Abstract
Background
Fatigue and the accompanying perception of effort are often heightened in Parkinson’s disease.
Objectives
To compare performance on three sense-of-effort tasks between patients with PD and matched neurologically normal control subjects.
Methods
Sixteen PD subjects and 16 normal subjects performed three tasks to assess sense of effort: self-ratings of effort using direct-magnitude estimation, generating pressures at various levels of effort, and sustaining a submaximal level of effort. The latter two tasks were done with handgrip and tongue elevation.
Results
Two of the three tasks successfully differentiated the groups. Subjects with PD provided significantly higher ratings of effort for general daily activities and for speech. During the constant-effort task, pressure curves decayed more rapidly for the PD subjects.
Conclusions
Performance by PD subjects on the constant-effort task resembled that by normal adults who were pre-fatigued in previous experiments. Results support greater than normal sense-of-effort related to fatigue in PD, and provide preliminary validation of a performance-based physiologic task to assess abnormal sense of effort in this population.
Keywords: Parkinson’s disease, Fatigue, Effort, Tongue, Hand, Assessment
1. Introduction
The presence and impact of fatigue and the related perception of increased effort in Parkinson’s disease (PD) are topics of increasing clinical and research interest. Most patients with PD include fatigue as one of their three most prominent symptoms, and claim that it substantially impacts quality of life [1–4], yet neurologists tend to underdiagnose fatigue as a presenting symptom of PD [5]. Clinically, fatigue can be described as an “overwhelming sense of tiredness, lack of energy, or feeling of exhaustion” [6]. The physiology literature defines fatigue as an inability to maintain a predetermined level of activity [7], along with the increased perception of effort that builds over the course of the activity [8]. Tests of endurance at maximal or submaximal output levels are most logically used to assess fatigue, because they require the individual to perform a task to exhaustion.
Previously, Solomon and colleagues [9,10] tested strength and endurance (at 50% of maximum strength) of handgrip and tongue elevation in a total of 36 patients with PD and 36 matched normal control subjects. A retrospective analysis of data from both studies revealed lower than normal tongue strength and endurance but no differences between PD and normal control subjects for handgrip performance [10]. These findings support the expected decrements in maximal performance, but only for the axial structure tested.
The clinical presentation of PD often includes differential impairments of axial and distal motor functions. Furthermore, pharmacological and neurosurgical treatments commonly improve general motor functions but have little or detrimental effects on speech [11–13]. The current research targets the tongue because it is motivated by the study of speech disorders, and the tongue is arguably the most active and important articulator for speech. The hand was included as a representative limb structure for comparison.
An interesting insight from the speech-language pathology literature relates to the level of effort PD patients must exert to improve their speech. Traditional treatment approaches for the speech and voice disorders (hypokinetic dysarthria, hypophonia) that occur in most patients with PD have met with minimal success [14]. One explanation for this appears to relate to their aberrant sense of effort. A popular current behavioral treatment emphasizes high levels of phonatory and respiratory effort, and targets normalization (‘recalibration’) of the perception of effort (re: vocal loudness) [15]. A consistent reduction in the sense of effort without a reduction in vocal loudness appears to be critical to the process of long-term voice and speech improvement [16].
The present study examined perception of effort by a rating scale as well as by handgrip and by tongue elevation tasks in persons with PD and neurologically normal control subjects. During data collection for the same 16 pairs of subjects reported previously [10], the study included additional tasks to quantify effort using three techniques. These tasks are of interest physiologically and clinically because they may provide quantitative indicators of fatigue without depending on maximal performance.
First, subjects rated their perception of effort using direct magnitude estimation (DME). DME allows participants to scale their perceptions without the underlying assumption of a linear distribution, as would be the case with equal-interval scales, and to avoid endpoint effects that can occur with equal-interval or visual-analog scales.
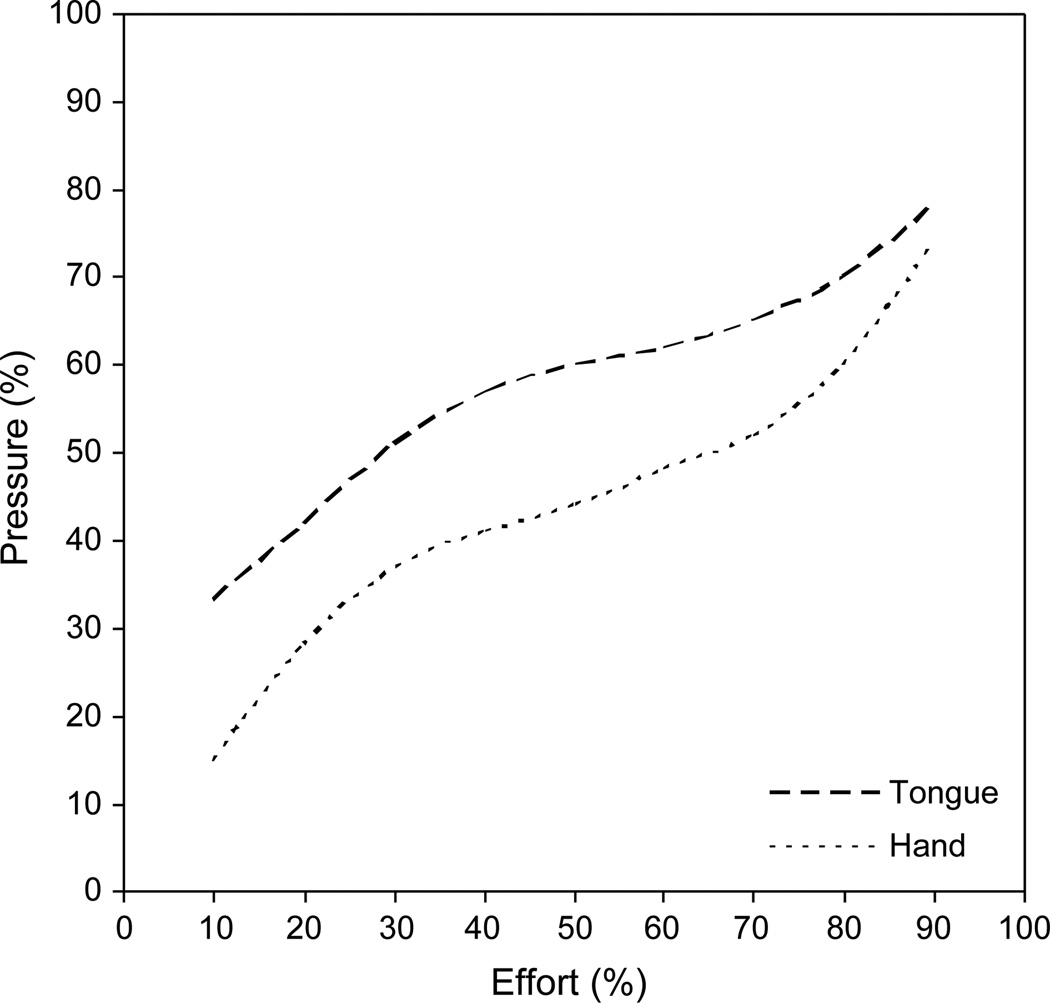
Second, subjects squeezed air-filled bulbs by the tongue and hand to various levels of effort. Previously, healthy young adults performed this task and demonstrated a systematic relationship between perceived effort and measured output that was represented by a 3rd-order polynomial curve [17]. This relationship for the tongue and hand is illustrated in Fig. 1; the curves represent the predicted mean data. Note the steeper slopes of both curves away from midrange effort levels (~ 50–80%), suggesting that the ability to discriminate effort levels at either extreme is more refined than within the midrange. Because speech is typically produced with low effort (10–25% of maximum [18, 19]), Somodi et al. [17] speculated that normal speech depends upon fine discrimination of effort perception. Patients with an abnormal effort sense may have difficulty with fine motor control needed for well-coordinated speech. Pilot data with PD patients revealed markedly aberrant responses on this task [20].
Fig. 1.
Estimated mean response curves for the percent-effort (%E) task for the tongue (dashed line) and hand (dotted line) based on 20 normal young adults (modified from Somodi et al. [17]).
Third, subjects exerted pressure on the bulbs at a constant, submaximal (50% of maximum) effort level. This constant-effort task was initially proposed as a physiologic indicator of fatigue, presumably of central origin. Fatigue is usually considered to be of either peripheral or central origin, referring to the locus of force failure in the nervous system. Central fatigue indicates failure with descending neural drive to the lower motor neuron pool, usually reflected by an awareness of effort. One tactic for implicating central fatigue is to rule out peripheral fatigue mechanisms, as has been done for patients with PD [21,22].
People are able to differentiate the perceptions of effort and output level (e.g. force, pressure, heaviness) [8,23,24]. Given that effort increases as task output level remains constant, output can be expected to decrease as effort remains constant. Indeed, when neurologically normal young adults were instructed to maintain a constant submaximal effort with the tongue and hand, the majority of trials were represented by an exponentially decaying function to a positive asymptote [25,26]. When these normal subjects acutely fatigued the tongue and hand, the curve decay was steeper, indicated by a smaller time constant. It is possible that patients who experience chronically fatiguing disorders may exhibit similar differences.
The purpose of this study was to compare performance on these three effort tasks between patients with PD and matched neurologically normal control subjects. Predictions included: (1) greater DME ratings of effort; (2) smaller terms in the polynomial equations for the sense-of-effort task, reflecting less differentiation of pressure across effort levels (i.e. flatter extremes), and (3) smaller time constants (i.e. steeper exponential decay of pressure) on the constanteffort task for PD subjects. Each of these findings would be consistent with greater-than-normal sense of effort, presumably corresponding with abnormal fatigue.
2. Methods
2.1. Subjects
Sixteen adults (ages 54–84, 12 men and 4 women) who had Parkinson’s disease (PD) and 16 adults with normal neurologic histories participated in this study. Subjects in each group were matched one-to-one for sex, age (± 3 years), weight (± 12 kg), and height (± 9 cm). PD severity while optimally medicated ranged from Hoehn & Yahr Stages 2–4, and UPDRS-Part 3 (Motor Examination) scores 18–46.5 (see Appendix for individual scores). Subjects scored ‘highly probable normal’ for dementia [27]. These persons participated in a larger investigation, and were described in more detail previously [10]. Subjects provided written informed consent, as approved by the Institutional Review Board of the University of Iowa.
2.2. Instrumentation
The Iowa Oral Performance Instrument (IOPI, Blaise Medical) was used for all measures of the tongue and hand. Pressure exerted on air-filled bulbs was detected and displayed digitally and by an LED display on the IOPI, and output to a laptop computer via a custom-designed hardware interface (18.4 mV/kPa amplification; A:D 8-bit conversion; 88 Hz sampling rate). The output is linear up to at least 250 kPa, well within the functional range needed for this study. The tongue bulb was a pliable airfilled plastic bulb, with dimensions 3 × 1.5 × 1 cm. The hand bulb was a 10-mL rubber syringe bulb filled with water that surrounded a 1-mL air-filled pipette bulb. Tubing from each bulb was coupled with the IOPI’s pressure transducing circuitry.
2.3. Procedures
Testing for control participants occurred in a clinic, office, or home setting. PD participants were tested in their homes or, in three cases, a home-like environment to reduce anxiety and transportation concerns. Data were collected during an optimal-response portion of their drug cycle (30– 90 min after levodopa ingestion). The role of antiparkinsonism medications on the sense of effort is not known, although one study indicated that levodopa did not affect perceived exertion and effort during a continuous exercise task in persons with PD [28].
Sense of effort was assessed three ways: (1) direct magnitude estimation (DME), (2) percent effort (%E), and (3) constant effort (CE). Additionally, the determination of strength was necessary to establish a target performance level for the CE task. The %E and CE tasks were performed by the tongue and preferred hand. The order of using the tongue and hand for these tasks alternated between participants. The order of the tasks was fixed so that the groups had similar experiences before each task, allowing for direct comparison of tasks across groups. Differential carry-over of task effects between groups cannot be ruled out, however.
2.3.1. Direct magnitude estimation (DME)
Participants verbally provided a rating for perception of effort in general (i.e. for performing activities of daily living) and effort for speaking. They were instructed to use direct magnitude estimation (DME) with 100 as the modulus, or reference value, for ‘no particular effort.’ An example of a direct multiplier was provided (e.g. If you generally feel twice as much effort as you would if you were feeling no particular effort, then you’d say ‘200’.).
2.3.2. Strength (Pmax)
Tongue strength was assessed by asking the participant to ‘squeeze as hard as you can’ by elevating the tongue blade against the IOPI tongue bulb, placed posterior to the maxillary alveolar ridge and lengthwise along the hard palate. The incisors rested lightly on the attached tubing, serving to stabilize the jaw in a position found to be optimal for this measure [29]. For the hand, the IOPI hand-bulb was placed in the palm with the fingers wrapped around it in a fist configuration. The best performance (highest pressure) over three trials was used.
2.3.3. Percent effort (%E)
Participants briefly squeezed the IOPI bulbs at 10 levels of effort, from 10 to 100% in 10% increments, randomly ordered without replacement, according to the procedures described by Somodi et al. [17]. The investigator instructed the participant to ‘squeeze to x% of your maximum effort.’ A cardboard replica of a thermometer marked at 10% increments was presented as a visual aid in the interpretation of effort levels. A sliding red bar was adjusted on the thermometer for each effort level requested during this task. Subjects performed this task three times for each of the 10 effort levels. No feedback was provided.
2.3.4. Constant effort (CE)
The CE task was conducted according to the method published previously [25]. Subjects squeezed the bulb at a level corresponding to 50% Pmax, viewing the LED display on the IOPI to achieve the desired starting level. Subjects then were instructed to ‘close your eyes and keep the level of effort the same.’ The investigator terminated trials once a pressure asymptote was achieved, usually after 30–60 s. The CE task was conducted three times each in alternating order with the tongue and the hand, with rest periods provided between each trial. Occasionally, extra trials (totaling six for the hand, 32 for the tongue) were recorded if the pressure signal appeared too variable or if the subject reported difficulty with the task.
2.4. Data reduction and analysis
2.4.1. Direct magnitude estimation
Ratings for generalized and speech-related effort ranged from 100 to infinity; ratings of ‘infinity’ were assigned a value of 1000 for analysis purposes, because no ratings exceeded this value and the analysis depended only on ranks. An analysis of variance on ranked data (Kruskal–Wallis procedure) compared group DME ratings for general-effort and speech-effort (α = .05). Significant differences were examined for multiple-pairwise comparisons with the Tukey Test.
2.4.2. Percent effort
The digitized pressure data were calibrated and displayed (in kPa). The pressure generated for each effort was determined by a peak-picking algorithm (software by DATAQ, Akron, OH) and verified against values recorded from the digital display on the IOPI during data-collection sessions. The pressure data were calculated as a percentage of the subject’s Pmax and plotted against the targeted effort level to create a curve for each trial.
A random coefficient regression analysis [30] was used to fit a 3rd-order polynomial equation, y = a0 + a1x + a2x2 + a3x3, where y equals the percentage of the maximum pressure and x equals the percentage of effort attempted. This equation modeled the relationship of percent maximum pressure on the target effort level studied previously [17] (Fig. 1), and was examined for its appropriateness to fit the present data by testing for the significance of the cubic term (H0: a3 = 0). The fitted regression curves for the two structures (hand and tongue) and two subject groups (PD and control) were compared within each pair of subjects. A large-sample F-test approximation compared structures and groups (α = .05); caution is warranted when interpreting these results because of the relatively small sample size.
2.4.3. Constant effort
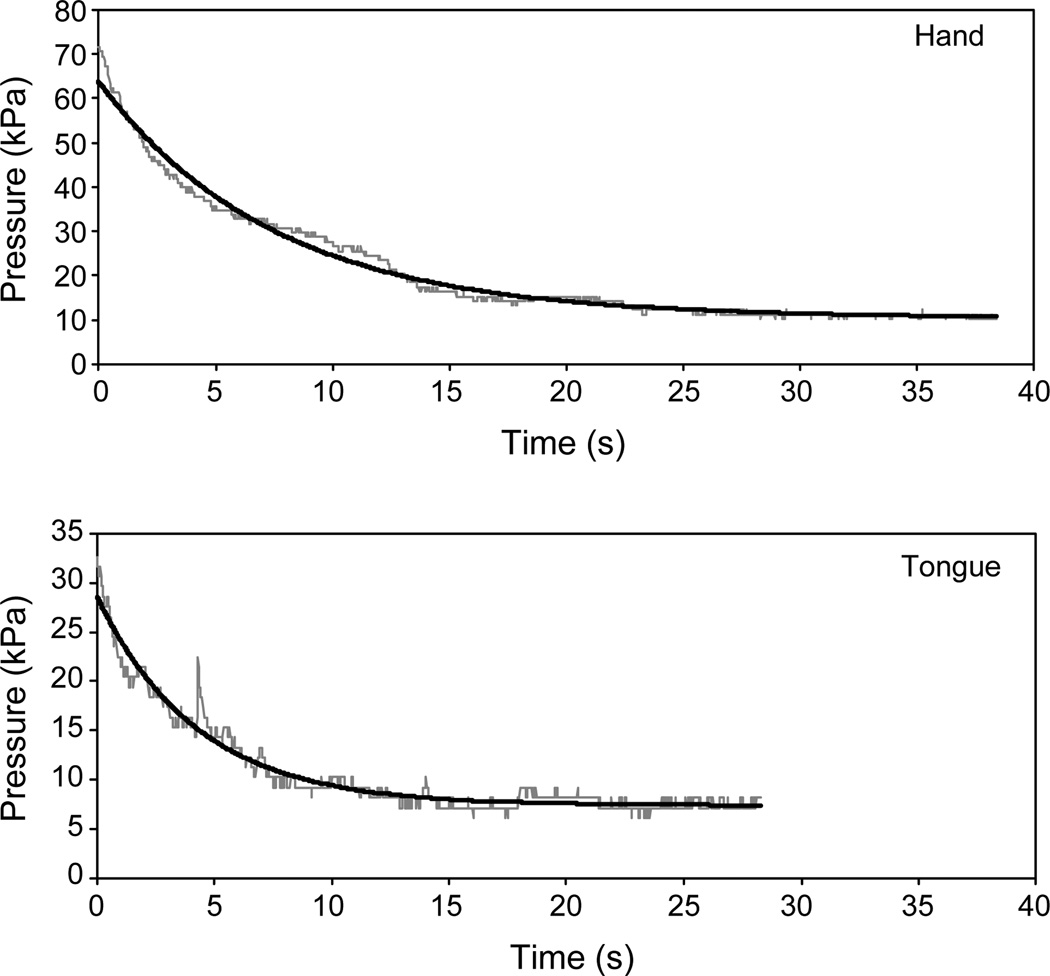
CE trials resulted in pressure-by-time curves usually characterized by an exponential decay to a non-zero asymptote. Fig. 2 provides an example of CE trials for the tongue and hand by one PD subject. The raw pressure data are plotted in gray. As described previously [25], the curves were fitted to the equation F(t) = e−at + b + c, where a represents the rate of pressure decay, c is the asymptote or residual pressure, and b is the antilog of e − c, from which the y-intercept [the value of F(t) at t = 0] can be determined. These fitted functions for the two trials are plotted in black in Fig. 2. Estimation of the parameters a and b differed from the previous study in that the first half of the pressure curve was used rather than two points at the beginning and two points in the middle of the file. The parameter c was estimated, as before, from the data in the last 5 s of the file. Modifications in parameter estimation for the optimization algorithm were made to accommodate the higher sampling rate (88 vs 4 Hz), which resulted in greater jitter in the pressure signal.
Fig. 2.
Constant-effort trials at 50% of maximum effort performed by the hand (A) and the tongue (B) by one subject with Parkinson’s disease. The raw pressure signal is plotted in gray, and the fitted exponential curve is black. The time constants (TC) for the exponential functions are 7.5 s (r2 = .98) for the hand, and 4.3 s (r2 = .96) for the tongue.
The time constant (TC), defined as the inverse of the parameter a in the fitted exponential equation, was used to characterize the rate of declining pressure early in each trial as effort was held constant. The TC essentially represents the amount of time it takes for the pressure curve to decrease to approximately 1/3rd of its total excursion. For the trials illustrated in Fig. 2, TCs were 7.5 s for the hand and 4.3 s for the tongue. TCs from multiple trials generated by each subject were averaged for analysis. Because the TC data for the PD tongue and hand, and the control tongue, were normally distributed (Kolmogorov–Smirnov distribution, p > .05), the TC data were analyzed with repeated-measures ANOVA with group, structure, and trial as within-subjects factors. Group was defined as a within-subjects factor to allow for paired comparisons of matched participants. Including structure and trial allowed hand vs tongue comparisons and examination of trial-order effects within individuals.
3. Results
3.1. Direct magnitude estimation
DME ratings of generalized effort and speaking effort were significantly greater for the participants with PD than for the control subjects (Table 1). Pairwise comparisons revealed significant differences (p < .05) between groups for both generalized effort and speech-related effort, but no significant differences between the two effort descriptors within each subject group.
Table 1.
Summary and inferential statistics for PD and control participants’ ratings of effort for general daily activities and effort for speech based on direct magnitude estimation (DME). The modulus, or reference value, for ‘no particular effort’ was 100
| Parkinson’s disease | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| Effort | Median | 25%–75% C.I | Median | 25%–75% C.I | ||||
| General | 192.5 | 137.5–300.0 | 105.0 | 100.0–127.5 | ||||
| Speech | 200.0 | 145.0–350.0 | 100.0 | 100.0–100.0 | ||||
| Kruskal–Wallis one way ANOVA on ranks: H(df=3)=31.176, p<0.001 | ||||||||
| Pairwise comparison (Tukey) | Difference of ranks | q | ||||||
| PD vs control | ||||||||
| General effort | 312.0 | 4.189* | ||||||
| Speech effort | 466.0 | 6.257* | ||||||
| General vs speech effort | ||||||||
| PD | 17.0 | 0.228 | ||||||
| Control | 137.0 | 1.840 | ||||||
p < .05.
Note. The multiple comparisons on ranks do not include an adjustment for ties.
3.2. Percent effort
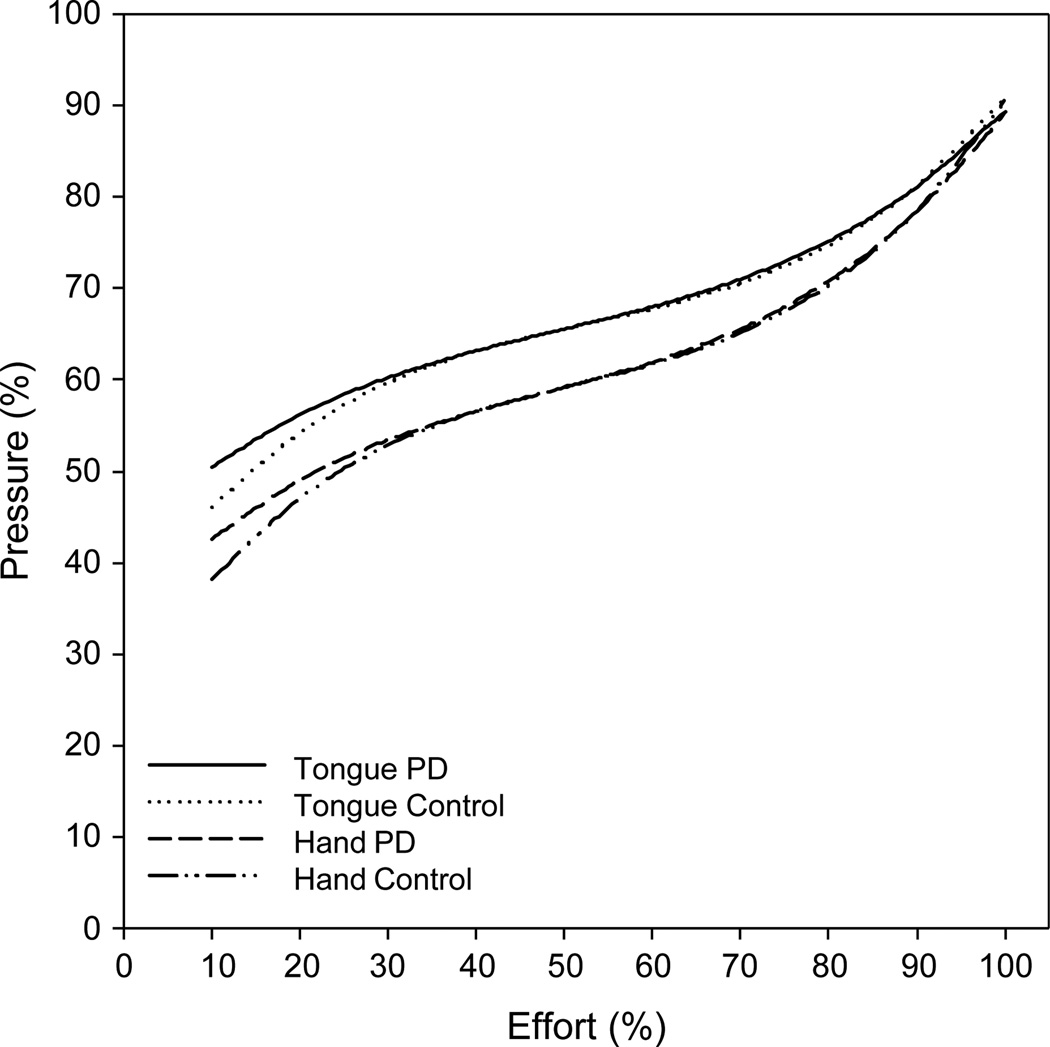
Fig. 3 illustrates the predicted mean data for the %E task performed by the hand and tongue for the two groups of subjects. The data sets from each group and each structure fit the 3rd-order polynomial equation. The curves appear similar between structures and, especially, groups.
Fig. 3.
Third-order polynomial curve fitted to the pressure data (relative strength, as a percentage of maximum pressure) for mean perceptions of various levels of effort by the hand and tongue for subjects with Parkinson’s disease and neurologically normal control subjects.
Testing the fitted regression curves for a trial effect revealed no statistically significant differences (F = 1.35; df = 8,8; p = .339). Therefore, trial was removed from the model for further analysis. Statistically significant differences were not detected for the main effects of structure (F = 1.47; df = 4,12; p = .271) or group (F = 0.51; df = 4,12; p = .732), nor was there a significant group × structure interaction (F = 0.20; df = 4,12; p = .934).
3.3. Constant effort
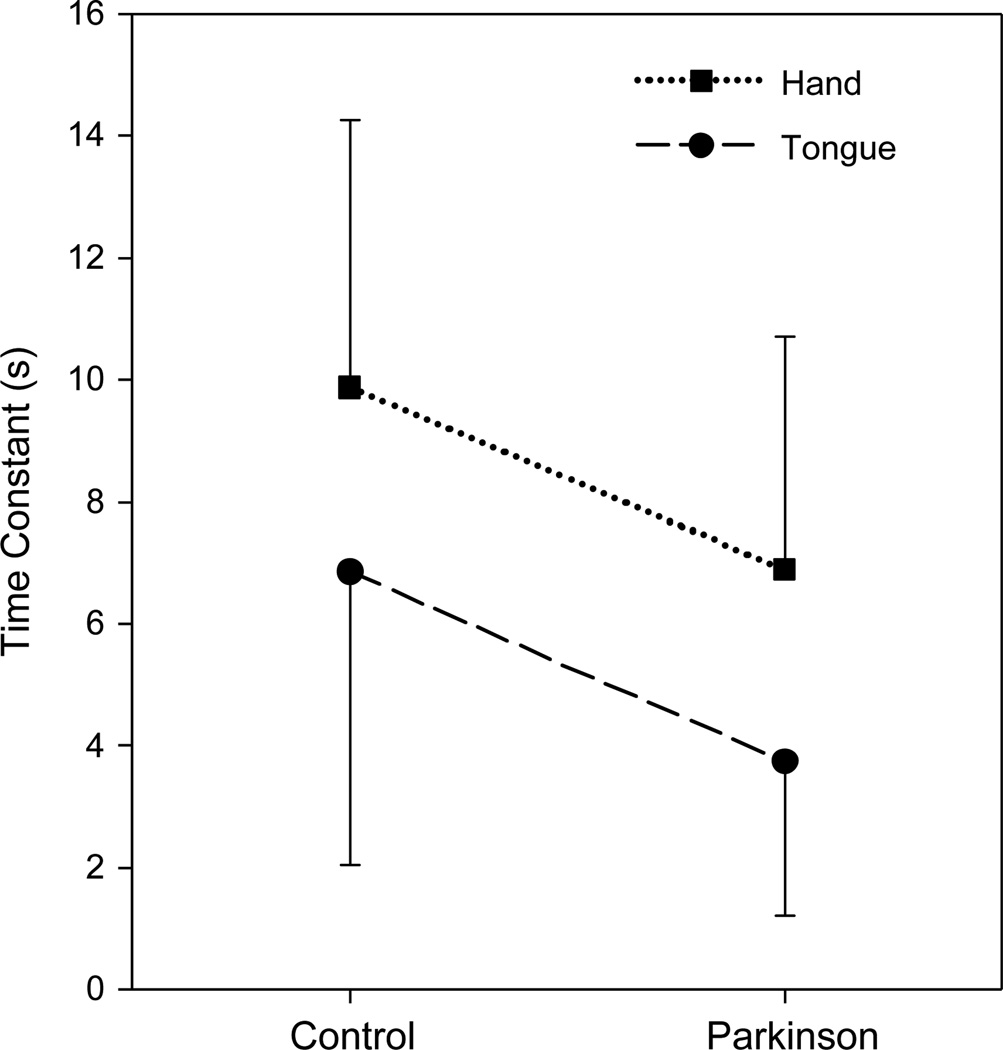
Time constants did not vary systematically across trials when trial, group, and structure were included as within-subjects factors in a RM-ANOVA (F = 0.763; df = 2,9; p = .494). Therefore, TC data were averaged across trials and the ANOVA was repeated with group and structure as within-subjects factors. TC was significantly smaller for the subjects with PD than for the control subjects when collapsed across structures (F = 8.076; df = 1,15; p = .012). In addition, TC was significantly smaller for the tongue than for the hand when collapsed across subject groups (F = 21.061; df = 1,15; p < .001). Fig. 4 illustrates both of these main effects clearly. No significant interaction between group and structure was detected (F = 0.028; df = 1,15; p = .870).
Fig. 4.
Time constants (mean, in seconds) generated from fitting a declining exponential curve with a positive asymptote to the data obtained during a constant-effort task at 50% of maximum effort. Error bars represent 1 standard deviation.
These findings indicate that pressure dropped more quickly during the constant-effort task performed by PD subjects than by control subjects, and when performed by the tongue than by the hand. Despite the relatively small sample size, the observed statistical power was acceptable (.757) for the main effect of group, and high (.987) for the main effect of structure.
4. Discussion
This study investigated the hypothesis that sense of effort would be impaired in persons with PD. This was expected because fatigue is common in PD, and sense of effort increases as fatigue ensues. Two of the three sense-of-effort tasks used in this study differentiated subjects with PD from neurologically intact control subjects matched for age, sex, height, and weight. In the task involving self-ratings of effort using a DME paradigm, subjects with PD provided significantly higher ratings of effort for general daily activities and for speech. This result is consistent with a growing body of literature indicating that increased levels of effort and fatigue are prevalent in PD and that persons with the disease report that fatigue affects specific activities of daily living [1–4].
The second assessment task, for which subjects squeezed the bulb to match a particular level of effort (percentage effort, %E), did not differentiate the subject groups. Likewise, Stelmach et al. [31] used a similar task with elbow flexion, and performance by PD and normal control groups did not differ significantly. Important to the validity of the task, the present findings replicated the previously demonstrated 3rd-order polynomial relationship between perceived effort and the pressure actually exerted by the tongue and hand [17]. The observed function is characterized by relatively larger differences in pressure at the effort extremes and little difference in pressure across the midrange effort levels. Similarly, Pincivero et al. [32] described a relatively linear relationship in the midrange, but not at the extremes, of perceived exertion for knee-extension in normal adults. Another major finding from the study by Somodi et al. [17] was that higher levels of effort corresponded with lower pressure values for the hand than for the tongue. They interpreted this finding as evidence for a reduced sense of effort for the tongue, perhaps relating to the mechanisms required for fine motor control tasks. This difference for structure was not replicated in the present data.
Pilot data were reported previously using the %E task with three parkinsonian subjects, each with symptoms of generalized fatigue [20]. One patient generated relatively low pressures to reflect a wide variety of effort levels with both the tongue and hand. The remaining two patients generated the expected relationship between pressure and effort with the hand, but higher-than-expected pressures across the effort range with the tongue. In addition, variability on the task, especially when performed with the tongue, was substantially greater than expected based on the results published by Somodi et al. [17]. The present data confirmed the preliminary indication of increased variability of performance in PD, but also revealed a wider spread in the data for the normal control subjects (based on 95% confidence intervals; not illustrated in Fig. 3). Furthermore, pressures were elevated more than expected, especially at the low-end of the effort range, for both groups of subjects (Figs. 1 and 3).
The most apparent difference between the present control subjects and the normal adults tested previously was age. The women and men in the study by Somodi et al. [17] averaged 21 and 27 years of age and none was older than 50, whereas the present subjects averaged 69 years of age, and ranged in age from 54 to 84. Perhaps younger healthy adults are more sensitive to and aware of differences in relative strength than older adults. Interestingly, Weiffenbach et al. [33] reported diminished perception of local pressure applied to the dorsal tongue with aging despite no other significant changes in oral sensation (i.e. taste, temperature, viscosity). This diminished tactile sensation may correspond with decreased reliance on general sensation for normal motor function by older adults.
The final task used in this study, for which subjects were asked to maintain a constant sense of effort (CE) that corresponded to 50% of maximum effort until asked to stop (for ~ 30–90 s), succeeded in differentiating the groups. The PD subjects’ TCs for the CE task were significantly shorter than those for the control subjects, indicating that the pressure curves decreased more quickly for the PD subjects. As expected, the TCs for the tongue were significantly shorter than for the hand.
The TC results for the control group were remarkably similar to those reported previously for neurologically normal young adults [25,26]. Furthermore, the average TC for the PD tongue (3.7 s) was extremely close to those reported previously for normal young adults after they had acutely fatigued the tongue by repetitive maximal efforts (2.7 s [25], 3.9 s [26]). Similarly, the PD subjects’ average TC for the hand (6.9 s) was similar to that reported previously for one group of normal subjects after they had acutely fatigued the hand (7.8 s [25]). The similarity between time constants resulting from fatigued structures in normal subjects and rested structures in PD subjects is striking and provocative. It is reasonable to interpret these data as evidence that the TC variable reveals some aspect of fatigue in persons who experience chronic neurogenic fatigue.
Tasks designed to assess sense of effort, purported to correspond with descending motor drive, are expected to reveal central fatigue processes. This statement must be qualified with a disclaimer that it is not possible to specify the locus of fatigue with this or any other purely behavioral task. Still, the robustness of the time-constant results for normal young adults for two body structures, and the intriguing similarity between results for PD patients who report fatigue and neurologically normal subjects after inducing acute fatigue, merit further attention. An initial hypothesis is that abnormal fatigue in PD is related to central mechanisms because of the pathophysiology of the disease.
The potential role of the basal ganglia in central fatigue is receiving increasing attention in the literature. Chaudhuri and Behan [34] cite evidence of reduced motivation and higher perceived effort in PD, and include the basal ganglia as a component in a current model of central fatigue [35]. This model is consistent with other fatiguing disorders as well, because of their pathogenesis in the cortico-striato-thalamic circuit. Brain-imaging studies revealed involvement of the prefrontal cortex in patients with chronic fatigue syndrome [36], and in the prefrontal cortex and thalamus in patients with fatigue associated with multiple sclerosis [37]. Abnormal metabolic findings have also been reported in the prefrontal cortex and basal ganglia (putamen) in patients with fatigue and multiple sclerosis [38].
Rossi et al. [39] examined PD subjects for peripheral mechanisms of fatigue. Based on (tibialis anterior) muscle biopsies from a small group of PD subjects, they found signs of reduced peripheral fatigability, such that type I fibers had increased prevalence and tended to hypertrophy. These observations were corroborated by EMG findings from a larger group of PD subjects compared to normal control subjects; PD subjects had smaller changes in the rate of nerve conduction velocity and median frequency of the power spectrum during a course of stimulated contraction. These data suggest that muscle changes in PD are not a cause, but rather a response to central mechanisms of fatigue. Additional evidence for the role of the basal ganglia in the sense of effort derives from abnormal weight-matching performance by patients with Huntington’s disease [40]. These studies provide support to the hypothesis that fatigue in PD is a central phenomenon, and that the smaller TC from the current CE task could reflect central fatigue.
One potential complication in the interpretation of the CE results relates to the procedure of removing visual feedback. Vaillancourt et al. [41] demonstrated that normal control and PD subjects generally were able to maintain a sustained submaximal force (not effort) when provided with visual feedback. This task is similar to the endurance task described earlier, except that trials were terminated at 20 s. Interestingly, when subjects were asked to maintain force after removal of visual feedback, the PD subjects’ force signals decayed more rapidly than did those of the control group. The authors interpreted this difference as a decay in motor memory in PD. It is possible that neurologically normal persons have a more robust internal model of force production than do persons with PD. The CE task, in contrast, includes repeated instructions to concentrate specifically on effort throughout the trials. This procedure should reduce complications due to memory decay as well as confusion between force and effort.
In addition to providing information on fatigue and the basic mechanisms underlying fatigue in PD, the DME and CE tasks may have clinical utility. First, the DME rating scale clearly revealed differences in effort perception between the two subject groups. Whether this scale is a more sensitive or accurate reflection of self-perceived fatigue than other types of rating scales was not addressed in this study. Future research should compare and attempt to cross-validate various types of rating scales for the assessment of fatigue in PD. The CE task holds promise for eventual clinical use because the characteristic decreasing- exponential curve has now been demonstrated for subjects with PD as well as in neurologically normal older adults. Performance on this task by the subjects with PD differed from that by the normal control subjects, and resembled performance by previously studied young adults who were fatigued experimentally. This exciting observation suggests that the CE task may be useful as a physiological indicator of fatigue. As such, it may eventually replace effortful and aversive endurance procedures for this purpose. Maximum performance tasks, such as endurance, have been criticized for being excessively variable and for being susceptible to low motivation, pain aversion, and motor instability [42,43]. Nonetheless, before the constant-effort task can be implemented clinically, measurement and interpretive issues remain. Specifically, future studies should assess the impact of visual feedback, memory, and attention on performance of the CE task. Also, the sensitivity and specificity of the measure must be demonstrated to establish its validity.
In summary, this research provides support that effort perceptions are aberrant in persons who have PD. Two of the three tasks used support this conclusion for a group of subjects who previously demonstrated normal endurance on a handgrip task and somewhat lower than normal endurance on a tongue-elevation task. PD subjects rated themselves as using greater-than-normal effort for general activities as well as for speech, and they performed a constant-effort task with the hand and tongue with a more steeply sloping loss of pressure than did the matched control subjects. The constant-effort task may reveal the presence of fatigue in the subjects with PD, particularly deriving from central activation. If the constant-effort task is robust in revealing such distinctions from normal performance in a variety of fatigue-related disease populations, then it may succeed as a physiologic indicator of fatigue.
Acknowledgements
This research was supported by the American Speech-Language-Hearing Foundation and by Grants No. P60 DC00976 and R03 DC06096 from the National Institutes on Deafness and Other Communication Disorders. Data collection took place at the University of Iowa and was approved by their Institutional Review Board for Human Subjects Research. We gratefully acknowledge Douglas VanDaele and Bridget Zimmerman for their technical assistance, and Erich Luschei for his scientific guidance. The view expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or U.S. Government.
Appendix
UPDRS, Part 3—Motor Examination scores
| Subject code | PD | Control |
|---|---|---|
| A | 18.0 | 1.0 |
| B | 35.5 | 5.0 |
| C | 36.0 | 1.0 |
| D | 37.0 | 3.0 |
| E | 25.5 | 7.0 |
| F | 30.0 | 0.0 |
| G | 27.0 | 8.5 |
| H | 24.0 | 8.0 |
| I | 39.5 | 0.0 |
| J | 33.0 | 2.0 |
| K | 37.5 | 2.0 |
| L | 36.0 | 2.0 |
| M | 40.5 | 0.5 |
| N | 36.0 | 7.0 |
| O | 46.5 | 11.0 |
| P | 44.5 | 3.5 |
Note. Previously published UPDRS, Part 3 scores for these subjects were based on a total score of 56, derived by averaging subitems (e.g. right hand, left hand) within each item (e.g. finger taps) [10]; scores were recalculated according to common practice, based on a total of 108, weighting each subitem equally.
References
- 1.Friedman JH, Freidman H. Fatigue in Parkinson’s disease: a nine-year follow-up. Mov Disord. 2001;16:1120–1122. doi: 10.1002/mds.1201. [DOI] [PubMed] [Google Scholar]
- 2.Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson’s disease. Neurology. 2003;60:1119–1124. doi: 10.1212/01.wnl.0000055868.06222.ab. [DOI] [PubMed] [Google Scholar]
- 3.Herlofson K, Larsen JP. The influence of fatigue on health-related quality of life in patients with Parkinson’s disease. Acta Neurol Scand. 2003;107:1–6. doi: 10.1034/j.1600-0404.2003.02033.x. [DOI] [PubMed] [Google Scholar]
- 4.Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA. Nonmotor fluctuations in Parkinson’s disease: frequent and disabling. Neurology. 2002;29:408–413. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 5.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2002;8:193–197. doi: 10.1016/s1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 6.Krupp LB, Pollina DA. Mechanisms and management of fatigue in progressive neurological disorders. Curr Opin Neurol. 1996;9:456–460. doi: 10.1097/00019052-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Edwards RHT. Human muscle function and fatigue. In: Porter R, Whelan J, editors. Human muscle fatigue: physiological mechanisms. London: Pitman; 1981. pp. 1–18. [Google Scholar]
- 8.Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- 9.Solomon NP, Lorell DM, Robin DA, Rodnitzky RL, Luschei ES. Tongue strength and endurance in mild to moderate Parkinson’s disease. J Med Speech Lang Pathol. 1995;3:15–26. [Google Scholar]
- 10.Solomon NP, Robin DA, Luschei ES. Strength, endurance, and stability of the tongue and hand in Parkinson disease. J Speech Lang Hear Res. 2000;43:256–267. doi: 10.1044/jslhr.4301.256. [DOI] [PubMed] [Google Scholar]
- 11.Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, Cornu P, Damier P, Agid Y. Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatr. 2000;68:595–600. doi: 10.1136/jnnp.68.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krack P, Pollak P, Limousin P, Hoffmann D, Benazzouz A, Le Bas JF, Koudsie A, Benabid AL. Opposite motor effects of pallidal stimulation in Parkinson’s disease. Ann Neurol. 1998;43:180–192. doi: 10.1002/ana.410430208. [DOI] [PubMed] [Google Scholar]
- 13.Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: a review of the literature. J Commun Disord. 2000;33:59–88. doi: 10.1016/s0021-9924(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 14.Sarno MT. Speech impairment in Parkinson’s disease. Arch Phys Med Rehabil. 1968;49:269–275. [PubMed] [Google Scholar]
- 15.Ramig LO, Countryman S, Thompson L, Horii Y. A comparison of two forms of intensive speech treatment for Parkinson disease. J Speech Hear Res. 1995;38:1232–1251. doi: 10.1044/jshr.3806.1232. [DOI] [PubMed] [Google Scholar]
- 16.Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, Thompson LL. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow, up. J Neurol Neurosurg Psychiatr. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somodi LB, Robin DA, Luschei ES. Sense of effort in relation to the motor control of the tongue. Brain Lang. 1995;51:371–382. doi: 10.1006/brln.1995.1066. [DOI] [PubMed] [Google Scholar]
- 18.Barlow SM, Burton MK. Ramp-and-hold force control in the upper and lower lips: developing new neuromotor assessment applications in traumatically brain injured adults. J Speech Hear Res. 1990;33:660–675. doi: 10.1044/jshr.3304.660. [DOI] [PubMed] [Google Scholar]
- 19.Searl J. Comparison of transducers and intraoral placement options for measuring lingua-palatal contact pressure during speech. J Speech Lang Hear Res. 2003;46:1444–1456. doi: 10.1044/1092-4388(2003/112). [DOI] [PubMed] [Google Scholar]
- 20.Solomon NP, Robin DA, Lorell DM, Rodnitzky RL, Luschei ES. Tongue function testing in Parkinson’s disease: indications of fatigue. In: Till J, Yorkston K, Beukelman D, editors. Motor speech disorders: advances in assessment and treatment. Baltimore: Paul H. Brookes; 1994. pp. 147–160. [Google Scholar]
- 21.Hwang WJ, Lin TS. Evaluation of fatigue in Parkinson’s disease patients with stimulated single fiber electromyography. Acta Neurol Scand. 2001;104:271–274. doi: 10.1034/j.1600-0404.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 22.Yanagawa S, Shindo M, Yanagisawa N. Muscular weakness in Parkinson’s disease. Adv Neurol. 1990;53:259–269. [PubMed] [Google Scholar]
- 23.Burgess PR, Jones JF. Perceptions of effort and heaviness during fatigue and during the size-weight illusion. Somatosens Mot Res. 1997;14:189–202. doi: 10.1080/08990229771051. [DOI] [PubMed] [Google Scholar]
- 24.McCloskey DI, Eberling P, Goodwin GM. Estimation of weights and tensions and apparent involvement of a ‘sense of effort’. Exp Neurol. 1974;42:220–232. doi: 10.1016/0014-4886(74)90019-3. [DOI] [PubMed] [Google Scholar]
- 25.Solomon NP, Robin DA, Mitchinson SI, VanDaele DJ, Luschei ES. Sense of effort and the effects of fatigue in the tongue and hand. J Speech Hear Res. 1996;39:114–125. doi: 10.1044/jshr.3901.114. [DOI] [PubMed] [Google Scholar]
- 26.Solomon NP, Drager KDR, Luschei ES. Sustaining a constant effort by the tongue and hand: effects of acute fatigue. J Speech Hear Res. 2002;45:613–624. doi: 10.1044/1092-4388(2002/049). [DOI] [PubMed] [Google Scholar]
- 27.Eslinger PJ, Damasio AR, Benton AL. The Iowa Screening Battery for Mental Decline. Iowa City: Department of Neurology, The University of Iowa College of Medicine; 1984. [Google Scholar]
- 28.LeWitt PA, Bharucha A, Chitrit I, Takis C, Patil S, Schork MA, Pichurko B. Perceived exertion and muscle efficiency in Parkinson’s disease: l-dopa effects. Clin Neuropharm. 1994;17:454–459. doi: 10.1097/00002826-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Solomon NP, Munson B. The effect of jaw position on measures of tongue strength and endurance. J Speech Lang Hear Res. 2004;47:584–594. doi: 10.1044/1092-4388(2004/045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonesh EF, Carter RL. Efficient interference for random-coefficient growth curve models with unbalanced data. Biometrics. 1987;43:617–628. [PubMed] [Google Scholar]
- 31.Stelmach GE, Teasdale N, Phillips J, Worringham CJ. Force production characteristics in Parkinson’s disease. Exp Brain Res. 1989;76:165–172. doi: 10.1007/BF00253633. [DOI] [PubMed] [Google Scholar]
- 32.Pincivero DM, Dixon PT, Coelho AJ. Knee extensor torque, work, and EMG during subjectively graded dynamic contractions. Muscle Nerve. 2003;28:54–61. doi: 10.1002/mus.10393. [DOI] [PubMed] [Google Scholar]
- 33.Weiffenbach JM, Tylenda CA, Baum BJ. Oral sensory changes in aging. J Gerontol. 1990;45:M121–M125. doi: 10.1093/geronj/45.4.m121. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 36.Okada T, Tanaka M, Kuratsune H, Watanbe Y, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004;4:14. doi: 10.1186/1471-2377-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, Comi G. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage. 2002;15:559–567. doi: 10.1006/nimg.2001.1011. [DOI] [PubMed] [Google Scholar]
- 38.Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W, Plohmann A, Dellas S, Maguire RP, Missimer J, Radu EW, Steck A, Leenders KL. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology. 1997;48:1566–1571. doi: 10.1212/wnl.48.6.1566. [DOI] [PubMed] [Google Scholar]
- 39.Rossi B, Siciliano G, Carboncini MC, Manca ML, Massetani R, Viacava P, Muratorio A. Muscle modifications in Parkinson’s disease: myoelectric manifestations. Electroenceph Clin Neurophys. 1996;101:211–218. doi: 10.1016/0924-980x(96)94672-x. [DOI] [PubMed] [Google Scholar]
- 40.Lafargue G, Sirigu A. Sensation of effort is altered in Huntington’s disease. Neuropsychologia. 2002;40:1654–1661. doi: 10.1016/s0028-3932(02)00033-7. [DOI] [PubMed] [Google Scholar]
- 41.Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson’s disease. Neuropsychologia. 2001;39:1410–1418. doi: 10.1016/s0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- 42.Vøllestad NK. Measurement of human muscle fatigue. J Neurosci Meth. 1997;74:219–227. doi: 10.1016/s0165-0270(97)02251-6. [DOI] [PubMed] [Google Scholar]
- 43.Kent RD, Kent JF, Rosenbek JC. Maximum performance tests of speech production. J Speech Hear Disord. 1987;52:367–387. doi: 10.1044/jshd.5204.367. [DOI] [PubMed] [Google Scholar]