Abstract
Background and Aims
Large floral displays have opposing consequences for animal-pollinated angiosperms: they attract more pollinators but also enable elevated among-flower self-pollination (geitonogamy). The presence of sterile flowers as pollinator signals may enhance attraction while allowing displays of fewer open fertile flowers, limiting geitonogamy. The simultaneous contributions of fertile and non-fertile display components to pollinator attraction and reproductive output remain undetermined.
Methods
The simultaneous effects of the presence of sterile flowers and fertile-flower display size in two populations of Leopoldia comosa were experimentally assessed. Pollinator behaviour, pollen removal and deposition, and fruit and seed production were compared between intact plants and plants with sterile flowers removed.
Key Results
The presence of sterile flowers almost tripled pollinator attraction, supplementing the positive effect of the number of fertile flowers on the number of bees approaching inflorescences. Although attracted bees visited more flowers on larger inflorescences, the number visited did not additionally depend on the presence of sterile flowers. The presence of sterile flowers improved all aspects of plant performance, the magnitude of plant benefit being context dependent. During weather favourable to pollinators, the presence of sterile flowers increased pollen deposition on stigmas of young flowers, but this difference was not evident in older flowers, probably because of autonomous self-pollination in poorly visited flowers. Total pollen receipt per stigma decreased with increasing fertile display size. In the population with more pollinators, the presence of sterile flowers increased fruit number but not seed set or mass, whereas in the other population sterile flowers enhanced seeds per fruit, but not fruit production. These contrasts are consistent with dissimilar cross-pollination and autonomous self-pollination, coupled with the strong predispersal inbreeding depression exhibited by L. comosa populations.
Conclusions
Sterile flowers enrich pollination quality by promoting pollen export and import, while limiting the mating costs of geitonogamy associated with large fertile displays.
Keywords: Anthophora, cross-pollination, geitonogamy, fertile floral display, mating cost, Leopoldia comosa, non-fertile flowers, outcrossing, pollen deposition, pollen quality, pollen removal, sterile flowers
INTRODUCTION
Multi-flowered plants experience conflicting selection on the number of flowers that they display simultaneously (Klinkhamer and de Jong, 1993; Harder and Barrett, 1996). Large displays are beneficial, because they promote pollinator attraction (Ohashi and Yahara, 2001). However, simultaneous exposure of many flowers can also increase the incidence of self-pollination among flowers (geitonogamy: Harder and Barrett, 1995; Karron et al., 2004), which can have negative effects owing to reduced pollen export (pollen discounting: Harder and Barrett, 1995; Karron and Mitchell, 2012), disabled ovules in species with ovarian self-incompatibility (Sage et al., 1999; Vaughton and Ramsey, 2010) and inbreeding depression in self-compatible species (Charlesworth and Charlesworth, 1987). Adaptive resolution of these opposing influences generally favours simultaneous display of only a subset of a plant's flowers (Klinkhamer and de Jong, 1993; Harder et al., 2001) as a consequence of selection on the underlying controls on display size, namely the number of flowers opened per day (anthesis rate) and their longevity (Harder and Johnson, 2005). This conflict should also commonly select for mitigating adaptations that allow plants to enhance attractiveness with limited mating costs. For example, for species with vertical inflorescences that are pollinated by upward-moving bees, the attractive benefit of a large display can be realized with limited geitonogamy and pollen discounting if female and male functions are segregated among lower and upper flowers, respectively, as a consequence of dichogamy or monoecy (Harder et al., 2000). Other traits that have been proposed to allow large, attractive displays with limited mating costs include heterostyly and inclusion of showy, non-sexual organs as display components (Klinkhamer and de Jong, 1993; Harder and Barrett, 1996). Examples of the latter adaptation include the maintenance of flowers that have ceased sexual function and contribute to pollinator signalling, but are seldom visited because they have altered colour in association with limited nectar, and the presence of showy bracts or sterile flowers at the inflorescence periphery.
Peripheral sterile flowers occur sporadically among monocots (e.g. Bellevalia, Lachenalia, Leopoldia, Muscari; Asparagaceae, Hyacintheae) and eudicots (e.g. Viburnum, Adoxaceae; Hydrangea, Hydrangeaceae; Dichrostachys, Mimosoideae), being most common in the Asteraceae (see Anderberg et al., 2007). To date, all studies of their pollination function have considered eudicot species with largely flat, circular inflorescences with the sterile flowers arrayed around the circumference. In these species, the presence of sterile flowers typically increases pollinator attraction, pollen removal and/or seed production (see Krannitz and Maun, 1991 for an exception), compared with that of plants from which peripheral flowers are naturally (Lack, 1982) or experimentally absent (Lack, 1982; Stuessy et al., 1986; Krannitz and Maun, 1991; Englund, 1994; Nielsen et al., 2002; Jin et al., 2010), supporting Darwin's (1877) hypothesis that sterile flowers promote attraction. To date, no study of the pollination function of sterile flowers has accounted for the influence of the number of fertile flowers on plant performance, so that the simultaneous contributions of the two flower types to attraction remains to be determined.
Unlike previously studied cases of sterile marginal flowers, the cases within the monocot tribe Hyacintheae all involve terminal sterile flowers on vertical racemes (e.g. Fig. 1A). This architectural difference could influence the function of sterile flowers. For species with peripheral sterile flowers, the extent of the inflorescence is fully established before anthesis, so the positions of the sterile flowers in the inflorescence circumference remain relatively fixed while the inflorescence interacts with pollinators. In contrast, the acropetal racemes of ‘hyacinth’ species with terminal sterile flowers elongate as new flowers open above old flowers. Consequently, as flowering progresses within an inflorescence, sterile flowers are presented higher and new fertile flowers (formed by upper buds) are closer to the sterile flowers than the initial fertile flowers (formed by lower buds). Furthermore, for bee-pollinated species, fertile flowers could contribute to attraction, as bees begin foraging low on vertical inflorescences (Harder et al., 2001; Keasar et al., 2006), where these flowers are located.
Fig. 1.
(A) Leopoldia comosa inflorescence showing, from bottom to top, open fertile flowers, buds of fertile flowers and sterile flowers. (B) A female Anthophora balearica bee visiting a fertile flower. Photos by C. L. Morales.
In this study, we assessed the effects of the presence of sterile and fertile flowers on pollinator attraction, pollination and female reproductive output by Leopoldia comosa in two natural populations. As in previous studies of the function of sterile flowers, we experimentally removed them from selected plants to replicate the typical phenotype in angiosperms. However, we also took advantage of the extensive within-population variation in the number of open fertile flowers per stem (fertile floral display size) to quantify their additional, perhaps interacting, effects on pollinator attraction and female success. Although the experiments in the two populations were designed and implemented independently (Mallorca, C.L.M. & A.T.; France, L.D.H.), we present our results jointly to illustrate the scope of variation in the influences of sterile flowers on pollination function.
MATERIALS AND METHODS
Leopoldia comosa Parl. (Asparagaceae; syn. Muscari comosum Mill.) is a spring-flowering geophyte native to Mediterranean regions, which propagates entirely via sexual reproduction (Garrido-Ramos et al., 1998). Reproductive plants produce a raceme of up to 100 lower, fertile urceolate flowers and up to 50 upper sterile flowers (Fig. 1A). Each fertile flower produces six ovules, with two ovules in each of three locules. As an inflorescence expands, all flower buds are initially dark purple, but as fertile flowers open they turn light brown with yellow pedicels, whereas fully formed sterile flowers do not open and they and their expanded pedicels turn bright lavender (Fig. 1A). Fertile flowers are pollinated primarily by long-tongued bees, especially Anthophora spp. (Fig. 1B), but they are also visited by bombyliid flies. Experienced bees do not inspect sterile flowers, but fly directly to the fertile flowers when approaching an inflorescence (L. D. Harder, pers. observ.). In contrast, bombyliid flies first explore sterile flowers before feeding on lower fertile flowers (also see Knoll, 1921; Keasar et al., 2006).
The pollination effects of the sterile flowers of L. comosa were studied during 2002 and 2007 in a population in southern France and another on Mallorca, Spain, respectively (see Table 1 for a summary of the respective experiments). In both populations, we estimated floral longevity, and its influence on fertile display size (Harder and Johnson, 2005), and assessed fruit and seed set by fertile flowers of plants with and without sterile flowers. In the French population we also assessed the effects of sterile flowers on pollen deposition and removal, whereas in the Mallorcan population we additionally measured the degree of pollinator dependence and the effects of sterile and fertile flowers on pollinator visitation and seed mass.
Table 1.
Response variables measured at each locality for the two experimental treatments
| Response variable | Locality |
|---|---|
| (1) Removal of sterile flowers | |
| Fertile flower longevity | France, Spain |
| Pollinator visitation | Spain |
| Pollen removal | France |
| Pollen deposition | France |
| Fruit set | France, Spain |
| Seed set | France, Spain |
| Seed mass | Spain |
| (2) Hand-pollinations | |
| Fruit set | Spain |
| Seed set | Spain |
Field methods
Southern France
Flowering phenology and the pollination effects of sterile flowers of L. comosa were studied on a grassy terrace above Madières, France (43°51′17″N, 3°33′55″E, elevation 250 m), during 2002. Twenty-three pairs of plants were selected on 24 April, when some lower fertile flowers were open, but most fertile and all sterile flowers remained unexpanded. Members of each pair were selected for similar inflorescence size and were separated by <1 m, usually <0·5 m. The uppermost open fertile flower on each plant was removed to allow identification of flowers that opened subsequently and were exposed during the experimental period. One plant in each pair was selected randomly and its sterile flowers and associated inflorescence rachis were removed (clipped plants); the remaining pair member was left intact (intact plants).
On 29 April and 5 May we counted fertile flowers that had opened above the removed flowers on all inflorescences to quantify display size and underlying aspects of floral phenology. All new fertile flowers provided information about anthesis rate since 24 April and the open fertile flowers and all sterile flowers represented display size. If anthesis rate (r; flowers per day) and longevity (L; days) of fertile flowers were constant between samples, the fertile display size would be D = rL (Harder and Johnson, 2005), so the average longevity of fertile flowers during the two sample periods (24–29 April, 29 April – 5 May) can be estimated for each sample inflorescence as L = D/r. The second sampling period included two rainy, cold days and a cool windy day, which probably reduced pollinator activity.
On 29 April and 5 May we also collected fertile flowers to estimate the effects of sterile flowers on pollen removal and deposition. On each day we collected the second and sixth uppermost fertile flowers from each inflorescence (i.e. the second youngest and the second oldest open flowers, on average) and preserved them individually in 70 % ethanol in microcentrifuge tubes. Later, we counted the pollen remaining in the second uppermost fertile flowers with an Elzone 5380 particle analyser (Micromeritics Instrument Corp., Norcross, GA, USA) as described by Harder (1990). These relatively young fertile flowers were used to index pollen removal, because they should have been exposed to pollinators for similar periods, but should not have been depleted of pollen, so that the average number of remaining pollen grains should also indicate the average rate of pollen removal. We also removed the stigmas from the second and sixth uppermost fertile flowers and counted the deposited pollen grains at 100× magnification after staining with basic fucsin.
On 16 May we assessed the fruit set by all fertile flowers on all infructescences and collected up to three fruits per infructescence to quantify seed set, which were preserved individually in 70 % ethanol in microcentrifuge tubes. One fruit was collected to represent each of three periods: the fertile flowers exposed before the experiment, and those exposed during the two sample periods (each demarcated by fertile flowers removed on 24, 29 April and 5 May). The position of each collected fruit counted upward from the lowermost fertile flower was recorded, as it might affect the access of fruits to maternal resources and thereby influence fruit and seed set. Expanded or expanding ovaries were recorded as successful fruits.
Mallorca
The capacity for self-pollination and self-fertilization, and pollinator and reproductive responses to removal of sterile flowers were studied in a grassland population at sea level in Parc Natural de s'Albufera, Mallorca, Spain (39°46′31″N, 3°07′45″E) during spring 2007. This study began on 21 April, when some lower fertile flowers had opened, but most fertile and all sterile flowers remained unexpanded.
We evaluated self-compatibility and estimated the capacity for autonomous self-pollination with 17 plants, which we bagged on 21 April to exclude pollinator visits, after we removed all open and senescent fertile flowers. Between 27 April and 4 May, we applied three treatments to alternate open fertile flowers on each plant: hand cross-pollination, hand self-pollination and no hand pollination (to assess autonomous self-pollination). Cross-pollinated flowers received pollen from an average of five donor plants located >20 m from the focal plant, whereas hand self-pollinated flowers received their own pollen and pollen from other fertile flowers of the same individual. After pollination, all plants were rebagged until 15 May, when fruit set was assessed. On 16 June we collected all ripe fruits for seed counting.
We assessed the effects of the presence of sterile flowers on pollinator visits and female reproductive success with 120 additional plants selected on 21 April. Senescent fertile flowers were removed and the rachis on each plant was marked with a permanent marker immediately above the uppermost open fertile flower to distinguish flowers that opened subsequently and were exposed during the experimental period. From half of the plants, selected randomly, we removed the sterile flowers and associated inflorescence rachis, leaving the remaining plants intact. After all fertile flowers withered, we removed the sterile flowers from intact plants to avoid possible post-pollination effects through resource allocation from sterile to fertile flowers during fruit development.
From 24 to 27 April we observed pollinator visits to clipped and intact plants in random order during 78 5-min periods. Due to extensive herbivory by snails (Theba pisana) on fertile flowers, sterile flowers and the inflorescence rachis, some individual plants were lost during the dates of pollinator observations and were replaced by new individuals when possible. In total, observation periods involved 111 individual plants. When two or more plants were located <2 m from each other (hereafter, a ‘flowering patch’), they were observed simultaneously (mean = 4·6 plants observed per period, range = 1–13). During each observation period we recorded the time of day, treatment of the focal plant, the numbers of open (fertile display size) and senescent flowers, the number of pollinators that visited fertile flowers and the number of flowers visited per pollinator. When feasible during simultaneous observations of two or more plants, we recorded the inflorescence treatment of each plant visited by individual pollinators and the sequence of plants visited.
On 8 and 9 May, after flowering, we counted all fertile flowers that had opened after the onset of the experiment. Herbivores had eaten developing fruits on 38 plants for which we had observed pollinators, so to avoid further losses we removed all affected fruits and bagged all inflorescences. On 6 and 13 June, we assessed infructescences for final fruit set and collected ripe fruits. We counted the seeds in a random subset of fruits (up to eight fruits per plant) and weighed a sample of counted seeds (up to five seeds per plant to the nearest 0·1 mg), recording whether the fertile flowers that produced the seeds were open before or after sterile flowers were clipped (as indicated by their position relative to the ink mark on the rachis).
We used another set of 13 intact and 12 clipped inflorescences, which were bagged on 27 April, to test whether the removal of sterile flowers caused unintended effects on either pollinator attraction by affecting fertile flower size, or reproductive output, via resource reallocation. Between 1 and 5 May we measured the length and diameter of two fertile flowers per individual and hand-outcrossed 2–8 flowers before rebagging the inflorescences. On 16 June we collected the ripe fruits and their seeds. Removal of sterile flowers did not significantly affect flower size (repeated-measures ANOVA: length, F1,26·1 = 0·01, P > 0·9; diameter, F1,26·4 = 2·17, P > 0·15), fruit set (generalized linear model, G1 = 0·42, P > 0·5) or mean seed number per fruit (ANOVA, F1,11 = 0·02, P > 0·85) of hand-outcrossed flowers.
Statistical methods
Analyses of pollinator behaviour and fruit and seed production involved either generalized linear models (McCullagh and Nelder, 1989: SAS proc Genmod or proc Glimmix; SAS Institute Inc., 2009) or general linear models (Kutner et al., 2005: SAS proc Mixed; SAS Institute Inc., 2009) that accounted for repeated measurement of individual plants (Fitzmaurice et al., 2004) when necessary. All analyses of open-pollinated plants compared intact and clipped plants, with treatment as a categorical factor. Response variables included pollinator visits per 5 min, fertile flowers visited per inflorescence by individual pollinators, pollen remaining in anthers, pollen receipt and fruit production by open-pollinated plants. The last analysis also considered ln(fertile flower number) as a covariate, in which case the initial analysis also included the interaction between treatment and ln(fertile flower number), which was subsequently excluded if it did not explain significant variation (α = 0·05). The paired design used in the French population was accounted for by recognizing pair as a random factor. Generalized linear models that considered binomial distributions (fruit/flower and seed/ovule following hand-pollination, and open pollination in France) used logit-link functions, whereas those that considered Poisson (pollinator visits per 5 min) or negative-binomial distributions (fertile flowers visited per inflorescence, pollen receipt, fruit and seed number following open-pollination) used ln-link functions. Least-squares means presented for these analyses are back-transformed and so are associated with asymmetric standard errors (termed l.s.e. and u.s.e. for the upper and lower standard error, respectively). For analyses with ln-link functions, a partial regression coefficient (b) equal to 1 for an ln-transformed covariate indicates a proportional relation. Statistical tests for generalized linear models without repeated measures (fruit production by open-pollinated plants) involved likelihood-ratio (G) tests, whereas generalized estimating equations and score statistics (T) were employed with repeated measures to accommodate compound-symmetric variance–covariance matrices (Liang and Zeger, 1986). Analysis of anthesis rate (ln-transformed), floral longevity, inflorescence display size, pollen remaining in anthers (ln-transformed) and seed mass involved general linear, repeated-measures models that used a compound-symmetric variance–covariance matrix and Kenward and Roger's (1997) method to adjust the denominator degrees of freedom of F-tests to account for the measured lack of independence caused by repeated measurement.
Our study considers directional predictions that removal of sterile flowers will diminish the attractiveness of plants to pollinators, thereby reducing fertile flower visitation, the rate of pollen removal and deposition, and fruit and seed production. We represented these expectations statistically by considering corresponding one-tailed null hypotheses for tests involving the experimental treatment. The associated probability of obtaining a test statistic owing to sampling error alone will be denoted P(1).
RESULTS
Display characteristics
Intact and clipped inflorescences in the French population had similar characteristics, other than the imposed absence of sterile flowers for manipulated inflorescences. Intact plants produced an average (± s.e.) of 36·0 ± 4·15 fertile flowers and 33·5 ± 2·15 sterile flowers (n = 23) and produced equivalent numbers of fertile flowers to their paired clipped plants (34·7 ± 2·80; paired t-test, t20 = 0·65, P > 0·5). For intact plants, the number of sterile flowers correlated weakly with the number of fertile flowers (r18 = 0·445, P < 0·05). On average, L. comosa inflorescences opened about two fertile flowers per day, with faster anthesis during the first sampling period (24–29 April; 2·5 ± 0·09 fertile flowers per day) than during the cooler second period (29 April – 5 May; 1·7 ± 0·6 fertile flowers per day: F1,77 = 51·34, P < 0·001, based on ln-transformed data). Therefore, the second- and sixth-uppermost fertile flowers used to measure pollen removal and receipt were roughly 1 and 3 d old, respectively. Individual fertile flowers lasted about 3·5 d, with briefer longevity during the first sampling period (3·3 ± 0·13 d) than during the second (4·1 ± 0·13 d: F1,61·5 = 23·97, P < 0·001), regardless of inflorescence fertile flower production. Together, these characteristics generated daily displays of about 7·4 fertile flowers, with larger displays on 29 April (mean = 7·8 fertile flowers, l.s.e. = 0·37, u.s.e. = 0·39) than on 5 May (6·9 fertile flowers, l.s.e. = 0·32, u.s.e. = 0·34; F1,63·3 = 4·02, P < 0·05, based on ln-link function). Fertile display size varied positively with total fertile flower production (0·776 ± 0·090; t28·4 = 74·68, P < 0·001). None of the preceding aspects of flowering phenology differed significantly between intact and clipped inflorescences (P > 0·05 in all cases).
Plants in the Spanish population displayed more fertile flowers (10·4 flowers, l.s.e. = 0·42, u.s.e. = 0·44) than those in the French population. This difference resulted even though fertile flowers also lasted 3·3 d (s.e. = 0·09 d, 31 plants) in the Mallorca population. During pollinator observations, fertile display size did not differ significantly between intact and clipped plants (F1,109·8 = 0·5, P > 0·4). However, fewer of the fertile flowers exposed to pollinators during the entire experiment remained to be assessed for fruit set on clipped plants (mean = 9·0 fertile flowers, l.s.e. = 1·05, u.s.e. = 1·19) than on intact plants (mean = 13·1 fertile flowers, l.s.e. = 1·47, u.s.e. = 1·65; F1,65 = 4·80, P < 0·05), perhaps because of differential florivory by snails.
Pollinator visitation
Anthophora bees accounted for 88·4 % of approaches to plants and 97·5 % of visits to flowers recorded during pollinator observations in the Mallorcan population. During the 28 trials when bees could choose between intact and clipped inflorescences, they significantly preferred intact inflorescences. These trials involved almost equal numbers of intact (n = 103) and clipped (n = 105) inflorescences, and yet the observed bees visited 43·6 % of the intact inflorescences (l.s.e. = 6·7 %, u.s.e. = 7·0 %), but only 18·6 % of the clipped inflorescences (l.s.e. = 4·3 %, u.s.e. = 5·3 %; T1 = 12·81, P(1) < 0·001). This difference arose primarily because 89·3 % (l.s.e. = 7·4 %, u.s.e. = 4·6 %) of the first inflorescences that bees visited in a patch were intact (comparison to no preference, T1 = 12·04, P(1) < 0·001). In contrast, during subsequent inflorescence visits bees exhibited only a weakly significant preference for intact inflorescences (mean = 57·6 %, l.s.e. = 4·5 %, u.s.e. = 4·4 %; comparison to no preference, T1 = 2·81, P(1) < 0·05; comparison of first and subsequent visits, T1 = 8·73, P < 0·005). These results were unaffected by the total number of plants observed simultaneously or the combination of the two treatments within patches (P > 0·1 in all cases).
Observations of individual plants revealed several effects of floral display on visitation by Anthophora bees. Fertile display size and the presence of sterile flowers influenced the attractiveness of plants independently (interaction, T1 = 0·80, P > 0·3). Plants with many open fertile flowers attracted more Anthophora than those with smaller displays (Fig. 2A; T1 = 4·80, P(1) < 0·025). Pollinator attraction increased proportionally with fertile display size (b ± s.e. = 0·922 ± 0·410; comparison with β = 1, t130 = 0·18, P > 0·85). In addition, the presence of sterile flowers enhanced bee attraction by 267 %, as intact plants attracted an average of 2·9 bees h−1 (l.s.e. = 0·37, u.s.e. = 0·42), compared with only 1·1 bees h−1 (l.s.e. = 0·28, u.s.e. = 0·37) for clipped plants (Fig. 2A; T1 = 12·12, P(1) < 0·001). In contrast, although attracted bees visited more fertile flowers on inflorescences with many fertile flowers (Fig. 2B: T1 = 8·39, P < 0·005), the number visited did not additionally depend on the presence or absence of sterile flowers (T1 = 0·02, P(1) > 0·85: overall mean = 5·6 fertile flowers, l.s.e. = 1·87 flowers, u.s.e. = 2·79 flowers). The proportion of fertile flowers visited per bee tended to decrease with increasing fertile display size (Fig. 2B), although the partial regression coefficient for ln(fertile flower number) was not quite significantly less than 1 (0·700 ± 0·164: t25 = 1·83, P = 0·079). Comparison of a subset of neighbouring pairs of intact and clipped plants led to qualitatively similar conclusions.
Fig. 2.
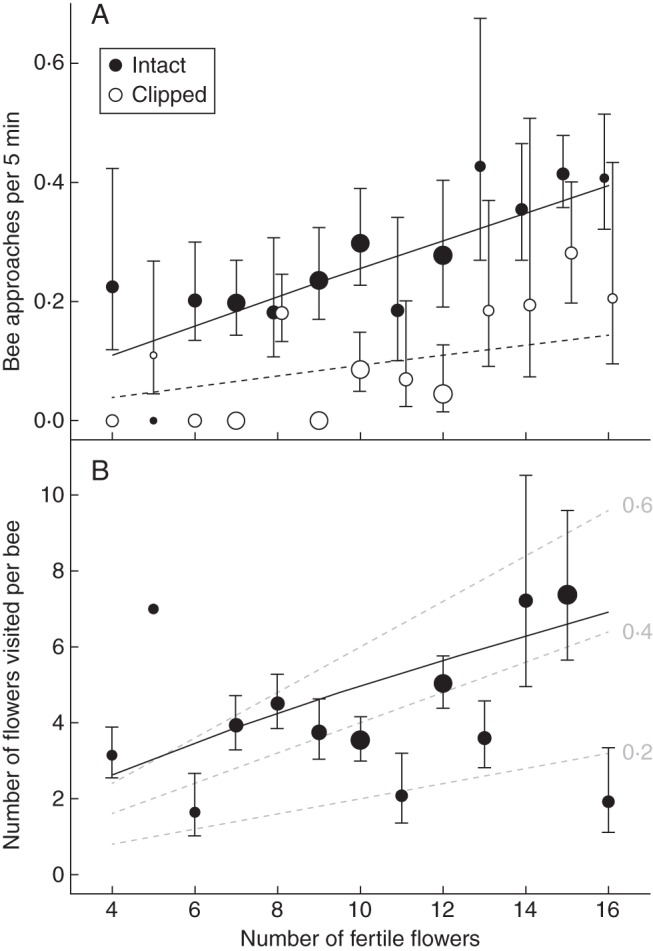
Relations between the (A) mean (±s.e.) attractiveness of Leopoldia comosa inflorescences and (B) mean (±s.e.) number of fertile flowers visited by attracted bees to fertile display size in the Mallorcan population. In (A), the results are presented separately for intact inflorescences and those from which the sterile flowers had been removed, and symbol size indicates the number of 5-min observation periods represented by an observation, ranging from 2 (smallest) to 22 (largest). In (B), the solid line illustrates the regression relation, the grey lines depict the indicated proportions of flowers visited, and symbol size indicates the number of bees contributing to a mean, ranging from 1 (smallest) to 8 (largest). Standard errors are asymmetric as data are ln-transformed.
Pollination, fruit and seed production
Based on the hand-pollination experiments in the Mallorcan population, L. comosa is self-compatible and self-pollinates autonomously, but self-pollination results in limited seed production. Overall, 19·3 % (l.s.e. = 6·22, u.s.e. = 8·23) of hand-selfed flowers and those that we did not hand-pollinate on bagged inflorescences set fruit, with no difference between these treatments (T1 = 0·24, P > 0·6), indicating complete capacity for autonomous selfing. In contrast, 78·5 % (l.s.e. = 10·75, u.s.e. = 7·89) of hand-crossed flowers set fruit, significantly exceeding fruit set by self-pollinated flowers (T1 = 6·76, P < 0·01). Likewise, for flowers that set fruit, an average of 17·2 % (l.s.e. = 3·42, u.s.e. = 4·07) of ovules set seeds following self-pollination compared with 31·7 % (l.s.e. = 5·20, u.s.e. = 5·70) following cross-pollination (T1 = 4·79, P < 0·05). Thus, the total percentage of ovules setting seeds per flower was seven times higher in hand-crossed flowers (24·8 %) than in hand-selfed flowers (3·3 %). Seeds from six hand-crossed fruits were heavier (mean ± s.e. = 7·5 ± 0·55 mg) than those from nine autonomously selfed fruits (6·3 ± 0·52 mg), but seeds from three hand-selfed fruits did not differ in mass from either extreme (6·6 ± 0·77 mg: F2,57·9 = 4·08, P < 0·025).
The display of sterile and fertile flowers had heterogeneous effects on pollination in the French population. Many of the second-uppermost fertile flowers had no pollen on their stigmas, which we interpret as evidence that they had not been visited, but the incidence of such unvisited flowers differed between sampling dates (Fig. 3A). Specifically 78·4 % of stigmas collected on 29 April had received pollen, compared with only 49·8 % on 5 May (T1 = 6·20, P < 0·025). In addition, the presence of sterile flowers significantly enhanced the incidence of pollination of second-uppermost fertile flowers on 29 April (T1 = 3·01, P(1) < 0·05), but not on 5 May (T1 = 0·67, P(1) > 0·5: Fig. 3A). These effects were unaffected by the number of fertile flower buds above the open flowers (T1 = 0·47, P > 0·4). The amount of pollen remaining in anthers of these same flowers differed correspondingly between sampling dates (F1,40·7 = 14·92, P < 0·001: 29 April, mean = 4713 grains, l.s.e. = 401·4, u.s.e. = 438·8; 5 May, mean = 6981 grains, l.s.e. = 575·3, u.s.e. = 627·0) and between flowers with or without pollen on their stigmas (F1,67·1 = 8·77, P < 0·005: pollinated, mean = 4713, l.s.e. = 401·4, u.s.e. = 438·8; unpollinated, mean = 6981, l.s.e. = 575·3, u.s.e. = 627·0), but was not additionally affected by the presence of sterile flowers (F1,21·6 = 0·43, P(1) > 0·25) or fertile display size (F1,61·9 = 0·07, P > 0·7). The sixth-uppermost fertile flowers on inflorescences had all received some pollen. Pollen receipt by these flowers did not depend on either the presence of sterile flowers (F1,61·3 = 0·07, P(1) > 0·75) or sampling date (F1,60·1 = 0·07, P > 0·75); however, it declined significantly with fertile display size (F1,74·3 = 12·98, P < 0·001: b ± s.e. for ln[fertile flower number] = –1·297 ± 0·360; Fig. 3B).
Fig. 3.
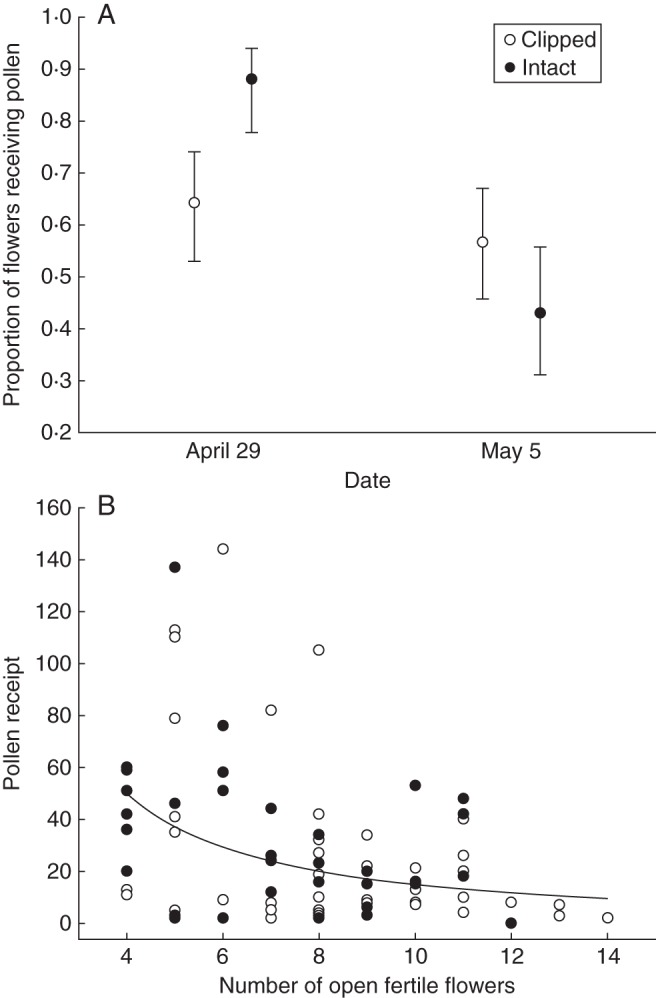
Influences on pollen receipt in the French Leopoldia comosa population, including (A) the mean (±s.e.) proportion of second-uppermost fertile flowers that had received pollen on the two sampling dates, and (B) the relation between the number of pollen grains received by sixth-uppermost flowers to the number of fertile flowers displayed on intact and clipped inflorescences.
Fruit and seed production in the French population responded differently to the removal of sterile flowers. Fruit number increased disproportionately with the number of fertile flowers exposed during the experimental period (F1,34 = 85·17, P < 0·001: b ± s.e. for ln[fertile display] = 1·446 ± 0·157: test of β = 1, t34 = 2·84, P < 0·01), but was not further affected by the number of fertile flower buds (F1,33 = 3·55, P > 0·05). Fruit number did not differ between clipped and intact plants (F1,36 = 1·17, P(1) > 0·25). Overall, 52·5 % of fertile flowers exposed during the experimental period set fruit. However, flowers exposed between 24 and 29 April set more fruits (mean = 5·6 fruits, l.s.e. = 0·31, u.s.e. = 0·33) than those exposed between 29 April and 5 May (mean = 3·6 fruits, l.s.e. = 0·39, u.s.e. = 0·43; F1,34 = 17·17, P < 0·001), after adjustment for variation in fertile flower number. Seed production by experimental fertile flowers varied negatively with the number of fruits produced by flowers exposed prior to the experimental period (b ± s.e. for ln[fruit number], –0·309 ± 0·101: F1,29 = 9·45, P < 0·005) and positively with a fruit's position within the inflorescence counted upward from the bottom fertile flower (b ± s.e. for ln[position], 0·314 ± 0·121: F1,15 = 6·72, P < 0·05), but not with fruit position counted from the top fertile flower (F1,15 = 2·11, P > 0·1). In isolation from these effects, fruits on intact inflorescences produced an average of one more seed (mean = 4·8 seeds, l.s.e. = 0·33, u.s.e. = 0·36: 79·5 % seed set) than those on clipped inflorescences (3·8 seeds, l.s.e. = 0·33, u.s.e. = 0·38: 64·0 % seed set), which represents a 26 % benefit (F1,29 = 3·12, P(1) < 0·05).
For open-pollinated plants in the Mallorcan population, removal of sterile flowers affected fruit number, but not seed number per fruit (T1 = 0·11, P(1) > 0·7) or seed mass (F1,71·3 = 0·49, P(1) > 0·4). Intact plants realized 68·6 % fruit set, and 66·1 % seed set. Fruit production increased somewhat differently with the number of fertile flowers sampled for intact (b ± s.e. = 1·008 ± 0·111) and clipped plants (1·431 ± 0·143; treatment × ln[fertile flower number], G1 = 5·62, P(1) < 0·01), primarily because among small plants clipped individuals set fewer fruits than intact plants (Fig. 4). Fertile display size correlated positively with the number of fertile flowers sampled for fruit production (r64 = 0·584, P < 0·001) and did not additionally affect fruit number (G1 = 0·24, P < 0·6), seeds per fruit (T1 = 0·82, P > 0·3) or seed mass (F1,62·8 = 1·71, P > 0·1). On average, seeds from distal fruits weighed 11·6 % less (7·6 ± 0·18 mg) than basal fruits within infructescences (8·6 ± 0·19 mg: F1,530 = 99·19, P < 0·001).
Fig. 4.
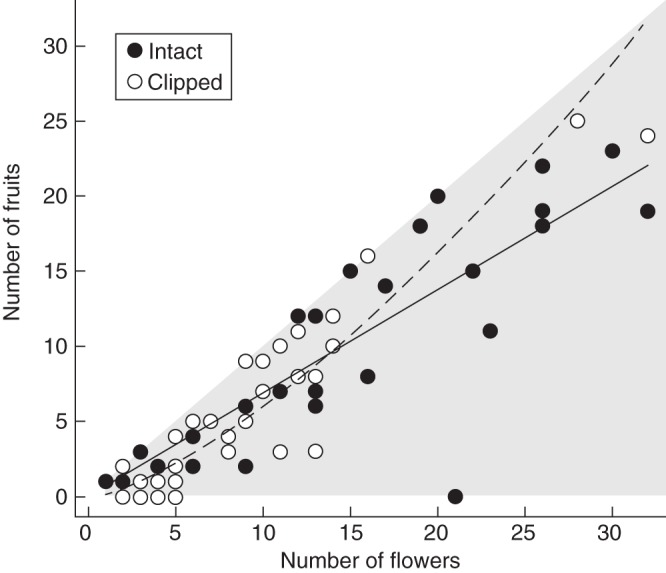
Relations between fruit production and the number of sampled fertile flowers for intact (solid symbols and solid line) plants and for plants from which the sterile flowers had been removed (open symbols, dashed line) in the Mallorcan population. The grey area depicts possible outcomes.
DISCUSSION
As Darwin (1877) proposed, the presence of sterile flowers in Leopoldia comosa inflorescences greatly enhances their attractiveness to pollinators, almost tripling the bee approaches to inflorescences compared with plants with only fertile flowers (Fig. 2A). Interestingly, sterile flowers seem primarily to enhance long-distance attraction, increasing attraction of bees most strongly as they arrived at patches, rather than during subsequent visits within patches. This change in behaviour also indicates that bees were not simply exhibiting an overall preference for the most common phenotype in the population. Such dependence of the contribution of sterile flowers to attractiveness on a pollinator's proximity to inflorescences seems to be a general feature of the attractive benefits of sterile flowers, as it also explains contradictory results from previous studies of eudicot species. In particular, pollinator preference or reproductive output declined in response to removal of all of a plant's sterile flowers (Stuessy et al., 1986; Englund, 1994; Nielsen et al., 2002; Jin et al., 2010), but not when only a fraction of them were removed (Krannitz and Maun, 1991). A similar contrast is evident in studies of the absence of female ray florets from gynomonoecious Asteraceae (Abbott and Irwin, 1988; Andersson, 1991, 1996, 2008). Together, these results suggest that the attractive benefit of sterile flowers is greatest in low-density situations. This context dependence also has a temporal component, as revealed by the heterogeneous effect of sterile flowers on the proportion of pollinated day-old fertile flowers between sampling periods with contrasting weather conditions, and probably contrasting pollinator activity in the French population (Fig. 3A).
In contrast to their enhancement of pollinator attraction, sterile flowers did not affect the number of fertile flowers visited per attracted bee (see Herrera, 1997, for a similar conclusion concerning showy bracts). As a consequence, inflorescences should have experienced equivalent geitonogamy and associated negative implications, regardless of the presence of sterile flowers. This result is completely consistent with an elaboration of Darwin's hypothesis (Harder and Barrett, 1996), namely that sterile flowers enhance attraction without imposing mating costs that would accrue if, instead, display size was enhanced by the addition of fertile flowers (see Harder and Barrett, 1995; Karron and Mitchell, 2012). That individual bees visited more fertile flowers on inflorescences with many fertile flowers (Fig. 2B) illustrates that large fertile displays probably experience more geitonogamy and associated mating costs than small displays.
The pollination results observed in the French population are partially consistent with the behaviour displayed by pollinators in Mallorca. Pollen removal from first-day fertile flowers was not directly altered by removal of sterile flowers, but instead depended on whether a fertile flower had been visited (i.e. received pollen), which in turn was enhanced by the presence of sterile flowers when the weather was favourable for pollinator foraging. This result indicates that the fertile flowers used to measure pollen removal were collected too young, so that removal did not depend on visitation frequency, as affected by the display of sterile and fertile flowers. Note also that only half of fertile flowers in the French population were pollinated during their first day, which is probably much lower than in the Mallorcan population, where intact plants attracted on average three bees per hour. Unlike the incidence of pollination of young fertile flowers, the stigmatic pollen loads of flowers exposed to pollinators for most of their functional lives were unaffected by the presence of sterile flowers and whether they were exposed during favourable or inclement weather (Fig. 3A). This disparity is consistent with autonomous self-pollination having contributed an appreciable proportion of the pollen on stigmas of old fertile flowers, obscuring effects of floral display on cross-pollination. This contribution was probably largest for poorly visited fertile flowers, as they would have more pollen remaining in their anthers, providing a larger source of self-pollen. According to this interpretation, display characteristics primarily affected pollination quality, rather than quantity, in this population. The decline in total pollen receipt with the number of fertile flowers in an inflorescence (Fig. 3B) probably reflects the similar, and commonly observed, declining relation for the proportion of open flowers visited by individual bees (Fig. 2B) (reviewed by Ohashi and Yahara, 2001). This result illustrates a cost of large fertile displays, in addition to elevated geitonogamy, which would be most important in populations in which pollen receipt limits seed production.
The post-pollination consequences of geitonogamy on female success depend on whether a species is self-compatible and, if so, the severity of inbreeding depression (see Lloyd, 1992; Harder and Barrett, 1996). Leopoldia comosa is capable of self-fertilization, although only Ss = 3·3 % of all ovules set seed following saturated self-pollination, compared with Sx = 24·8 % for hand cross-pollination. That this contrast arose from inbreeding depression during seed development, rather than partial self-incompatibility, is evident from the different effects of self- and cross-pollination for the 11 plants subject to hand pollination. Although ten of these plants set fruit following cross-pollination (only one flower was pollinated on the remaining plant), only five plants set fruit following self-pollination, with fruit set by the latter plants ranging from 14 to 100 % (mean = 49 %, CV = 62·0 %). This heterogeneous response to self-pollination seems more consistent with genomic differences in the presence and severity of deleterious alleles, which are responsible for inbreeding depression, than extensive variation among plants in self-incompatibility (see Seavey and Bawa, 1986). According to this interpretation, the Mallorcan population of L. comosa is subject to strong inbreeding depression during seed production, namely (Sx – Ss)/Sx = 0·87 (assumes that seed production is ovule limited: Harder et al., 2012). We assume that such inbreeding depression is a general characteristic of this species, and that it strongly influences selection for cross-pollination mechanisms, such as the production of sterile flowers.
Differences in fruit and seed set between the populations suggest greater limitation of female reproductive output by both insufficient and inadequate pollination in the French population. Fertile flowers on intact plants in the Mallorcan population were 30 % more likely to produce a fruit than those in the French population, where fruit production also declined 35 % for flowers exposed during inclement weather. Both results are consistent with more limited pollinator service in the French population, which would have diminished both female and male performance. In the Mallorcan population, removal of sterile flowers reduced fruit production only for small plants (Fig. 4), suggesting that large clipped plants were sufficiently attractive by virtue of their displays of fertile flowers alone to import sufficient cross-pollen to maximize fruit set [see Andersson (1996) for similar responses by bisexual disc florets to the removal of female ray florets from gynomonoecious Senecio jacobaea]. In such populations with abundant pollinators, the attractive benefit of sterile flowers may be realized primarily through plants' siring ability. The capacity of L. comosa for autonomous self-pollination (also see Garrido-Ramos et al., 1998) may provide some reproductive assurance against limited cross-pollination by maintaining ovule fertilization. However, most viable seeds of this species are cross-fertilized (see Ruiz Rejón et al., 1982, 1988), probably because severe inbreeding depression during seed development constrains the contribution of autonomous autogamy to seed production. Accordingly, the greater seed production by flowers on intact inflorescences in the French population indicates higher-quality pollination of these inflorescences than of clipped inflorescences, even though the numbers of pollen grains on stigmas did not differ significantly. Such quality effects probably did not arise in the Mallorcan population, because proportionately more pollen was involved in cross-pollination.
The preceding interpretation of sterile flowers as a mechanism to promote cross-fertilization provides a consistent explanation for our heterogeneous results between populations and among response variables. According to this interpretation, by enhancing pollinator attraction without the need to expose many fertile flowers simultaneously, inclusion of sterile flowers in floral displays improves pollen export and import, while enriching pollination quality by limiting both geitonogamy and autonomous selfing. As the proximity of open fertile flowers to the sterile flowers did not affect the incidence of pollen receipt, fruit set or seed number per fruit in the French population, this interpretation probably applies generally, regardless of whether sterile flowers are produced marginally in flat inflorescences, as in previous studies, or terminally on vertical inflorescences, as in L. comosa. Like all cross-promotion mechanisms, effective functioning of sterile flowers requires that pollinators are relatively abundant in the environment (see Harder and Thomson, 1989; Harder and Barrett, 1996). Under such conditions, severe inbreeding depression accentuates the adaptive benefits of outcrossing mechanisms as both maternal and paternal parent (Harder and Aizen, 2010).
ACKNOWLEDGEMENTS
We thank Dale Hensley for field assistance, David Gibbs and David Baldock for bee identification, the staff of the s'Albufera Natural Reserve for logistical support and permits, and Randall Mitchell and an anonymous reviewer for comments on the manuscript. This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina and Programa Iberoamericano de Ciencia y Tecnología para el desarrollo [XII-6 to C.L.M.] and the Natural Sciences and Engineering Research Council of Canada [L.D.H].
LITERATURE CITED
- Abbott RJ, Irwin JA. Pollinator movements and the polymorphism for outcrossing rate at the ray floret locus in groundsel, Senecio vulgaris L. Heredity. 1988;60:295–298. [Google Scholar]
- Anderberg AA, Baldwin BG, Bayer RG, et al. Compositae. In: Kadereit JW, Jeffrey C, editors. The families and genera of vascular plants, Volume 8, Asterales. Berlin: Springer; 2007. pp. 61–588. [Google Scholar]
- Andersson S. Floral display and pollination success in Achillea ptarmica (Asteraceae) Holarctic Ecology. 1991;14:186–191. [Google Scholar]
- Andersson S. Floral display and pollination success in Senecio jacobaea (Asteraceae): interactive effects of head and corymb size. American Journal of Botany. 1996;83:71–75. [Google Scholar]
- Andersson S. Pollinator and non pollinator selection on ray morphology in Leucanthemum vulgare (oxeye daisy, Asteraceae) American Journal of Botany. 2008;95:1072–1078. doi: 10.3732/ajb.0800087. [DOI] [PubMed] [Google Scholar]
- Charlesworth D, Charlesworth B. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics. 1987;18:237–268. [Google Scholar]
- Darwin CR. The different forms of flowers on plants of the same species. London: J. Murray; 1877. [Google Scholar]
- Englund R. Male and female reproductive success in the hermaphroditic shrub Viburnum opulus (Caprifoliaceae). Sweden: Uppsala University; 1994. PhD thesis. [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, NJ: Wiley-Interscience; 2004. [Google Scholar]
- Garrido-Ramos MA, Jamilena M, de la Herrán R, Ruiz Rejón C, Camacho JPM, Ruiz Rejón M. Inheritance and fitness effects of a pericentric inversion and a supernumerary chromosome segment in Muscari comosum (Liliaceae) Heredity. 1998;80:724–731. [Google Scholar]
- Harder LD. Pollen removal by bumble bees and its implications for pollen dispersal. Ecology. 1990;71:1110–1125. [Google Scholar]
- Harder LD, Aizen MA. Floral adaptation and diversification under pollen limitation. Philosophical Transactions of the Royal Society, B. 2010;365:529–543. doi: 10.1098/rstb.2009.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. Mating cost of large floral displays in hermaphrodite plants. Nature. 1995;373:512–515. [Google Scholar]
- Harder LD, Barrett SCH. Pollen dispersal and mating patterns in animal-pollinated plants. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York: Chapman and Hall; 1996. pp. 140–190. [Google Scholar]
- Harder LD, Johnson SD. Adaptive plasticity of floral display size in animal-pollinated plants. Proceedings of the Royal Society of London, Series B. 2005;272:2651–2657. doi: 10.1098/rspb.2005.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Thomson JD. Evolutionary options for maximizing pollen dispersal of animal-pollinated plants. American Naturalist. 1989;133:323–344. [Google Scholar]
- Harder LD, Barrett SCH, Cole WW. The mating consequences of sexual segregation within inflorescences of flowering plants. Proceedings of the Royal Society of London B. 2000;267:315–320. doi: 10.1098/rspb.2000.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Williams NM, Jordan CY, Nelson WA. The effects of floral design and display on pollinator economics and pollen dispersal. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge: Cambridge University Press; 2001. pp. 297–317. [Google Scholar]
- Harder LD, Hobbhahn N, Richards SA. How depressed? Estimates of inbreeding effects during seed development depend on reproductive conditions. Evolution. 2012;66:1375–1386. doi: 10.1111/j.1558-5646.2011.01515.x. [DOI] [PubMed] [Google Scholar]
- Herrera J. The role of colored accessory bracts in the reproductive biology of Lavandula stoechas. Ecology. 1997;78:494–504. [Google Scholar]
- Jin B, Wang L, Wang J, et al. The structure and roles of sterile flowers in Viburnum macrocephalum f. keteleeri (Adoxaceae) Plant Biology. 2010;12:853–862. doi: 10.1111/j.1438-8677.2009.00298.x. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany. 2012;109:563–570. doi: 10.1093/aob/mcr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ, Holmquist KG, Bell JM, Funk B. The influence of floral display size on selfing rates in Mimulus ringens. Heredity. 2004;92:242–248. doi: 10.1038/sj.hdy.6800402. [DOI] [PubMed] [Google Scholar]
- Keasar T, Pollak G, Arnon R, Cohen D, Shmida A. Honesty of signaling and pollinator attraction: the case of flag-like bracts. Israel Journal of Plant Sciences. 2006;54:119–128. [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Klinkhamer PGL, de Jong TJ. Attractiveness to pollinators: a plant's dilemma. Oikos. 1993;66:180–184. [Google Scholar]
- Knoll F. Insekten und Blumen: experimentelle Arbeiten zur Vertiefung unserer Kenntnisse u“ber die Wechselbeziehungen zwischen Pflanzen und Tieren. Heft 1: II. Bombylius fuliginosus und die Farbe der Blumen. Abhandlungen der Zoologische-Botanische Gesellschaft in Wien. 1921;12:17–116. [Google Scholar]
- Krannitz PG, Maun MA. An experimental study of floral display size and reproductive success in Viburnum opulus: importance of grouping. Canadian Journal of Botany. 1991;69:394–399. [Google Scholar]
- Kutner MG, Nachtscheim CJ, Neter J, Li W. Applied linear statistical models. 5th edn. New York: McGraw-Hill Irwin; 2005. [Google Scholar]
- Lack AJ. Competition for pollinators in the ecology of Centaurea scabiosa L and Centaurea nigra L. III. Insect visits and the number of successful pollinations. New Phytologist. 1982;91:321–339. [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lloyd DG. Self- and cross-fertilization in plants. II. The selection of self-fertilization. International Journal of Plant Sciences. 1992;153:370–380. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. London: Chapman & Hall; 1989. [Google Scholar]
- Nielsen LR, Philipp M, Siegismund HR. Selective advantage of ray florets in Scalesia affinis and S. pedunculata (Asteraceae), two endemic species from the Galápagos. Evolutionary Ecology. 2002;16:139–153. [Google Scholar]
- Ohashi K, Yahara T. Behavioural responses of pollinators to variation in floral display size and their influences on the evolution of floral traits. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge: Cambridge University Press; 2001. pp. 274–296. [Google Scholar]
- Ruiz Rejón M, Perez L, Pascual L, Ruiz Rejón CL, Lopez D, Oliver JL. An estimate of the outcrossing rate in natural populations of Muscari comosum (Liliaceae). Preliminary note. Isozyme Bulletin. 1982;15:106. [Google Scholar]
- Ruiz Rejón C, Lozano R, Ruiz Rejón M. Genetic variability in Muscari comosum (Liliaceae). III. Enzyme polymorphism in European and Canarian populations. Anales de la Estación Experimental de Aula Dei. 1988;19:143–150. [Google Scholar]
- Sage TL, Strumas F, Cole WM, Barrett SCH. Differential ovule development following self- and cross-pollination: the basis of self-sterility in Narcissus triandrus (Amaryllidaceae) American Journal of Botany. 1999;86:855–870. [PubMed] [Google Scholar]
- SAS Institute Inc. SAS OnlineDoc® 9·2. Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- Seavey SR, Bawa KS. Late-acting incompatibility in angiosperms. Botanical Review. 1986;52:195–219. [Google Scholar]
- Stuessy TF, Spooner DM, Evans KA. Adaptive significance of ray corollas in Helianthus grosseserratus (Compositae) American Midland Naturalist. 1986;115:191–197. [Google Scholar]
- Vaughton G, Ramsey M. Floral emasculation reveals pollen quality limitation of seed output in Bulbine bulbosa (Asphodelaceae) American Journal of Botany. 2010;97:174–178. doi: 10.3732/ajb.0900183. [DOI] [PubMed] [Google Scholar]



