Abstract
During September 2004, 4 adult northern myotis, Myotis septentrionalis, were collected from LeFlore County, Oklahoma (n = 2), and Logan (n = 1) and Yell (n = 1) counties, Arkansas, and their feces examined for coccidian parasites. Three of 4 bats (75%) were passing oocysts of Eimeria spp. Oocysts of Eimeria tumlisoni n. sp. were ovoidal, 17.6 × 16.8 (16–19 × 14–18) μm with a shape index of 1.0 (1.0–1.1). A micropyle and oocyst residuum were absent, although 1–2 bilobed polar granules were often present. Sporocysts were ovoidal, 10.5 × 5.9 (9–12 × 5–7) μm with a shape index of 1.8 (1.6–2.0). A Stieda body was present, but sub–Stieda and para–Stieda bodies were absent. A sporocyst residuum was present consisting of compact to dispersed granules between the sporozoites. The sporozoites were elongate, with subspherical anterior refractile body and spherical posterior refractile body; a nucleus was not discernable. This is the second coccidian reported from this host and the first instance of a bat coccidian reported from Oklahoma. We also document a new geographic record for Eimeria catronensis in Oklahoma, and provide an emended description.
During the last decade, there has been a keen interest in the study of coccidia (Apicomplexa) of chiropterans in Arkansas (McAllister et al., 2001, 2004, 2011; McAllister and Upton, 2009). A redescription of Eimeria macyi Wheat, 1975 was provided by McAllister et al. (2001) from tricolored (=eastern pipistrelle) bats, Perimyotis subflavus, and Eimeria catronensis Scott and Duszynski, 1997, was reported in northern myotis, Myotis septentrionalis (McAllister et al., 2004) from the state. In addition, new eimerians have been documented from eastern red bats, Lasiurus borealis (McAllister and Upton, 2009) and P. subflavus (McAllister et al., 2011). To our knowledge, however, no coccidians have ever been reported from any of the 21 species of bats (Caire et al., 1989) currently known from adjacent Oklahoma. Herein, we provide a description of a new species of Eimeria from M. septentrionalis from Oklahoma, and information on 2 additional eimerians from M. septentrionalis in Arkansas and Oklahoma.
MATERIALS AND METHODS
During September 2004, 2 adult M. septentrionalis were collected with standard nylon mist nets (3 × 6–12 m, mesh size = 2.5 cm) from LeFlore County, Oklahoma. In addition, a single adult M. septentrionalis each was collected with same method from Logan and Yell counties, Arkansas. Specimens were placed in individual cloth collecting bags and allowed to defecate. They were banded and released unharmed at their collection sites. Fresh feces were collected from the bags and placed in individual vials containing 2.5% (w/v) aqueous potassium dichromate (K2Cr2O7) and examined for coccidia by light microscopy after flotation in Sheather’s sugar solution (specific gravity = 1.30). Samples were allowed to complete sporulation at room temperature (23 C) in Petri dishes containing a shallow layer of 2.5% (w/v) K2Cr2O7 for 5 days. Samples were stored at uncontrolled temperatures and not examined again until they were ~2,665–days–old and sent to RSS for further examination. Sporulated oocysts were again isolated by flotation (as above), examined using an Olympus BX53 compound microscope, and measurements were taken on 20 oocysts (n. sp.) using Olympus cellSens digital image acquisition software and reported in micrometers (μm) with means followed by the ranges in parentheses. Photomicrographs were taken with an Olympus DP73 digital camera using Nomarski interference–contrast optics. Descriptions of oocysts and sporocysts follow guidelines of Wilber et al. (1998) as follows: oocyst length (L) and width (W), their ranges and ratios (L/W), micropyle (M), oocyst residuum (OR), polar granules (PG), sporocyst length (L) and width (W), their ranges and ratio (L/W), Stieda body (SB), substieda body (SSB), parastieda body (PSB), sporocyst residuum (SR), sporozoites (SP), refractile bodies (RB), and nucleus (N). Photovouchers of sporulated oocysts were accessioned into the United States National Parasite Collection (USNPC), Beltsville, Maryland. Herein, we follow van Zyll de Jong (1979) who elevated M. septentrionalis to full species status rather than a subspecies of Keen’s myotis, Myotis keenii. Bat common names follow Wilson and Reeder (2005).
RESULTS
Three of 4 (75%) of the M. septentrionalis were found to be passing oocysts of eimerian coccidia. Each infected bat was passing a different species of Eimeria, 1 of which is described herein.
DESCRIPTION
Eimeria tumlisoni n. sp
Diagnosis
Oocyst shape ovoidal; bilayered wall, ~1.3 (1.1–1.7) thick, rough outer layer yellowish brown ~ 2/3 thickness, inner layer smooth; L × W 17.6 × 16.8 (16–19 × 14–18); L/W 1.0 (1.0–1.2); M and OR absent; 1–2 PG often present, bilobed, centrally located. Sporocysts ovoidal, L × W 10.5 × 5.9 (9–12 × 5–7); L/W 1.8 (1.6–2.0); wall single–layered and smooth; button–like SB present, SSB and PSB absent; SR present consisting of compact to dispersed granules between SZ. SZ elongate with subspherical anterior RB and spherical posterior RB; N not discernable.
Taxonomic summary
Type host: Myotis septentrionalis (Trouessart, 1897), northern myotis (Chiroptera: Vespertilionidae), adult, collected on 21 September 2004 by David A. Saugey. Host banded and released.
Type locality: USA: Oklahoma, LeFlore County, near entrance of Bear Den Cave, Ouachita National Forest, 16.1 km NE of Talihina (34.47460°N, 94.54357°W).
Prevalence: One of 4 (25%) of the M. septentrionalis examined; 0/1 Logan County, Arkansas; 0/1 Yell County, Arkansas; 1/2 (50%) LeFlore County, Oklahoma.
Type specimens: Phototypes of sporulated oocysts deposited as USNPC 105303.
Sporulation: Exogenous. All oocysts were passed unsporulated or partially sporulated and became fully sporulated within 5 days at ca. 23 C.
Prepatent and patent periods: Unknown.
Site of infection: Unknown. Oocysts recovered from feces.
Endogenous stages: Unknown.
Pathology: Unknown.
Etymology: The specific epithet is given in honor of Dr. Renn Tumlison, Professor of Biology, Henderson State University, Arkadelphia, Arkansas, for his contributions to the ecology and natural history of Arkansas and Oklahoma mammals.
Remarks
To our knowledge, no other bat eimerian has been described with a combination of characters descriptive of the new species. However, the new species is most similar in size and shape (and with other bat eimerians having a rough outer oocyst wall) to E. macyi Wheat, 1975 from P. subflavus in Alabama (Wheat, 1975) and Arkansas (McAllister et al., 2001). However, it can be distinguished from E. macyi by being smaller in oocyst L × W (17.6 × 16.8 vs. 19 × 17.6 [Alabama isolate] and 22.2 × 20.5 [Arkansas isolate] μm) and sporocyst L × W (10.5 × 5.9 vs. 11 × 7 [Alabama isolate] and 12.4 × 8.3 [Arkansas isolate] μm), and by not possessing a sub–Stieda body, as does E. macyi. The only other eimerian that is similar in size and shape and also has a rough outer oocyst wall is Eimeria sealanderi McAllister and Upton, 2009, from L. borealis (McAllister and Upton, 2009), but it also possesses an oocyst residuum, which is not present in the new species.
EMENDED DESCRIPTION
Eimeria catronensis Scott and Duszynski, 1997
Diagnosis
Ellipsoidal oocysts (n = 2) of an eimerian with a smooth outer wall matching the description of Eimeria catronensis (Scott and Duszynski, 1997) were found in a single bat.
Taxonomic summary
Host: Myotis septentrionalis, adult, collected on 21 September 2004 by David A. Saugey. Host banded and released.
Locality: USA: Oklahoma, LeFlore County, near entrance of Bear Den Cave, Ouachita National Forest, 16.1 km NE of Talihina (34.47460°N, 94.54357°W).
Prevalence: One of 4 (25%) M. septentrionalis; 0/1 Logan County, Arkansas; 0/1 Yell County, Arkansas; 1/2 (50%) LeFlore County, Oklahoma.
Specimens deposited: Photovoucher of sporulated oocyst deposited as USNPC 105304.
Sporulation: Exogenous. All oocysts were passed unsporulated or partially sporulated and became fully sporulated within 5 days at ca. 23 C.
Site of infection: Unknown. Oocysts recovered from feces.
Remarks
Oocysts of our isolate of E. catronensis measured 17.7 × 12.8 μm (L/W = 1.4) and sporocysts were 8.2 × 5.7 μm (L/W = 1.4). A micropyle was located near the more pointed end of the oocyst (Fig. 5). Eimeria catronensis appears to be the only bat coccidian known to definitively possess a micropyle (see Duszynski, 2002). Bray (1958) and Mandal and Nair (1973) stated that a micropyle was present in Eimeria levinei from the gland–tailed free–tailed bat, Chaerephon bemmeleni, from Liberia, and in Eimeria andamanensis from the black–bearded tomb bat, Taphozous melanopogon from India, respectively; however, possession of this structure was considered doubtful by Duszynski (2002) and we concur.
Figures 5–6.
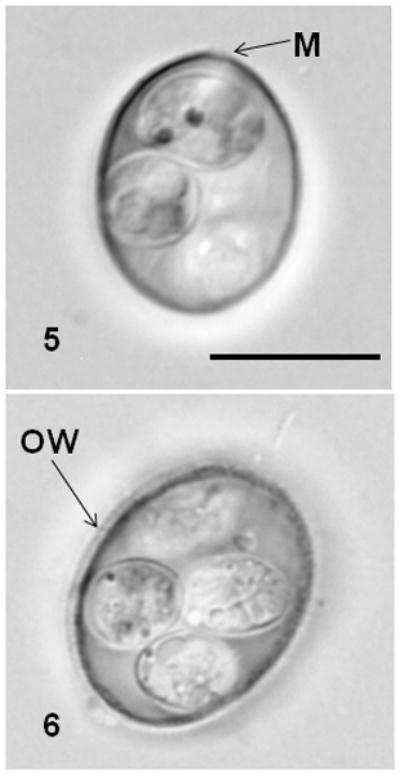
Nomarski-interference contrast photomicrographs of oocysts of Eimeria catronensis (5) showing micropyle (M) and Eimeria sp. (6) showing rough oocyst wall (OW). Bar = 10 μm for both figures.
This coccidian has now been reported from 3 species of vespertilionid bats and 4 widely separated localities in the U.S.A. (see Table I). However, this is the first time that E. catronensis has been reported from a bat collected in Oklahoma.
TABLE I.
Comparative data on Eimeria catronensis from 3 species of vespertilionid bats from various localities.
| Host | Oocyst L × W (L/W)* | Sporocyst L × W (L/W)* | Locality | Reference |
|---|---|---|---|---|
| Myotis lucifugus | 22.2 × 14.8 (1.5) | 8.1 × 6.6 (1.2) | New Mexico | Scott and Duszynski (1997) |
| 20.2 × 13.8 (1.5) | 7.8 × 6.7 (1.2) | Wyoming | Seville and Gruver (2004) | |
| Myotis septentrionalis | 19.9 × 14.3 (1.4) | 9.1 × 6.7 (1.4) | Arkansas | McAllister et al. (2004) |
| 17.7 × 12.8 (1.4) | 8.2 × 5.7 (1.4) | Oklahoma | This report | |
| Myotis yumanensis† | – | – | New Mexico | Scott and Duszynski (1997) |
Average measurements in μm (ratio).
Measurements not provided for this host.
Eimeria sp
Diagnosis
Sporulated ovoidal oocysts (n = 3) of an unknown eimerian were found in a single M. septentrionalis.
Taxonomic summary
Host: Myotis septentrionalis, adult, collected on 17 September 2004 by David A. Saugey. Specimen banded and released.
Locality: USA: Arkansas, Logan County, Mount Magazine State Park, vicinity Benefield Picnic Area (35.16395°N, 93.646917°W).
Prevalence: One of 4 (25%) M. septentrionalis; 1/1 Logan County, Arkansas; 0/1 Yell County, Arkansas; 0/2 (0%) LeFlore County, Oklahoma.
Specimens deposited: Photovoucher of sporulated oocyst deposited as USNPC 105305.
Sporulation: Exogenous. All oocysts were passed unsporulated or partially sporulated and became fully sporulated within 5 days at ca. 23 C.
Site of infection: Unknown. Oocysts recovered from feces.
Remarks
Oocysts of this eimerian possessed a rough outer wall (Fig. 6) and measured 16.7 × 15.3 μm (L/W = 1.1) while sporocysts were 8.7 × 5.8 μm (L/W = 1.4). Too few oocysts were available for study and additional samples must be obtained before this species can be identified.
DISCUSSION
Duszynski (2002) provided a taxonomic summary on the coccidian parasites of bats of the world. No coccidia had been previously reported from M. septentrionalis until McAllister et al. (2004) reported 4 of 20 (20%) from Montgomery and Polk counties, Arkansas, with E. catronensis. In addition, the only bat species ever examined for coccidia, to our knowledge, from Oklahoma was 10 Brazilian free–tailed bats, Tadarida brasiliensis from Major County and a single cave myotis, Myotis velifer from Woodward County (McAllister et al., 2004); however, none was passing oocysts. Therefore, we report the first bat coccidians from the state.
As noted by McAllister et al. (2011) and reiterated again here, it is currently difficult, to impossible, for most individuals on their own to survey bats for coccidia in the Ozark and Ouachita National Forests of Arkansas and Oklahoma with the restriction by the USDA Forest Service being placed on collections. Nearly all caves and mines are closed due to fear of spreading white–nose syndrome, caused by the fungus Geomyces destructans (see Frick et al., 2010). Alternatively, we suggest that an agreement be made between federal and state personnel currently holding valid scientific collection permits and those responsible for bat surveys with coccidiologists who wish to continue studying bat coccidia. Many bats remain to be surveyed from both states and additional new species of coccidia are predicted if potential hosts can be examined in the future.
Figures 1–3.
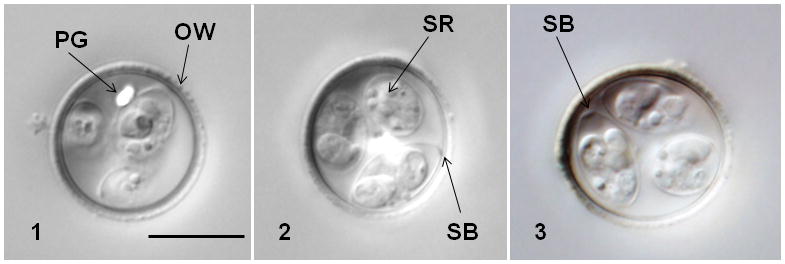
Nomarski-interference contrast photomicrographs of oocysts of Eimeria tumlisoni (1–3) showing rough outer oocyst wall (OW), polar granule (PG), Stieda body (SB), and sporocyst residuum (SR). Bar = 10 μm for all figures.
Figure 4.
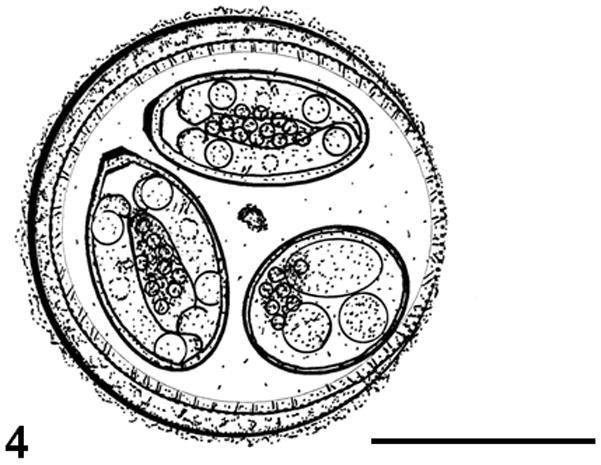
Composite line drawing of an oocyst of Eimeria tumlisoni. Bar = 10 μm.
Acknowledgments
We especially want to thank David A. Saugey, formerly of the USDA Forest Service, Ouachita National Forest, Jessieville, Arkansas, for providing fecal samples from bats. Further appreciation is extended to Patricia A. Pilitt (USNPC) for expert curatorial assistance. This project was supported in part by grants from the National Center for Research Resources (5P20RR016474–12) and the National Institute of General Medical Sciences (8 P20 GM103432–12) from the National Institutes of Health.
LITERATURE CITED
- Bray RS. On the parasitic Protozoa of Liberia. I. Coccidia of some small mammals. Journal of Protozoology. 1958;5:81–83. [Google Scholar]
- Caire W, Tyler JD, Glass BP, Mares MA. Mammals of Oklahoma. University of Oklahoma Press; Norman, Oklahoma: 1989. p. 567. [Google Scholar]
- Duszynski DW. Coccidia (Apicomplexa: Eimeriidae) of the mammalian order Chiroptera. Special Publication of the Museum of Southwestern Biology. 2002;5:1–45. [Google Scholar]
- Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GR, Butchkoski CM, Kunz TH. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010;329:679–682. doi: 10.1126/science.1188594. [DOI] [PubMed] [Google Scholar]
- Mandal AK, Nair KN. A new species of coccidium from Taphozous melanopogon Temminck (Mammalia: Chiroptera) from Andaman Islands. Proceedings of the Indian Academy of Sciences. 1973;77B:243–246. [Google Scholar]
- McAllister CT, Upton SJ. Two new species of Eimeria (Apicomplexa: Eimeriidae) from eastern red bats, Lasiurus borealis (Chiroptera: Vespertilionidae), in Arkansas and North Carolina. Journal of Parasitology. 2009;95:991–993. doi: 10.1645/GE-1967.1. [DOI] [PubMed] [Google Scholar]
- McAllister CT, Upton SJ, Trauth SE, Allard DW. A redescription of Eimeria macyi (Apicomplexa: Eimeriidae) from the eastern pipistrelle, Pipistrellus subflavus (Mammalia: Chiroptera), from Arkansas. Journal of the Arkansas Academy of Science. 2001;55:181–183. [Google Scholar]
- McAllister CT, Upton SJ, Trauth SE, Allard DW, Bursey CR. Parasites (Coccidia, Trematoda, Nematoda) from selected bats of Arkansas. Journal of the Arkansas Academy of Science. 2004;58:133–135. [Google Scholar]
- McAllister CT, Upton SJ, Trauth SE, Allard DW, Bursey CR, Burt S, Seville RS, Robison HW. A new species of Eimeria (Apicomplexa: Eimeriidae) from the eastern pipistrelle, Perimyotis subflavus (Chiroptera: Vespertilionidae), from Arkansas. Journal of Parasitology. 2011;97:896–898. doi: 10.1645/GE-2761.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DT, Duszynski DW. Eimeria from bats of the world: Two new species from Myotis spp. (Chiroptera: Vespertilionidae) Journal of Parasitology. 1997;83:495–501. [PubMed] [Google Scholar]
- Seville RS, Gruver J. Species of Eimeria (Apicomplexa: Eimeriidae) from bats (Chiroptera: Vespertilionidae) in central Wyoming. Journal of Parasitology. 2004;90:348–351. doi: 10.1645/GE-122R. [DOI] [PubMed] [Google Scholar]
- van Zyll de Jong CG. Distribution and systematic relationships of long–eared Myotis in western Canada. Canadian Journal of Zoology. 1979;57:987–994. [Google Scholar]
- Wheat BE. Eimeria macyi sp. n. (Protozoa: Eimeriidae) from the eastern pipistrelle, Pipistrellus subflavus, from Alabama. Journal of Parasitology. 1975;61:920–922. [PubMed] [Google Scholar]
- Wilber PG, Duszynski DW, Upton SJ, Seville RS, Corliss JO. A revision of the taxonomy and nomenclature of the Eimeria spp. (Apicomplexa: Eimeriidae) from rodents in the Tribe Marmotini (Sciuridae) Systematic Parasitology. 1998;39:113–135. [Google Scholar]
- Wilson DE, Reeder DM, editors. A taxonomic and geographic reference. 3. Johns Hopkins University Press; Baltimore, Maryland: 2005. Mammal species of the world; p. 2142. [Google Scholar]


