Abstract
Background and Aims
Despite their toxicity, reactive oxygen species (ROS) play important roles in plant cell signalling pathways, such as mediating responses to stress or infection and in programmed cell death, at lower levels. Although studies have indicated that hydrogen peroxide (H2O2) promotes seed germination of several plants such as Arabidopsis, barley, wheat, rice and sunflower, the role of H2O2 in soybean seed germination is not well known. The aim of this study therefore was to investigate the relationships between ROS, plant hormones and soybean seed germination.
Methods
An examination was made of soybean seed germination, the expression of genes related to ethylene biosynthesis, endogenous ethylene contents, and the number and area of cells in the root tip, using N-acetylcysteine, an antioxidant, to counteract the effect of ROS.
Key Results
H2O2 promoted germination, which N-acetylcysteine suppressed, suggesting that ROS are involved in the regulation of soybean germination. H2O2 was produced in the embryonic axis after imbibition. N-Acetylcysteine suppressed the expression of genes related to ethylene biosynthesis and the production of endogenous ethylene. Interestingly, ethephon, which is converted to ethylene, and H2O2 reversed the suppression of seed germination by N-acetylcysteine. Furthermore, morphological analysis revealed that N-acetylcysteine suppressed cell elongation at the root tip, and this suppression was also reversed by ethephon or H2O2 treatments, as was the case in germination.
Conclusions
In soybean seeds, ROS produced in the embryonic axis after imbibition induce the production of endogenous ethylene, which promotes cell elongation in the root tip. This appears to be how ROS regulate soybean seed germination.
Keywords: Ethylene, reactive oxygen species, seed germination, Glycine max.
INTRODUCTION
Seed germination is an important process in plant development and the process is complicated by several factors. Recently, as one of such factors, the relationship between seed germination and reactive oxygen species (ROS) in species such as Arabidopsis thaliana (Liu et al., 2010; Leymarie et al., 2012), sunflower (Oracz et al., 2007), wheat (Ishibashi et al., 2008), cress (Müller et al., 2009a) and barley (Ishibashi et al., 2010a; Bahin et al., 2011) has been reported.
In general, ROS such as O2−, hydrogen peroxide (H2O2) and •OH cause oxidative damage to lipids, proteins and nucleic acids. Indeed, seed deterioration is due in part to peroxidation of membrane lipids by ROS and the resulting leakiness of the membranes (Sung and Jeng, 1994; Bailly et al., 1998). Seed longevity is enhanced through elimination of ROS by overaccumulated ROS scavengers in transgenic seeds (Lee et al., 2010; Zhou et al., 2012). However, they also play various important roles in cellular signalling in plants, notably acting as regulators of growth and development, programmed cell death, hormone signalling, and responses to biotic and abiotic stresses (Mittler et al., 2004). In seed physiology, several studies have reported that exogenous H2O2 promotes seed germination in many plants (Chien and Lin, 1994; Fontaine et al., 1994). Furthermore, the production of H2O2 during the early imbibition period has been demonstrated in seeds of soybean (Puntarulo et al., 1988), maize (Hite et al., 1999), wheat (Caliskan and Cuming, 1998) and Zinnia elegans (Ogawa and Iwabuchi, 2001). On this basis, ROS produced after imbibition appear to regulate seed germination. Indeed, in barley seeds, NADPH oxidase, which is one of the major sources of ROS, acts as a key enzyme in germination and subsequent seedling growth (Ishibashi et al., 2010a). In pea seeds, H2O2 accelerates germination and stimulates the early growth of seedlings (Barba-Espin et al., 2010). In contrast, exogenous antioxidants, which act as ROS scavengers, significantly suppressed seed germination in several species (Ogawa and Iwabuchi, 2001; Ishibashi and Iwaya-Inoue, 2006).
Plant hormones, which are one of such factors, are important in the regulation of seed dormancy and germination (Koornneef et al., 2002; Finkelstein, 2004). The interactions among abscisic acid (ABA), gibberellins, ethylene, brassinosteroids, auxins and cytokinins in regulating the interconnected molecular processes that control dormancy release and germination have been reported (Kucera et al., 2005). There are many reports on the interaction of ROS with plant hormones in plant. In guard cells, ROS are considered second messengers in the ABA transduction pathway (Wang and Song, 2008), and exogenous ABA leads to an increase in H2O2 in guard cells (Pei et al., 2000) regulating ion channels leading to stomatal closure (Schroeder et al., 2001). In addition, ethylene receptor ETR1 plays an important role in guard cell ROS signalling and stomatal closure (Desikan et al., 2005). In seed physiology, exogenous H2O2 increased ABA catabolism by enhancing the expression of CYCP707A genes, played a major role in ABA catabolism and enhanced gibberellic acid (GA) biosynthesis genes in Arabidopsis dormant seeds (Liu et al., 2010). ROS regulated the expression of ethylene response factor ERF1, a component of the ethylene signalling pathway in sunflower seed germination (Oracz et al., 2009). In barley dormant seed, H2O2 enhanced GA synthesis genes such as GA20ox1 rather than repression of ABA signalling in embryo (Bahin et al., 2011). Recently, we have also shown that ROS regulate the induction of α-amylase through gibberellin–ABA signalling in barley aleurone cells (Ishibashi et al., 2012).
In soybean seeds, ROS are produced in the embryonic axis during germination (Puntarulo et al., 1988, 1991), and the production and scavenging of ROS during ageing were related to vigour and cell death, respectively, during accelerated ageing of the embryonic axis (Tian et al., 2008). In addition, low temperatures led to oxidative stress and lipid peroxidation caused by ROS in the embryonic axis (Posmyk et al., 2001). Although the negative role of ROS in soybean seed is now well documented, there have been fewer studies of a positive role of ROS in soybean seed. We therefore investigated the effects of ROS and antioxidants on soybean seed germination to clarify the role of ROS in germination.
MATERIALS AND METHODS
Plant material
Soybean (Glycine max ‘Fukuyutaka’) seeds were obtained from the Nakahara Seed Product Co. Ltd (Fukuoka, Japan), and only intact seeds were used.
Germination test
For each replicate, 20 soybean seeds between two filter papers were placed in a Petri dish (diameter: 9 cm). Then, 12 mL distilled water, 100 mm H2O2, 100 mm mannitol (added as an osmotic solute), N-acetylcysteine (NAC, at 10, 25 or 50 mm), or ethephon (at 1, 10, 100 or 300 p.p.m.) were added to each plate. The plates were incubated at 25 °C in the dark, and the number of germinating seeds was counted for 3 d. Seeds were considered to have germinated when the radicle protruded through the seed coat. The results presented are the means of the germination percentages obtained for five replicates per treatment.
Localization and content of H2O2
Hand-cut longitudinal sections of seeds treated with distilled water or 25 mm NAC for 24 h were incubated in 1 mg L−1 3,3′-diaminobenzidine stain at room temperature for 1 h. H2O2 was visualized as deposits of dark brown stain under a stereomicroscope (Stemi DV4; Zeiss, Oberkocken, Germany). H2O2 content was measured according to the method of Oracz et al. (2007) using a peroxidase-based assay with 3-dimethylaminobenzoic acid and 1·3 mm 3-methyl-2-benxothiazolidone hydrazine (O'Kane et al., 1996). The results presented are the means of the H2O2 contents obtained using three replicates.
Ethylene production
For each treatment, 20 soybean seeds were placed in a 9-cm-diameter Petri dish and germinated as described above. Each plate was then sealed with cling film, and a 1·0-mL gas sample after imbibition for 24 and 48 h was removed from the headspace using a gas-tight syringe. The samples were assayed on a gas chromatograph (GC-4000; GL Science, Tokyo, Japan) with a flame ionization detector and a column packed with Porapak-Q (GC-4000; GL Science, Tokyo, Japan). Temperature was maintained at 150 and 50 °C for infector/detector and oven, respectively. Ethylene was quantified by comparison of peak areas with those produced by known amounts of ethylene. Ethylene production was normalized by dividing the content by the number of seeds in each plate.
RT-PCR analysis
Embryonic axis samples were collected after imbibition for 12 or 24 h and frozen in liquid nitrogen. The frozen materials were ground to a fine powder in liquid nitrogen using a mortar and pestle, and total RNA was extracted by using the SDS–phenol–LiCl method (Chirgwin et al., 1979). cDNA was synthesized from total RNA (1 µg) with ReverTra Ace reverse transcriptase (Toyobo, Osaka, Japan) according to the manufacturer's protocol. cDNA (1 µL) was amplified in a reaction mixture containing 10 µL of Go Taq Green Master Mix (Promega, Madison, WI, USA), 0·1 µL each of 100 µm forward and reverse primers (Supplementary Data Table S1) and 8·8 µL of water. The amplification was conducted using a PC-320 Program Temp Control System (Astec, Fukuoka, Japan) as follows: 1 min at 94 °C; 25–30 cycles of 15 s at 94 °C, 30 s at 58–60 °C and 30 s at 72 °C; and a final 5 min at 72 °C. The resulting PCR products were visualized by FluorChem (Cell Bioscience, Santa Clara, CA, USA) after electrophoresis in 1·5 % agarose gels and staining with ethidium bromide.
Embryonic axis growth and longitudinal sections
The embryonic axis of seedlings was photographed with a stereomicroscope with a built-in digital camera (Stemi DV4), and we then measured the distance from the root tip to the hypocotyl base using the linear measurement tool of Adobe Acrobat Reader (http://get.adobe.com/reader/) (Adobe Systems Inc., San Jose, CA, USA). The segments of embryonic axis tissue were immediately placed in formalin–acetic acid–alcohol fixative solution. Serial longitudinal sections, 20 µm thick, were cut using a cryostat microtome (Cryostat HM 505 E; Microm GmbH, Walldorf, Germany). The sections were stained for 1 min in 0·1 % (w/v) toluidine blue and then gently washed for 2 min with distilled water.
Cell number and size in the embryonic axis
The contrast and tone of the digital images of the longitudinal sections were adjusted in the GIMP image editing software (http://www.gimp.org/). Each cell edge was manually drawn on a tracing desk. These traces were scanned on a digital scanner and then trimmed in GIMP. The size and number of the cells in the embryonic axis were determined with ImageJ software (http://rsb.info.nih.gov/ij/). Cell areas were calculated by using the ‘Analyse Particles’ function with a particle size cut-off threshold of 100 pixels (a pixel has an image area of 1·197 µm2).
RESULTS
Effect of H2O2 and NAC on soybean seed germination and localization of H2O2 during soybean seed germination
NAC, which acts as an antioxidant, suppressed soybean seed germination, and the effect increased with increasing concentration (Fig. 1A). In contrast, H2O2 significantly promoted germination compared with the control (Fig. 1B). This result was in accordance with results of pea and barley seeds (Barba-Espin et al., 2010; Ishibashi et al., 2010a). The germination of seeds treated with distilled water (the control) reached approx. 70 % after 24 h of imbibition, whereas that of seeds treated with 25 mm NAC was approx. 20 %. Because the suppression of germination by 25 mm NAC was significant after 18 and 24 h, we used 25 mm NAC in the subsequent experiments. The germination of seeds treated with 100 mm mannitol was almost the same as that in the control, suggesting that the suppression or promotion caused by NAC or H2O2 was not caused by their osmolality. In addition, germination of seeds treated with a solution containing both NAC and H2O2 showed a significant reversal of the inhibitory effects caused by NAC alone (Fig. 1B).
Fig. 1.
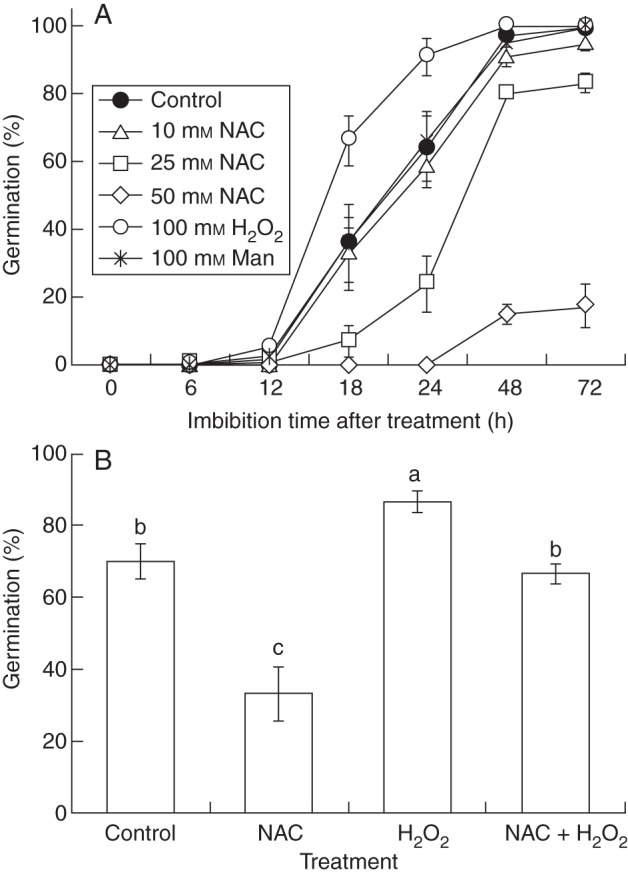
The effects of hydrogen peroxide (H2O2) and N-acetylcysteine (NAC) on soybean seed germination. (A) Change of germination with time of soybean seeds treated with distilled water (control), and with 10, 25, or 50 mm NAC, 100 mm H2O2 or 100 mm mannitol (Man). (B) Germination of soybean seeds treated with distilled water (control), 25 mm NAC, 100 mm H2O2 or 25 mm NAC + 100 mm H2O2 for 24 h. Bars with different letters differ significantly (P < 0·05, Tukey's test, n = 5).
We stained the seeds with 3,3′-diaminobenzidine to assay the accumulation of H2O2. Seeds treated with distilled water clearly showed H2O2 accumulation in the embryonic axis but not in the cotyledon (Fig. 2A, B), as reported previously (Puntarulo et al., 1988, 1991). On the other hand, NAC-treated seeds accumulated little H2O2. In addition, the H2O2 content in embryonic axis in the control was significantly higher than that in the NAC treatment (Fig. 2C).
Fig. 2.
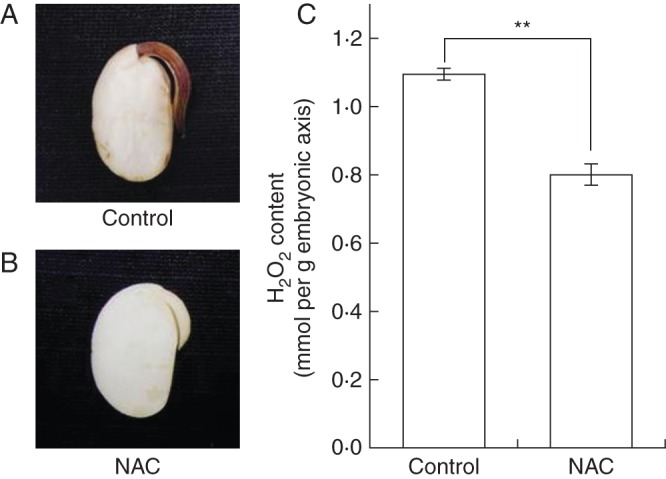
The production of hydrogen peroxide (H2O2) in the soybean embryonic axis after imbibition. (A, B) ROS accumulation monitored by staining with 1 mg mL−1 3,3′-diaminobenzidine. (C) H2O2 contents in the embryonic axis of germinating seeds treated with distilled water or 25 mm N-acetylcysteine (NAC) for 24 h. (**P < 0·05, Student's test, n = 3).
NAC suppresses ethylene production during soybean seed germination
We investigated the relationship between the stimulation of ethylene production by ROS and seed germination (Fig. 3), because both ROS and ethylene can stimulate germination and overcome dormancy in many species (Kępczyński and Kępczyńska, 1997; Bailly et al., 2008). 1-Aminocyclopropane-1-carboxylic acid (ACC) is a direct precursor of ethylene during biosynthesis (Wang et al., 2002). ACC synthase is a key enzyme in ethylene biosynthesis, and the ACS gene belongs to a multi-gene family whose members are regulated by a complex network of developmental and environmental signals that respond to both internal and external stimuli (Johnson and Ecker, 1998). Tucker et al. (2010) reported 21 ACS-like sequences (the GmACS family) in the soybean genomic sequence database. Of these sequences, we focused on GmACS2e, GmACS6a and GmACS9b. GmACS2e is a homologue of the injury-induced AtACS genes in Arabidopsis (Broekaert et al., 2006). GmACS6a and GmACS9b show high homology to VrACS1 and VrACS6, whose expression is promoted by H2O2 or auxin in mungbean (Vigna radiata) hypocotyls (Song et al., 2007).
Fig. 3.
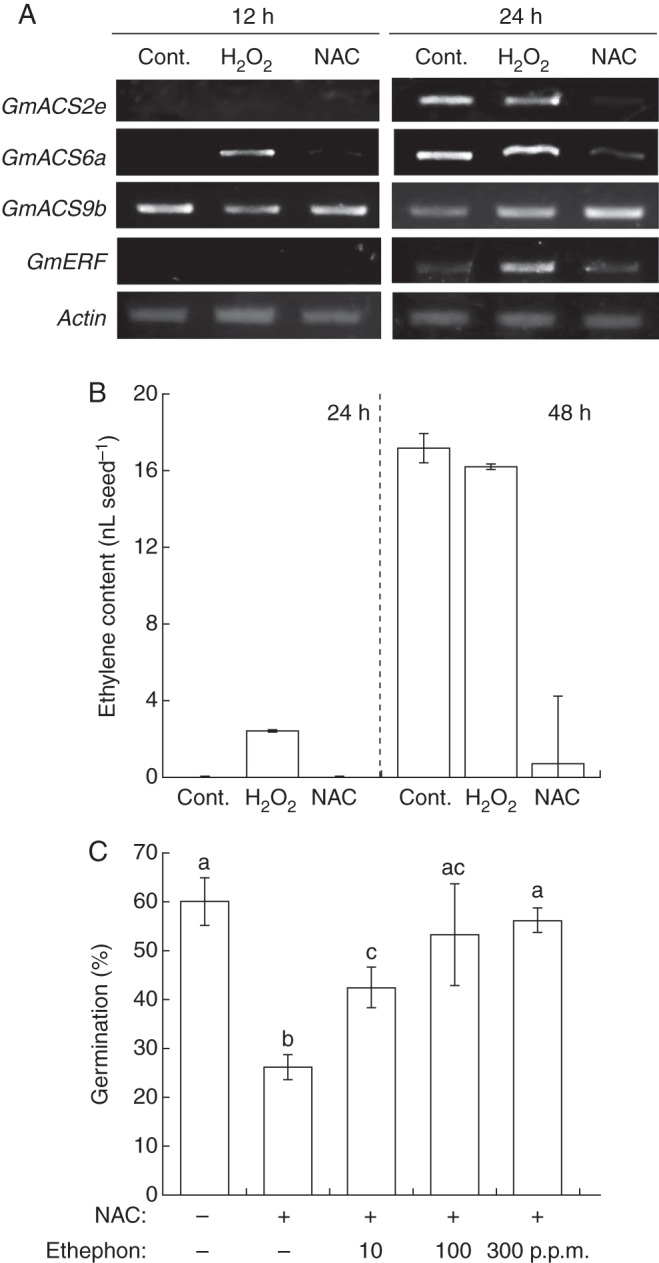
Hydrogen peroxide (H2O2) promotes biosynthesis of ethylene during soybean seed germination. (A) Effect of H2O2 (100 mm) and N-acetylcysteine (NAC; 25 mm) on the expression of genes related to ethylene biosynthesis in embryonic axis and (B) production of ethylene in soybean seed after imbibition. (C) Germination of seeds treated with distilled water (Cont.), 25 mm NAC and 25 mm NAC + 10, 100 or 300 p.p.m. ethephon for 24 h. Bars with different letters differ significantly (P < 0·05, Tukey's test, n = 5).
In the expression of GmACS genes, GmACS9b was hardly affected by any of the treatments (Fig. 3A). Control increased the expression of GmACS2e and GmACS6a at 24 h but not at 12 h after imbibition. H2O2 treatment induced the expression of GmACS6a within 12 h of imbibition. On the other hand, NAC treatment suppressed the expression of GmACS2e and GmACS6a at both 12 and 24 h after imbibition. It also induced the expression of GmERF, which acts downstream of ethylene signals, after 24 h. Ethylene production increased significantly in the H2O2 treatment but not in the control and NAC treatments after 24 h (Fig. 3B). It did not differ significantly between the control and H2O2 treatments after 48 h (17·2 and 16·2 nL per seed, respectively). However, the ethylene content in soybean seeds treated with NAC was much lower after 48 h (0·69 nL per seed). Treatment of seeds with NAC plus ethephon (which is converted to ethylene) significantly increased germination compared with treatment with NAC alone, and the two higher levels of ethephon increased germination in the presence of NAC to levels that did not differ significantly from those in the control (Fig. 3C).
NAC suppresses cell elongation in the root tip after imbibition
The length of the embryonic axis in seeds treated with NAC was significantly less than the control (Fig. 4). However, the length in seeds treated with NAC + H2O2 or NAC + ethephon did not differ significantly from the control, indicating that H2O2 and ethephon reversed the inhibitory effect of NAC on the growth of the embryonic axis. Konings and Jackson (1979) reported that a certain amount of endogenous ethylene is necessary for root growth, but that excess ethylene decreased cell elongation in the roots. We therefore carried out a morphological analysis of the embryonic axis (Supplementary Data Fig. S1). In the morphological analysis, we found major structural differences among the treatments at the root tip (Fig. 5A, B). The total area of the root tip in seeds treated with NAC was significantly smaller than the control (Fig. 5C). However, the ethephon treatment fully counteracted the effects of NAC, producing a root tip area that was not significantly different from the control, but the H2O2 treatment could not fully counteract the effects of NAC; although the root area was significantly greater in the H2O2 treatment than in the NAC treatment, the root tip area remained significantly smaller than in the control. Moreover, the number of cells in the root tip did not differ significantly among the four treatments (Fig. 5D). The size of cells in root tips treated with NAC was significantly lower (by approx. 42 %) than the control. Ethephon completely counteracted the effect of NAC, but although H2O2 significantly decreased the adverse effects of NAC, cell size remained significantly smaller than the control (Fig. 5E). It seems likely that there was insufficient H2O2, not that something about the experimental conditions prevented this ROS from stimulating ethylene production because H2O2 completely counteracted the effect of NAC on expression of GmACS2e and GmACS6a (Supplementary Data Fig. 2S).
Fig. 4.
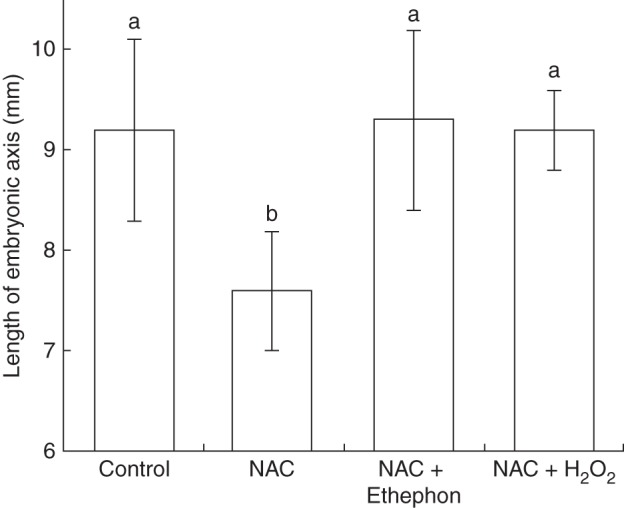
Effect of N-acetylcysteine (NAC) on the length of the embryonic axis during soybean seed germination. Seeds were treated with distilled water (control), 25 mm N-acetylcysteine (NAC), 25 mm NAC + 300 p.p.m. ethephon, or 25 mm NAC + 100 mm H2O2 for 24 h. Bars with different letters differ significantly (P < 0·01, Tukey's test, n = 10).
Fig. 5.
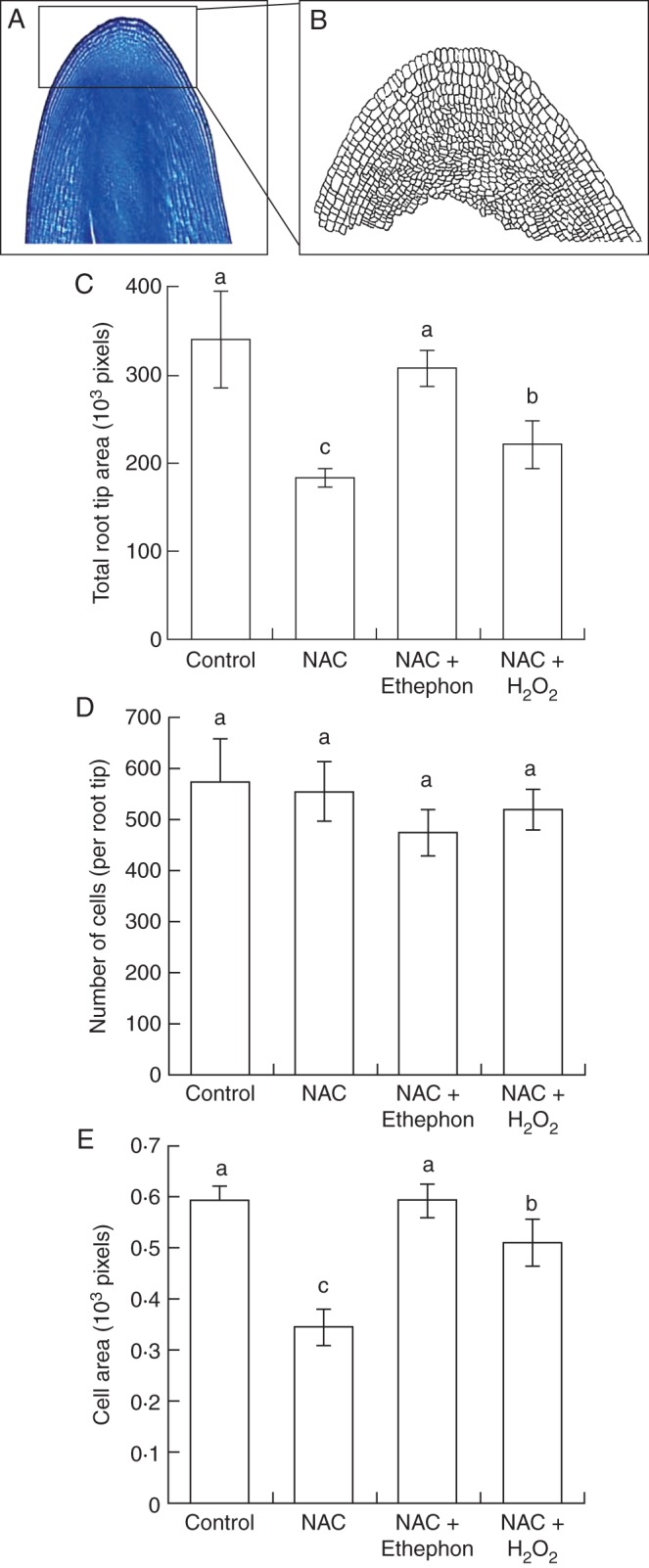
Number and area of cells in the root tip during soybean seed germination. Seeds were treated with distilled water, 25 mm N-acetylcysteine (NAC), 25 mm NAC + 300 p.p.m. ethephon, or 25 mm NAC + 100 mm H2O2. (A) Staining of the embryonic axis by toluidine blue. (B) Trace of cells in the root tip. (C) Total root tip area, (D) number of cells and (E) cell area of the root tip, based on traces that were measured in ImageJ software. Bars with different letters differ significantly (P < 0·05, Tukey's test, n = 5).
DISCUSSION
Recent studies of seed physiology have described the relationships between ROS and seed germination, dormancy and after-ripening (Bailly et al., 2008; Müller et al., 2009b; Oracz et al., 2009; Liu et al., 2010; Ishibashi et al., 2010a; Bahin et al., 2011; Leymarie et al., 2012). Here, we confirmed that soybean seed germination was regulated through ethylene production in response to ROS. The first line of evidence supporting this conclusion was the suppression of germination by NAC, an antioxidant that suppressed H2O2 content in the embryonic axis (Fig. 2) and decreased seed germination (Fig. 1A). In addition, exogenous H2O2 counteracted the suppression of germination by NAC (Fig. 1B). These results suggest that NAC suppressed the germination of soybean seed by inhibiting H2O2 production in the embryonic axis after imbibition. Puntarulo et al. (1988) reported the production of H2O2 during early imbibition in soybean seeds, and subsequently found that the activities of the enzymes involved in H2O2 metabolism (e.g. superoxide dismutase, catalase, peroxidase, and glutathione and ascorbate peroxidases) changed markedly in the soybean embryonic axis during germination (Puntarulo et al., 1991). However, the role of H2O2 in soybean seed germination remained unclear.
Ethylene was originally regarded as a plant stress hormone, because its synthesis is induced by a variety of stress signals, such as mechanical wounding (Kende, 1993), exposure to a range of chemicals and metals, drought, extreme temperatures and pathogen infection (Johnson and Ecker, 1998). In seed biology, ethylene clearly participates in the germination of certain seeds (Matilla and Matilla-Vazquez, 2008) and increases radicle emergence under unfavourable conditions (Abeles, 1986; Kozarewa et al., 2006). In addition, there have been numerous reports of a relationship between ROS and ethylene: in legumes, ROS and ethylene are part of a Nod factor-induced signal cascade that is important for the initiation of nodule primordia (D'Haeze et al., 2003). In winter squash (Cucurbita maxima), CmACS1 is inhibited by diphenylene iodonium, which blocks the superoxide-generating enzyme NADPH oxidase (Watanabe et al., 2001). In mungbean hypocotyls, exogenous ACC (a precursor that is easily converted to ethylene) did not affect ROS production, but hypocotyls exposed to H2O2 showed high ethylene accumulation as a result of activation of ethylene biosynthesis enzymes (Song et al., 2007). Thus, we hypothesized that ethylene production in soybean seeds would be affected by ROS generated after imbibition, as our results confirmed (Fig. 3).
Although expression levels of GmACS9b remained high in all treatments, those of GmACS6a and GmACS2e were very low in the NAC treatment but increased in the control and H2O2 treatments, especially GmACS6a, expression of which was promoted within 24 h after imbibition in the H2O2 treatment (Fig. 3A). In Arabidopsis and tomato, expression of specific members of the ACS gene family is rapidly induced by O3 (Vahala et al., 1998; Overmyer et al., 2000; Nakajima et al., 2001; Moeder et al., 2002; Tamaoki et al., 2003). Although the stimulation of ethylene synthesis by environmental stress involves the generation of ROS, there is also a regulatory mechanism by which ROS regulate ethylene biosynthesis (Surplus et al., 1998; Qin et al., 2008). We found that the production of endogenous ethylene in soybean seeds after imbibition was promoted by H2O2 and suppressed by NAC (Fig. 3B). Other researchers have reported that ethylene sensitivity is a major factor in the germination of dormant Arabidopsis seeds (Beaudoin et al., 2000; Ghassemian et al., 2000; Gallardo et al., 2002; Siriwitayawan et al., 2003). In our study, expression of GmERF (which acts downstream of ethylene signalling) was induced by H2O2, but only after 24 h (Fig. 3A), and the suppression of seed germination by NAC could be mitigated or eliminated by ethephon treatment (Fig. 3C). These results indicate that ethylene production in response to the ROS produced after imbibition was involved in the germination of soybean seeds.
Several hypotheses have been proposed to explain the mechanisms of ethylene action in germinating seeds (Esashi, 1991; Kępczyński and Kępczyńska, 1997; Matilla and Matilla-Vazquez, 2008). For example, the promotion of radial cell expansion is a primary response to ethylene during seed germination (Kucera et al., 2005). In addition, ROS are involved in cell elongation; for example, the requirement for ROS during elongation of growing apical cells in root hairs has been demonstrated through the use of NADPH oxidase mutants (Foreman et al., 2003; Carol and Dolan, 2006). The production of ROS such as O2− has been detected in the expansion zone of maize leaf blades, and results in cell-wall loosening (Rodríguez et al., 2002). In our study, NAC reduced the length of the embryonic axis, but the addition of ethephon or H2O2 counteracted the reduction by NAC (Fig. 4), suggesting that ethylene produced in response to ROS after imbibition regulated the length of the embryonic axis. Furthermore, morphological analysis revealed that the ethylene produced in response to ROS in the embryonic axis (root tip and zone of elongation) induced cell hypertrophy (Fig. 5E, Supplementary Data Fig. S3B) but not hyperplasia (Fig. 5D, Supplementary Data Fig. S3A). Our results suggest that the ethylene produced in response to ROS regulates the length of the embryonic axis by increasing the size of root tip cells without increasing their number, and thereby regulated soybean seed germination.
Konings and Jackson (1979) proposed that a certain amount of endogenous ethylene is necessary to maintain root growth. Increasing the concentration of endogenous ethylene leads to increased growth until a threshold concentration is reached; thereafter, further increases in the ethylene concentration reduce growth. We previously reported that pre-treatment with H2O2 promoted the germination of soybean seeds but delayed emergence (Ishibashi et al., 2010b). The emergence was delayed by lateral growth of the hypocotyls in response to ethylene produced in response to the H2O2. Furthermore, Zheng and Inoue (1989) reported that when hypocotyl elongation during emergence was inhibited by soil particles and crust under field conditions, the seedlings produced ethylene, and their hypocotyls thickened and became able to break the crust. In soybean, the production of H2O2 in seeds after imbibition might govern the growth from seed germination to emergence through the production of ethylene.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by a Grant-in-Aid for Scientific Research from the JSPS to Y.I. (No. 24780014).
LITERATURE CITED
- Abeles FB. Role of ethylene in Lactuca sativa cv. ‘Grand Rapids’ seed germination. Plant Physiology. 1986;81:780–787. doi: 10.1104/pp.81.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahin E, Bailly C, Sotta B, Kranner I, Corbineau F, Leymarie J. Crosstalk between reactive oxygen species and hormonal signaling pathways regulates grain dormancy in barley. Plant Cell and Environment. 2011;34:980–993. doi: 10.1111/j.1365-3040.2011.02298.x. [DOI] [PubMed] [Google Scholar]
- Bailly C, Benamar A, Corbineau F, Come D. Free radical scavenging as affected by accelerated ageing and subsequent priming in sunflower seeds. Physiologia Plantarum. 1998;104:646–652. [Google Scholar]
- Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biologies. 2008;331:806–814. doi: 10.1016/j.crvi.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Barba-Espin G, Diaz-Vivancos P, Clemente-Moreno MJ, et al. Interaction between hydrogen peroxide and plant hormones during germination and the early growth of pea seedlings. Plant Cell and Environment. 2010;33:981–994. doi: 10.1111/j.1365-3040.2010.02120.x. [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;12:1103–1115. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekaert WF, Delaure SL, De Bolle MFC, Cammue BPA. The role of ethylene in host–pathogen interactions. Annual of Review Phytopathology. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- Caliskan M, Cuming AC. Spatial specificity of H2O2-generating oxalate oxidase gene expression during wheat embryo germination. Plant Journal. 1998;15:165–171. doi: 10.1046/j.1365-313x.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. Journal of Experimental Botany. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- Chien CT, Lin TP. Mechanism of hydrogen peroxide in improving the germination of Cinnamomum camphora seed. Seed Science Technology. 1994;22:231–236. [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;24:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Bright J, Harrison H, Weir I, Hooley R, Neill SJ. A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiology. 2005;137:831–834. doi: 10.1104/pp.104.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Haeze W, De Rycke R, Mathis R, et al. Reactive oxygen species and ethylene play a positive role in lateral root base nodulation of a semiaquatic legume. Proceedings of the National Academy of Sciences USA. 2003;100:11789–11794. doi: 10.1073/pnas.1333899100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esashi Y. Ethylene and seed germination. In: Mattoo AK, Suttle JC, editors. The plant hormone ethylene. Boca Raton, FL: CRC Press; 1991. pp. 133–157. [Google Scholar]
- Finkelstein RR. The role of hormones during seed development and germination. In: Davies PJ, editor. Plant hormones-Biosynthesis, signal transduction, action! Dordrecht: Kluwer; 2004. pp. 513–537. [Google Scholar]
- Fontaine O, Huault C, Pavis N, Billard JP. Dormancy breakage of Hordeum vulgare seeds: effects of hydrogen peroxide and scarification on glutathione level and glutathione reductase activity. Plant Physiology and Biochemistry. 1994;2:677–683. [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Gallardo K, Job C, Groot SPC, et al. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiology. 2002;129:823–837. doi: 10.1104/pp.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hite DR, Auh C, Scandalios JG. Catalase activity and hydrogen peroxide levels are inversely correlated in maize scutella during seed germination. Redox Report. 1999;4:29–34. doi: 10.1179/135100099101534710. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Iwaya-Inoue M. Ascorbic acid suppresses germination and dynamic states of water in wheat seeds. Plant Production Science. 2006;9:172–175. [Google Scholar]
- Ishibashi Y, Yamamoto K, Tawaratsumida T, Yuasa T, Iwaya-Inoue M. Hydrogen peroxide scavenging regulates germination ability during wheat (Triticum aestivum L.) seed maturation. Plant Signaling and Behavior. 2008;3:183–188. doi: 10.4161/psb.3.3.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y, Tawaratsumida T, Zheng SH, Yuasa T, Iwaya-Inoue M. NADPH oxidases act as key enzyme on germination and seedling growth in barley (Hordeum vulgare L.) Plant Production Science. 2010a;13:45–52. [Google Scholar]
- Ishibashi Y., Zheng SH, Arima S. Effect of seed pre-treatment with hydrogen peroxide in soybean seed germination and seedling growth. Coastal Bioenvironment. 2010b;15:55–60. [Google Scholar]
- Ishibashi Y, Tawaratsumida T, Kondo K, et al. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiology. 2012;158:1705–1714. doi: 10.1104/pp.111.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annual Review of Genetics. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- Kępczyński J, Kępczyńska E. Ethylene in seed dormancy and germination. Physiologia Plantarum. 1997;101:720–726. [Google Scholar]
- Kende H. Ethylene biosynthesis. Annual Review Plant Physiology and Plant Molecular Biology. 1993;44:283–307. [Google Scholar]
- Konings H, Jackson MB. A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation. Zeitschrift für Pflanzenphysiologie. 1979;92:385–397. [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Current Opinion in Plant Biology. 2002;5:33–36. doi: 10.1016/s1369-5266(01)00219-9. [DOI] [PubMed] [Google Scholar]
- Kozarewa I, Cantliffe DJ, Nagata RT, Stoffella PJ. High maturation temperature of lettuce seeds during development increased ethylene production and germination at elevated temperatures. Journal of the American Society for Horticultural Science. 2006;131:564–570. [Google Scholar]
- Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15:281–307. [Google Scholar]
- Lee YP, Baek KH, Lee HS, Kwak SS, Bang JW, Kwon SY. Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. Journal of Experimental Botany. 2010;61:2499–2506. doi: 10.1093/jxb/erq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leymarie J, Vitkauskaité G, Hoang HH, et al. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant and Cell Physiology. 2012;53:96–106. doi: 10.1093/pcp/pcr129. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ye N, Liu R, Chen M, Zhang J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. Journal of Experimental Botany. 2010;61:2979–2990. doi: 10.1093/jxb/erq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla AJ, Matilla-Vazquez MA. Involvement of ethylene in seed physiology. Plant Science. 2008;175:87–97. [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends in Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Moeder W, Barry CS, Tauriainen AA, et al. Ethylene synthesis regulated by biphasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiology. 2002;130:1918–1926. doi: 10.1104/pp.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. In vivo cell wall loosening by hydroxyl radicals during cress (Lepidium sativum L.) seed germination and elongation growth. Plant Physiology. 2009a;150:1855–1865. doi: 10.1104/pp.109.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytologist. 2009b;184:885–897. doi: 10.1111/j.1469-8137.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- Nakajima N, Matsuyama T, Tamaoki M, et al. Effects of ozone exposure on the gene expression of ethylene biosynthetic enzymes in tomato leaves. Plant Physiology and Biochemistry. 2001;39:993–998. [Google Scholar]
- Ogawa K, Iwabuchi M. A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant and Cell Physiology. 2001;42:286–291. doi: 10.1093/pcp/pce032. [DOI] [PubMed] [Google Scholar]
- O'Kane D, Gill V, Boyd P, Burdon RH. Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta. 1996;198:366–370. doi: 10.1007/BF00620053. [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Farrant JM, et al. ROS production and protein oxidation as novel mechanism of seed dormancy alleviation. Plant Journal. 2007;50:452–465. doi: 10.1111/j.1365-313X.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C. The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key actors of cellular signalling during germination. Plant Physiology. 2009;150:494–505. doi: 10.1104/pp.109.138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, et al. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, et al. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Posmyk MM, Corbineau F, Vinel D, Bailly C, Côme D. Osmoconditioning reduces physiological and biochemical damage induced by chilling in soybean seeds. Physiologia Plantarum. 2001;111:473–482. doi: 10.1034/j.1399-3054.2001.1110407.x. [DOI] [PubMed] [Google Scholar]
- Puntarulo S, Sánchez RA, Boveris A. Hydrogen peroxide metabolism in soybean embryonic axes at the onset of germination. Plant Physiology. 1988;86:626–630. doi: 10.1104/pp.86.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntarulo S, Galleano M, Sanchez RA, Boveris A. Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. Biochimica et Biophysica Acta. 1991;1074:277–283. doi: 10.1016/0304-4165(91)90164-c. [DOI] [PubMed] [Google Scholar]
- Qin YM, Hu CY, Zhu YX. The ascorbate peroxidase regulated by H2O2 and ethylene is involved in cotton fiber cell elongation by modulating ROS homeostasis. Plant Signaling and Behavior. 2008;3:194–196. doi: 10.4161/psb.3.3.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez A, Grunberg K, Taleisnik E. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiology. 2002;129:1627–1632. doi: 10.1104/pp.001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- Siriwitayawan G, Geneve RL, Downie AB. Seed germination of ethylene perception mutants of tomato and Arabidopsis. Seed Science Research. 2003;13:303–314. [Google Scholar]
- Song YJ, Joo JH, Ryu HY, Lee JS, Bae YS, Nam KH. Reactive oxygen species mediate IAA-induced ethylene production in mungbean (Vigna radiata L.) hypocotyls. Journal of Plant Biology. 2007;50:18–23. [Google Scholar]
- Sung JM, Jeng T. Lipid peroxidation and peroxide-scavenging enzymes associated with accelerated aging of peanut seed. Physiologia Plantarum. 1994;91:51–55. [Google Scholar]
- Surplus SL, Jordan BR, Murphy AM, Carr JP, Thomas B, Mackerness SAH. Ultraviolet-B-induced responses in Arabidopsis thaliana: role of salicylic acid and reactive oxygen species in the regulation of transcripts encoding photosynthetic and acidic pathogenesis-related proteins. Plant Cell and Environment. 1998;21:685–694. [Google Scholar]
- Tamaoki M, Matsuyama T, Kanna M, et al. Differential ozone sensitivity among Arabidopsis accessions and its relevance to ethylene synthesis. Planta. 2003;216:552–560. doi: 10.1007/s00425-002-0894-2. [DOI] [PubMed] [Google Scholar]
- Tian X, Song S, Lei Y. Cell death and reactive oxygen species metabolism during accelerated ageing of soybean axes. Russian Journal of Plant Physiology. 2008;55:33–40. [Google Scholar]
- Tucker ML, Xue P, Yang R. 1-Aminocyclopropane-1-carboxylic acid (ACC) concentration and ACC synthase expression in soybean roots, root tips, and soybean cyst nematode (Heterodera glycines)-infected roots. Journal of Experimental Botany. 2010;61:463–472. doi: 10.1093/jxb/erp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahala J, Schlagnhaufer CD, Pell EV. Induction of an ACC synthase cDNA by ozone in light grown Arabidopsis thaliana leaves. Physiologia Plantarum. 1998;103:45–50. [Google Scholar]
- Wang KLC, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14(suppl.):S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Song CP. Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytologist. 2008;178:703–718. doi: 10.1111/j.1469-8137.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Seo S, Sakai S. Wound-induced expression of a gene for 1-aminocyclopropane-1-carboxylate synthase and ethylene production are regulated by both reactive oxygen species and jasmonic acid in Cucurbita maxima. Plant Physiology and Biochemistry. 2001;39:121–127. [Google Scholar]
- Zheng SH, Inoue J. Changes in elongation force, stem thickness and evolution of ethylene accompanied with growth of soybean seedlings. Japanese Journal of Crop Science. 1989;58:357–363. [Google Scholar]
- Zhou Y, Chu P, Chen H, et al. Overexpression of Nelumbo nucifera metallothioneins 2a and 3 enhances seed germination vigor in Arabidopsis. Planta. 2012;235:523–537. doi: 10.1007/s00425-011-1527-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


