Abstract
Assessment of nonspeech tongue function is common in speech-language pathology. This paper reviews techniques used to determine tongue strength and endurance, and describes a constant-effort task. These techniques are intended to reveal and quantify the presence of weakness or fatigue of the tongue. The consequences of performing these tasks with and without a bite block, used to fix jaw position, are considered. Whether nonspeech tongue impairment is associated with speech dysfunction in Parkinson’s disease is another topic of interest. Past studies indicated reduced tongue strength and endurance in Parkinson’s disease, but these measures did not correlate with speech measures. It was hypothesized that weakness and fatigue need to be impaired to a “critical” level before speech is perceptibly affected. To examine whether experimentally induced tongue fatigue affects speech, normal speakers performed prolonged strenuous tongue exercise. Speech deteriorated following these exercises. A new investigation examines whether 1 hour of speech-like tongue exercise (rapid syllable repetitions) affects dysarthric speech. Preliminary data from 6 participants with Parkinson’s disease, 1 person with bulbar ALS, and 6 neurologically normal control subjects indicate that sentences sound more precise but less natural after the exercises. Surprisingly, results did not differ significantly between the groups. Continued collection of data and refinement of tasks will contribute to our understanding of the potential relationships between weakness, fatigue, and speech.
Keywords: tongue strength, endurance, effort, bite blocks, Parkinson’s disease, dysarthria
INTRODUCTION
Speech-language pathologists routinely assess oromotor structure and function in an attempt to identify contributing factors underlying a client’s speech or swallowing disorders. Standard approaches to such assessment are few, and most are based on subjective ratings. The author’s research over the past decade has focused on issues related to nonspeech assessment of tongue function and how the results might relate to disordered speech in neurogenic populations.
Nonspeech oromotor assessment often includes measures of strength, range of motion, rhythmicity, target accuracy, and coordination. Testing involves a variety of tasks, and results are based on visual, auditory, or tactile perception by a clinician. The objectivity and reliability of testing should be improved by using standardized instructions and quantitative measures.
This paper reviews studies that assessed tongue strength in order to reveal weakness, and tongue endurance as an indictor of tongue fatigue. An additional task reviewed is the “constant-effort” task, which is being tested as another indicator of fatigue. Within each of these task discussions, the effect of using bite blocks to stabilize the jaw will be considered. Finally, the review will focus on the impact of tongue fatigue on normal and disordered speech. It is premature to offer “final words” on these issues, but implications for future research and clinical practice will be suggested.
STRENGTH TESTING
A basic function of muscle is to exert force with or without effecting movement. The maximal force that a muscle or group of muscles can exert is interpreted as the strength of that structure. In the case of normal activity of the tongue, groups of intrinsic and extrinsic muscles work together to shift and contort the tongue into an amazing number of positions and shapes. It is not known how much tongue strength is required to accomplish certain important functions like speech and oral swallowing. It is generally believed that producing speech requires relatively low levels of strength (Barlow & Abbs, 1986; Searl, 2003), and clearing the oral cavity of highly viscous foods requires somewhat more (Nicosia et al., 2000). Before entertaining the possibility that a client’s speech or swallowing is affected by tongue weakness, it is important to determine and document that the tongue is actually weak.
This line of research does not make the assumption that tongue weakness causes dysarthria or dysphagia. Instead, it addresses the more basic question of whether the tongue is weak in some disorders. If weakness is detected, then correlations between tongue weakness and dysarthria are explored in an attempt to identify preliminary associations between tongue weakness and dysarthria. This particular line of research has not delved into oral swallowing, but it is an obvious extension for future work.
The most common clinical method for assessing tongue strength is by using a tongue depressor. The clinician typically asks the client to push the tongue against a tongue depressor held vertically a few centimeters in front of the client’s lips. Lateralization can also be tested, by having the client push the tongue against the tongue depressor positioned to the right and left of the lips. The examiner rates the tongue as having normal protrusive and lateralization strength or as being mildly, moderately, or severely weak. There are no norms for this test, and ratings are necessarily based on the clinician’s experience.
Instruments are available that can provide objective measures of tongue strength, and can provide numeric data to quantify function (e.g., Hayashi et al., 2002). The instrument that has been used in much of the research reviewed here is the Iowa Oral Performance Instrument (IOPI; Robin & Luschei, 1992). This instrument displays pressure data digitally as well as on an LED array, and it contains a timer. The data can be sent out to a computer or other display device, but by itself, it is small enough to fit into the pocket of a lab coat.
The tongue bulb, an oblong, air-filled, soft plastic bulb, is placed along the hard palate for the tongue to push up against. Compressing the air within the bulb increases pressure which is sensed by the IOPI’s pressure-transducing circuitry. The outer shell of the hand bulb is rubber and fits comfortably in the palm of the hand. The same air-filled bulb that is used for the tongue is contained within the hand bulb and is surrounded by water. Squeezing the hand bulb essentially compresses only the air, making the assessments between the hand and the tongue comparable. Not surprisingly, the handgrip generates greater pressure than does tongue elevation by severalfold.
Standard procedure involves instructing participants to briefly (2–3 seconds) squeeze the bulb as hard as possible, accompanied by a motivating command to “Squeeze!” They repeat this task twice more. The greatest pressure generated from the three trials is taken as their maximum pressure (Pmax), or strength.
In a recent study, Clark, Henson, Barber, Stierwalt, and Sherrill (2003) directly compared tongue strength assessments done with a tongue depressor (protrusion and lateralization) and with the IOPI (elevation). Results from 63 clinic patients and across 2 experienced clinicians and 9 student clinicians revealed a weak-to-moderate correlation (r = .541) between clinical ratings of tongue weakness and the IOPI maximum tongue-strength assessment. (They reported similar results when using the average of the three strength trials.) There was substantial variability in the data, such that only the groups rated as “normal” and “severely weak” avoided overlap of the IOPI results. Surprisingly, the student clinicians’ ratings correlated more strongly with the IOPI measurement than did the experienced clinicians’ ratings (.696 vs. .395). Furthermore, this study attempted to relate functional aspects of oral-phase swallowing to these subjective and objective measures of tongue strength. Results revealed weak to moderate correlations between clinical tongue-strength ratings and several of the swallowing parameters. Correlations generally were less strong between the IOPI results and the oral-swallowing parameters, perhaps because the clinical assessments included a tongue lateralization task.
Factors Affecting Strength Measures
Some factors that can affect tongue strength measures include instructions, external motivation, number of trials, feedback, and tongue and jaw position. This review focuses on tongue and jaw position. For example, the position of the IOPI bulb in the mouth has differed between studies. The standard position of the tongue bulb involves placing it behind the maxillary alveolar ridge so that the entire bulb is placed within the oral cavity and the tongue’s superior surface can contact the bulb’s entire inferior surface. Alternatively, Clark et al. (2003) positioned the bulb such that its midsection was in contact with the alveolar ridge, thereby allowing a portion of the bulb to protrude beyond the teeth. The tongue-bulb position used by Clark et al. can underestimate maximal pressure because of distention of the bulb in free space. One should be aware of such methodological differences when comparing results across studies.
For certain research questions or clinical measures, one may wish to vary bulb position within the mouth. One such application is to assess tongue elevation at a relatively anterior position compared to a more posterior position (cf. Robbins, Levine, Wood, Roecker, & Luschei, 1995). At least one pressure-sensing system is designed with a multi-channel tongue bulb array so that the pressure exerted by the tongue can be assessed at several anterior-to-posterior sites as well as on the right and left for symmetry (Kay Elemetrics Digital Swallowing Workstation).
Tongue-to-sensor position is an important consideration when measuring strength, not only because the distribution of pressure or force across the device can vary, but because the length of the tongue muscle fibers can affect the maximal output generated. For example, using a tongue protrusion task, Bu Sha, England, Parisi, and Stobel (2000) demonstrated that normal adults generated the greatest protrusal force when the force transducer was placed 2.5 cm from the incisors within the oral cavity. Notable is that even at its most elongated position, the tongue was never tested while protruding beyond the teeth, as is routinely done with tongue depressors.
Biomechanical position of the jaw also can affect measures of tongue strength. Various instruments have provided a block against which participants rest their teeth with the intent of fixing jaw position to isolate tongue movement (Hayashi et al., 2002; Palmer & Osborn, 1940; Solomon, 2000; Thompson, Murdoch, & Stokes, 1995). Solomon and Munson (2004) reported differences in tongue-elevation strength using the IOPI with and without bite blocks. Bite blocks were custom-made out of dental putty to be 2, 5, 10, and 15 mm in height, and were placed between the molars on one side. Tongue strength was assessed to be the greatest when no bite block was used, but did not differ significantly from the measure taken with a 2-mm bite block. Strength measures decreased incrementally as bite-block height increased. Therefore, the authors recommended using no bite block or a very small bite block when assessing tongue-elevation strength.
ENDURANCE TESTING
Endurance can be defined as the duration for which a prescribed amount of work can be performed, either by repeated or sustained activity. To understand how best to measure endurance, one must appreciate the meaning of fatigue. There are several meanings or components of fatigue. Fatigue is classically defined as “a failure to maintain the required or expected force” (Edwards, 1981, p. 1). Thus, it appears that a simple endurance task can directly reflect fatigue. Fatigue, however, has been defined and studied in diverse ways. Enoka and Stuart (1992) advanced this intentionally nonspecific and broad definition: “fatigue is a general concept intended to denote an acute impairment of performance that includes both an increase in the perceived effort necessary to exert a desired force and an eventual inability to produce this force” (p. 1631). This definition acknowledges effort as a critical component to the fatigue process, and has inspired the use of effort-assessment techniques (cf. Solomon, Robin, Lorell, Rodnitzky, & Luschei, 1994).
Anecdotal clinical reports commonly claim that fatigue affects speech and swallowing, yet endurance is rarely tested in speech pathology for the purpose of understanding fatigue (however, see Gommerman & Hodge, 1995). Schedulers are warned to arrange appointments at times when the client is less likely to be fatigued, and session length is reduced to allow clients time to rest or recover. Unexplained reductions in function often are attributed to fatigue. Clinical methods used to assess fatigue usually include interview, rating scales or questionnaires, and perhaps a speech “stress test.” The stress test requires the patient to talk for several minutes without resting; Duffy (1995) recommends a counting task. This test is used primarily to test for neuromuscular junction dysfunction, as in myasthenia gravis.
Endurance assessment is commonplace in physical therapy, and similar tasks can be used in speech pathology. For example, the client can squeeze the IOPI bulb at 50% of Pmax (determined during the strength assessment) as long as possible. The client watches the light display on the IOPI, set so that the center light of the 9-LED array represents this submaximal pressure level. Other levels can, and have, been tested as well, but the 50% Pmax level provides results of a reasonable duration in most cases (normally about 30 s for tongue elevation, 60 s for handgrip). During the trial, the examiner provides spirited verbal encouragement and instructs the participant to decide when to stop the trial (e.g., “Keep it there as long as you can; quit if you have to!”). Trials can be timed during the trial or measured off-line from an external recording device, usually a computer with appropriate signal-processing hardware and software. Solomon et al. (2000) developed the following rules for measurement. Timing begins when the pressure meets or exceeds 50% Pmax and terminates when either (1) the pressure drops precipitously, (2) Pmax is maintained between 40–50% Pmax for 2 or more seconds, or (3) Pmax stays below 40% Pmax for at least 0.5 s. These rules allow for transient changes in pressure or variations in pressure related to oscillations associated with certain movement disorders (e.g., the tremor that often occurs with Parkinson’s disease).
Factors Affecting Endurance Measures
Endurance tasks can be difficult to interpret because performance is affected by a myriad of factors. For example, a person’s motivation, tolerance to pain, and competitive spirit will determine how long he or she is willing to sustain the task. Especially for persons with movement disorders, the ability to sustain a task with sufficient stability may affect successful task performance. Also, if strength is abnormally reduced, the endurance trials will be performed at lower pressure levels than expected. The individual’s own performance determines the pressure level. Although such normalization is intended to make the trials comparable, this assumption is untested.
One methodological issue addressed by Solomon and Munson (2004) is whether or not stabilizing the jaw with a bite block will improve tongue stability for the endurance task. Ten normal young adults demonstrated no significant difference in a single trial of the standard tongue endurance task when using or not using a 5-mm bite block. However, there was one outlying data point that, once removed, allowed an effect to emerge such that endurance was longer when no bite block was used. This result must be interpreted with caution because of small sample size and the small number of trials.
The number of trials that can be attempted is severely limited by the need for recovery. Participants performed no more than two trials per session, and these were separated by at least 15 minutes. In addition, performing the task with a bite block in place sometimes resulted in unproductive masseter activity, which could contribute to overall task-related fatigue. Given the trial-to-trial variability that occurs with endurance tasks, it is important but often impractical to collect a sufficient number of trials for valid endurance testing. The desire for other, less strenuous behavioral tests led to the exploration and development of the constant-effort task for the tongue and hand using the IOPI.
CONSTANT-EFFORT TASK: AN ALTERNATIVE FATIGUE ASSESSMENT?
Based on the premise that effort increases as force is exerted (Enoka & Stuart, 1992), it follows that force (or pressure) will decrease if the sense of effort is maintained. Studies have demonstrated that people can perceive force and effort separately (Burgess & Jones, 1997; Enoka & Stuart, 1992). When participants are asked to maintain a constant sense of effort, the force or pressure output may be interpretable as an indicator of fatigue.
Instructions for the “constant-effort” task are key, emphasizing that effort must be kept the same, unlike when pressure is maintained for an endurance task. An analogy is helpful – for example, “If you held a 10-lb weight out to your side, you could do it initially, but it would quickly become harder and harder to do until you couldn’t do it anymore. Your task is to do whatever you need to do to make sure that holding that weight doesn’t get any harder. You should also concentrate on not letting it get any easier. You must keep your effort the same.” They are then instructed to achieve a certain pressure level on the IOPI (usually 50% of Pmax) by looking at the LED display, to close their eyes, and then to keep the level of effort the same. The examiner reminds the participant throughout the trial to “Keep the effort the same; don’t let it get any easier or any harder to do.” The examiner decides, while watching an on-line data display, when to stop the trial based on whether the residual pressure has stabilized (see below). Because the task is not performed to exhaustion, it is less aversive than the endurance task and can be repeated several times within a session. Rest periods are provided between trials, lasting generally 3 minutes. This duration is based on experience, but is otherwise arbitrary.
The constant-effort task has been applied to elbow flexion (Eason, 1959; Jones & Hunter, 1983), handgrip (Cain & Stevens, 1971; Solomon, Drager, & Luschei, 2002; Solomon & Robin, submitted; Solomon, Robin, Mitchinson, VanDaele, & Luschei, 1996), and tongue elevation (Solomon et al., 1996, 2002; Solomon & Robin, submitted). Each of these studies has provided evidence that output decreases exponentially. The rate at which the output decreases with constant effort is hypothesized to reveal fatigue processes. The analysis procedure involves fitting the pressure curve with an equation that includes a single exponential term. The curve begins at a prescribed level and then drops exponentially to a positive asymptote (i.e., residual pressure). The time constant (the inverse of a in the equation F(t) = e−at+b +c) indicates the steepness of the initial portion of the curve. The time constant essentially represents the amount of time it takes for the pressure to decrease about 2/3rds of the way to the residual pressure.
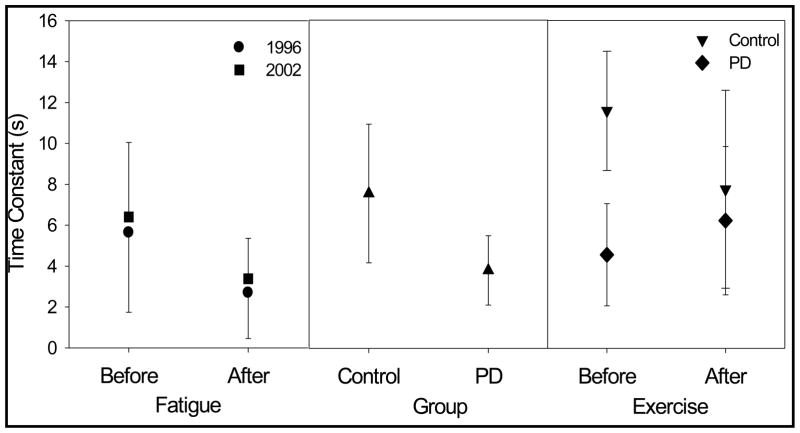
Neurologically normal young adults from two studies generated time constants for the tongue that averaged approximately 6 seconds (Solomon et al., 1996, 2002). When the tongue was exercised to the point of exhaustion (fatigue was defined as the inability to achieve 70% Pmax over three consecutive trials of attempted brief maximal-effort contractions), the time constant decreased to 3 seconds on average. These data for the tongue are plotted in the left panel of Figure 1. For the handgrip, constant effort trials had time constants of approximately 10 seconds when rested and 7 seconds when fatigued. Thus, it appeared that the reduction in time constant, which indicates faster pressure decay, reflected post-exercise fatigue. The authors contended that the very short duration of the initial pressure decay was unlikely a result of peripheral processes and probably reflected central nervous system factors (Solomon et al., 2002).
Figure 1.
Mean (error bars = SD) time constants derived from fitting a curve with a single exponential term to the constant-effort pressure curves. All data are for the tongue performing the constant-effort task beginning at 50% of maximum pressure (strength), and then removing visual feedback with participants concentrated on maintaining a constant sense of effort. The left panel illustrates data from two separate groups of young neurologically normal adults performing the task before and after a fatiguing task (repetitive brief maximal efforts to exhaustion; Solomon et al., 1996, 2002). The middle panel contains results from 16 pairs of matched participants with PD and without neurologic disease (Solomon & Robin, submitted). The right panel plots preliminary data from 6 participants in each of the PD and control groups from an ongoing study. In this study, participants performed the constant-effort task, with a bite block placed unilaterally between the molars, before and after 1 hour of rapid syllable repetitions containing lingual-alveolar consonants and diphthongs (speech-like exercise).
In a currently active study, normal older adults (control subjects) perform the constant-effort task with a bite block in place. Data from 6 control subjects are available to date. Unfortunately, the exponential model has been less successful at describing these data. Furthermore, the average time constant from the current control group is inexplicably longer than previous results. This result is illustrated by comparing prior data from a group of neurologically normal older adults, shown in the middle panel of Figure 1, to the present control-subject data (Before Exercise) shown in the right panel. The remainder of the results illustrated will be described in the following section. Whether the bite block interfered with the successful implementation of the constant-effort task is currently under consideration.
TASK PERFORMANCE IN PARKINSON’S DISEASE
Weakness and especially fatigue are gaining attention as common symptoms and impairments in Parkinson’s disease (PD). Studies examining performance on strength, endurance, and constant-effort tasks using the tongue and hand in persons with PD have been published and are ongoing. Analysis of data combined from two studies (Solomon, Lorell, Robin, Rodnitzky, & Luschei 1995; Solomon, Robin, & Luschei, 2000) revealed reduced tongue strength and endurance in participants with PD as compared to matched neurologically normal control subjects. Results for each of the two studies were inconsistent, indicating that the effect is too weak to be detected without a large enough sample size. In these studies, the PD and control groups did not differ significantly for strength and endurance of the hand, despite the combined analysis. The difference in findings between the tongue and hand could be attributed to differential effects of the disease process or medications on the limbs and speech structures. Performance variables or sampling error could contribute to the discrepancy in findings as well.
Performance of the constant-effort task was tested in 16 adults with PD and 16 neurologically normal control subjects matched for age, sex, height, and weight (Solomon & Robin, submitted). Interestingly, when they were instructed to keep effort constant, the PD group produced pressure curves with shorter time constants, reflecting a faster decline in pressure. In fact, as plotted in the center panel of Figure 1, the time constants for the tongue in the PD group were remarkably similar to those published earlier for normal young adults who had been fatigued experimentally and whose data are plotted in Figure 1’s left panel (Solomon et al., 1996, 2002). This observation supported the hypothesis that the time constant derived from the constant-effort task reflected a state of fatigue in the group of PD participants.
As described previously, the constant-effort task currently is included in a new study that again compares performance by PD and control participants. In this study, data are collected several times before, at 10-minute intervals during, and after a 60-minute syllable-repetition task that is intended to exercise the tongue and perhaps to induce fatigue. Data from 6 PD subjects and 6 control subjects comprise the preliminary results plotted in the right panel of Figure 1. Time constants before the exercise task were similar for this new group of PD subjects as for the previously studied PD participants. The discrepancy between the control group’s results across studies was discussed previously in the context of using a bite block. Comparing the before- and after-exercise data reveals an unexpected pattern of results. The average time constant for the control group decreased after the speech-loading task. Oddly, however, the average time constant increased for the PD group. These results may be consistent with an increase in overall energy and attention rather than a fatigue-related process, as suggested in the next section.
TONGUE WEAKNESS, TONGUE FATIGUE, AND SPEECH
Fatigue often is presumed to affect speech, but there is little evidence to support this assertion. (An exception to this is the well-documented clinical entity of vocal fatigue.) An initial consideration of this topic involved examining data for negative correlations of tongue strength and endurance with perceptual or temporal characteristics of speech in persons with PD. Experienced speech-language pathologists listened to extemporaneous speech samples (picture descriptions and spontaneous monologues) and rated them for overall speech defectiveness and speech imprecision. Speech also was measured for rate (minus pauses). No significant correlations were found between either of the nonspeech tongue function measures (strength and endurance) and any of the speech measures (Solomon et al., 1995, 2000). Evidence of substantial tongue weakness and dysarthria in another disorder, namely amyotrophic lateral sclerosis (ALS) (DePaul & Brooks, 1993; Langmore & Lehman, 1994), led us to speculate that weakness must reach some threshold before speech articulation is impaired (Solomon et al., 2000). What that “critical level” of weakness is remains unspecified.
To more directly address the potential effects of tongue fatigue on speech, 8 normal young adults engaged in a tongue-loading activity. The activity consisted of maximal-effort tongue elevations for 6 seconds, resting for 4 seconds, and then repeating this cycle until reaching a fatigue criterion (the inability to reach 50% Pmax at any time during three consecutive exercise cycles). The median amount of time participants spent performing this activity was 51 minutes. The speakers produced syllable repetitions (fast and slow) and sentences loaded with lingual-alveolar consonants before and after the fatiguing exercise. Speech precision of sentence production, judged by 8 experienced listeners and two larger groups of inexperienced listeners, decreased for each speaker in the study. The timing of speech did not change, and although certain acoustic characteristics of lingual-alveolar consonants changed, these were in a direction opposite than expected. Post-hoc perceptual and acoustic analyses revealed that the most obvious changes seemed to have been for high vowels and diphthongs.
In the new study described previously, participants with primarily bulbar amyotrophic lateral sclerosis (ALS) are being recruited as well as PD and control subjects. Furthermore, in addition to performing the constant effort task at 10-minute intervals during the 60-minute speech-like exercise task, participants are reciting sentences. The sentences, listed in Table 1, contain many lingual-alveolar consonants, high vowels, and diphthongs. Three sentences include 5 repetitions of /s/, /ʃ/ and /t/ in VCV′ contexts and three instances of stressed /i/, and two sentences each contain 3 repetitions of /ɔɪ/ or /aɪ/.
TABLE 1.
Sentence Stimuli
| The two silly teachers enjoy teasing the seal. |
| You should really show Tina how to shine the shoes. |
| I said the shellfish would be too salty by Saturday. |
| Let the boy enjoy a toy again. |
| They fly up high in the sky again. |
Note. Consonantal targets are indicated in boldface; vowel and diphthong targets are underscored.
All tasks are performed with a unilateral custom-made 3–4 mm bite block. The purpose of using the bite block is to isolate tongue movement so that the jaw cannot compensate for altered tongue function as the exercise task proceeds. Because it was important to know how the bite blocks affected the specific speech stimuli for this study before embarking on the fatigue portion of this project, a separate study of the effect of using bite blocks on these sentences was conducted. A key result of that study was that sentences produced with bite blocks were perceived as less natural than those produced freely (Solomon, Makashay, & Munson, 2004). This was especially true for the sentences loaded with consonants rather than diphthongs. Acoustic results supported this finding. Differences were particularly apparent for /s/.
For the current study of the effects of prolonged tongue exercise in persons with disordered speech, results are available for 6 control, 6 PD, and 1 ALS participants. The final baseline productions of the five experimental sentences were compared to the same sentences produced after all six 10-minute intervals of speech-like exercises. The exercise task consisted of randomly varying strings of the syllables /ti/, /si/, /ʃi/, /ɔɪ/ and /aɪ/, repeated as fast as possible. The task was intended to exercise the tongue to some level of fatigue without requiring persons with fatiguing neurological disorders to put forth maximal effort to a point of exhaustion.
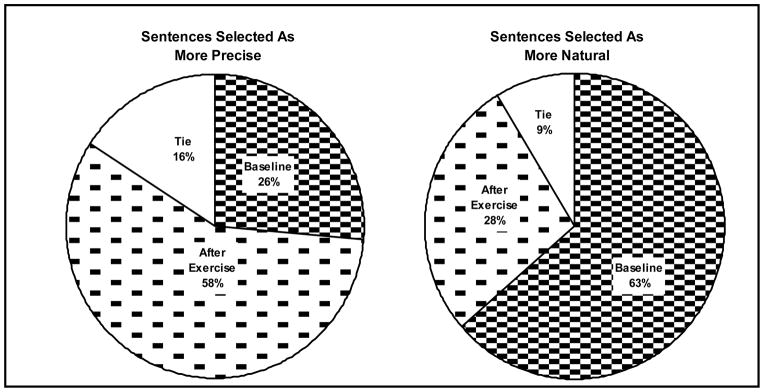
Pairs of sentences were played to three normal-hearing listeners who selected the sentence in each pair that sounded more precise. The experiment was repeated with the instruction to select the more natural-sounding sentence. There were no between-group differences, so the results, were combined for all 13 participants. Figure 2 illustrates the results of these paired-comparison analyses. Surprisingly, listeners selected the sentences read after the exercise task as being more precise. Despite this, they more often perceived these same sentences as sounding less natural.
Figure 2.
The percentage of sentences selected as More Precise (left) and More Natural (right) after six 10-minute sets of speech-like tongue exercises with a bite block in place. Data from 6 PD, 1 ALS, and 6 control participants were combined because of no discernable differences between groups.
These preliminary data do not support the hypothesis that persons with fatiguing neurologic disorders are more susceptible to changes in their speech following speech-like tongue exercises than neurologically normal subjects. The finding that speech more often sounded more precise after the exercises was unexpected, but may reflect a transient effect of the preceding fast-talking equal-stress task. This repetitive activity might have increased overall energy and speech rate, which could be perceived as increased precision. The finding that these sentences sounded less natural may be directly related to the increased precision, because super-precise speech is not typical. Clearly, more data are needed before drawing any conclusions, especially with only one participant with ALS. In addition, the speech-like exercise task used in this study may be reconsidered for its adequacy in eliciting a fatigue effect.
CONCLUSIONS
The studies reviewed in this article address assessment procedures for nonspeech tongue function. Instrumentation and procedures that allow quantitative documentation of tongue strength and endurance are described. Although the procedures appear simple and straightforward, various methodological issues can confound results. For example, using a bite block to prevent the jaw from moving can result in less than maximal measures of tongue strength and endurance.
The endurance task is particularly susceptible to performance issues because the client is required to sustain an activity to the point of exhaustion. A less strenuous task, that of sustaining a constant sense of effort, is described as an alternative indicator of fatigue. This task has been used in several studies, but requires further testing and validation before it can be applied clinically. For example, preliminary evidence indicates that bite blocks may interfere with successful performance of this task.
The primary clinical population studied with these tasks has been Parkinson’s disease. The studies reviewed here revealed modestly but significantly lower than normal tongue strength and endurance, but no correlation between these measures and speech impairment. Interestingly, a group of persons with PD performed similarly on the constant-effort task to young healthy adults after tongue fatigue had been induced through exercise. Based on this observation, it is tempting to conclude that fatigue is revealed by the constant-effort task. However, such a conclusion is guarded because of variability in performance across subjects and trials.
To more directly address the impact of tongue fatigue on speech, a previous study induced tongue fatigue through rigorous exercise in normal speakers. Speech, sampled by reading sentences weighted with lingual-alveolar consonants, deteriorated after the exercise. However, preliminary data from an ongoing investigation have not supported the hypothesis that moderate speech-like tongue exercise will affect dysarthric speech. Neither, for that matter, did these exercises differentially affect speech produced by normal and dysarthric speakers.
Specific clinical implications from this work are premature, but certain logical principles are supported. First, nonspeech tongue function should be objectively documented and shown to be impaired before including improvement of these functions as a treatment goal. Second, assessments must be repeatable over several trials. This is problematic for certain tasks that are in themselves fatiguing, like the endurance task. For such assessments to be clinically useful, it is best if the task can be repeated over several sessions to allow adequate rest. Third, high quality recordings of controlled and spontaneous speech tasks are highly recommended for documenting change with intervention. Routine collection of clinical data can be examined retrospectively to justify intervention, document functional decline with disease, and support or refute various treatment strategies.
Ample clinical anecdotes exist to suggest that fatigue is an important factor in disordered speech and swallowing. Research programs, such as the ones described in this article, strive to provide stable, valid, and easy-to-implement measures of strength and fatigue of the tongue. In turn, these techniques will improve clinical databases and help demonstrate treatment effectiveness.
Acknowledgments
The author gratefully acknowledges support for this research from the American Speech-Language-Hearing Foundation (New Investigator Grant), and Grants P60 DC00976 and R03-DC06096 (current) from the National Institute on Deafness and Other Communication Disorders. The opinions or assertions contained herein are the private views of the Author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense. This manuscript is based on an invited presentation at the 32nd Annual Convention of the International Association of Orofacial Myology in Valley Forge, PA, in June 2004.
References
- Barlow SM, Abbs JH. Fine force and position control of select orofacial structures in the upper motor neuron syndrome. Experimental Neurology. 1986;94:699–713. doi: 10.1016/0014-4886(86)90248-7. [DOI] [PubMed] [Google Scholar]
- Bu Sha BF, England SJ, Parisi RA, Stobel RJ. Force production of the genioglossus as a function of muscle length in normal humans. Journal of Applied Physiology. 2000;88:1678–1684. doi: 10.1152/jappl.2000.88.5.1678. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Jones JF. Perceptions of effort and heaviness during fatigue and during the size-weight illusion. Somatosensory & Motor Research. 1997;14:189–202. doi: 10.1080/08990229771051. [DOI] [PubMed] [Google Scholar]
- Cain WS, Stevens JC. Effort in sustained and phasic handgrip contractions. American Journal of Psychology. 1971;84:52–65. [PubMed] [Google Scholar]
- Clark HM, Henson PA, Barber WD, Stierwalt JAG, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairment. American Journal of Speech-Language Pathology. 2003;12:40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- DePaul R, Brooks BR. Multiple orofacial indices in amyotrophic lateral sclerosis. Journal of Speech and Hearing Disorders. 1993;45:37–44. doi: 10.1044/jshr.3606.1158. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders. St. Louis: Mosby; 1995. [Google Scholar]
- Eason G. The surface electromyogram (EMG) gauges subjective effort. Perceptual and Motor Skills. 1959;9:359–361. [Google Scholar]
- Edwards RHT. Human muscle function and fatigue. In: Porter R, Whelan J, editors. Human muscle fatigue: Physiological mechanisms. London: Pitman; 1981. pp. 1–18. [Google Scholar]
- Enoka RM, Stuart DG. Neurobiology of muscle fatigue. Journal of Applied Physiology. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Gommerman SL, Hodge MM. Effects of oral myofunctional therapy on swallowing and sibilant production. International Journal of Orofacial Myology. 1995;21:9–22. [PubMed] [Google Scholar]
- Hayashi R, Tsuga K, Hosokawa R, Yoshida M, Sato Y, Akagawa Y. A novel handy probe for tongue pressure measurement. International Journal of Prosthodontics. 2002;15:385–388. [PubMed] [Google Scholar]
- Jones LA, Hunter IW. Force and EMG correlates of constant effort contractions. European Journal of Applied Physiology and Occupational Physiology. 1983;51:75–83. doi: 10.1007/BF00952540. [DOI] [PubMed] [Google Scholar]
- Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research. 1994;37:28–37. doi: 10.1044/jshr.3701.28. [DOI] [PubMed] [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55:M634–640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- Palmer MF, Osborn CD. A study of tongue pressures of speech defective and normal speaking individuals. Journal of Speech Disorders. 1940;52:133–140. [Google Scholar]
- Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. Journal of Gerontology: Medical Sciences. 1995;50A:M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- Robin DA, Luschei ES. IOPI: Iowa Oral Performance Instrument, Model 1.5, Reference Manual. Oakdale, IA: Breakthrough, Inc; 1992. [Google Scholar]
- Searl JP. Comparison of transducers and intraoral placement options for measuring lingua-palatal contact pressure during speech. Journal of Speech, Language, and Hearing Research. 2003;46:1444–1456. doi: 10.1044/1092-4388(2003/112). [DOI] [PubMed] [Google Scholar]
- Solomon NP. Changes in normal speech after fatiguing the tongue. Journal of Speech, Language, and Hearing Research. 2000;43:1416–1428. doi: 10.1044/jslhr.4306.1416. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Drager KDR, Luschei ES. Sustaining a constant effort by the tongue and hand: Effects of acute fatigue. Journal of Speech, Language, and Hearing Research. 2002;45:613–624. doi: 10.1044/1092-4388(2002/049). [DOI] [PubMed] [Google Scholar]
- Solomon NP, Lorell DM, Robin DA, Rodnitzky RL, Luschei ES. Tongue strength and endurance in mild to moderate Parkinson’s disease. Journal of Medical Speech-Language Pathology. 1995;3:15–26. [Google Scholar]
- Solomon NP, Makashay MJ, Munson B. Spectral characteristics of speech with fixed jaw displacements [Abstract] Journal of the Acoustical Society of America. 2004;115:2431. [Google Scholar]
- Solomon NP, Munson B. The effect of jaw position on measures of tongue strength and endurance. Journal of Speech, Language, and Hearing Research. 2004;47:584–594. doi: 10.1044/1092-4388(2004/045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NP, Robin DA. Perceptions of effort during handgrip and tongue elevation in Parkinson’s disease. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NP, Robin DA, Lorell DM, Rodnitzky RL, Luschei ES. Tongue function testing in Parkinson’s disease: Indications of fatigue. In: Till J, Yorkston K, Beukelman D, editors. Motor speech disorders: Advances in assessment and treatment. Baltimore: Paul H. Brookes; 1994. pp. 147–160. [Google Scholar]
- Solomon NP, Robin DA, Luschei ES. Strength, endurance, and stability of the tongue and hand in Parkinson disease. Journal of Speech, Language, and Hearing Research. 2000;43:256–267. doi: 10.1044/jslhr.4301.256. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Robin DA, Mitchinson SI, VanDaele DJ, Luschei ES. Sense of effort and the effects of fatigue in the tongue and hand. Journal of Speech and Hearing Research. 1996;39:114–125. doi: 10.1044/jshr.3901.114. [DOI] [PubMed] [Google Scholar]
- Thompson EC, Murdoch BE, Stokes PD. Tongue function in subjects with upper motor neuron type dysarthria following cerebrovascular accident. Journal of Medical Speech-Language Pathology. 1995;3:27–40. doi: 10.3109/13682829509087244. [DOI] [PubMed] [Google Scholar]