | 1·9 ± 2·5 | 0·2 ± 0·6 | 0 | |
| Vegetation | 27·5 | 22·1 ± 2·9 | 0 | 0 | 0 | |
| Ayora | Gap | 65·0 | 51·9 ± 10·9 | 2·1 ± 2·5 | 1·1 ± 1·6 | 0 |
| Brushing | 56·0 | 33·9 ± 7·7 | 0·6 ± 1·7 | 0·2 ± 1·1 | 0 | |
| Vegetation | 25·9 | 21·5 ± 2·8 | 0 | 0 | 0 | |
Temperatures were recorded over 63 d (from 21 July to 21 September) at a 1 cm depth of the soil profile.
Seed bank dynamics
Passage of fire marginally reduced seed viability in Ulex from 99·3 ± 1·2 % (mean ± s.d.) to 92·7 ± 4·8 %, but not significantly (Student's t-test, n = 3, F = 2·79, P = 0·081). In Rosmarinus, fire significantly reduced viability from 90·2 ± 1·1 to 40·2 ± 24·6 % (F = 14·69, P = 0·025). Cistus seeds showed no significant changes (F = 2·62, P = 0·399), and the initial viability of 94·2 ± 1·6 % changed to 91·4 ± 4·9 %.
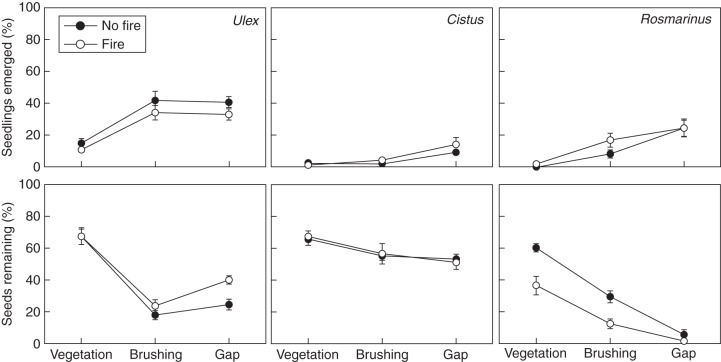
Seedling emergence occurred mainly in the first year after fire. Of the total emergences, 77 % for Ulex, 74 % for Rosmarinus and 93 % for Cistus occurred in the first year. In addition, this emergence varied in accordance with treatments. For Ulex, gap and brushing had a significant effect on increasing seedling emergence (regard vegetation treatment as the baseline; Table 3, Fig. 2). Fire exposure also had a significant effect (P = 0·022, Table 3) and reduced emergence by approx. 5 % compared with treatments without fire (Fig. 2). For Cistus, only gap had a significant effect (Table 3, Fig. 2), while for Rosmarinus gap and brushing affected seedling emergence (Table 3).
Table 3.
Parameters estimated by the GLMM models for seedlings emerged and seeds remaining ungerminated in soil
| Species | Fixed effects | Estimate | s.e. | d.f. | t-value | P-value |
|---|---|---|---|---|---|---|
| Seedlings emerged | ||||||
| Ulex | Intercept | –1·747 | 0·214 | 44 | –8·163 | < 0·001 |
| Brushing | 1·418 | 0·210 | 40 | 6·748 | < 0·001 | |
| Gap | 1·377 | 0·210 | 40 | 6·547 | < 0·001 | |
| Fire | –0·340 | 0·143 | 44 | –2·375 | 0·022 | |
| Cistus | Intercept | –4·285 | 0·395 | 44 | –10·824 | < 0·001 |
| Brushing | 0·516 | 0·456 | 40 | 1·133 | 0·264 | |
| Gap | 1·858 | 0·414 | 40 | 4·489 | < 0·001 | |
| Fire | 0·406 | 0·207 | 44 | 1·963 | 0·056 | |
| Rosmarinus | Intercept | –6·301 | 1·819 | 42 | –3·464 | 0·001 |
| Brushing | 3·745 | 1·804 | 40 | 2·076 | 0·044 | |
| Gap | 5·013 | 1·789 | 40 | 2·801 | 0·007 | |
| Fire | 2·518 | 1·841 | 42 | 1·367 | 0·178 | |
| Brushing × fire | –1·672 | 1·880 | 42 | -0·889 | 0·379 | |
| Seeds remaining ungerminated | ||||||
| Ulex | Intercept | 0·743 | 0·191 | 42 | 3·891 | 0·004 |
| Brushing | –2·270 | 0·293 | 40 | –7·725 | < 0·001 | |
| Gap | –1·866 | 0·279 | 40 | –6·692 | < 0·001 | |
| Fire | –0·010 | 0·237 | 42 | –0·043 | 0·965 | |
| Brushing × fire | 0·363 | 0·363 | 42 | 1·001 | 0·322 | |
| Gap × fire | 0·734 | 0·338 | 42 | 2·166 | 0·036 | |
| Cistus | Intercept | 0·696 | 0·147 | 45 | 4·718 | < 0·001 |
| Brushing | –0·446 | 0·206 | 40 | –2·166 | 0·036 | |
| Gap | –0·613 | 0·205 | 40 | –2·983 | 0·004 | |
| Rosmarinus | Intercept | 0·445 | 0·205 | 44 | 2·168 | 0·035 |
| Brushing | –1·343 | 0·214 | 40 | –6·271 | < 0·001 | |
| Gap | –3·359 | 0·381 | 40 | –8·821 | < 0·001 | |
| Fire | –1·033 | 0·204 | 44 | –5·040 | < 0·001 | |
P-values <0·05 are highlighted in bold.
Fig. 2.
Fate of the seeds placed inside the containers in the different microhabitats and for the three species. Bars represent the standard error (n = 15).
Persistence of the soil seed bank also varied in accordance with treatment. For Ulex, gap and brushing negatively affected the percentage of remaining seeds (Table 3). There was also an interaction between fire and gap that increased the seeds remaining (Table 3, Fig. 2). For Cistus, gap and brushing had a significant effect (Table 3), whereas for Rosmarinus gap, brushing and fire negatively affected the percentage of remaining seeds (Table 3, Fig. 2).
DISCUSSION
Microhabitat disturbance by vegetation depletion may induce significant soil heating during summer periods. In the gaps which opened by fire, or even in brushing treatments, daily temperature fluctuations may exceed the thresholds for stimulating seed germination in some MB species. Very few studies have identified the lower thresholds for breaking seed dormancy; however, in some ‘obligate seeders’ (i.e. species of the Cistaceae, Lamiaceae and Leguminoseae families), germination can be seen to be enhanced after being exposed to sustained temperatures of between 40 and 60 °C (for some examples, see Trabaud and Casal, 1989; Baeza and Roy, 2008). In addition, this syndrome has also been observed for Leguminoseae species from Australian fire-prone areas (Auld and O'Connell, 1991; Tieu et al., 2001; Santana et al., 2010b). Consequently, germination in heat-stimulated species cannot be understood by taking into account only fire temperatures. It is worth noting that although soil experiences its maximum temperatures during passage of fire (80–100 °C), durations above the stimulating temperature thresholds (40–60 °C) were short (a few minutes) if compared with those experienced in the subsequent summer (a few hours every day).
The relevance of microhabitat modification is reflected in the seedbank dynamics observed in this study. The gaps opened by fire and, to a lesser extent, the brushing treatment were the most significant factors in determining both seedling emergence and the amount of seeds remaining in soil. Direct exposure to fire did not enhance seedling emergence in any species; seedling emergence was even reduced by fire in U. parviflorus. These results indicate that daily temperature fluctuation could, in some cases, have a greater influence than fire temperatures during the germination and emergence of heat-stimulated species. In line with this, Trabaud and Casal (1989) observed for R. officinalis that, while treatments mimicking fire temperatures gave negligible results, the highest germination rates were noted in those exposed to 40 and 60 °C for 24 h. For U. parviflorus, Baeza and Roy (2008) reported that daily exposures (12 h/12 h) to alternating temperatures of 15/45 °C resulted in similar germination rates (approx. 80 %) to treatments mimicking fire temperatures. It should be taken into account, however, that the intensity of fire temperatures may modulate the specific influence of daily temperature fluctuations. In this study, the emergence of C. albidus seedlings was low and was mainly determined by the opening of gaps, but fire temperatures were low and of short duration [in comparison with other experimental fires in similar shrublands (Baeza et al., 2002a; De Luis et al., 2004) or natural fires with more severe environmental conditions]. In contrast, Céspedes et al. (2012) observed that germination in C. albidus was clearly stimulated by fire after an experimental fire. After a wildfire, Ferrandis et al. (1999) found that for three species of Cistaceae (Halimium ocymoides, C. ladanifer and C. salvifolius), the direct effect of fire was mainly responsible for seed germination (approx. 70 % of the total seed bank). However, these authors suggested that there were other environmental factors involved in seed softening (mainly temperature fluctuation) since seed bank depletion was always >90 %. Therefore, despite daily temperature fluctuations possibly playing a decisive role in seed bank dynamics, further studies are required to determine their specific level of influence, in terms of fire temperature intensity, as well as possible complex interactions with other direct fire cues (e.g. smoke). Indeed, this modulated response may differ according to species since, for example, U. parviflorus and R. officinalis viability was sensitive to these low intensity temperatures, unlike C. albidus.
The important role of microhabitat modification (and the subsequent daily temperature fluctuation in summer) supports the hypothesis of climate being a decisive selective pressure in MB obligate seeders (Pausas et al., 2006; Baeza and Roy, 2008). Breakage of seed dormancy by summer temperatures may enhance plant fitness via different mechanisms. For example, it may avoid germination and seedling establishment failures before and during a dry summer period. This response is also critical in the species' ability to detect vegetation gaps, where not only is competition with other species poor, but the inhibiting low red/far-red ratio noted under canopies was absent (Gorsky et al., 1998; Rees, 1997). Nonetheless, and despite this possible relevance, there are very few studies available which have investigated the role of seasonal climatic patterns on the seed germination and seedling emergence of MB species. After an experimental fire performed in October (autumn), De Luis et al. (2008) found that Cistaceae (Helianthemum marifolium and C. albidus) and Fabaceae species (Ononis fruticosa and U. parviflorus) displayed a bimodal pattern of germination, with the first peak just after burning and a second one in the following autumn (after summer). Baeza and Roy (2008) noted a similar pattern in U. parviflorus after an autumn brushing. Similarly, in fire-prone ecosystems from Australia, Ooi et al. (2012) also found that Leguminoseae species with physical dormancy germinate readily after being exposed to summer temperature fluctuation. The scarcity of studies that determine the role of seasonal climatic patterns in seed bank dynamics of fire-prone areas is surprising, especially when considering that physical and other dormancy types in arid systems and other less fire-prone systems depend on season-long high temperature fluctuations being broken (Baskin and Baskin, 1998; Ooi et al., 2009).
The seeding capacity of MB species evolved during the Quaternary period and concomitantly with the Mediterranean climate and frequent fires (Pausas et al., 2006; Saura-Mas and Lloret, 2007). In this sense, a question that arises in this study is whether the post-fire seeding trait is an adaptation to fire or, if instead, it is an exaptation from the species' ability to respond to seasonal temperature fluctuations (for a clear explanation of these concepts, see Bradshaw et al., 2011; Keeley et al., 2011). Studies that have worked with a wide range of MB species have denied that germination is exclusively linked to fire heat (Buhk and Hesen, 2006; Luna et al, 2007); i.e. post-fire recruitment was probably the result of seeds being tolerant to fire rather than being stimulated directly by it. Most of the species studied, including the three herein, were able to germinate in response to moderate pulses of heat (approx. 80 °C for a few minutes), but the germination of only a few species was stimulated by fire peak temperatures (100–120 °C). Even these high temperatures were deleterious (in terms of seed viability) in most species (Baeza et al., 2002b; Buhk and Hesen, 2006; Luna et al., 2007; Reyes and Trabaud, 2009). Additionally, several germination experiments applying other fire products, such as smoke and charred wood, obtained poor results in MB flora (with only some response in the Lamiaceae species; Keeley and Baer-Keeley, 1999; Reyes and Trabaud, 2009; Moreira et al., 2010). This limited evidence for fire-cued germination in MB species supports the results obtained in this study, thus suggesting that climatic factors are a decisive selective pressure on the post-fire seeding trait (but see Lloret et al., 2005; Moreira et al., 2012). These results contrast with those obtained in studies into other Mediterranean-type ecosystems, such as the Californian chaparral, where direct fire cues are essential for the germination of main seeder species (Tyler, 1995; Keeley and Fotheringham, 2000). In this case, it is postulated that the rise of seeding capacity pre-dates the appearance of the Mediterranean climate, during the Tertiary period and under other fire-prone climatic conditions (Pausas et al., 2006).
In summary, the response of MB seeder species to seasonal temperature fluctuations suggests that seeding capacity may have evolved in response to a wide range of disturbances, and not exclusively to fire (Ackerly, 2004). The seeder trait is associated with species that act as opportunistic colonizers (Verdú, 2000); thus, it may also have evolved in response to the long history of disturbance in the MB by either human use or herbivory.
ACKNOWLEDGEMENTS
We thank J. G. Alday for his help in the data analysis. V.M.S. has been supported by a VAli + d post-doctoral grant awarded by the Generalitat Valenciana. This research has been carried out as part of the RESILIEN (CGL 2011-30515-C02-02) and Consolider-Ingenio 2010 (GRACCIE CSD2007-00067) projects. CEAM is supported by the Generalitat Valenciana.
LITERATURE CITED
- Ackerly D. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecological Monographs. 2004;74:25–44. [Google Scholar]
- Auld TD, O'Connell MA. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Australian Journal of Ecology. 1991;16:53–70. [Google Scholar]
- Baeza MJ. Aspectos ecológicos y técnicas de control de combustible (roza y quema controlada) en matorrales con alto riesgo de incendio dominados por Ulex parviflorus(Pourr.) Spain: Universidad de Alicante; 2001. PhD Thesis (available online at http://rua.ua.es/dspace/handle/10045/3223. ) [Google Scholar]
- Baeza MJ, Roy J. Germination of an obligate seeder (Ulex parviflorus) and consequences for wildfire management. Forest Ecology and Management. 2008;256:685–693. [Google Scholar]
- Baeza MJ, Vallejo VR. Ecological mechanisms involved in dormancy breakage in Ulex parviflorus seeds. Plant Ecology. 2006;183:191–205. [Google Scholar]
- Baeza MJ, De Luis M, Raventós J, Escarré A. Factors influencing fire behaviour in shrublands of different stand ages and the implications for using prescribed burning to reduce wildfire risk. Journal of Environmental Management. 2002;65:199–208. doi: 10.1006/jema.2002.0545. [DOI] [PubMed] [Google Scholar]
- Baeza MJ, Raventós J, Escarré A. Ulex parviflorus germination after experimental burning: effects of temperature and soil depth. In: Trabaud L, Prodon R, editors. Fire and biological processes. Leiden: Backhuys Publishers; 2002. pp. 83–91. [Google Scholar]
- Baskin C, Baskin J. Seeds, ecology, biogeography and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Bond WJ, van Wilgen BW. Fire and plants. New York: Chapman & Hall; 1996. [Google Scholar]
- Bradshaw S, Dixon KW, Hopper SD, Lambers H, Turner SR. Little evidence for fire-adapted plant traits in Mediterranean climate regions. Trends in Plant Science. 2011;16:69–76. doi: 10.1016/j.tplants.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Buhk C, Hensen I. ‘Fire seeders’ during early post-fire succession and their quantitative importance in south-eastern Spain. Journal of Arid Environments. 2006;66:193–209. [Google Scholar]
- Céspedes B, Torres I, Luna B, Pérez B, Moreno J. Soil seed bank, fire season, and temporal patterns of germination in a seeder-dominated Mediterranean shrubland. Plant Ecology. 2012;213:383–393. [Google Scholar]
- Clemente AS, Rego FC, Correia OA. Seed bank dynamics of two obligate seeders, Cistus monspeliensis and Rosmarinus officinalis, in relation to time since fire. Plant Ecology. 2007;190:175–188. [Google Scholar]
- De Luis M, Baeza MJ, Raventós J, González-Hidalgo JC. Fuel characteristics and fire behaviour in mature Mediterranean gorse shrublands. International Journal of Wildland Fire. 2004;13:79–87. [Google Scholar]
- De Luis M, Raventós J, Wiegand T, González-Hidalgo JC. Temporal and spatial differentiation in seedling emergence may promote species coexistence in Mediterranean fire-prone ecosystems. Ecography. 2008;31:620–629. [Google Scholar]
- Di Castri F, Goodall DW, Specht RL. Mediterranean-type shrublands. Amsterdam: Elsevier Scientific Publishing Company; 1981. [Google Scholar]
- FAO. Soil map of the world. Revised legend. World soil resources report. Vol. 60. Rome: 1988. [Google Scholar]
- Ferrandis P, Herranz JM, Martínez-Sánchez JJ. Effect of fire on hard-coated Cistaceae seed banks and its influence on techniques for quantifying seed banks. Plant Ecology. 1999;144:103–114. [Google Scholar]
- Gorski T, Gorska K, Rybicki J. Studies on the germination of seeds under leaf canopy. Flora. 1978;167:289–299. [Google Scholar]
- Herrera CM. Historical effects and sorting processes as explanations for contemporary ecological patterns: character syndromes in Mediterranean woody plants. American Naturalist. 1992;140:421–446. [Google Scholar]
- Keeley JE. Resilience of Mediterranean shrub communities to fires. In: Dell B, Hopkins AJM, Lamont BB, editors. Resilience in Mediterranean-type ecosystems. Dordrecht: Dr W. Junk Publishers; 1986. pp. 95–112. [Google Scholar]
- Keeley JE, Baer-Keeley M. Role of charred wood, heat-shock, and light in germination of postfire phrygana species from the eastern Mediterranean basin. Israel Journal of Plant Sciences. 1999;47:11–16. [Google Scholar]
- Keeley JE, Fotheringham CJ. Role of fire in regeneration from seed. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CABI; 2000. pp. 311–330. [Google Scholar]
- Keeley JE, Pausas JG, Rundel PW, Bond WJ, Bradstock RA. Fire as an evolutionary pressure shaping plant traits. Trends in Plant Science. 2011;16:406–411. doi: 10.1016/j.tplants.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Keeley JE, Bond WJ, Bradstock RA, Pausas JG, Rundel PW. Fire in Mediterranean ecosystems: ecology, evolution and management. New York: Cambridge University Press; 2012. [Google Scholar]
- Ladd PG, Crosti R, Pignatti S. Vegetative and seedling regeneration after fire in planted Sardinian pinewood compared with that in other areas of Mediterranean-type climate. Journal of Biogeography. 2005;32:85–98. [Google Scholar]
- Lloret F, Esteban H, Vayreda J, Terradas J. Fire regenerative syndromes of forest woody species across fire and climatic gradients. Oecologia. 2005;146:461–468. doi: 10.1007/s00442-005-0206-1. [DOI] [PubMed] [Google Scholar]
- Luna B, Moreno JM, Cruz A, Fernández-González F. Heat-shock and seed germination of a group of Mediterranean plant species growing in a burned area: an approach based on plant functional types. Environmental and Experimental Botany. 2007;60:324–333. [Google Scholar]
- Moreira B, Tormo J, Estrelles E, Pausas JG. Disentangling the role of heat and smoke as germination cues in Mediterranean basin flora. Annals of Botany. 2010;105:627–635. doi: 10.1093/aob/mcq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira B, Tavsanoglu Ç, Pausas JG. Local versus regional intraspecific variability in regeneration traits. Oecologia. 2012;168:671–677. doi: 10.1007/s00442-011-2127-5. [DOI] [PubMed] [Google Scholar]
- Ooi MKJ, Auld TD, Denham AJ. Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology. 2009;15:2375–2386. [Google Scholar]
- Ooi MKJ, Auld TD, Denham AJ. Projected soil temperature increase and seed dormancy response along an altitudinal gradient: implications for seed bank persistence under climate change. Plant and Soil. 2012;353:289–303. [Google Scholar]
- Paula S, Pausas JG. Burning seeds: germinative response to heat treatments in relation to resprouting ability. Journal of Ecology. 2008;96:543–552. [Google Scholar]
- Pausas JG, Verdú M. Plant persistence traits in fire-prone ecosystems of the Mediterranean basin: a phylogenetic approach. Oikos. 2005;109:196–202. [Google Scholar]
- Pausas JG, Keeley JE, Verdú M. Inferring differential evolutionary processes of plant persistence traits in Northern Hemisphere Mediterranean fire-prone ecosystems. Journal of Ecology. 2006;94:31–39. [Google Scholar]
- Quintana JR, Cruz A, Fernandez-Gonzalez F, Moreno JM. Time of germination and establishment success after fire of three obligate seeders in a Mediterranean shrubland of central Spain. Journal of Biogeography. 2004;31:241–249. [Google Scholar]
- Rees M. Seed dormancy. In: Crawley M, editor. Plant ecology. London: Blackwell Science; 1997. pp. 214–238. [Google Scholar]
- Reyes O, Trabaud L. Germination behaviour of 14 Mediterranean species in relation to fire factors: smoke and heat. Plant Ecology. 2009;202:113–121. [Google Scholar]
- Roy J, Sonié L. Germination and population dynamics of Cistus species in relation to fire. Journal of Applied Ecology. 1992;29:647–655. [Google Scholar]
- Santana VM, Baeza MJ, Marrs RH, Vallejo VR. Old-field secondary succession in SE Spain: can fire divert it? Plant Ecology. 2010;211:337–349. [Google Scholar]
- Santana VM, Bradstock RA, Ooi MKJ, Denham AJ, Auld TD, Baeza MJ. Effects of soil temperature regimes after fire on seed dormancy and germination in six Australian Fabaceae species. Australian Journal of Botany. 2010;58:539–545. [Google Scholar]
- Santana VM, Baeza MJ, Vallejo VR. Fuel structural traits modulating soil temperatures in different species patches of Mediterranean basin shrublands. International Journal of Wildland Fire. 2011;20:668–677. [Google Scholar]
- Santana VM, Baeza MJ, Maestre FT. Seedling establishment along post-fire succession in Mediterranean shrublands dominated by obligate seeders. Acta Oecologica. 2012;39:51–60. [Google Scholar]
- Saura-Mas S, Lloret F. Leaf and shoot water content and leaf dry matter content of Mediterranean woody species with different post-fire regenerative strategies. Annals of Botany. 2007;99:545–554. doi: 10.1093/aob/mcl284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos CA, Georghiou K, Kadis C, Pantazi C. Cistaceae: a plant family with hard seeds. Israel Journal of Botany. 1992;41:251–263. [Google Scholar]
- Tieu A, Dixon KW, Meney KA, Sivasithamparam K. The interaction of heat and smoke in the release of seed dormancy in seven species from southwestern Australia. Annals of Botany. 2001;88:259–265. [Google Scholar]
- Traba J, Azcarate M, Peco B. From what depth do seeds emerge? A soil seed bank experiment with Mediterranean grassland species. Seed Science Research. 2004;14:297–303. [Google Scholar]
- Trabaud L, Casal M. Réponses des semences de Rosmarinus officinalis à différents traitements simulant une action de feu. Acta Oecologica. Oecologia Applicata. 1989;10:355–363. [Google Scholar]
- Tyler C. Factors contributing to postfire seedling establishment in chaparral: direct and indirect effects of fire. Journal of Ecology. 1995;83:1009–1020. [Google Scholar]
- Verdú M. Ecological and evolutionary differences between Mediterranean seeders and resprouters. Journal of Vegetation Science. 2000;11:265–268. [Google Scholar]
- Verdú M, Traveset A. Early emergence enhances plant fitness: a phylogenetically controlled meta-analysis. Ecology. 2005;86:1385–1394. [Google Scholar]