Abstract
Bone morphogenetic protein-3 (BMP) has been identified as a negative regulator in the skeleton as mice lacking BMP3 have increased bone mass. To further understand how BMP3 mediates bone formation, we created transgenic mice overexpressing BMP3 using the type I collagen promoter. BMP3 transgenic mice displayed spontaneous rib fractures that were first detected at E17.0. The fractures were due to defects in differentiation of the periosteum and late hypertrophic chondrocytes resulting in thinner cortical bone with decreased mineralization. As BMP3 modulates BMP and activin signaling through ActRIIB, we examined the ribs of ActRIIB receptor knockout mice and found they had defects in late chondrogenesis and mineralization similar to BMP3 transgenic mice. These data suggest that BMP3 exerts its effects in the skeleton by altering signaling through ActRIIB in chondrocytes and the periosteum, and this results in defects in bone collar formation and late hypertrophic chondrocyte maturation leading to decreased mineralization and less bone.
Keywords: BMP3, ActRIIB, periosteum, ribs, chondrocytes
INTRODUCTION
Bone morphogenetic proteins (BMPs) are expressed in skeletal tissues and deposited in bone and cartilage where they act as regulators of skeletal development and maintenance of bone mass in adults. BMPs and other members of the transforming growth factor-beta (TGF-β) superfamily transduce signals through complexes of type I and type II serine/threonine kinase receptors. BMP ligands selectively use the type I receptors Alk2, Alk3, and Alk6 and the type II receptor, BMPR-II while sharing the other type II receptors, ActRIIA and ActRIIB with activin-like ligands. Upon BMP binding, type II receptors phosphorylate type I receptors. The activated type I receptor subsequently phosphorylates receptor regulated Smads (R-Smads 1, 5, 8) that then bind to Smad4 (Shi and Massague, 2003; Massague et al., 2005). This complex translocates into the nucleus and regulates the transcription of target genes.
BMP signaling is essential for multiple aspects of chondrogenesis and endochondral bone formation. BMPs are required at the earliest stages of skeletogenesis for mesenchymal cell compaction and condensation and to maintain Sox9 expression in the embryonic limb (Pogue and Lyons, 2006; Barna and Niswander, 2007). At later stages of endochondral ossification, BMPs and their receptors promote chondrocyte proliferation and survival and are necessary for the completion of hypertrophic differentiation, transducing signals in the growth plate through Smad1 and Smad5 (Kobayashi et al., 2005; Yoon et al., 2006; Retting et al., 2009). For proper bone development to occur, BMP activity must be precisely controlled. BMP signaling is regulated by ligand antagonists such as noggin, chordin, or gremlin that bind to BMPs and prevent them from interacting with their receptors (Balemans and Van Hul, 2002), and by receptor antagonists like inhibin and BMP3 that bind to type II receptors preventing BMP proteins from initiating signal transduction on target cells (Wiater and Vale, 2003; Gamer et al., 2005; Allendorph et al., 2007). Although considerable information exists on the effects of ligand antagonists on bone formation, there is little data available on what role receptor antagonists, in particular BMP3, play in skeletal development. Previously, we showed that BMP3 is a negative regulator of bone formation as adult BMP3 null mice display an increased bone mass phenotype (Daluiski et al., 2001).
To further our understanding of how BMP3 influences bone formation, we created transgenic mice overexpressing BMP3 using the 3.2-kb collagen I promoter (Rossert et al., 1995). BMP3 transgenic mice developed spontaneous rib fractures starting at embryonic day (E) 17.0 that resulted from defects in bone collar formation and late hypertrophic chondrocyte differentiation. Endochondral ossification was delayed in rib and resulted in thinner cortical bone that had decreased mineralization. When we analyzed the ribs of ActRIIB null mice, we found that they had a similar phenotype to the BMP3 transgenics, suggesting that BMP3 can exert its effects on the skeleton by altering BMP signaling through ActRIIB.
RESULTS
BMP3 Marks the Developing Periosteum and Osteoblasts
The expression of BMP3 in limb bones and ribs was examined by section in situ hybridization. In the developing skeleton at E14.5, BMP3 expression was restricted to the perichondrium that outlines the central region of the forming bone (Fig. 1A,B). At E16.5, BMP3 localized to the inner layer of the forming periosteum where the osteoprogenitors are located and was also strongly expressed by newly differentiated osteoblasts in the center of the bone (Fig. 1C,D). In E16.5 rib, BMP3 was highly expressed in osteoblasts and in the periosteum that surrounds the bone (Fig. 1E,F). In addition, BMP3 was detected in the perichondrium in a region that surrounds the hypertrophic chondrocytes with expression decreasing distally going from prehypertrophic to proliferating chondrocyte zones of the growth plate (Fig. 1E,F). At all stages analyzed in long bone and rib, BMP3 was not detected in chondrocytes. The dynamic localization pattern of BMP3 in the bone collar and osteoblasts suggested to us that it may play a unique role in regulating bone formation.
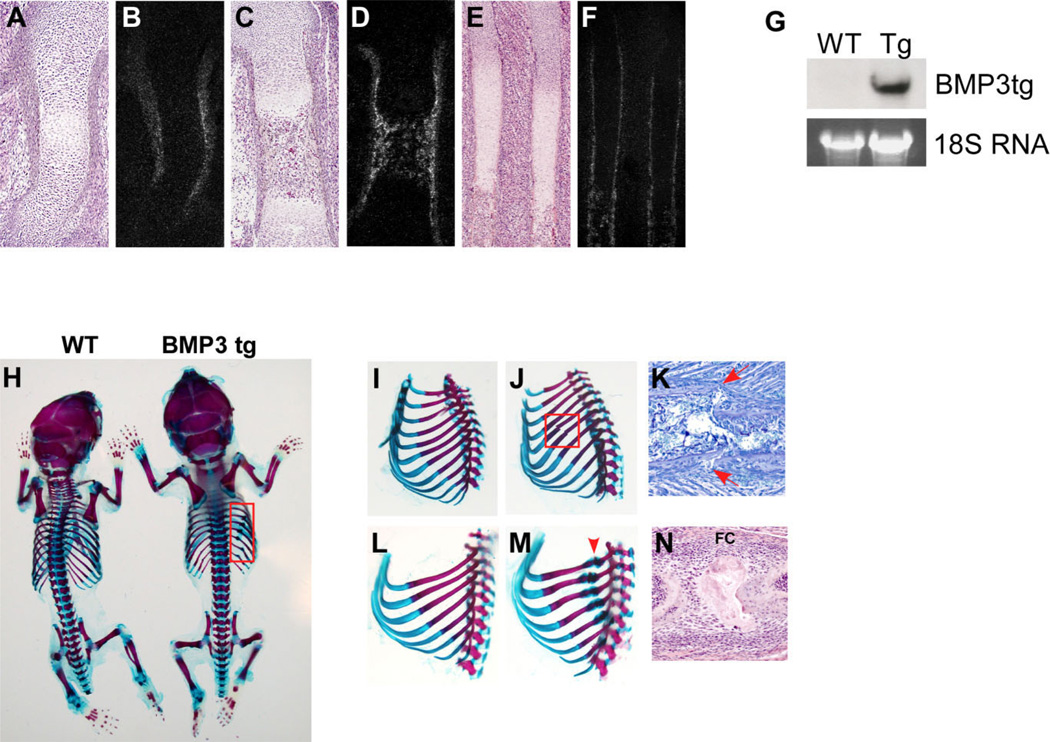
Fig. 1.
Bone morphogenetic protein-3 (BMP3) transgenic mice have spontaneous rib fractures. A–F: Radioactive in situ hybridization for BMP3 in long bones and ribs of embryonic day (E) 14.5 and E16.5 embryos. A,B: In the E14.5 humerus, BMP3 localizes to the perichondrium outlining the forming bone. C,D: In the E16.5 femur, BMP3 is detected in the inner layer of the periosteum and is also expressed by newly differentiated osteoblasts in the center of the bone. E,F: In E16.5 ribs, BMP3 is strongly expressed in the periosteum and by osteoblasts. BMP3 is also found in the perichondrium in a restricted area that surrounds the hypertrophic chondrocytes and part of the prehypertrophic chondrocyte zone. G: RNA was extracted from pooled long bones of 2 week old wild-type (WT) and BMP3 transgenic (Tg) mice and hybridized with a probe specific for the human BMP3 transgene (BMP3tg). The 18S rRNA band was used as a loading control. H: Alizarin Red and Alcian Blue staining of whole skeletons from newborn wild-type and BMP3 transgenic mice. No obvious skeletal patterning or development differences are detected but BMP3 transgenic mice display multiple spontaneous rib fractures (boxed area). I–N: Whole-mount ribs from E17.0 and Newborn mice stained with Alizarin Red and Alcian Blue. I: E17.0 wild-type ribs do not have any breaks. J: The E17.0 ribs from BMP3 transgenic mice have two fractures (boxed area). K: Toluidine blue stained rib from E17.5 BMP3 transgenic animal showing new fracture (arrows). L: Ribs from newborn wild-type mice appear normal and have no breaks. M: Ribs from newborn BMP3 transgenic animals display healing fractures (arrowhead points to healing breaks with fracture calluses) in most of the ribs. N: Hematoxylin and eosin stained rib from a newborn BMP3 transgenic animal showing the healing fracture and fracture callus (FC).
BMP3 Transgenic Mice Have Multiple Rib Fractures and Defects in Chondrocyte Maturation and Bone Collar Formation
The effects of increased BMP3 expression in the skeleton were analyzed using transgenic mice in which a human BMP3 transgene was driven by the 3.2-kb fragment of the mouse α (1) I collagen promoter. This targets BMP3 expression to the perichondrium, periosteum, osteoprogenitors, osteoblasts, odontoblasts, and tendon starting at E14.5 (Rossert et al., 1995). Two independent transgenic lines with analogous phenotypes were generated and one was studied in detail and described here. Expression of the transgene in the skeleton was confirmed by Northern blot analysis of RNA extracted from pooled long bones (femur, tibia, humerus, radius, and ulna) using a probe specific for the BMP3 transgene. The BMP3 transgene was detected in bone from 2-week-old transgenic mice and not wild-type littermate controls (Fig. 1G).
BMP3 transgenic mice were viable and survived into adulthood. Analysis of whole skeletal preparations of newborn BMP3 transgenic mice showed no obvious defects in skeletal patterning or development as the shape, location, and size of all skeletal elements appeared normal (Fig. 1H). However, the BMP3 transgenic mice displayed multiple spontaneous rib fractures along both sides of the rib cage (Fig. 1H,M). Breaks in the BMP3 transgenic ribs first occurred at E17.0 in two or three ribs located in the center of the rib cage (n = 4; Fig. 1J). At birth, most ribs had fractured and were in the process of healing as indicated by the presence of a fracture callus (Fig. 1M,N). By 2 weeks of age, the BMP3 transgenic ribs had healed (data not shown).
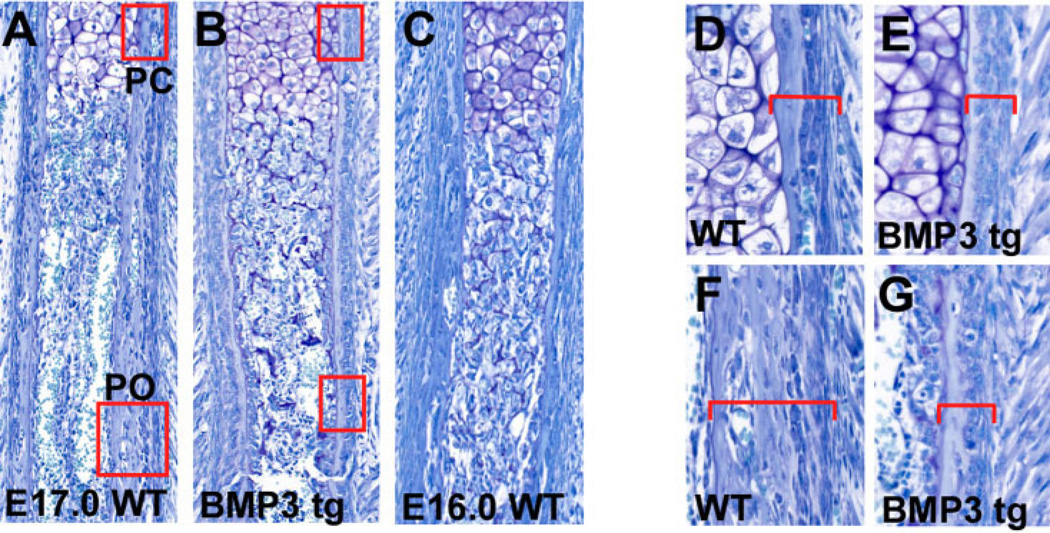
We next performed detailed histological analysis of BMP3 transgenic mice from E16.0 to newborn and found that increased BMP3 expression affected the proper formation of the bone collar. At E17.0, the rib perichondrium and periosteum of mice overexpressing BMP3 was thinner and more cellular when compared with wild-type controls (n = 3; Fig. 2A–G). At this stage, the differentiation of the bone collar of BMP3 transgenic ribs appeared delayed, and resembled the bone collar seen in a wild-type E16.0 rib (compare Fig. 2B and C).
Fig. 2.
Ribs from bone morphogenetic protein-3 (BMP3) transgenic mice have defects in bone collar formation. A,B: Toluidine blue stained ribs from E17.0 wild-type (WT) and BMP3 transgenic (BMP3 tg) mice. The perichondrium (boxed area, PC) and periosteum (boxed area, PO) of the BMP3 transgenic rib are thinner and more cellular than the wild-type rib. C: The BMP3 transgenic rib appears delayed in its differentiation and resembles the E16.0 rib shown. D,E: Higher power view of the boxed regions in A and B showing the morphology of the perichondrium (brackets) of wild-type and BMP3 transgenic ribs at E17.0. F,G: Higher power view of the boxed regions in A and B showing the periosteum (brackets) of E17.0 wild-type and BMP3 transgenic ribs.
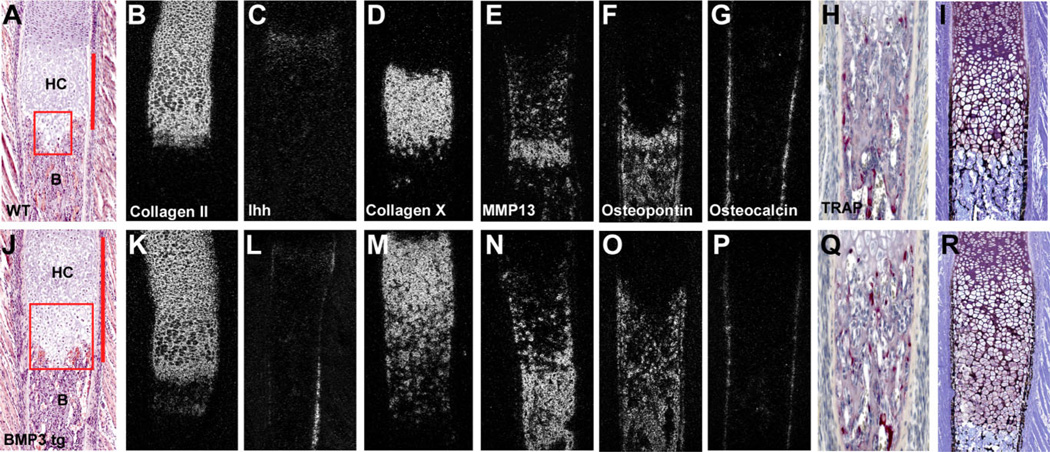
The perichondrium and periosteum are important structures for controlling proper cartilage growth and differentiation during bone formation (Long and Linsenmayer, 1998; Di Nino et al., 2001; Colnot et al., 2004). The defects in the bone collar of BMP3 transgenics prompted us to look for changes in chondrocyte maturation in the ribs. We found that the growth plate region of E17.0 ribs in BMP3 transgenics was not significantly different from wild-type controls; however, the ribs of newborn BMP3 transgenic mice had expanded zones of hypertrophic chondrocytes. These zones were 34% longer (control rib = 0.40 ± 0.05 mm; BMP3 tg rib = 0.60 ± 0.04 mm; n = 5 each; P < 0.0001) than those of wild-type control ribs and contained increased numbers of cells with tightly packed nuclei, a characteristic feature of late hypertrophic chondrocytes (Fig. 3A,J). The expansion of the hypertrophic zone in the BMP3 transgenic ribs was not due to changes in cell proliferation, apoptosis, or angiogenesis (data not shown) but appeared to be a result of defects in chondrogenesis that led to an accumulation of terminally differentiated chondrocytes. In situ hybridization analysis of molecular markers of chondrocyte maturation confirmed these results. Newborn BMP3 transgenic ribs had increased levels and expanded expression domains of type II collagen and type X collagen as well as the late hypertrophic chondrocyte markers, MMP13 and osteopontin (Fig. 3B–O). Ribs from BMP3 transgenics also displayed decreased levels of osteocalcin indicative of defects in osteoblasts differentiation (Fig. 3G,P). When we looked for the presence of osteoclasts using TRAP staining, we saw no obvious changes in osteoclast number, location, or appearance in BMP3 transgenic ribs when compared with wild-type controls, suggesting the defects in bone formation we observed were not coupled to changes in osteoclast formation or activity (Fig. 3H,Q). Further histological analysis of newborn mice revealed that BMP3 transgenic ribs had thinner cortical bone with less mineral when compared with controls as seen by Von Kossa staining (n = 3; Fig. 3I,R). Of interest, our in situ hybridization studies on chondrocyte and osteoblast markers also revealed a consistent up-regulation of Ihh along the periosteum of BMP3 transgenic ribs (Fig. 3C,L). This may be a direct effect of BMP3 overexpression altering rib periosteal function or may reflect the fracture healing response in the broken ribs, as Ihh has been shown to play a role in early periosteal bone repair (Le et al., 2001).
Fig. 3.
A–R: Ribs of bone morphogenetic protein-3 (BMP3) transgenic mice have defects in chondrocyte maturation and mineralization. A–J: Hematoxylin and eosin stained ribs from newborn wild-type and BMP3 transgenic mice. BMP3 transgenic mice have an expansion of the hypertrophic chondrocyte (HC) zone (compare lengths indicated by red bars) and increased numbers of late hypertrophic chondrocytes (indicated by red boxed areas containing cells with dark, tightly packed nuclei). B, bone. B–G,K–P: Radioactive in situ hybridization analysis of markers for chondrogenesis and osteogenesis from ribs of newborn wild-type (B–G) and BMP3 transgenic mice (K–P) at birth. B,K: BMP3 transgenic ribs have expanded zones of type II collagen. C,L: BMP3 transgenic ribs have normal levels of Ihh in chondrocytes but increased levels in the periosteum. D,M: BMP3 transgenic ribs have an expanded expression domain of type X collagen. E,N: BMP3 transgenic ribs have increased levels and expanded expression of MMP13. F,O: BMP3 transgenic ribs have increased expression of osteopontin. G,P: BMP3 transgenic ribs have decreased expression of osteocalcin. H,Q: TRAP stained newborn wild-type and BMP3 transgenic ribs show no difference in the osteoclast population (red staining). I,R: Von Kossa staining of ribs from newborn wild-type and BMP3 transgenic mice. BMP3 transgenic ribs have thinner cortical bone with less mineral.
No Obvious Defects in Endochondral Bone Formation in Long Bones of BMP3 Transgenic Mice
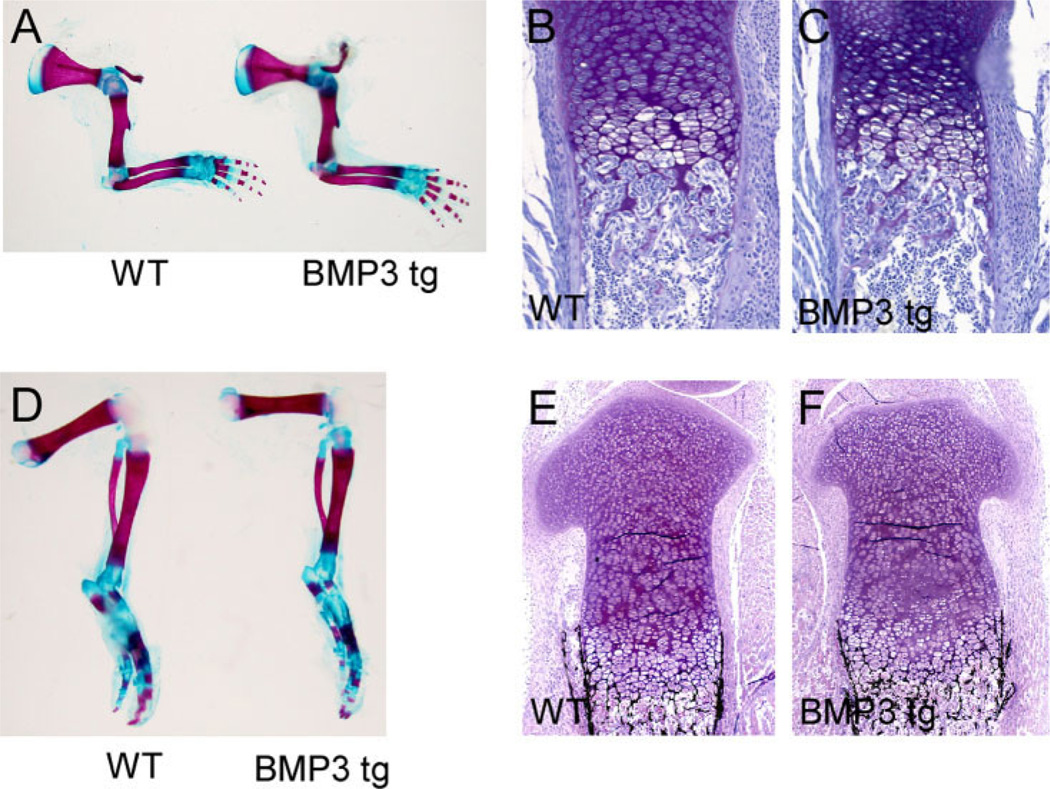
Skeletal preparations of wild-type and BMP3 transgenic mice revealed no similar spontaneous fracture phenotype in the forelimbs or hindlimbs at any stage examined (E16.0, E17.0, E18.5, and newborn; Fig. 4A,D). In addition, histological analysis of newborn BMP3 transgenic radius and tibia showed no significant changes in growth plate chondrocytes, trabecular bone or cortical bone when compared with wild-type littermates (n = 3 at each stage; Fig. 4B–F).
Fig. 4.
Long bones of bone morphogenetic protein-3 (BMP3) transgenic mice have no significant defects in endochondral bone formation. A,D: Whole-mount forelimbs and hindlimbs from newborn wild-type and BMP3 transgenic mice stained with Alizarin Red and Alcian Blue. No fractures or obvious defects in bone formation are observed. B,C: Toluidine blue staining of proximal radius from newborn mice showing no significant differences between wild-type control and BMP3 transgenic bones. E,F: Von Kossa staining of proximal tibia from newborn mice showing no significant differences in bone formation or mineralization between wild-type and BMP3 transgenic animals.
Ribs From ActRIIB Knockout Mice Have a Similar Phenotype to the BMP3 Transgenic Mice
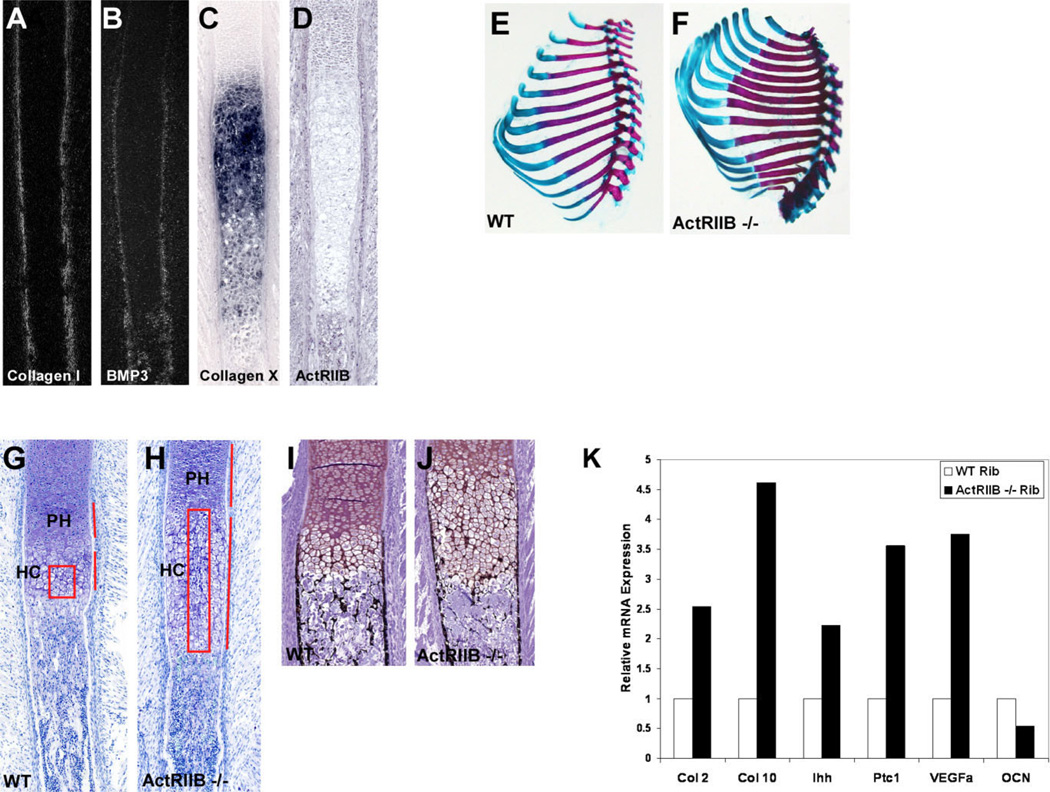
Previous studies have shown that BMP3 acts by binding to ActRIIB, a type II receptor for both BMPs and activin (Gamer et al., 2005; Allendorph et al., 2007). In the BMP3 transgenic mice, we hypothesized that increased levels of BMP3 in the skeleton alter signaling by blocking other ligands from binding to ActRIIB, creating a phenotype similar to loss of ActRIIB availability. To test this, we analyzed ribs from the ActRIIB receptor null mice focusing on the areas most affected by excess BMP3 expression. First, we examined the localization pattern of ActRIIB in developing ribs in comparison to BMP3 to determine if these factors were expressed near one another and could influence each other’s activity in bone. In E16.5 rib, ActRIIB was found in the prehypertrophic and proliferating chondrocytes but not in the type X collagen–expressing hypertrophic chondrocytes (Fig. 5C,D). ActRIIB was also detected in the developing perichondrium, periosteum, and in osteoblasts where it colocalized with BMP3 and type I collagen (Fig. 5A,B,D).
Fig. 5.
Ribs from ActRIIB knockout mice have defects in chondrocyte maturation and mineralization similar to BMP3 transgenic ribs. Expression pattern analysis of ActRIIB in comparison to BMP3, type I collagen, and type X collagen in wild-type E16.5 ribs using radioactive (A,B) and nonradioactive (C,D) in situ hybridization. A: Type I collagen marks the perichondrium and the periosteum and is also detected in the osteoblasts. B: BMP3 is detected in the periosteum, part of the perichondrium and in the osteoblasts. C: Type X collagen is expressed by hypertrophic chondrocytes. D: ActRIIB localizes to the prehypertrophic and proliferating chondrocytes and is also found in the perichondrium, periosteum, and osteoblasts. Its expression overlaps with that of type I collagen and BMP3. E,F: Alizarin Red and Alcian Blue staining of ribs from newborn wild-type (WT) and ActRIIB−/− mice showing the characteristic two extra pairs of ribs in the ActRIIB knockout animal. G,H: Toluidine Blue staining of newborn wild-type (WT) and ActRIIB−/− ribs. Ribs from ActRIIB−/− mice have elongated zones of prehypertrophic (PH) and hypertrophic chondrocytes (HC; compare lengths indicated by red bars in G and H) and increased numbers of late hypertrophic chondrocytes in the central region of the rib (compare boxed areas). I,J: Von Kossa staining of newborn wild-type and ActRIIB−/− ribs. Ribs from ActRIIB−/− mice have decreased mineral and thinner cortical bone in comparison to wild-type mice. K: Quantitative polymerase chain reaction (QPCR) analysis of chondrocyte and bone markers in wild-type (WT) and ActRIIB−/− ribs at E18.5.
Next we performed histological analysis on ribs from ActRIIB receptor null mice. ActRIIB knockout mice have axial skeletal patterning anomalies that result in vertebral transformations and extra pairs of ribs (Oh and Li, 1997). These mice have abnormally positioned heart and pulmonary arteries, as well as lung and kidney defects that result in perinatal lethality (Oh and Li, 1997). Analysis of ribs from skeletal preparations of newborn mice showed the characteristic extra pairs of ribs but no other abnormalities (Fig. 5E,F). Histological analysis of these ribs identified the presence of an elongated zone of hypertrophic chondrocytes that was 42% longer (control rib = 0.36 ± 0.02 mm; ActRIIB knockout rib = 0.62 ± 0.03 mm; n = 4 each; P < 0.0001) than the wild-type control. In addition, ActRIIB knockout ribs had increased numbers of late hypertrophic chondrocytes throughout the central region as well as an expanded region of prehypertrophic chondrocytes (n = 3; Fig. 5G,H). Von Kossa staining of newborn ribs showed decreased mineralization and thinner cortical bone in ActRIIB knockout ribs when compared with wild-type control ribs (n = 3; Fig. 5I,J). Examination of ribs from ActRIIB knockout and control embryos at E16.5–E17.5 did not reveal any obvious defects in the formation of the bone collar that were similar to the BMP3 transgenic mice at these earlier stages (data not shown). These histological results were confirmed by quantitative polymerase chain reaction (QPCR) analysis of ActRIIB knockout and wild-type ribs at E18.5. Ribs lacking ActRIIB showed increased levels of the chondrocyte markers type II collagen, Ihh, type X collagen, and VEGFa and decreased expression of the osteoblast marker, osteocalcin (Fig. 5K). The phenotype of the ribs in the ActRIIB knockout mice is similar to what we observed in the ribs of the BMP3 trangenic mice suggesting that the effects we see when BMP3 is overexpressed are due in part to blocking ActRIIB receptor utilization.
DISCUSSION
Although BMP3 is expressed in a specific and dynamic localization pattern in developing bone, how it influences the process of skeletal development is not known. To further define a role for BMP3 in the skeleton, we made transgenic mice expressing BMP3 using the type I collagen promoter. BMP3 transgenic mice had defects in bone collar formation, late hypertrophic chondrocyte maturation and mineralization that resulted in spontaneous rib fractures and thinner cortical bone. We hypothesize that BMP3 affects these skeletal cells through its target receptor, ActRIIB, as ActRIIB knockout mice had a similar rib phenotype with defects in chondrocyte maturation and mineralization. Our results suggest BMP3 functions by regulating signaling through ActRIIB-expressing cells in the skeleton to ensure proper endochondral ossification.
The phenotype we observed in the BMP3 transgenic mice appears to be due to both increased levels of BMP3 and from changing the extent of BMP3 expression along the length of the perichondrium. In the BMP3 transgenic mice, expanding this expression domain places BMP3 in contact with ActRIIB-expressing prehypertrophic and proliferating chondrocytes and enables BMP3 to affect late chondrocyte maturation by altering signaling through Act-RIIB. The increased levels of BMP3 in BMP3 transgenic mice may also be affecting signaling through ActRIIA, a lower affinity receptor for BMP3 that is also expressed in chondrocytes (Nohno et al., 1993; Matzuk et al., 1995). This may also be one of the reasons the phenotype we observed in the ribs of the BMP3 transgenic mice is not an exact phenocopy of that seen in the ribs of the ActRIIB knockouts (no bone collar defects or fractures). Analysis of ribs and long bones from ActRIIA knockout mice will be critical for understanding the role of individual type II receptors in the skeleton as BMP3 is able to bind to ActRIIA with very low affinity. Unfortunately, the consequences of loss of both receptors on bone formation cannot be studied at this time, as the ActRIIA/ActRIIB double knockout mice are embryonic lethal (Song et al., 1999), and awaits the future generation of floxed alleles for these receptors.
The perichondrium and periosteum have been shown to be important for proper cartilage growth, differentiation, and endochondral ossification. When the perichondrium is removed from developing bone, chondrocytes differentiate up to the late hypertrophic stage but do not undergo terminal differentiation or programmed cell death, resulting in expanded hypertrophic cartilage zones (Long and Linsenmayer, 1998; Di Nino et al., 2001; Colnot et al., 2004). We observed similar effects on chondrocytes in the BMP3 transgenic ribs where the increased expression of BMP3 altered the formation of and signaling from the perichondrium and periosteum. The perichondrium exerts its negative influence on chondrocyte maturation in part through Twist-1 regulation of Runx2 activity in perichondrial cells where FGF18 is a target (Hinoi et al., 2006). On the basis of our results, BMP3 may also be a target for Runx2 regulation in the perichondrium. BMP3 does not appear to be upstream of Twist-1, Runx2, or FGF18 as we did not detect any significant changes in the expression of these factors in ribs of BMP3 transgenic animals (data not shown). It is likely that the perichondrium negatively regulates chondrocyte maturation through more than one mechanism and this may include BMP3 controlling signaling through ActRIIB. The phenotype we observed in the BMP3 transgenic mice is similar to that seen in BMP3 infected chick limbs where expanded zones of hypertrophic chondrocytes and altered perichondrial formation also occur (Gamer et al., 2008). Taken together, these data suggest that the periosteum and the perichondrium and its adjacent chondrocytes are important targets for BMP3 action.
The defects in chondrocyte maturation we observed in the BMP3 transgenic and ActRIIB knockout ribs were similar to those reported for Col2-Cre Bmpr1a conditional knockout bones where altered BMP signaling in chondrocytes results in bones with elongated zones of hypertrophic chondrocytes, delayed terminal chondrocyte differentiation, and increased levels of type X collagen, MMP13, and osteopontin (Yoon et al., 2006). This concordance in phenotype suggests that Bmpr1a and ActRIIB (and perhaps ActRIIA) may be an important signaling complex during chondrocyte maturation and endochondral ossification. These data coupled with the lack of effect on osteoclasts in the BMP3 transgenic bones indicate that the BMP3 transgenic phenotype most likely results from BMP and not activin signaling defects (Fuller et al., 2000; Gaddy-Kurten et al., 2002).
The ribs are the most severely affected bones in the BMP3 transgenic mice. The simplest explanation for this difference is that ribs are one of the thinner bones in the skeleton, making them more susceptible to fracture when the periosteum and cortical bone have been altered as in the BMP3 transgenic mice. Another possibility is that signaling through Act-RIIB may be more important for proper endochondral ossification in ribs than in other bones due to differential type II BMP receptor expression. Precedence for this idea comes from recent studies by Guenther et al. (2008) showing that BMP5 plays a specific role in rib development but not in other bones in the forming skeleton.
The results presented here confirm a role for BMP3 as a negative regulator of skeletogenesis as transgenic mice with excess BMP3 have decreased bone formation and mice lacking BMP3 have increased bone mass, and highlight the unique physiological function BMP3 has in the skeleton. Through its ability to act as a receptor antagonist, BMP3 modulates the availability of ActRIIB in skeletal cells thus controlling BMP activity in a specific manner. This property of BMP3 provides the skeleton with an additional means for restricting osteogenic BMP signaling during chondrogenesis and endochondral ossification.
EXPERIMENTAL PROCEDURES
Mouse Strains
To generate transgenic mice expressing BMP3 under the control of the 3.2-kb fragment of the mouse α(1) I collagen promoter, the coding region of human BMP3 (1.4 kb) was substituted for the lacZ gene in the plasmid pJ320 (kindly provided by Dr. Benoit de Crombrugghe, University of Texas, MD Anderson Cancer Center, Houston, TX). Positive founders were identified by Southern blot analysis of EcoRV digested tail DNA. Founder mice were bred to wild-type FVB mice to generate transgenic lines. Heterozygous mice were intercrossed to generate homozygous offspring which were identified by Southern blot analysis and PCR. Two independent transgenic lines were analyzed with identical results.
ActRIIB knockout mice were kindly provided by Dr. Paul Oh (University of Florida College of Medicine, Gainesville, FL) and described previously (Oh and Li, 1997).
In Situ Hybridization
Whole-mount in situ hybridization was performed with digoxigenin-labeled antisense mouse BMP3 probe (Daluiski et al., 2001) as previously described (Brent et al., 2003). Section in situ hybridization with radiolabeled probes was performed as described (Lanske et al., 1996; Bandyopadhyay et al., 2006). 35S-labeled complementary RNA probes were transcribed from plasmids encoding BMP3, type II collagen (Kohno et al., 1984), type X collagen (Jacenko et al., 1993), Ihh (Bitgood and McMahon, 1995), MMP13 (Yamagiwa et al., 1999), osteopontin (Oldberg et al., 1986), and osteocalcin (Desbois et al., 1994). Section in situ hybridization with digoxigenin-labeled probe for ActRIIB (Matzuk et al., 1995) was performed as described (Chandler et al., 2007).
Skeletal Preparations
For whole skeletal analysis, staged embryos (the day the vaginal plug was observed was considered embryonic day 0.5–E0.5) and newborn mice were prepared and stained with Alizarin Red and Alcian Blue to identify mineralized bone and cartilage as previously described (McLeod, 1980).
Histological and Immunohistochemical Analyses
Limbs and ribs were dissected and fixed in 4% paraformaldehyde, decalcified in EDTA/PVP, paraffin embedded, sectioned, and stained with hematoxylin and eosin, Toluidine Blue and Von Kossa using standard methods. For measurement analysis of the height of the hypertrophic chondrocyte zones in the ribs, the sixth rib was chosen from newborn wild-type and BMP3 transgenic mice for comparison. Immunohistochemical detection of apoptosis, cell proliferation, or changes in angiogenesis was performed on paraffin-embedded ribs from wild-type and BMP3 transgenic newborn mice (n = 3 for each) without antigen unmasking. For analysis of apoptosis, the In Situ Cell Death Detection kit, POD (Roche) was used according to the manufacturer’s instructions. For analysis of cell proliferation, immunohistochemistry for proliferation cell nuclear antigen (PCNA) was performed using anti-PCNA antibody (Zymed) according to the manufacturer’s instructions. For analysis of vascular invasion and angiogenesis, sections were incubated with antibody to PECAM (BD Pharmigen) and signal detected using the Tyramide Signal Amplification Kit (Perkin Elmer Life Sciences).
Northern Blot and QPCR Analysis
Total RNA was isolated from long bones of 2-week-old BMP3 transgenic and wild-type mice (n = 3) or from ribs of E18.5 wild-type and ActRIIB knockout mice (n = 3 for each age) with Trizol reagent (Invitrogen) according to the manufacturer’s instructions. For Northern blot analyses, equal amounts (20 µg) of total RNA from pooled long bones were loaded on formaldehyde agarose gels following denaturation and transferred onto nylon membranes. Membranes were hybridized with 32P-labeled cDNA probe for human BMP3 transgene (SphI-HindIII fragment of full length hBMP3 cDNA). For QPCR, following Trizol isolation, total RNA was cleaned up using the RNeasy kit (Qiagen) following the manufacturers instructions for on-column DNase treatment and cDNA synthesized using the Roche Transcriptor First Strand cDNA Synthesis kit (Roche). QPCR was performed by using the Roche LightCycler 480 Real-time PCR system with probe-based detection (Universal Probe Library; Roche). Values were normalized to β-actin using the 2-ΔΔCt method (Livak and Schmittgen, 2001). Experiments were performed in triplicate.
ACKNOWLEDGMENTS
We thank Kuni Tsuji and Eleni Gagari for technical assistance with genotyping and phenotypic analysis of BMP3 transgenic mice as well as helpful discussions. This work was supported by AR50174 from NIAMS/NIH (V.R.).
REFERENCES
- Allendorph GP, Isaacs MJ, Kawakami Y, Belmonte JC, Choe S. BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry. 2007;46:12238–12247. doi: 10.1021/bi700907k. [DOI] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna M, Niswander L. Visualization of cartilage formation: insight into cellular properties of skeletal progenitors and chondrodysplasia syndromes. Dev Cell. 2007;12:931–941. doi: 10.1016/j.devcel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Chandler RL, Chandler KJ, McFarland KA, Mortlock DP. Bmp2 transcription in osteoblast progenitors is regulated by a distant 3’ enhancer located 156.3 kilobases from the promoter. Mol Cell Biol. 2007;27:2934–2951. doi: 10.1128/MCB.01609-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269:55–69. doi: 10.1016/j.ydbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- Desbois C, Hogue DA, Karsenty G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem. 1994;269:1183–1190. [PubMed] [Google Scholar]
- Di Nino DL, Long F, Linsenmayer TF. Regulation of endochondral cartilage growth in the developing avian limb: cooperative involvement of perichondrium and periosteum. Dev Biol. 2001;240:433–442. doi: 10.1006/dbio.2001.0471. [DOI] [PubMed] [Google Scholar]
- Fuller K, Bayley KE, Chambers TJ. Activin A is an essential cofactor for osteoclast induction. Biochem Biophys Res Commun. 2000;268:2–7. doi: 10.1006/bbrc.2000.2075. [DOI] [PubMed] [Google Scholar]
- Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology. 2002;143:74–83. doi: 10.1210/endo.143.1.8580. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Nove J, Levin M, Rosen V. BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Biol. 2005;285:156–168. doi: 10.1016/j.ydbio.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Ho V, Cox K, Rosen V. Expression and function of BMP3 during chick limb development. Dev Dyn. 2008;237:1691–1698. doi: 10.1002/dvdy.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther C, Pantalena-Filho L, Kingsley DM. Shaping skeletal growth by modular regulatory elements in the Bmp5 gene. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000308. e1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoi E, Bialek P, Chen YT, Rached MT, Groner Y, Behringer RR, Ornitz DM, Karsenty G. Runx2 inhibits chondrocyte proliferation and hypertrophy through its expression in the perichondrium. Genes Dev. 2006;20:2937–2942. doi: 10.1101/gad.1482906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacenko O, LuValle P, Solum K, Olsen BR. A dominant negative mutation in the alpha 1 (X) collagen gene produces spondylometaphyseal defects in mice. Prog Clin Biol Res. 1993;383B:427–436. [PubMed] [Google Scholar]
- Kobayashi T, Lyons KM, McMahon AP, Kronenberg HM. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci U S A. 2005;102:18023–18027. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K, Martin GR, Yamada Y. Isolation and characterization of a cDNA clone for the amino-terminal portion of the pro-alpha 1(II) chain of cartilage collagen. J Biol Chem. 1984;259:13668–13673. [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Le AX, Miclau T, Hu D, Helms JA. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res. 2001;19:78–84. doi: 10.1016/S0736-0266(00)00006-1. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long F, Linsenmayer TF. Regulation of growth region cartilage proliferation and differentiation by perichondrium. Development. 1998;125:1067–1073. doi: 10.1242/dev.125.6.1067. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. doi: 10.1038/374356a0. [DOI] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Nohno T, Noji S, Koyama E, Myokai F, Ohuchi H, Nishikawa K, Sumitomo S, Taniguchi S, Saito T. Expression patterns of the activin receptor IIA and IIB genes during chick limb development. Prog Clin Biol Res. 1993;383B:705–714. [PubMed] [Google Scholar]
- Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11:1812–1826. doi: 10.1101/gad.11.14.1812. [DOI] [PubMed] [Google Scholar]
- Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Curr Top Dev Biol. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129:1421–1432. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Song J, Oh SP, Schrewe H, Nomura M, Lei H, Okano M, Gridley T, Li E. The type II activin receptors are essential for egg cylinder growth, gastrulation, and rostral head development in mice. Dev Biol. 1999;213:157–169. doi: 10.1006/dbio.1999.9370. [DOI] [PubMed] [Google Scholar]
- Wiater E, Vale W. Inhibin is an antagonist of bone morphogenetic protein signaling. J Biol Chem. 2003;278:7934–7941. doi: 10.1074/jbc.M209710200. [DOI] [PubMed] [Google Scholar]
- Yamagiwa H, Tokunaga K, Hayami T, Hatano H, Uchida M, Endo N, Takahashi HE. Expression of metalloproteinase-13 (Collagenase-3) is induced during fracture healing in mice. Bone. 1999;25:197–203. doi: 10.1016/s8756-3282(99)00157-x. [DOI] [PubMed] [Google Scholar]
- Yoon BS, Pogue R, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. BMPs regulate multiple aspects of growth-plate chondrogenesis through opposing actions on FGF pathways. Development. 2006;133:4667–4678. doi: 10.1242/dev.02680. [DOI] [PubMed] [Google Scholar]