Abstract
Background and aims
The protocarnivorous plant Paepalanthus bromelioides (Eriocaulaceae) is similar to bromeliads in that this plant has a rosette-like structure that allows rainwater to accumulate in leaf axils (i.e. phytotelmata). Although the rosettes of P. bromelioides are commonly inhabited by predators (e.g. spiders), their roots are wrapped by a cylindrical termite mound that grows beneath the rosette. In this study it is predicted that these plants can derive nutrients from recycling processes carried out by termites and from predation events that take place inside the rosette. It is also predicted that bacteria living in phytotelmata can accelerate nutrient cycling derived from predators.
Methods
The predictions were tested by surveying plants and animals, and also by performing field experiments in rocky fields from Serra do Cipó, Brazil, using natural abundance and enriched isotopes of 15N. Laboratory bioassays were also conducted to test proteolytic activities of bacteria from P. bromelioides rosettes.
Key Results
Analyses of 15N in natural nitrogen abundances showed that the isotopic signature of P. bromelioides is similar to that of carnivorous plants and higher than that of non-carnivorous plants in the study area. Linear mixing models showed that predatory activities on the rosettes (i.e. spider faeces and prey carcass) resulted in overall nitrogen contributions of 26·5 % (a top-down flux). Although nitrogen flux was not detected from termites to plants via decomposition of labelled cardboard, the data on 15N in natural nitrogen abundance indicated that 67 % of nitrogen from P. bromelioides is derived from termites (a bottom-up flux). Bacteria did not affect nutrient cycling or nitrogen uptake from prey carcasses and spider faeces.
Conclusions
The results suggest that P. bromelioides derive nitrogen from associated predators and termites, despite differences in nitrogen cycling velocities, which seem to have been higher in nitrogen derived from predators (leaves) than from termites (roots). This is the first study that demonstrates partitioning effects from multiple partners in a digestion-based mutualism. Despite most of the nitrogen being absorbed through their roots (via termites), P. bromelioides has all the attributes necessary to be considered as a carnivorous plant in the context of digestive mutualism.
Keywords: Animal–plant interactions, nutrient flux, nitrogen cycling, digestive mutualisms, multiple partners, carnivorous plants, Paepalanthus bromelioides, Latrodectus geometricus, Isoptera, stable isotopes of 15N, rupestrian fields
INTRODUCTION
Nutrients are randomly distributed in the environment, and their amounts are inferior to the amounts required by organisms (Chapin, 1980). Moreover, nutrients can be in forms that plants generally cannot absorb directly (e.g. organic nitrogen). Species can cope with a low concentration of nutrients by investing in alternative strategies to obtain and conserve resources (e.g. Pereira et al., 2012). In fact, several plant species evolved convergent adaptations to the carnivorous habit (Butler and Ellison, 2007; Chase et al., 2009). This alternative mode of nutrient acquisition and allocation for the photosynthetic process is present in approx. 700 monocot and dicot species (Król et al., 2012). It has been suggested that carnivory in plants has developed in response to the need to survive under harsh environmental conditions. For example, in sunny and wet habitats that are nutrient poor, especially in nitrates and phosphates, the benefits from investing in carnivory exceed the costs (Givnish et al., 1984; Ellison and Gotelli, 2002; Butler and Ellison, 2007; Lambers et al., 2008; Millett et al., 2012). The nutritional supply provided by the addition of prey decreases photosynthetic stress and, consequently, nutrient uptake can be allocated to reproduction and development (Givnish et al., 1984; Farsnworth and Ellison, 2008).
By definition, a carnivorous plant is able to absorb nutrients derived from animals trapped on their surface and must bear morphological, physiological and behavioural adaptations for prey attraction, adhesion (i.e. capture), prey digestion especially by secreting hydrolysing enzymes themselves, and absorption (Givnish et al., 1984). In contrast, plant species that inhabit sunny, wet and nutrient-poor environments and that can trap prey but do not display all the attributes necessary for carnivory (e.g. production of proteolytic enzymes) (e.g. Darnowski et al., 2006) are called protocarnivores (Givnish et al., 1984). It has been suggested that many species that possess sticky glands (i.e. glandular trichomes), such as Geranium viscosissimum and Potentilla arguta, and are able to absorb nutrients on their surface are protocarnivores (Spomer, 1999). In the tank-bromeliad Catopsis berteroniana (Bromeliaceae), prey digestion is mediated by bacteria in the phytotelmata and has been classified by Frank and O'Meara (1984) as protocarnivore. However, some species such as Brocchinia reducta (Bromeliaceae), which have an indirect digestion process mediated by the microbial community present in their phytotelmata, are also classified as legitimate carnivores because they possess adaptations for attracting prey (Givnish et al., 1984).
Anderson and Midgley (2003) proposed that the definition of carnivory be extended to include plants that do not require conventional organs to attract and digest prey, as long as these plants sustain a persistent mutualism with a species-specific predator. This animal–plant interaction is termed digestive mutualism and is indicated by plants that capture or attract prey and provide suitable foraging sites for predators, which in turn improve plant fitness by providing nutrients through defecation into the tank or upon the leaves, and thereby provide easily digestible nutrients that are easily absorbed by the plant surface (Ellis and Midgley, 1996; Romero et al., 2006). According to this new definition, the plant Roridula gorgonias is considered to be a carnivore because it sustains the digestive mutualist Pameridea roridulae (Hemiptera) (Anderson and Midgley, 2003).
Paepalanthus bromelioides (Eriocaulaceae) is a plant-like Bromeliaceae that lives in well-lit, nutrient-poor habitats (rocky fields) in the highlands of southeastern Brazil (Supplementary Data Fig. S1). These habitats are characterized by strong erosion, and frequently receive nutrient input after natural or man-made fires (Rizzini, 1997). This plant has some carnivorous traits, such as UV reflection by leaves, and a central tank (or phytotelmata) containing acidic mucilaginous liquid, which possibly function to attract and digest nutrients from arthropods, respectively (Jolivet and Vasconcellos-Neto, 1993). In addition, these plants have leaves covered by wax which makes the surface slippery to insects, and bear hydrophilous trichomes at the base of their leaves which possibly function to absorb nutrients from arthropods (Jolivet and Vasconcellos-Neto, 1993; Figueira et al., 1994). However, P. bromelioides plants were classified as protocarnivores because apparently they are not able to trap prey, despite the fact that the presence of lubricant liquid might cause some little insects to stick. In addition, their digestive process seems to be carried out only by bacterial degradation (Jolivet and Vasconcellos-Neto, 1993; Figueira et al., 1994), although this assumption was not empirically investigated; the low pH (3·5–5·2) of the liquid was attributed to bacterial activity (Figueira et al., 1994), and apparently no proteolytic enzyme is secreted by the plant.
However, in rosette leaves, several predators, including ants, scorpions, harvestmen and especially spiders, find foraging sites, shelter, humidity and suitable microclimates for protection from high temperatures (Figueira and Vasconcellos-Neto, 1991) and thus are plausible digestive mutualists of P. bromelioides. The web spiders Latrodectus geometricus (Theridiidae) and Alpaida quadrilorata (Araneidae) build their webs above plant tanks, whereas the active hunters Arachosia proseni (Anyphaenidae) capture invertebrates that find shelter in rosette leaves. A considerable amount of prey carcasses and spider faeces are deposited upon the P. bromelioides leaves and into their rosettes (Figueira et al., 1994; Supplementary Data Fig. S1; G. Q. Romero, pers. obs.), which might be degraded by aquatic detritivorous larvae. Thus, the predators may compensate for the low prey capture ability of plants, by canalizing prey carcasses and faeces into the plant rosette. In addition, the poorly developed stem and roots of this plant are frequently wrapped by a compact termite mound of cylindrical shape (Supplementary Data Fig. S1 and S2) measuring up to 30 cm tall, which is constructed by at least four termite genera (Velocitermes, Nasutitermes, Armitermes and Spinitermes). These termite mounds are formed naturally by erosion and deposition of particles on the mound walls (Figueira and Vasconcellos-Neto, 1991) (Supplementary Data Fig. S1), and are more compact (few cells and galleries) than typical termite nests. Generally there are no galleries linking the mounds with cellulosic sources (e.g. tree trunks), meaning that the main nutrient source for termites is derived from dead leaves of P. bromelioides (Figueira, 1989; Figueira and Vasconcellos-Neto, 1991). Termites contribute to nutrient cycling through the grinding, decomposition, humification and mineralization of cellulosic resources, which might also improve plant nutrition acquired through roots (e.g. Breznak and Brune, 1994). In addition, the P. bromelioides roots are strictly associated with arbuscular mycorrhiza, which are considered to be highly absorbent (Pagano and Scotti, 2009).
The plants of P. bromelioides might be considered to be carnivorous plants based on the definition by Anderson and Midgley (2003) if there was evidence that these plants absorb nutrients from the biotic activities of mutualists (e.g. termites, predators or bacteria) which, in turn, might accelerate cycling of organic nutrients released by predators (e.g. faeces or prey carcasses). We tested these predictions in field experiments using isotopic methods of 15N and laboratory bioassays to test proteolytic activities of bacteria. The following questions were addressed: (1) How much nitrogen of P. bromelioides is derived from predator debris (faeces or carcasses) and from insects that are attracted to the rosette and eventually fall into them? (2) Do bacteria accelerate nutrient cycling derived from predators (faeces or carcasses), thus improving P. bromelioides nutrition? (iii) How much nitrogen of P. bromelioides is derived from termite activities?
MATERIALS AND METHODS
Study area
This study was conducted at the reserve of Morro da Pedreira (19 °17'S, 43 °53'W), a property owned by Dr G. W. Fernandes, at Santana do Riacho city (Serra do Cipó), Minas Gerais state, Brazil. This reserve is located in a mountainous region (elevation >1000 m a.s.l.), characterised by quartzitic rock fields (Espinhaço complex). The vegetation in this area consists of herbs and small shrubs, which grow on rocks or nutrient-poor soils with a low capacity for water retention (Giulietti et al., 1987). The mean annual rainfall is 1500 mm, with average temperatures varying from 17·4 to 19·8 °C.
Nitrogen flux from predators and prey to the plant
Field survey – 15N natural abundance
First, we investigated the isotopic profile of Paepalanthus bromelioides Silveira (Eriocaulaceae) in relation to reference plant species, including carnivorous plants and plants that do not maintain nutritional associations with animals. We collected two new, expanded leaves from ten individuals of P. bromelioides not associated with termite mounds. We also collected new, expanded leaves from some reference plants found in higher abundance in the study area to trace the isotopic profile of the vegetation. We used more than one reference plant to control for natural variations in the 15N distribution in the soil because these plants can vary in root depth and thus vary in their levels of nitrogen uptake. Processes involved in mycorrhiza–plant associations can alter the amounts of 15N in plants (Hobbie et al., 2000). Because P. bromelioides maintains this type of association (Pagano and Scotti, 2009), we used only reference plants associated with mycorrhizae. The plants that met this requirement were Lychnophora sp. (Asteraceae; n = 5 plants), Barbacenia sp. (Velloziaceae; n = 10) and Hyptis sp. (Lamiaceae; n = 10) (Pagano and Scotti, 2009). In addition, we collected leaves from two carnivorous plants inhabiting the study area, Drosera hirtella (n = 10) and D. aff. villosa (n = 5), for comparative analyses. These surveys were conducted in two different areas, but the material was homogenized for each area for the analyses.
The natural abundance of 15N from insects collected using sweep net sampling in the vegetation close to rosettes of P. bromelioides (12 leafhopper, 39 ants, 11 flies, six hemipterans, 12 grasshoppers, one butterfly, three wasps and three beetles) were pooled within the two areas surveyed (n = 2 samples). We also collected faecal samples from the spiders Alpaida quadrilorata (Araneidae), Arachosia proseni (Anyphaenidae) and Latrodectus geometricus (Theridiidae) (n = 9). Each faecal sample consisted of 3–5 individuals of A. quadrilorata, A. proseni and L. geometricus diluted in distilled water (150 µL). In addition, we collected 20 faecal pellets from ten individuals of a grasshopper species associated with P. bromelioides rosettes; these samples were pooled in a single sample, homogenized and diluted in distilled water. Spiders (two A. quadrilorata, one A. proseni and one L. geometricus) were also analysed isotopically (n = 4).
Field experiment
To quantify nitrogen flux from animals to P. bromelioides leaves, we applied labelled faeces (15N) of L. geometricus spiders onto rosettes growing naturally in the field. To obtain spider faeces, 70 females collected in urban areas from São José do Rio Preto city (São Paulo state) were kept in glass vials (7 cm in diameter, 10 cm in height). Each spider was fed with 15N-labelled Tenebrio molitor (Coleoptera) larvae every 2 d, which was sufficient time for capture and ingestion of larvae to occur (information obtained from prior bioassays). The T. molitor larvae were reared with a sub-stratum composed of mouse feed (Labina-Purina®) and cassava flour. The sub-stratum was sprinkled with a solution of enriched ammonium sulfate [(15NH4)2SO4, 10 atom % excess, Cambridge Isotope Laboratories, MA, USA], in the proportion of 100 gsubstrate/20 mLsolution. The substratum was oven-dried for 24 h at 60 °C and then homogenized. Every 2 d, the labelled faeces were collected with a micropipette and diluted in distilled water (150 µL); these samples were kept frozen until their application in the field.
To determine the amount of nitrogen derived from prey carcasses and insects that eventually fall inside the rosette, we applied labelled (15N; labelled as described above) T. molitor carcasses, which were fed upon by the spiders, and T. molitor larvae directly onto the rosettes. The carcasses from the spiders (kept in vials) and larvae were collected every 2 d and frozen until application.
Plants of P. bromelioides that were similar in size (i.e. rosette diameter and height) were randomly selected to receive the following treatments: (1) L. geometricus faeces (n = 10 plants); (2) T. molitor carcasses (n = 10); (3) T. molitor larvae (n = 10); and (4) control plants (i.e. no addition of labelled material; n = 10). Each plant was at least 3 m away from its nearest neighbour. Applications occurred every 2 d; in each application, the first treatment group received three faecal samples (1 mg each, total of 3 mg), the second treatment group received a sample of T. molitor carcass (3 mg each) and the third treatment group received T. molitor larvae (3 mg). Overall, 30 applications were made from 11 December 2009 to 5 March 2010. At the end of the experiment, we surveyed three young leaves (first, second and third nodes) from each plant for isotopic analyses.
The role of bacteria in nitrogen cycling and flux
To verify the presence of proteolytic bacteria in the tanks of P. bromelioides, which might mineralize animal compounds (e.g. guanine and chitin), we randomly surveyed samples from the central tank of P. bromelioides in the field (n = 15). With the aid of sterile forceps and a heat source to prevent contamination, we surveyed samples from the liquid in P. bromelioides tanks and inoculated them into separate Petri dishes containing agar (n = 15). In the laboratory, we isolated bacterial colonies in medium supplemented with: (1) protein (skim milk; n = 15); (2) guanine (n = 15); (3) chloramphenicol at 20 mg mL−1 (n = 15), 40 mg mL−1 (n = 15) and 60 mg mL−1 (n = 15); and (4) tetracycline at 20 mg mL−1 (n = 15), 40 mg mL−1 (n = 15) and 60 mg mL−1 (n = 15). These bioassays were conducted at a food engineering laboratory in the Microbiology Department of the State University of São Paulo (UNESP).
In the field, plants of P. bromelioides were randomly selected to receive the following treatments: (i) L. geometricus faeces plus 10 mL of distilled water (n = 10); (2) L. geometricus faeces plus 10 mL of tetracycline solution (20 mg mL−1 in distilled water) (n = 10); (3) Tenebrio carcasses plus 10 mL of distilled water (n = 10); and (4) Tenebrio carcasses plus 10 mL of tetracycline solution (20 mg mL−1 in distilled water) (n = 10). Each plant was at least 3 m away from its nearest neighbour. Only tetracycline was used in this experiment because its effect is similar to that of chloramphenicol in the inhibition of bacterial colonies, i.e. the diameter of inhibition halos caused by tetracycline and chloramphenicol did not differ (F1,28 = 0·001, P = 0·96) (see Supplementary Data Fig. S3). Moreover, tetracycline is one of the most powerful antibiotics in the control of vegetation pests (Agrios, 2005), and its addition did not have any apparent effects on plant physiology based on the observation that there were no changes in leaf appearance (e.g. colour) or incidences of plant death. In each application, the plants from the first and second treatment (groups 1 and 2 above) received three faecal samples (1 mg each), and plants from the third and fourth treatment (groups 3 and 4 above) received a sample of Tenebrio carcass (3 mg). Overall, 25 applications of nitrogen sources were made between 16 January and 5 March 2010. Tetracycline was applied four times (i.e. once every 2 weeks), from 16 January to 1 March 2010. At the end of the experiment, three young leaves (i.e. from the first, second and third nodes) from each plant were collected for isotopic analyses.
Nitrogen flux from soil (termite mounds) to the plant
Field survey – 15N natural abundance
To verify if P. bromelioides benefit nutritionally from their associations with termite mounds, we surveyed two new, expanded leaves of P. bromelioides that were either associated (n = 9) or not associated with termite mounds (n = 10). We also collected three termites from five independent termite mounds for isotopic analyses.
Field experiment
To quantify nitrogen flux from termite mounds to P. bromelioides via roots, we used strips of cardboard to simulate deposition of vegetal material in the soil. The strips were rectangular with a total area of 50 cm2 and 2 g in mass. Thirty plants of P. bromelioides that were similar in size (i.e. similar rosette diameter and height as well as associated termite mound height) were randomly chosen to receive the following treatments: (1) eight 15N-labelled strips of cardboard and insecticide solution (n = 10); (2) eight 15N-labelled strips of cardboard and no insecticide (n = 10); and (3) eight non-labelled strips of cardboard and no insecticide (n = 10). Each plant was at least 8 m away from its nearest neighbour. Each termite mound from the first treatment received 20 mL of Termitox insecticide (10 mL L−1 distilled water), which was applied twice (17 January and 16 February 2010) to four surface holes that were 2 cm deep. This insecticide is used in the control of termites and Attini ants (Link and Link, 2008). At the end of the experiment, we verified that the mounds that had received insecticide had a density of on average 0·4 termites per plant (± 0·30 s.e.), while the mounds that did not receive insecticide had a density of on average 16·05 termites per plant (± 4·60 s.e.), indicating that the method used was effective. For all mounds, we excavated four entrances at a 45 º angle with respect to the soil surface to prevent damaging the roots; these entrances were deep enough to insert the whole cardboard strips. Overall, we inserted eight strips in each mound throughout the experiment; the first four strips were inserted on 17 January and the remaining four strips were inserted in the same holes on 2 March 2010.
The cardboard strips were 15N labelled by immersion for 5 s in a solution of 5 g L−1 ammonium sulfate [(15NH4)2SO4, 10 atom % excess, from Cambridge Isotope Laboratories]. The non-labelled cardboard strips were immersed for 5 s in distilled water. At the end of the experiment, we collected three young leaves (i.e. first, second and third nodes) from each plant. We also collected random samples of mound soils (non-labelled) for isotopic analyses.
Isotopic and statistical analyses
The leaves collected were rinsed under running water for 3 min to remove small particles. Soon after collection, the leaves were frozen at –18 °C and then transported to the laboratory in ice-filled polypropylene boxes. In the laboratory, the leaves were oven-dried at 60 °C for 24 h and then ground to a fine powder using a micro-knife mill (Marconi, Piracicaba, Brazil). Samples of labelled and non-labelled cardboard were dried by similar procedures. Faecal samples were concentrated by centrifuging (13 000 r.p.m.) for 30 min, and then oven-dried at 50 °C for 20 min and ground manually. Spiders and insects were oven-dried and ground manually.
The δ15N values { = [(15N:14Nsample/15N:14Nstandard) – 1] × 1000 (‰ vs. At-air)} from leaves, faeces, spiders, insects, soil and cardboard were determined using an isotopic ratio mass spectrometer (IRMS) (20–20 mass spectrometer; PDZ Europa, Sandbach, UK, after sample combustion to N2 at 1000 °C by the on-line elemental analyser PDZ Europa ANCA-GSL) in the Stable Isotope Facility (UC-Davis).
The percentage of nitrogen (%N) in plants derived from faeces, carcasses, larvae and termites was determined using linear mixing model equations from two sources for a single element (δ15N; Phillips and Gregg, 2001). In our model, we added known fractionation rates for nitrogen during plant metabolic and assimilation processes. According to Phillips and Gregg (2001), the percentage contribution of a source (fA) can be expressed as follows:
| (1) |
where fA is the fraction (%) of N of Paepalanthus derived from a given source (e.g. faeces, cascass, larvae or termite), δM is the isotopic ratio of Paepalanthus that received a given source, and δA and δB are the isotopic ratios of potential N sources (δA = faeces, carcasses, larvae or termites; δB = soil). The Δδ15N values used in the linear mixing models were based on the nitrogen sources in McCutchan et al. (2003): for plants that received nitrogen-rich materials (e.g faeces), we used a value of +3·3 ± 0·26 ‰ (mean ± s.e.); for plants which received nitrogen-poor material (e.g carcasses, larvae or termites), we used a value of +1·4 ± 0·2 ‰.
The δ15N values for samplings and experiments had a probability distribution that closely resembled the Gaussian distribution (Shapiro–Wilk normality test; P ≥ 0·2). However, the variances were not homogeneous (Levene's test; P < 0·05) even after data transformations. Therefore, we used one-way analyses of variance (ANOVAs) for global comparisons and Tamhane's T2 post-hoc tests for paired comparisons between treatments. Tamhane's tests are appropriated for unequal sample sizes and heterogeneous variances (Hochberg and Tamhane, 2011). The percentage contributions from different sources (e.g. faeces, carcasses, larvae or termites) were statistically compared using one-way ANOVA and Tukey post-hoc tests for paired comparisons.
RESULTS
Our results of the natural abundance of 15N showed that leaves of P. bromelioides had a similar isotopic signature to D. hirtella, but that the δ15N values were lower in P. bromelioides compared with those of D. aff. villosa. However, Paepalanthus and the Drosera species had higher δ15N values than the reference, non-carnivorous plants, Barbacenia sp., Lychnophora sp. and Hyptis sp. (Fig. 1, F5,49 = 36·3, P < 0·001). Furthermore, the leaves from rosettes of P. bromelioides that received spider faeces as well as Tenebrio carcasses and larvae had higher δ15N values compared with the leaves from the control rosettes (Fig. 2A, F3,34 = 33·3, P < 0·001). However, the P. bromelioides leaves that received faeces had higher δ15N values than those receiving carcasses and larvae (Fig. 2A); leaves from P. bromelioides that received larvae and carcasses had similar values of δ15N (Fig. 2A). The %N (μg g−1) from the leaves did not differ among the four treatments (F3,34 = 1·23, P = 0·314).
Fig. 1.
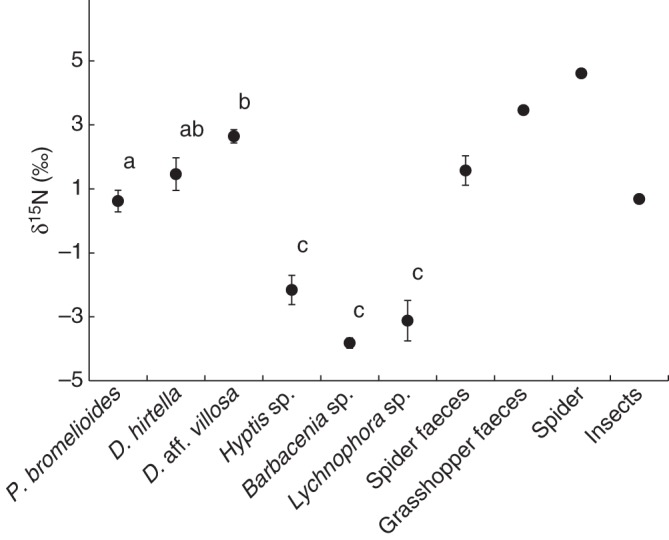
Average δ15N values indicating natural nitrogen abundance in Paepalanthus bromelioides, Drosera hirtella, Drosera aff. villosa, Hyptis sp., Lychnophora sp., Barbacenia sp., spider and grasshopper faeces, and tissues of spiders and insects. Bars indicate standard errors, and different letters indicate significant differences (P < 0·05; ANOVA/Tamhane's T2 post-hoc test; α = 0·05).
Fig. 2.
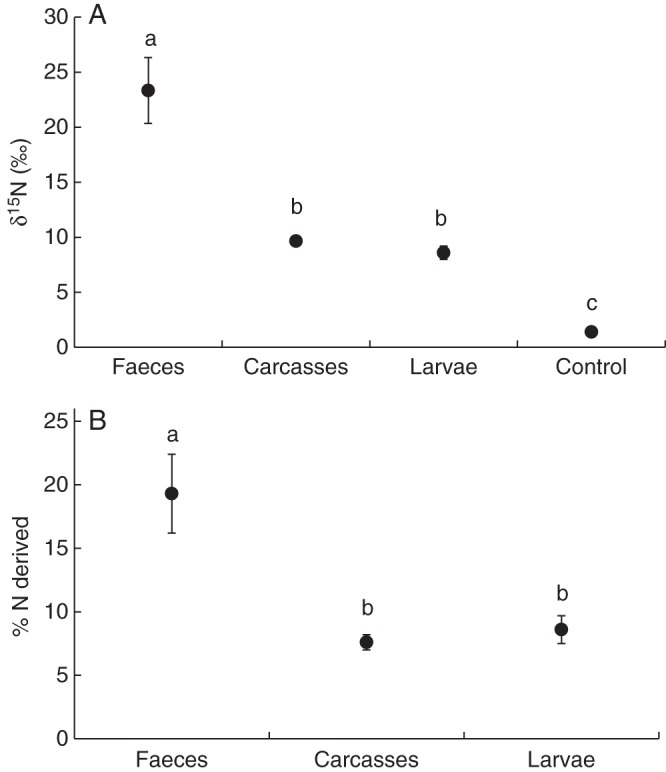
(A) Average δ15N values of Paepalanthus bromelioides leaves that received labelled faeces of the spider Latrodectus geometricus, labelled carcasses and labelled Tenebrio larvae, and control (no addition of labelled material). Bars indicate standard errors, and different letters indicate significant differences (P < 0·05; ANOVA/Tukey post-hoc test; α = 0·05). (B) The percentage of nitrogen of P. bromelioides derived from L. geometricus faeces, carcasses and Tenebrio larvae. Bars indicate standard errors, and different letters indicate significant differences (P < 0·05; ANOVA/Tamhane's T2 post-hoc test; α = 0·05).
The labelled faeces, carcasses and larvae had δ15N values of 99·1 ‰ (± 4·9 s.e.), 92·6 ‰ (± 4·0) and 68·8 ‰ (± 3·3), respectively. Linear mixing models of two sources showed that faeces contributed 19·0 ± 3·1 % (s.e.) to the total nitrogen of the plants, whereas carcasses and larvae contributed 7·5 ± 0·6 and 8·6 ± 1·1 %, respectively, to the plant nitrogen (Fig. 2B); these sources differed in their contribution to plant nitrogen (Fig. 2B, ANOVA: F2,25 = 9·92, P < 0·001).
Bacteria that were collected from the phytotelmata of P. bromelioides degraded guanine (main component of spider faeces) and protein (milk), probably by releasing proteolytic enzymes. From the 15 bacterial cultures analysed per treatment/bioassay, only two did not grow in protein medium, and five did not grow in guanine medium. The antibiotics chloramphenicol and tetracycline inhibited all bacterial cultures similarly (Supplementary Data Fig. S3), independent of the antibiotic concentration applied. In the field, experimental plants of P. bromelioides that received tetracycline absorbed 15N in similar proportions to plants that did not receive antibiotics; this finding was also observed in the presence of faeces and carcasses (Fig. 3, F3,39 = 14,6; P < 0·001), suggesting that bacteria may not contribute to nitrogen cycling in the P. bromelioides phytotelmata.
Fig. 3.
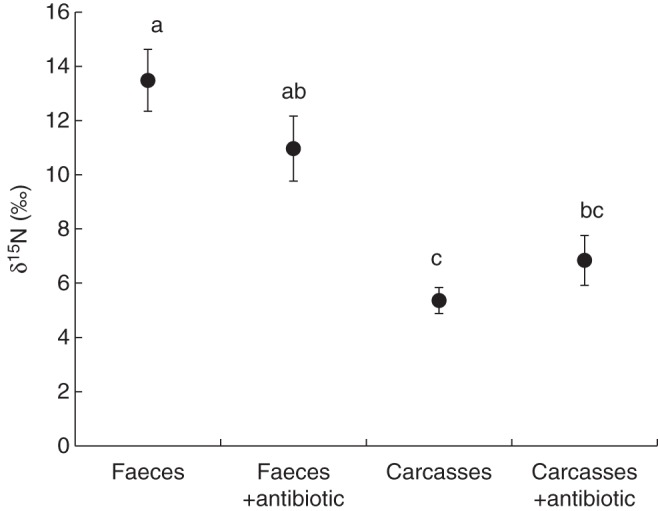
Average δ15N values of Paepalanthus bromelioides leaves exposed to labelled Latrodectus geometricus faeces, labelled faeces plus an antibiotic, labelled Tenebrio carcasses and labelled carcasses plus an antibiotic. Bars indicate standard errors, and different letters indicate significant differences (P < 0·05; ANOVA/Tamhane's T2 post-hoc test; α = 0·05).
Paepalanthus bromelioides rosettes associated with termite mounds had natural abundance δ15N values higher than those of rosettes not associated with termites (Fig. 4A, F1,18 = 9·11, P = 0·008). Linear mixing models from two sources for the data on natural abundance showed that termite mounds contributed 67 ± 51·2 % (s.e.) to the total nitrogen of the plants. The foliar N content (%) did not differ between P. bromelioides associated and not associated with termites (mean ± s.e.; associated with termites, 0·58 ± 0·02; not associated with termites, 0·60 ± 0·03; t-test for separate variances: t = 0·41, d.f. = 17, P = 0·68). During the experiments, termites fed on and degraded the cardboard strips efficiently (see Supplementary Data Fig. S2); the average δ15N value of termites inhabiting mounds with labelled cardboard (4·5 ± 1·7 ‰, n = 5) was higher than the values for those inhabiting mounds with non-labelled cardboard (2·5 ± 1·0 ‰, n = 5). Unlabelled cardboard had an average δ15N value of –14·6 ‰ (± 4·63 s.e.), and the δ15N value for cardboard after enrichment was 14 359·6 ± 603·3 ‰. The labelled strips had an average of 5·3 ± 0·3 (s.e.) μg of total nitrogen. The P. bromelioides plants were more isotopically enriched in the presence of labelled cardboard strips than those with non-labelled cardboard (Fig. 4B, F2,26 = 4·26, P = 0·026). However, δ15N values of plants that received insecticide did not differ from those of plants that did not receive insecticide (Fig. 4B). The mound soil samples of natural abundance (not enriched) had a δ15N value of 6·2 ± 0·5 ‰ (n = 5). δ15N values (natural abundance) among new, mature and dead leaves did not differ (Kruskall–Wallis; U = 4·31, P = 0·116).
Fig. 4.
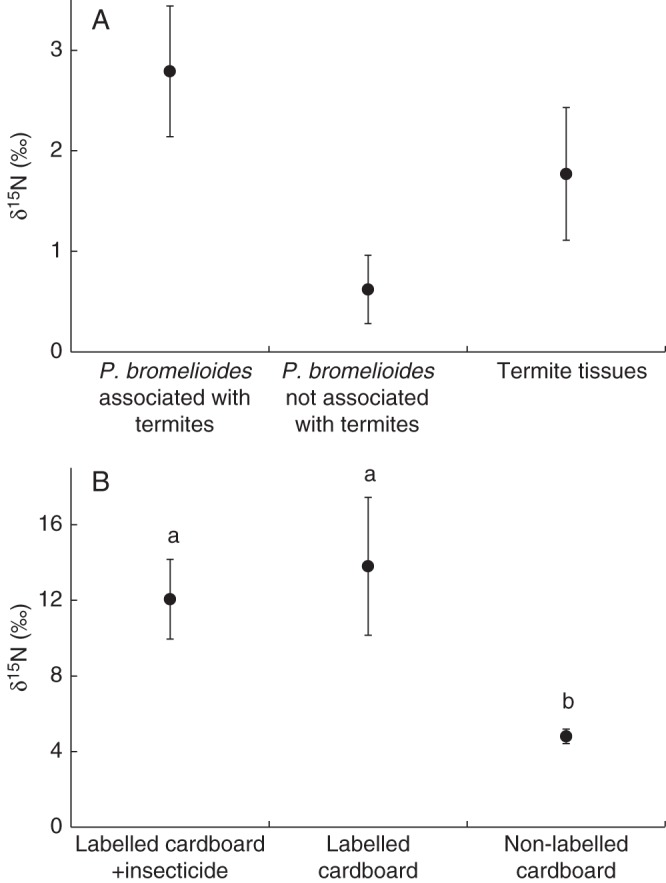
(A) Average δ15N values indicating natural nitrogen abundance of leaves of Paepalanthus bromelioides associated with termite mounds, and average δ15N values indicating natural nitrogen abundance of termite tissues. (B) Average δ15N values of leaves of P. bromelioides that received labelled cardboard plus insecticide, labelled cardboard without insecticide and non-labelled cardboard and no insecticide. Bars indicate standard errors, and different letters indicate significant differences (P < 0·05; ANOVA/Tamhane's T2 post-hoc test; α = 0·05).
DISCUSSION
Our results clearly show nutrient fluxes from predators associated with P. bromelioides and insects that eventually visit their rosettes to find food and shelter from harsh conditions. However, contrary to our expectations, the presence of bacteria did not affect nitrogen uptake by plants. Although our field experiment using tracer elements did not detect a nitrogen flux from termites to plants, isotopic methods of detection of 15N in natural abundance have suggested a strong nutrient input via termite–plant interactions. Thus, nitrogen seems to enter the system via rosettes through predator activities, with availability accelerated through a microfauna associated with the phytothelma (top-down flux), and via roots through termite activities (bottom-up fluxes).
Spider faeces contributed more to the nutrition of P. bromelioides than insect carcasses or larvae. These findings corroborate our prediction that predators, such as L. geometricus and A. quadrilorata (this last species being strictly associated with P. bromelioides in the study area), provide a portion of the required nutrients to P. bromelioides during its life cycle. It has been previously demonstrated that carnivorous plants can absorb faeces from shrews (Clarke et al., 2009) and bats (Grafe et al., 2011). This study is the first to demonstrate that carnivorous plants can take up debris from spiders. It is likely that this contribution observed for P. bromelioides is even higher because other sources released by predators (egg sacs and exuviae) can also be deposited inside the rosettes and absorbed by plants. In contrast to faeces, prey carcasses and insect larvae contributed less to P. bromelioides nutrition. These results were also reported in previous studies (Romero et al., 2006; Gonçalves et al., 2011) and can be explained by differences in chemical compounds among nitrogen sources. The spiders release in their faeces simple nitrogen compounds, such as hypoxanthine, uric acid, adenine and especially guanine (Foelix, 1996), which can be directly absorbed by the plants via specialized trichomes (e.g. Romero et al., 2006). On the other hand, carcasses and larvae are made up of complex chemical compounds, such as chitin, which must first be degraded into simple compounds before they can be absorbed by plants. Therefore, in this system the spiders appear to have two crucial functions: (1) to canalize nitrogen sources to the inside of rosettes by capturing prey and (2) to accelerate nutrient cycling by digesting their prey.
The presence of proteolytic bacteria that degrade guanine inside phytotelmata of P. bromelioides was observed in laboratory bioassays. However, our field experiments showed that the presence of these micro-organisms did not affect nitrogen absorption by the plants. Similar to other carnivorous plants, whose phytotelmata are inhabited by diverse heterotrophic organisms (Jaffe et al., 1992; Cochran-Stafira and Ende, 1998), P. bromelioides could also be inhabited by other organisms acting in nutrient cycling, and their effects could mask or surpass the bacterial effects (but see Butler et al., 2008). In fact, detritivorous larvae of Culicidae (Diptera) and Scirtidae (Coleoptera) were found in the P. bromelioides phytotelmata (A. L. Mendonça, pers. obs.). Although studies have suggested the importance of enzyme secretion by fungi and bacteria for nitrogen cycling in phytotelmata of Nepenthes and Sarracenia, to date no study has quantified the exact function of micro-organisms in the nutrition of carnivorous plants (Adlassnig et al., 2010).
Despite the fact that our tracer experiment using labelled cellulosic sources has not detected nitrogen flux from termite mounds to plants, our results on the natural abundance of 15N suggested a strong nitrogen input to P. bromelioides deriving from termite mounds (67 % of the total N of the plants). Similarly, based on the natural abundance of 15N, Leroy et al. (2009) showed that epiphytic bromeliads from French Guiana root on ant gardens (carton nest), and they derive a considerable amount of nitrogen from ant debris. The termites clearly consumed and digested the cardboard strips (see Supplementary Data Fig. S2). However, during our tracer experiment there was very little rain compared with the local mean (the authors, pers. obs.), implying that most of the nutrients provided by the termites may have been accumulated in the soil and, thus, poorly absorbed by the roots. Alternatively, P. bromelioides plants grow in association with termite mounds for >15–20 years, and it is likely that we have not detected nitrogen flux from soil to rosettes via termite activities due to the short period that the labelled cardboard was exposed to termites. Based on our results of the natural abundance of 15N, we conclude that the amount of plant nitrogen derived from termites is remarkable.
Although predatory activity is an additional source of nutrients for P. bromelioides (26·5 % of plant N derived from faeces and carcasses), our results are in contrast to values for the contribution by predatory insects found in Roridula gorgonias (70 %) (Anderson and Midgley, 2003) and in carnivorous plants that accumulate water, Darlingtonia californica (76 %) and Nepenthes mirabilis (61 %) (Schulze et al., 1997). Despite this, the contribution we report was similar to that of Bromelia balansae (Bromeliaceae) (18 %), a terrestrial plant that does not accumulate water and thus is likely to obtain all of its nitrogen from the soil. The values we report were also similar to those of the carnivorous plant Cephalotus follicularis (26 %), which absorbs nitrogen via its roots and even bears specialized organs for carnivory (Schulze et al., 1997). However, this absorption was higher than in Sarracenia purpurea (10 %), which takes up nitrogen in its organic and inorganic form (alternative pathway) via atmospheric deposition (Ellison and Gotelli, 2001; Karagatzides et al., 2009). Previous studies have classified P. bromelioides as protocarnivore (Jolivet and Vasconcellos-Neto, 1993; Figueira et al., 1994) due to its ability to absorb nutrients from dead animals (via trichomes) and because it possesses adaptations for attraction (i.e. UV reflection in the leaves) and capture of insects (i.e. wax on leaves, and a tank with viscous liquid). In this study, we showed that the prey digestion could be mediated by digestive mutualists. Therefore, we suggest that P. bromelioides can be considered a carnivorous plant, as described by Anderson and Midgley (2003).
The survival and evolution of P. bromelioides in its natural habitat were probably favoured by biotic relationships with diverse animal groups. Because P. bromelioides live in nutrient-poor environments (Giulietti et al., 1987), the maintenance of mutualism with associated predators and termites may represent a valuable alternative strategy for obtaining limiting elements. Whereas predator activity contributed 26·5 % of the plant nitrogen, termites contributed 67 %. Although spider-derived debris is degraded by bacteria, nutrients are not available more quickly for plants in the presence of these micro-organisms. Moreover, termites can also provide non-nutritional benefits to the plants, such as decreasing the amount of combustible material from the rosettes (protections against fire), acting as a thermal insulator, and building cohesive mounds that decrease nutrient loss caused by leaching (Figueira, 1989; Figueira and Vasconcellos-Neto, 1991). Although many studies have shown the ability of plants to absorb nutrients derived from animals (e.g. Treseder et al., 1995; Anderson and Midgley, 2003; Solano and Dejean, 2004; Romero et al., 2006; Leroy et al., 2009), this is the first study to test and quantify the provision of nutrients in a system involving multiple partners in a digestive mutualism. Even if most of the nitrogen was absorbed through the roots (via termites), our study suggested that digestive mutualism, together with the ability to attract, capture and absorb nutrients, fulfils the necessary conditions for P. bromelioides to be considered as a carnivorous plant.
SUPPLEMENTARY DATA
ACKNOWLEDEGMENTS
The authors thank M. F. Pareja, V. F. Farjalla, N. Chaffey and two anonymous reviewers for their helpful comments on the manuscript, H. A. Rocha Jr and A. L. Mendonça for help with data collection in the field, and A. Z. Gonçalves for help in the laboratory and with the mixing model equations. A.H.N. was supported by a graduate fellowship from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-08/56516-7). J.V.-N. was supported by a productivity grant from CNPq. G.Q.R. was supported by a productivity grant from CNPq and was the coordinator of a FAPESP grant that funded part of this study.
LITERATURE CITED
- Adlassnig W, Peroutka M, Lendl T. Review: traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Annals of Botany. 2011;107:181–194. doi: 10.1093/aob/mcq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrios GN. Plant pathology. 5th edn. Elsevier Academic Press; 2005. [Google Scholar]
- Anderson B, Midgley JJ. Digestive mutualism, an alternate pathway in plant carnivory. Oikos. 2003;102:221–224. [Google Scholar]
- Breznak JA, Brune A. Role of microorganisms in the digestion of lignocellulose by termites. Annual Review of Entomology. 1994;39:453–487. [Google Scholar]
- Butler JL, Ellison AM. Nitrogen cycling dynamics in the carnivorous northern pitcher plant Sarracenia purpurea. Functional Ecology. 2007;21:835–843. [Google Scholar]
- Butler JL, Gotelli NJ, Ellison AM. Linking the brown and green: nutrient transformation and fate in the Sarracenia microecosystem. Ecology. 2008;89:898–904. doi: 10.1890/07-1314.1. [DOI] [PubMed] [Google Scholar]
- Chapin FS., III The mineral nutrition of wild plants. Annual Review of Ecology and Systematics. 1980;11:233–260. [Google Scholar]
- Chase MW, Christenhusz MJM, Sanders D, Fay MF. Murderous plants: Victorian Gothic, Darwin and modern insights into vegetable carnivory. Botanical Journal of the Linnean Society. 2009;161:329–356. [Google Scholar]
- Clarke CM, Bauer U, Lee CiC, Tuen AA, Rembold K, Moran JA. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biology Letters. 2009;5:632–635. doi: 10.1098/rsbl.2009.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran-Stafira DL, von Ende CN. Integrating bacteria into food webs: studies with Sarracenia purpurea inquilines. Ecology. 1998;79:880–898. [Google Scholar]
- Darnowski DW, Carroll DM, Plachno B, Kabanoff E, Cinnamon E. Evidence of protocarnivory in Triggerplants (Stylidium spp.; Styliaceae) Plant Biology. 2006;8:805–812. doi: 10.1055/s-2006-924472. [DOI] [PubMed] [Google Scholar]
- Ellis AG, Midgley JJ. A new plant animal mutualism involving a plant with sticky leaves and a resident hemipteran insect. Oecologia. 1996;106:478–481. doi: 10.1007/BF00329705. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends in Ecology and Evolution. 2001;16:623–629. [Google Scholar]
- Ellison AM, Gotelli NJ. Nitrogen availability alters the expression of carnivory in the northern pitcher plant Sarracenia purpurea. Proceedings of the National Academy of Sciences, USA. 2002;99:4409–4412. doi: 10.1073/pnas.022057199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth EJ, Ellison AM. Prey availability directly affects physiology, growth, nutrient allocation, and scaling relationships among leaf traits in ten carnivorous plant species. Journal of Ecology. 2008;96:213–221. [Google Scholar]
- Figueira JEC. 1989 Associação entre Paepalanthus bromelioides Silv. (Eriocaulaceae), aranhas e térmitas. Msc Thesis, State University of Campinas (UNICAMP), Campinas, SP, Brazil. [Google Scholar]
- Figueira JEC, Vasconcellos-Neto J. Paepalanthus, cupins e aranhas. Ciência Hoje. 1991;13:20–26. [Google Scholar]
- Figueira JEC, Vasconcellos-Neto J, Jolivet P. Une nouvelle plante protocarnivore Paepalanthus bromelioides Silv. (Eriocaulaceae) du Brésil. Revue d'Écologie. 1994;49:3–9. [Google Scholar]
- Foelix RF. Biology of spiders. 2nd edn. New York: Oxford University Press; 1996. [Google Scholar]
- Frank JH, O'Meara GF. The bromeliad Catopsis berteroniana traps terrestrial arthropods but harbors Wyeomyia larvae (Diptera: Culicidae) Florida Entomologist. 1984;67:418–424. [Google Scholar]
- Giulietti AM, Menezes NL, Pirani JR, Meguro M, Wanderley MGL. Flora da Serra do Cipó, Minas Gerais: caracterização e lista de espécies. Boletim de Botânica da Universidade de São Paulo. 1987;9:1–151. [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. American Naturalist. 1984;124:479–497. [Google Scholar]
- Gonçalves AZ, Mercier H, Mazzafera P, Romero GQ. Spider-fed bromeliads: seasonal and interspecific variation in plant performance. Annals of Botany. 2011;107:1047–1055. doi: 10.1093/aob/mcr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe TU, Schöner CR, Kerth G, Junaidi A, Schöner MG. A novel resource–service mutualism between bats and pitcher plants. Biology Letters. 2011;7:436–439. doi: 10.1098/rsbl.2010.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie EA, Macko SA, Williams M. Correlations between foliar δ15N and nitrogen concentrations may indicate plant–mycorrhizal interactions. Oecologia. 2000;122:273–283. doi: 10.1007/PL00008856. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Tamhane A. Multiple comparison procedures. John Wiley & Sons; 2011. [Google Scholar]
- Jaffe K, Michelangeli F, Gonzalez JM, Miras B, Ruiz MC. Carnivory in pitcher plants of the genus Heliamphora (Sarraceniaceae) New Phytologist. 1992;122:733–744. [Google Scholar]
- Jolivet P, Vasconcellos-Neto J. Convergence chez les plantes carnivores. La Recherche. 1993;24:456–458. [Google Scholar]
- Karagatzides JD, Butler JL, Ellison AM. The pitcher plant Sarracenia purpurea can directly acquire organic nitrogen and short-circuit the inorganic nitrogen cycle. PLoS One. 2009;4:e6164. doi: 10.1371/journal.pone.0006164. http://dx.doi.org/10.1371/journal.pone.0006164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Król E, Płachno BJ, Adamec L, Stolarz M, Dziubińska H, Trębacz K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world. Annals of Botany. 2012;109:47–64. doi: 10.1093/aob/mcr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, III, Pons TL. Plant physiological ecology. 2nd edn. New York: Springer Science; 2008. [Google Scholar]
- Leroy C, Corbara B, Dejean A, Céréghino R. Potential sources of nitrogen in an ant-garden tank-bromeliad. Plant Signaling and Behavior. 2009;4:868–870. doi: 10.4161/psb.4.9.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link D, Link FM. Avaliação de doses de Fipronil, aplicadas em área total, no controle da formiga saúva, Atta sexdens piriventris, na cultura de milho. 53a Reunião Técnica Anual de Milho e 36a Reunião Técnica Anual de Sorgo-RTAMS. EMBRAPA CLIMA TEMPERADO. 2008 [Google Scholar]
- McCutchan JH, Lewis WM, Kendall C, McGrath CC. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos. 2003;102:378–390. [Google Scholar]
- Millett J, Svensson MB, Newton J, Rydin H. Reliance on prey-derived nitrogen by the carnivorous plant Drosera rotundifolia decreases with increasing nitrogen deposition. New Phytologist. 2012;195:182–188. doi: 10.1111/j.1469-8137.2012.04139.x. [DOI] [PubMed] [Google Scholar]
- Pagano MC, Scotti MR. A survey of the arbuscular mycorrhiza occurrence in Paepalanthus bromelioides and Bulbostylis sp. in rupestrian fields, Brazil. Micología Aplicada Internacional. 2009;1:1–10. [Google Scholar]
- Pereira CG, Almerana DP, Winter CE, Fritsch PW, Lambers H, Oliveira RS. Underground leaves of Philcoxia trap and digest nematodes. Proceedings of the National Academy of Sciences, USA. 2012;109:1154–1158. doi: 10.1073/pnas.1114199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Gregg JW. Uncertainty in source partitioning using stable isotopes. Oecologia. 2001;127:171–179. doi: 10.1007/s004420000578. [DOI] [PubMed] [Google Scholar]
- Rizzini CT. Tratado de fitogeografia do Brasil: aspectos ecológicos, sociológicos e florísticos. 2nd edn. São Paulo: Âmbito Cultural; 1997. [Google Scholar]
- Romero GQ, Mazzafera P, Vasconcellos-Neto J, Trivelin PCO. Bromeliad-living spiders improve host plant nutrition and growth. Ecology. 2006;87:803–808. doi: 10.1890/0012-9658(2006)87[803:bsihpn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Pate JS, Gillison AN. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- Solano PJ, Dejean A. Ant-fed plants: comparison between three geophytic myrmecophytes. Biological Journal of the Linnean Society. 2004;83:433–439. [Google Scholar]
- Spomer GG. Evidence of protocarnivorous capabilities in Geranium viscosissimum and Potentilla arguta and other sticky plants. International Journal of Plant Sciences. 1999;160:98–101. [Google Scholar]
- Treseder KK, Davidson DW, Ehleringer JR. Absorption of ant provided carbon dioxide and nitrogen by a tropical epiphyte. Nature. 1995;375:137–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


