Abstract
Assessment of tongue strength and endurance is common in research and clinical contexts. It is unclear whether the results reveal discrete function by the tongue or combined abilities of the tongue and jaw. One way to isolate the movement of the tongue is to constrain the jaw kinematically by using a bite block. In this study, 10 neurologically normal young adults performed tongue strength and endurance tasks without a bite block (“jaw-free”) and with bite blocks of various heights (2, 5, 10, and 15 mm for strength; 5 mm for endurance). Data signals included tongue pressure exerted on an air-filled bulb, surface electromyography (SEMG) from the superior tongue blade, and SEMG from 1 masseter. On average, tongue strength (pressure in kPa) was greatest with no bite block and generally decreased as bite blocks increased in height. Pairwise analyses revealed statistically significant differences for all but 3 comparisons (jaw-free to 2 mm, 2 to 5 mm, and 5 to 10 mm). After removing outlying data from 1 participant, tongue endurance at 50% of tongue strength was significantly greater without a bite block than with one. SEMG data did not differ significantly for the strength task across bite block conditions, but inspection of the individual data revealed a tendency for masseter activity to be lower when the jaw was unconstrained. These results suggest that maximal tongue strength and endurance are best assessed with an unconstrained mandible or with a very small bite block.
Keywords: tongue function, maximum performance tasks, bite block, assessment, surface electromyography
The assessment of oromotor abilities is a common component of most motor speech examinations, especially when cranial nerves or their central innervations are implicated. Clinical assessments typically include measures of strength, endurance, range of motion, and coordination of individual articulators. To assess tongue strength, clinicians commonly hold a tongue depressor beyond the lips and the patient pushes the tongue against the depressor. Strength is rated perceptually, often with a 3–5-point equal-appearing interval scale or with binary judgments of “normal” or “weak.” Such measures can provide rough diagnostic information for speech-language pathologists, neurologists, and other movement-disorders specialists. Performance on carefully selected tasks can help isolate cranial nerve dysfunction, as well as implicate side and site of neurologic lesion (Duffy, 1995). Clinicians also have become increasingly interested in oral-motor strength exercises for therapeutic purposes. Nonspeech oral-motor exercise programs are commonly recommended for speech and swallowing impairments in children and adults (e.g., Bahr, 2001; Dworkin, 1991).
Clinical instruments are available that allow for objective documentation of oral-motor performance. Several of these measure the pressure that is generated when an air-filled bulb is compressed by one of the articulators, particularly the tongue (Hayashi et al., 2002; Murdoch, Attard, Ozanne, & Stokes, 1995; Palmer & Osborn, 1940; Robin, Somodi, & Luschei, 1991). By pressing the tongue against the bulb, pressure increases within the bulb. Strength assessment involves brief maximal efforts, and endurance is often assessed with maximally sustained submaximal efforts (Robin, Solomon, Moon, & Folkins, 1997). It is sometimes assumed that the jaw should be stabilized during tongue function assessment to isolate muscles of mastication (Palmer & Osborn, 1940) and to prevent movement artifact (Thompson, Murdoch, & Stokes, 1995). Presumably, this would result in a more consistent and stable performance by the tongue.
Methods used to stabilize the jaw during tongue function assessment generally involve placing the teeth on some portion of the pressure-sensing instrument, causing interincisal separation of approximately 2–6 mm (Hayashi et al., 2002; Palmer & Osborn, 1940; Solomon, 2000; Thompson et al., 1995). It is possible to hold the tongue sensor in place with the hand as well, so that the teeth are not in contact with the instrument and the mandible is unconstrained. With this method, participants naturally tend to hold their mandible in some stable position to provide a base against which the tongue can develop leverage for pushing upward.
Studies of tongue strength have not systematically addressed whether the mandible should be placed in a standard position for tongue strength and endurance testing. A published footnote addressed whether the use of bite blocks to stabilize the jaw affects measures of tongue strength: Solomon (2000) cited previous laboratory pilot work (Robin & Luschei, unpublished observations) wherein 5-, 10-, and 15-mm bite blocks were constructed for 2 informed volunteers. Informal observations indicated that the 5- and 10-mm bite blocks did not appreciably affect tongue strength. The present study was conducted to revisit this topic and to measure tongue endurance in a group of naïve volunteers.
An additional contribution of this study is the simultaneous recording of surface electromyographic activity (SEMG) from the superior aspect of the anterior tongue dorsum (tongue blade) and from one masseter to sample muscle activation during the tongue strength and endurance tasks. Superficial masseter is a good representative of the jaw-closing muscles, although some task-specific activation occurs for deep masseter, and for anterior and posterior temporalis muscles as well (Blanksma & van Eijden, 1995). Robust recordings of superficial tongue muscle activity using the present technique have been reported previously (Solomon, Drager, & Luschei, 2002).
The extrinsic and intrinsic muscles of the tongue have complicated arrangements that allow for exquisite control of tongue position and shapes. Tongue postures, movements, and forces generated have been modeled biomechanically, and from these, inferences about muscular contributions have been made. For example, Kakita, Fujimura, and Honda’s (1985) finite element geometry model of the tongue predicts that the transverse intrinsic tongue muscle contributes to tongue elevation. Honda’s (1996) model of orthogonal extrinsic muscle pairs predicts that the posterior genioglossus (GGP) elevates the tongue dorsum by pulling the tongue root forward. Electromyographic data indicate strong activation of the genioglossus muscle upon tongue protrusion (Sauerland & Mitchell, 1975), an action that may be related to tongue elevation by virtue of anterior tongue base motion. Other well-elaborated models of tongue structure and deformation can predict muscular contributions to tongue retraction, lowering, and protrusion (Stone, Dick, Douglas, Davis, & Ozturk, 2000; Stone et al., 2001; Wilhelms-Tricarico, 1995, 1996) but have not yet modeled tongue elevation. Presumably, this action is accomplished by a combination of extrinsic and intrinsic muscle activation, predominantly the GGP and the transverse lingualis. In this study, the muscle recording sites were selected to represent the primary actions of interest: tongue elevation (transverse lingualis) and mandibular elevation (masseter). Despite the usefulness of bite blocks for stabilizing the jaw in a predetermined position, blocking the jaw does not necessarily determine or preclude jaw muscle activity. EMG data can reveal muscular activation patterns that are not apparent by studying or controlling postures and forces (Folkins & Zimmerman, 1981).
The technical issues of whether to stabilize the jaw, and in what position the jaw should be stabilized, to test maximal tongue strength and endurance were the motivations for this study. The primary goal of this research was to select an optimally sized bite block to yield maximal measures of tongue strength and tongue endurance at 50% of maximum strength. A related goal was to assess whether activity of a major muscle of mandibular elevation, the masseter, differed significantly across bite block conditions. The results can contribute to the standardization of these common nonspeech tongue assessment tasks and to an understanding of the masseter’s role in accomplishing these tasks.
Method
Participants
Ten neurologically normal adults (ages 20–38 years, M = 26.3 years), 8 women and 2 men, participated in this study. Participants reported no previous or present disorders involving speech, language, the jaw, or the temporomandibular joint. Candidates were excluded if they wore a dental appliance or were missing any incisor or first or second molar teeth. Additional exclusion criteria included playing brass or woodwind musical instruments, and special training in oration or diction within the past 5 years, on the basis of evidence that trumpet players and debaters had supranormal tongue endurance (Robin, Goel, Somodi, & Luschei, 1992). Participants passed a pure-tone hearing screening at 0.5, 1, 2, and 4 kHz at 20 dB HL in one ear or reported a normal audiometric evaluation within the past month. All of the participants were enrolled in larger studies on the effects of jaw fixation and lexical difficulty on speech production (Munson & Solomon, 2003), and on the impact of jaw position on speech acoustics and naturalness (Solomon, Makashay, & Munson, 2004). Participants received $10/hr for their participation. They were informed of the purposes and procedures of this research and were provided written consent.
Instrumentation
The Iowa Oral Performance Instrument (IOPI Model 1.5, Breakthrough, Oakdale, IA) sensed and displayed pressure (in kPa) exerted by the anterior dorsum of the tongue on an air-filled bulb (3 × 1.5 × 1 cm approximate dimensions) that was placed along the hard palate. An LED display on the IOPI provided visual feedback during the endurance task. An internal timer was activated manually to time endurance trials. These durations were later verified by measuring digitized computer records (sampling rate = 5 kHz) of the pressure signal (see criteria in Data Reduction and Analysis), which were output to a laboratory computer.
SEMG of the superior tongue blade was recorded via custom-made electrodes adhered to the inferior surface of an IOPI tongue bulb (described previously in Solomon et al., 2002). Three small pure platinum foil plates (2 × 3 mm) were configured so that the center electrode was the reference and the outer electrodes were positive and negative poles. Seven-strand stainless steel (Cooner Bioflex) wire was soldered to the electrodes. Because the electrodes were attached to the IOPI bulb and saliva was the conductivity medium, no special preparation of the tongue was needed.
For masseter SEMG recording, the skin over the masseter muscle belly and forehead was cleaned with an alcohol swab. Two pregelled disposable surface snap-disk electrodes (3M Red Dot or Vermed, 1-cm diameter) were placed 20 ± 3 mm apart (center-to-center) along an axis perpendicular to the presumed orientation of the muscle fibers of the masseter on the right side of the mandible. These were placed approximately 5 mm superior to the lower border of the mandible, with the more lateral electrode placed directly above the mandibular angle. A larger disposable reference electrode (Nicolet Biomedical, Madison, WI) was adhered to the forehead. We determined the adequacy of masseter recording by asking the participant to bite lightly. If the signal was poor, the electrodes were removed and reapplied to the same side or, in three cases, to the left masseter. Functional tasks were performed and recorded for electrode placement verification and calibration. These included 2–3 trials of maximal voluntary contraction (“clench your teeth as hard as you can”), light biting, pursing and retracting the lips, and moderate mandibular depression (“open your mouth halfway”). Placement was acceptable if activity was noted during the biting tasks, did not occur during mouth opening, and was minimal during facial muscle activity. Masseter activity could not be completely isolated from superficial facial muscle activity (e.g., platysma).
Electrode leads were coupled with an optically isolated EMG amplifier system (B466C Speech Physiology System, University of Iowa Bioengineering). Signals were low-pass filtered (8-pole) at 2.5 kHz. Additionally, signals passed through a 60-Hz notch filter and a 30-Hz high-pass filter inherent in the system. The SEMG data signals were digitized along with the pressure signal (Dataq Instruments, Akron, OH) to a laboratory computer (Gateway P5-133) at 5 kHz/channel.
Procedures
After placing the masseter electrodes on the skin and the IOPI bulb with its accompanying electrodes in the mouth, participants performed three brief (2–4 s) trials of tongue elevation to produce a maximal compressive force. The maximum pressure (Pmax) generated by the tongue was taken as tongue strength. The IOPI’s potentiometer was then set so that 50% of this strength value was the target level for the endurance assessment. Participants sustained this submaximal pressure level as long as possible given visual and spirited verbal feedback. A subsequent rest period of at least 15 min was provided, during which time four bite blocks were created.
Bite Blocks
The investigators constructed four bite blocks of different heights for each individual generally according to the procedures described by Netsell (1985) and Dworkin (1991). For each block, an appropriately sized cube of dental putty (Kerr Citricon) was prepared with the midportion of a long piece of dental floss embedded in the material. The soft block was placed unilaterally between the first molars. Participants then gently bit on a tongue depressor placed vertically between the central incisors so that their molars made an impression on the block. Tongue depressors were trimmed for interincisal distances of 3, 6, 11, and 17 mm so that the resulting bite blocks approximated 2, 5, 10, and 15 mm in height. The bite block was removed after 10–20 s, after which it set to a hard rubber consistency on a tray before being used in the study. When the bite blocks were used in the study, the free ends of the dental floss remained outside of the mouth and were used to assist with removal and as a safety tether. The bite blocks were placed on the right molars for 7 of the participants and on the left side for the three participants whose left masseter was used for SEMG recording (Participants 3, 6, and 9).
Data Collection
The participants first performed the initial tongue strength and endurance trials without a bite block (“jaw free”) as practice trials. They then performed three strength trials for each of five bite block conditions (jaw-free, 2 mm, 5 mm, 10 mm, 15 mm) and one endurance trial for each of two bite block conditions (jaw-free, 5 mm). The order of conditions was quasi-randomized across participants. Approximately half of the experimental trials were performed during each of two sessions on separate days. Because tongue fatigue has the potential to affect additional nonspeech tongue function trials as well as speech production (Solomon, 2000), endurance trials were conducted last in each session. When additional endurance trials were required (i.e., for practice or due to data collection errors), they were followed by rest periods of at least 15 min. A constraint on the randomization of conditions was that tongue strength in the jaw-free and 10-mm bite block conditions were scheduled on separate days to accommodate the research design for a parallel study (Munson & Solomon, 2003). Additional speech tasks, including syllable repetitions and oral reading of sentences, were performed in the jaw-free, 2-mm, and 5-mm bite block conditions for acoustic and perceptual analyses (Solomon et al., 2004).
Data Reduction and Analysis
The tongue strength values (the maximum pressure from three trials) that were noted by the experimenter during data collection sessions were confirmed by measuring the digitized records. Tongue endurance trials were analyzed from the digitized data according to criteria established previously (Solomon, Robin, & Luschei, 2000). Briefly, the trial began when pressure reached 50% Pmax and terminated when the pressure dropped precipitously, after the pressure was maintained between 40% and 50% Pmax for 2 s or after pressure dropped below 40% Pmax for 0.5 s. The latter two criteria allowed time to reestablish the target pressure level in response to a verbal cue or to tolerate minor fluctuations in the signal.
SEMG data from the superior tongue and the masseter were taken from the tongue strength trials in each bite block condition. The SEMG signals were zero-balanced by computationally removing the DC offset and then rectified. The mean of the rectified SEMG signals was used as an indicator of muscle activity during the tongue strength task. The mean was calculated over a 1-s interval that encompassed the peak pressure for the trial of interest. For the tongue, the output was adjusted according to the amplifier gain, and the data were expressed in mV. This procedure was preferred to normalizing the data to a maximal effort because all tongue trials were attempted maxima. For the masseter, the data were adjusted for amplifier gain, subtracted from the noise floor (1 s sample of minimal activity) and normalized to the greatest maximal voluntary contraction (MVC) by the masseter performed at the beginning of the session. This normalization procedure was used to account for day-to-day differences in electrode sensitivity, presumably related to placement variation.
All analyses included tests of normality and equal variance. If the data passed these tests, parametric procedures were used; otherwise nonparametric procedures were used. Parametric tests included paired t tests and repeated-measures analysis of variance (RM-ANOVA) followed by pairwise comparisons (Tukey test) as appropriate. Nonparametric tests included Wilcoxon signed-rank tests and Friedman’s repeated-measures ANOVAs on ranked data (F-RM-ANOVA). Analyses addressed tongue strength across the five bite block conditions (jaw free, 2, 5, 10, and 15 mm) and tongue endurance with and without a 5-mm bite block. Differences were considered statistically significant at p < .05.
In the assessment of measurement reliability of the mean SEMG data for the tongue and masseter during tongue-strength trials, one of five bite block conditions from each participant (20% of the data) was remeasured by the same and a different investigator. Pearson product–moment correlations were .999 for intrarater reliability and .998 for interrater reliability.
Results
Tongue Strength
Each participant provided tongue strength data without a bite block on at least two occasions; first as a practice trial, and later, randomized into the study as a bite block condition. Because of occasional SEMG data-collection problems, 5 of the participants returned for a third session during which 3 performed the jaw-free tongue-strength task once and 2 performed it twice. To assess for a practice effect, we compared the first (training) and last (experimental) trials for each participant using a paired-sample t test. The mean value across participants was 58.8 kPa for the first strength assessment and 61.6 kPa for the second (mean difference = 2.8 kPa; SD = 4.2 kPa). This difference was not statistically significant, t(9) = −2.213, p = .062. Because there was no practice effect, the experimental tongue-strength trial in the jaw-free condition was used for statistical analyses.
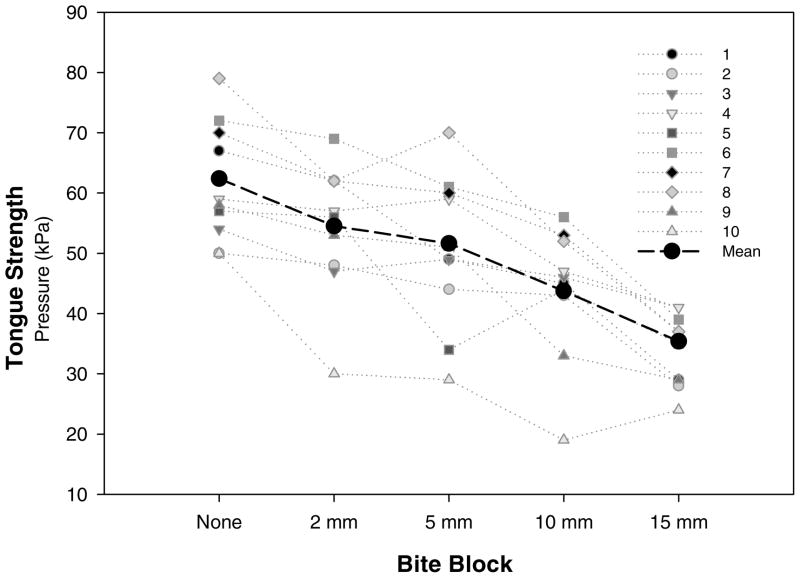
Figure 1 illustrates the tongue strength results in each bite block condition for each participant as well as the mean values across participants. On average, tongue strength values decreased as a bite block was added and as the bite block increased in height. The differences in mean values among the bite block conditions were greater than would be expected by chance, F(4, 9) = 34.0, p < .001 (RM-ANOVA). To identify the conditions that contributed to this result, we conducted a pairwise multiple comparison procedure (Tukey test). There was no significant difference in tongue strength when the jaw-free condition was compared with the 2-mm bite block, the 2–5-mm bite blocks, and the 5–10-mm bite blocks (see Table 1). Statistically significant differences in tongue strength resulted for all other comparisons.
Figure 1.
Individual and mean data for maximal tongue strength across five bite block conditions.
Table 1.
Results from a pairwise multiple comparison analysis for tongue strength among the bite block conditions.
| Bite block comparisons | q | p |
|---|---|---|
| Jaw free vs. 2 mm | 3.871 | .068 |
| 5 mm | 6.083 | .001* |
| 10 mm | 9.844 | <.001* |
| 15 mm | 15.263 | <.001* |
| 2 mm vs. 5 mm | 2.212 | .529 |
| 10 mm | 5.973 | .002* |
| 15 mm | 11.392 | <.001* |
| 5 mm vs. 10 mm | 3.761 | .081 |
| 15 mm | 9.180 | <.001* |
| 10 mm vs. 15 mm | 5.420 | .004* |
p < .05.
SEMG analysis for tongue strength revealed robust and discrete muscle activity sensed from the superior tongue blade and relatively low level masseter SEMG activity. The effect of practice was addressed by initially comparing the training and experimental (first and final) tongue strength trials in the jaw-free condition. Nonparametric analysis indicated no systematic SEMG difference between the training and experimental trials for the tongue (W = −13.0, p = .557) or the masseter (W = 22.0, p = .148).
Statistical analysis (F-RM-ANOVA) of average SEMG activity (mean measured over 1 s) failed to reveal significant differences among the bite block conditions for the tongue, χ2(4, N = 10) = 6.84, p = .144, or masseter, χ2(4, N = 10) = 4.23, p = .379. Although no systematic effects emerged from inferential statistical analysis, the summary data indicate a tendency for median masseter SEMG to be lowest in the jaw-free condition (13% MVC), higher for the 2-, 5-, and 10-mm bite blocks (22–26% MVC) and highest with the 15-mm bite block (31% MVC).
Tongue Endurance
As with tongue strength, one trial of the tongue endurance task was practiced during the first session without a bite block. Participants performed single trials of jaw-free tongue endurance at 50% of maximal strength during the randomized protocol as well. Five participants performed the endurance task without a bite block during one additional session because of procedural or equipment problems. To examine for a practice effect, we compared the first and final performances on this task. Data for this comparison were available for 9 participants. In one case, the second jaw-free trial was invalid because Participant 9 trapped air in the IOPI bulb by biting too hard on the tubing, an event that was readily apparent as a flatline data record. The mean endurance results for the two jaw-free trials were 33.19 s and 32.89 s; this group difference was not statistically significant, t(8) = 2.31, p = .971. Six participants demonstrated longer endurance during the practice trial (from 2 to 37 s), and 3 had longer trials during the experimental trial (from 24 to 33 s). Because of the substantial differences between trials and in accordance with the goal of obtaining measures of maximal performance, the best jaw-free endurance trial was used for the experimental comparison of endurance (jaw free vs. 5-mm bite block).
Tongue endurance at 50% of maximal pressure in the 5-mm bite block condition was available for 9 participants (missing for Participant 10 because of a recording error). Individual participants’ performance and the median data are plotted in Figure 2. The median duration across participants for the best jaw-free trial was 40.0 s and for the trial with a 5-mm bite block was 31.3 s. Endurance did not differ significantly between the bite block conditions (W = −17.0, p = .359). There was one outlier (Participant 1) whose endurance performance with the bite block was 2.8 SDs greater than the mean for the rest of the group. Reanalysis without the outlying data revealed a significant bite block effect for tongue endurance, t(7) = 2.62, p = .035, so that endurance was longer in the jaw-free condition.
Figure 2.
Individual and median data for tongue endurance (in seconds) at 50% of maximum tongue strength (Pmax) without and with a 5-mm bite block.
Discussion
The aim of this study was to ascertain whether a bite block was beneficial, detrimental, or inconsequential for assessment of tongue strength and endurance. The findings were intended to resolve issues about the importance of controlling jaw position when measuring tongue function in relative isolation. The primary goals of this study were to determine whether using a bite block improved maximal performance on tongue strength and endurance tasks, and if so, which height bite block provided the most benefit. The 10 young adults with normal oral structure and function who participated in this study generally demonstrated higher measures of tongue strength and endurance without a bite block. Consideration of specific aspects of these primary findings follows.
On average, the greatest pressure for the tongue strength assessment was generated when no bite block was used, although this result did not differ significantly from a very small bite block (2 mm). This bite block approximated the vertical distance between the molars in the jaw-free condition. Tongue strength measures also did not differ significantly when comparing 2-mm to 5-mm or 5-mm to 10-mm bite blocks. However, the differences were statistically significant for the jaw-free to 5-mm, 2-mm to 10-mm bite blocks, and other more extreme comparisons. These results indicate that providing a stabilizing structure for the jaw during tongue strength measures did not significantly affect results as long as jaw positions are roughly similar.
The larger bite blocks, and consequently the extent of jaw separation, appeared to interfere with the compressive pressure the tongue was able to exert. Available biomechanical models of tongue function do not address the issue of optimal biomechanical position during a maximal tongue-elevation maneuver (cf. Honda, 1996; Wilhelm-Tricarico, 1996). As reviewed previously, anterior carriage of the tongue is most likely the result of GGP contraction (Honda, 1996). Mandibular lowering, which could also involve anterior gliding of the mandible along the temporomandibular joint (Baragar & Osborn, 1984), could place the GGP vector at a mechanically disadvantageous position for generating maximal force in the desired direction.
Tongue endurance (sustaining 50% of maximum pressure as long as possible) was significantly longer without a 5-mm bite block than with it after removal of 1 participant’s outlying data. There was no obvious explanation for the inordinately long tongue endurance with a bite block for Participant 1. Extraordinarily long tongue endurance trials have been reported in persons trained in speech debate and trumpet playing (Robin et al., 1992), but this participant had no special speech or musical training. Her other two tongue-endurance trials, both without a bite block, were substantially shorter (17 s and 45 s) than her bite block trial. Training could have played a role because duration increased incrementally across the three trials she performed. A training effect was not apparent for the remaining participants.
A conclusion that tongue endurance is longer without a bite block should be viewed cautiously because the analysis was based on data from only 8 participants (missing one because of a recording error and removal of the one outlier). In addition, it is based on only one endurance trial in the bite block condition whereas the jaw-free result was the better of two trials. High trial-to-trial variability has been reported for endurance tasks (Lambert, Archer, & Evans, 2001; Lyons, Rouse, & Baxendale, 1993; Vøllestad, 1997; Walamies & Turjanmaa, 1993). Selecting the best performance from multiple endurance trials would improve task validity, but this strategy is complicated by the fact that the task itself can induce fatigue. Furthermore, the recovery period necessary to reverse this effect has not been determined. Most studies of tongue endurance allow only one trial per session for this reason (Robin et al., 1991, 1992; Solomon et al., 2000), but a few have allowed multiple trials separated by 1-min rest periods (Murdoch et al., 1995; Thompson et al., 1995). In the present study, no more than two endurance trials, which were separated by at least 15 min, were performed in a single session.
Another consideration when interpreting the endurance data is the target pressure, selected to be 50% of maximum pressure (i.e., strength) in each condition. Because the jaw-free strength data were, on average, about 10 kPa greater than those collected with a 5-mm bite block, endurance trials with the bite block were performed at ~5 kPa lower pressure. Use of a lower pressure level (i.e., with a bite block) would be expected to result in longer endurance times, but the opposite finding occurred. Others have cautioned against detailed interpretations of endurance trials when strength varies substantially across experimental conditions (Lyons et al., 1993; Ward, Theodoros, Murdoch, & Silburn, 2000), but the specific impact of the absolute pressure level at which the endurance trial is performed is unknown and did not affect the present results in the predicted direction.
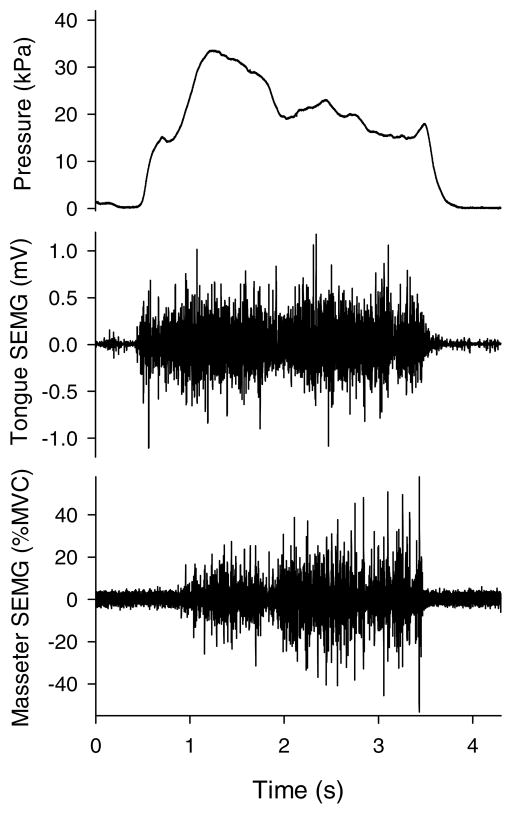
The second goal of this research was to sample surface electromyographic activity from the masseter and the anterior tongue with the intent of providing some insight regarding selected muscle contributions to the tongue-function tasks. One could reasonably argue that tongue strength was greater in the jaw-free condition because other muscles assisted the tongue. In particular, the mandibular elevators would be expected to contribute to the overall result. Although interpretive caution must be exercised because only one site for a mandibular elevator was sampled during this study and because there was no statistically significant difference between conditions, the masseter SEMG data tended to indicate lower activity without a bite block than with one. Without knowing the relative contributions of other synergistic and antagonistic muscles, no definitive conclusion can be reached, but it appears that the masseter was recruited to assist with the task and then activated further when jaw motion was prevented. Figure 3 illustrates this event. In this tongue strength trial with a 10-mm bite block in place, the participant quickly achieved Pmax (33 kPa) with obviously high tongue SEMG and with relatively low average masseter SEMG (13% MVC). Masseter activity then increased after the pressure peaked, perhaps in a futile attempt to increase tongue pressure. Unproductive masseter contraction also was reported previously by Folkins and Zimmerman (1981) during speech and speechlike tasks. Four normal speakers demonstrated discrete bursts of muscle activity by mandibular elevators that corresponded to syllable repetitions whether or not the jaw was free to move.
Figure 3.
Raw data signals during a tongue strength trial (10-mm bite block) by 1 participant. Data traces from top to bottom are tongue pressure, tongue surface electromyography (SEMG), and masseter SEMG.
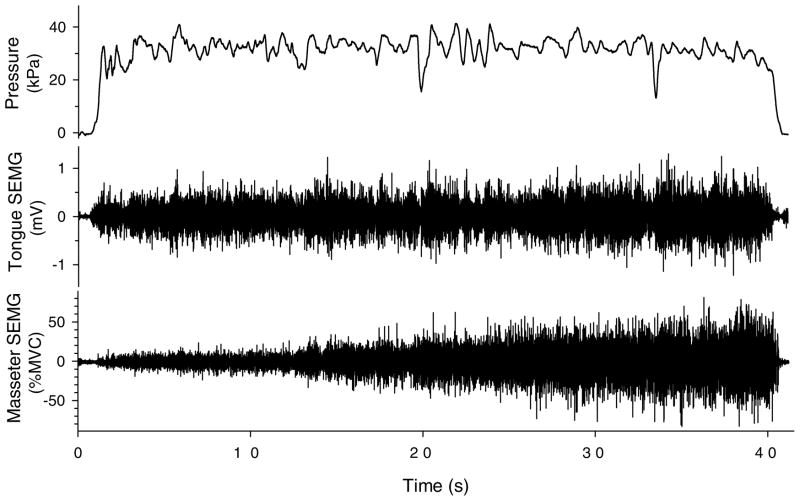
Another example of masseter recruitment with a bite block is illustrated for a tongue-endurance trial in Figure 4. During the endurance trial depicted, masseter SEMG increased steadily despite the presence of a 5-mm bite block. Perhaps this reflects overall motoneuronal excitability as effort increases, a process that could accelerate generalized fatigue of central origin rather than isolated central and peripheral fatigue of the tongue (Gandevia, 2001; McCloskey, 1981).
Figure 4.
Raw data from 1 participant’s tongue endurance trial (39 s) with a 5-mm bite block. Data traces from top to bottom are tongue pressure, tongue surface electromyography (SEMG), and masseter SEMG.
Previous experiments have examined the muscular effects of jaw opening and closing against external forces or during chewing (e.g., Abbink, van der Bilt, Bosman, & van der Glas, 1998; Blanksma & van Eijden, 1995), but none are known to examine the mandibular muscles during tasks designed to assess the tongue pushing against an external force (the hard palate). In this study, only one portion of one mandibular muscle was sampled. Other muscles undoubtedly contributed to the group of coordinative structures that could contribute to these and other nonspeech and speech tasks. Moore (1993) studied coordinative activity among three pairs of mandibular muscles during repetitive or similarly dynamic tasks and demonstrated plasticity of activation strategies. Moore called for future research to examine coordinative strategies of other orofacial structures, such as the tongue and lips, all of which have greater degrees of movement freedom than does the mandible.
The contribution of tongue muscles from sites other than the one sampled in this study for forceful tongue elevation is supported by the data illustrated in Figure 3. The tongue SEMG signal shows similar levels of activity in the beginning and later portions of the trial (between 0.5 and 2 s and between 2 and 3 s on the time axis) yet tongue pressure peaks early in the trial. The GGP probably contributed substantially to the initial part of the task. Future studies could include SEMG techniques that sample from the inferior surface of the tongue (Doble, Leiter, Knuth, Daubenspeck, & Bartlett, 1985; Scardella et al., 1993) in addition to the robust recordings obtained from the superior tongue surface. Multiple recording sites can reveal plasticity in the coordinative structures involved in orofacial function. To understand specific aspects of muscular activation for nonspeech tongue-function maximum performance tasks, researchers in future studies should sample several mandibular elevator and depressor muscles bilaterally along with various tongue muscles.
In summary, these data suggest that performing maximal voluntary contractions of tongue elevation yields similar results whether or not the jaw is unconstrained or stabilized unilaterally with a small (2-mm) bite block. Furthermore, tongue endurance at 50% of maximal strength was longer for most participants when the jaw was unconstrained. On the basis of these observations, it is reasonable to conclude that experimental protocols that strive to assess maximal tongue performance can either leave the mandible free to position itself or, if the results from the strength task can be extended to the endurance task, use a very small bite block. The SEMG data failed to show significantly different magnitudes of activity across the bite block conditions, but inspection of individual and summary data revealed a tendency for masseter activity to be somewhat greater with a bite block in place. This strategy, when used, could be considered biomechanically unproductive and physiologically inefficient because the neuromotor activation involved does not accomplish a biomechanical change.
Acknowledgments
Data collection was funded by the College of Liberal Arts at the University of Minnesota, while the first author was on the faculty. Data analysis and manuscript preparation were supported in part by Grant R03-DC06096 from the National Institute on Deafness and Other Communication Disorders. This research was presented in November 2003 at the annual convention of the American Speech-Language-Hearing Association in Chicago.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Department of the Army or the U.S. Department of Defense.
We gratefully acknowledge the guidance and inspiration of Erich Luschei and the assistance of Anne Benkusky, Nancy DeBoe, Matthew Makashay, Shayla Manthei, Katherine Sullivan, and Cyndie Swenson.
Contributor Information
Nancy Pearl Solomon, Walter Reed Army Medical Center, Washington, DC.
Benjamin Munson, University of Minnesota, Minneapolis.
References
- Abbink JH, van der Bilt A, Bosman F, van der Glas HW. A comparison of jaw-opener and jaw-closer muscle activity in humans to overcome an external force counteracting jaw movement. Experimental Brain Research. 1998;118:269–278. doi: 10.1007/s002210050281. [DOI] [PubMed] [Google Scholar]
- Bahr DC. Oral motor assessment and treatment: Ages and stages. Boston: Allyn and Bacon; 2001. [Google Scholar]
- Baragar FA, Osborn JW. A model relating patterns of human jaw movement to biomechanical constraints. Journal of Biomechanics. 1984;17:757–767. doi: 10.1016/0021-9290(84)90106-4. [DOI] [PubMed] [Google Scholar]
- Blanksma NG, van Eijden TM. Electromyographic heterogeneity in the human temporalis and masseter muscles during static biting, open/close excursions, and chewing. Journal of Dental Research. 1995;74:1318–1327. doi: 10.1177/00220345950740061201. [DOI] [PubMed] [Google Scholar]
- Doble EA, Leiter JC, Knuth SL, Daubenspeck JA, Bartlett D. A noninvasive intraoral electromyographic electrode for genioglossus muscle. Journal of Applied Physiology. 1985;58:1378–1382. doi: 10.1152/jappl.1985.58.4.1378. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. St. Louis, MO: Mosby Year Book; 1995. [Google Scholar]
- Dworkin JP. Motor speech disorders: A treatment guide. St. Louis, MO: Mosby Year Book; 1991. [Google Scholar]
- Folkins JW, Zimmerman GN. Jaw-muscle activity during speech with the mandible fixed. Journal of the Acoustical Society of America. 1981;69:1441–1445. doi: 10.1121/1.385828. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiological Review. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Tsuga K, Hosokawa R, Yoshida M, Sato Y, Akagawa Y. A novel handy probe for tongue pressure measurement. International Journal of Prosthodontics. 2002;15:385–388. [PubMed] [Google Scholar]
- Honda K. Organization of tongue articulation for vowels. Journal of Phonetics. 1996;24:39–52. [Google Scholar]
- Kakita Y, Fujimura O, Honda K. Computation of mapping from muscular contraction patterns to formant patterns in vowel space. In: Fromkin VA, editor. Phonetic linguistics. Orlando, FL: Academic Press; 1985. pp. 133–144. [Google Scholar]
- Lambert CP, Archer RL, Evans WJ. Muscle strength and fatigue during isokinetic exercise in individuals with multiple sclerosis. Medicine & Science in Sports & Exercise. 2001;33:1613–1619. doi: 10.1097/00005768-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Lyons MF, Rouse ME, Baxendale RH. Fatigue and EMG changes in the masseter and temporalis muscles during sustained contractions. Journal of Oral Rehabilitation. 1993;20:321–331. doi: 10.1111/j.1365-2842.1993.tb01614.x. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Corollary discharges: Motor commands and perception. In: Brookes VB, editor. Handbook of physiology: A critical comprehensive presentation of physiological knowledge and concepts. Section I: The nervous system. Bethesda, MD: American Physiological Society; 1981. pp. 1415–1448. [Google Scholar]
- Moore CA. Symmetry of mandibular muscle activity as an index of coordinative strategy. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson B, Solomon NP. The effects of lexical competition and jaw fixation on speech production [Abstract] The ASHA Leader. 2003;8:218. [Google Scholar]
- Murdoch BE, Attard MD, Ozanne AE, Stokes PD. Impaired tongue strength and endurance in developmental verbal dyspraxia: A physiological analysis. European Journal of Disorders of Communication. 1995;30:51–64. doi: 10.3109/13682829509031322. [DOI] [PubMed] [Google Scholar]
- Netsell R. Construction and use of a bite-block for the evaluation and treatment of speech disorders. Journal of Speech and Hearing Disorders. 1985;50:103–106. doi: 10.1044/jshd.5001.103. [DOI] [PubMed] [Google Scholar]
- Palmer MF, Osborn CD. A study of tongue pressures of speech defective and normal speaking individuals. Journal of Speech Disorders. 1940;52:133–140. [Google Scholar]
- Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: Relation to highly skilled movements. Journal of Speech and Hearing Research. 1992;35:1239–1245. doi: 10.1044/jshr.3506.1239. [DOI] [PubMed] [Google Scholar]
- Robin DA, Solomon NP, Moon JB, Folkins JW. Nonspeech assessment of the speech production mechanism. In: McNeil MR, editor. Clinical management of sensorimotor speech disorders. New York: Thieme Medical; 1997. pp. 49–62. [Google Scholar]
- Robin DA, Somodi LB, Luschei ES. Measurement of tongue strength and endurance in normal and articulation disordered subjects. In: Moore C, Yorkston KM, Beukelman DR, editors. Dysarthria and apraxia of speech: Perspectives on management. Baltimore: Paul H. Brookes; 1991. pp. 173–184. [Google Scholar]
- Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Texas Reports on Biology and Medicine. 1975;33:445–455. [PubMed] [Google Scholar]
- Scardella AT, Krawciw N, Petrozzino JJ, Co MA, Santiago TV, Edelman NH. Strength and endurance characteristics of the normal human genioglossus. American Review of Respiratory Disease. 1993;148:179–184. doi: 10.1164/ajrccm/148.1.179. [DOI] [PubMed] [Google Scholar]
- Solomon NP. Changes in normal speech after fatiguing the tongue. Journal of Speech, Language, and Hearing Research. 2000;43:1416–1428. doi: 10.1044/jslhr.4306.1416. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Drager KDR, Luschei ES. Sustaining a constant effort by the tongue and hand: Effects of acute fatigue. Journal of Speech, Language, and Hearing Research. 2002;45:613–624. doi: 10.1044/1092-4388(2002/049). [DOI] [PubMed] [Google Scholar]
- Solomon NP, Makashay MJ, Munson B. Spectral characteristics of speech with fixed jaw displacements [Abstract] Journal of the Acoustical Society of America. 2004;115:2431. [Google Scholar]
- Solomon NP, Robin DA, Luschei ES. Strength, endurance, and stability of the tongue and hand in Parkinson disease. Journal of Speech, Language, and Hearing Research. 2000;43:256–267. doi: 10.1044/jslhr.4301.256. [DOI] [PubMed] [Google Scholar]
- Stone M, Dick D, Douglas AS, Davis EP, Ozturk C. Modelling the internal tongue using principal strains. In: Hoole P, Mooshammer C, Honda M, editors. Proceedings of the Fifth Speech Production Seminar. Munich, Germany: Institut für Phonetik und Sprachliche Kommunikation; 2000. pp. 133–136. [Google Scholar]
- Stone M, Davis EP, Douglas AS, NessAiver M, Gullapalli R, Levine WS, Lundberg A. Modeling the motion of the internal tongue from tagged cine-MRI images. Journal of the Acoustical Society of America. 2001;109:2974–2982. doi: 10.1121/1.1344163. [DOI] [PubMed] [Google Scholar]
- Thompson EC, Murdoch BE, Stokes PD. Tongue function in subjects with upper motor neuron type dysarthria following cerebrovascular accident. Journal of Medical Speech-Language Pathology. 1995;3:27–40. [Google Scholar]
- Vøllestad NK. Measurement of human muscle fatigue. Journal of Neuroscience Methods. 1997;74:219–227. doi: 10.1016/s0165-0270(97)02251-6. [DOI] [PubMed] [Google Scholar]
- Walamies M, Turjanmaa V. Assessment of the reproducibility of strength and endurance handgrip parameters using a digital analyzer. European Journal of Applied Physiology & Occupational Physiology. 1993;67:83–86. doi: 10.1007/BF00377710. [DOI] [PubMed] [Google Scholar]
- Ward EC, Theodoros DG, Murdoch BE, Silburn P. Changes in maximum capacity tongue function following the Lee Silverman Voice Treatment Program. Journal of Medical Speech-Language Pathology. 2000;8:331–335. [Google Scholar]
- Wilhelms-Tricarico R. Physiological modeling of speech production: Methods for modeling soft-tissue articulators. Journal of the Acoustical Society of America. 1995;97:3085–3096. doi: 10.1121/1.411871. [DOI] [PubMed] [Google Scholar]
- Wilhelms-Tricarico R. A biomechanical and physiologically-based vocal tract model and its control. Journal of Phonetics. 1996;24:23–28. [Google Scholar]