Abstract
White mature adipocytes give rise to multipotent cells, so-called de-differentiated fat (DFAT) cells, when losing their fat in culture. The objective of this study was to examine the ability of DFAT cells to give rise to endothelial cells (ECs) in vitro and vivo. We demonstrate that mouse and human DFAT cells, derived from adipose tissue and lipospirate, respectively, initially lack expression of CD34, CD31, CD146, CD45 and pericyte markers, distinguishing them from progenitor cells previously identified in adipose stroma. The DFAT cells spontaneously differentiate into vascular ECs in vitro, as determined by real-time PCR, fluorescence activated cell sorting, immunostaining, and formation of tube structures. Treatment with bone morphogenetic protein (BMP)4 and BMP9, important in regulating angiogenesis, significantly enhance the EC differentiation. Furthermore, adipocyte-derived cells from Green Fluorescent Protein-transgenic mice were detected in the vasculature of infarcted myocardium up to 6 weeks after ligation of the left anterior descending artery in mice. We conclude that adipocyte-derived multipotent cells are able to spontaneously give rise to ECs, a process that is promoted by BMPs and may be important in cardiovascular regeneration and in physiological and pathological changes in fat and other tissues.
Keywords: Adipocyte-derived cells multipotent cells, endothelial cell differentiation, bone morphogenetic proteins, transgenic mice
1. INTRODUCTION
White mature adipocytes give rise to so-called de-differentiated fat (DFAT) cells when losing their fat in culture [1]. Mouse DFAT cells have been shown to be highly proliferative and differentiate into multiple mesenchymal lineages including fat, bone, cartilage, skeletal muscle and electrically active, beating cardiomyocytes [1–3]. Transplantation of DFAT cells into infarcted myocardium in rats was shown to promote capillary density in the infarcted area [4], suggesting that DFAT cells may support neoangiogenesis by contributing endothelial cells (ECs) or angiogenic factors. Indeed, previous studies have suggested a close relationship between adipocytes and ECs. Planat-Bernard et al. [5] demonstrated that adipocytes and ECs have common progenitor cells, Tang et al. [6] reported that adipocyte progenitor cells reside in the mural cell compartment of the adipose vasculature, and Gupta et al. [7] showed that preadipocytes exist as a subset of both pericytes and capillary ECs. Such progenitors may include microvascular pericytes, known to have many characteristics in common with mesenchymal stem cells [8]. However, it is not yet clear whether adipocytes or DFAT cells are able to give rise to ECs.
The importance of bone morphogenetic proteins (BMPs) in vascular development is increasingly being recognized. BMP4 promotes vascular networks and induces the activin-like kinase receptor (ALK)1 [9–11], which is activated by BMP9, a circulating BMP [12]. The activation of ALK1 is central in angiogenesis and EC differentiation [12], and absence of ALK1 causes embryonic lethality. BMP signaling also helps balance formation of vasculature versus organ-specific elements, as demonstrated in the lungs of mice lacking matrix Gla protein (MGP), a BMP inhibitor [13]. Thus, BMP signaling may be instrumental in directing EC differentiation in progenitor cells.
In this study, we provide evidence for a link between adipocytes and ECs. We demonstrate that DFAT cells derived from mouse and human adipocytes give rise to multipotent cells that initially lack expression of CD34, CD31, CD146, CD45 and pericyte markers, distinguishing them from progenitor cells previously identified in adipose stroma [14]. These DFAT cells spontaneously undergo mesenchymal-endothelial transitions, which is enhanced by treatment with BMP4 and BMP9. Finally we demonstrate that adipocyte-derived cells can be incorporated in the vasculature in infarcted myocardium in mice.
Together, our results support that adipocytes are able to give rise to ECs through DFAT cells, a process that is promoted by BMP4 and BMP9 and may be of central importance in cardiovascular regeneration and in physiological and pathological changes in fat and other tissue.
2. METHODS
2.1. Mice and human samples
AP2-Cre+/+;LacZ ROSA (R26R)+/+ double transgenic mice that expressed β-galactosidase (β-Gal) under the control of the aP2 promoter were generated as previously described [15], using aP2-Cre+/+ (strain B6.Cg-Tg(Fabp4-cre) 1Rev/J) mice and LacZ ROSA26+/+ (R26R) (strain B6.129S4-Gt(ROSA)26Sortm1Sor) reporter mice obtained from the Jackson Laboratory. For collection of mouse adipose tissue for cell isolation, transgenic or wild type C57BL6/J mice were euthanized at 8–10 weeks of age by inhalation of isoflurane (5–30%) and adipose tissues were collected postmortem.
Ligation of the left anterior descending artery in wild type C57BL6/J male mice was performed as previously described [16]. After LAD ligation, the mouse was closely monitored. Once the heart rate stabilized (approximately 15 minutes), a cocktail of 500,000 DFAT cells in alpha-minimum essential medium (MEM) supplemented with 1% ES cell qualified fetal bovine serum (Invitrogen) and 1% Methylcellulose (Sigma-Aldrich) was injected into the infarct zone. Green Fluorescent Protein (GFP)-expressing DFAT cells used for injection were prepared from transgenic mice that expressed GFP under the control of the human ubiquitin C promoter (strain C57BL/6-Tg(UBC-GFP)30Scha/J) obtained from the Jackson Laboratory. The cells were previously unpassaged and used approximately 10 days after adipocyte isolation. The chest was closed after the cell injection. The mice were sacrificed 2, 3, or 6 weeks after the procedure, and the heart was fixed for histological analysis. The hearts were analyzed for the presence of cells that co-expressed GFP and EC lineage markers by immunostaining.
The animal studies were reviewed by the Institutional Review Board and conducted in accordance with the animal care guidelines set by the University of California, Los Angeles. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publications No. 85–23, revised 1996). Samples of fresh human subcutaneous lipoaspirate were used for this study, but we were blinded to the identities, all characteristics, and the medical histories of the human subjects.
2.2. Isolation of adipocytes and culture of DFAT cells
Lipid-filled mature adipocytes were prepared from 2 grams of mouse subcutaneous adipose tissue or human fresh lipoaspirate (within 2–4 hours of lipoaspiration) as detailed in Supplemental Data and previously described [1, 3, 17]. The DFAT cells were cultured, treated and transfected with siRNA as described in the Supplemental Data.
2.3. Tube Formation Assay and β-Galactosidase (β-Gal) staining
Tube formation assays were performed with cells suspended in a mixture (1:1, volume) of rat tail collagen I gel and Matrigel™ (both BD Biosciences). Cultures were maintained for 1–4 weeks, and the medium was changed every 4–5 days. For β-Gal staining, cells were fixed in 0.05% glutaraldehyde and stained with β-Gal (X-Gal) solution at pH 8.1–8.5 for 4 hours at 37°C, and analyzed under phase contrast microscopy. The percentage of β-Gal positive cells was calculated after estimation of 10 different optical fields per culture well.
2.4. Flow Cytometric Analysis
The purity and size of the isolated adipocytes was assessed by fluorescence-activated cell sorting (FACS) using the lipophilic fluorescent dye Nile red as previously described [1, 3]. For characterization of the phenotype of DFAT cells, FACS analysis was performed after the first passage as previously described [1] using fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or Alexa Fluor 488 (AF-488)-conjugated anti-mouse or anti-human antibodies as detailed in Supplemental Data.
2.5. RNA analysis
RT-PCR and real-time PCR were performed as previously described [10], and as detailed in Supplemental Data.
2.6. Immunofluorescence
Cells grown in chamber slides were fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, blocked with 10% goat serum and 1% BSA in phosphate-buffered saline (PBS), and incubated over night at 4°C with the appropriate primary antibodies or non-specific IgG control antibodies, diluted 1:200 in 1% BSA in PBS as detailed in Supplemental Data. The next day, cells were incubated with secondary AF-488-conjugated (green fluorescence) or AF-594-conjugated (red fluorescence) goat anti-mouse or anti-rabbit secondary antibodies (Molecular Probes). The cells were washed with PBS, the nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich), and visualized by confocal or regular fluorescence microscopy. Lipids were stained with AdipoRed™ (Lonza) as per manufacturer’s instructions before immunofluorescence.
2.7. Immunoblotting
Immunoblotting was performed as previously described [18, 19]. Equal amounts of cellular protein were used. Blots were incubated with specific antibodies to VE-cadherin (400 ng/ml; Santa Cruz Biotechnology). β-Actin (1:5000 dilution; Sigma-Aldrich) was used as loading control.
2.8. Statistical Analysis
Data were analyzed for statistical significance by two-way analysis of variance with post hoc Tukey’s analysis using the GraphPad Instat® 3.0 software (GraphPad Software, San Diego, CA). P-values less than 0.05 were considered significant. All experiments were repeated a minimum of three times.
3. RESULTS
3.1. Preparation and characterization of DFAT cells
To determine if adipocyte-derived multipotent cells differentiate into ECs, we used adipocyte isolated from mouse adipose tissue or human lipoaspirate. The adipocytes were carefully washed a minimum of three times in phosphate-buffered saline as previously described [3]. The number of adipocytes with two or more nuclei was 1–2%, as determined by Nile Red and nuclear staining (data not shown), which is consistent with our and other’s previous observations [3, 20, 21]. It has been well documented that about 50% of the adipocytes adhere and enter the cell cycle after placement in culture. They divide in an apparent asymmetrical fashion where the lipid droplet stays with one of the dividing cells (see references [1, 4] for figures and videos). Once free from lipid droplets, after 5–7 days, the cells are referred to as dedifferentiated fat (DFAT) cells and continue to divide and form colonies. The findings suggest that the DFAT cells are generated from the adipocytes and not from cells attached to the adipocytes.
To ensure that other, non-adipocyte, cells were not contaminating the mouse or human adipocyte isolates, we examined expression of a number of surface markers and lineage specific markers. Peroxisome proliferator-activated receptor (PPAR)-γ, an adipogenic marker, was detected in the original adipocytes and in DFAT cells on day 7 when adipogenic redifferentiation has been described [1], We were unable to detect expression of CD34 (EC progenitors), CD45 and CD90 (leukocytes, hematopoietic progenitors), CD133 (neuroepithelial progenitors) CD14 or CD11b (human and mouse macrophages, respectively), CD31 (ECs), alpha-smooth muscle actin (ASMA, smooth muscle cells), and CD140b, neuron-glia antigen 2 (NG2) and CD146 (pericytes) as determined by RT-PCR (Figure 1A for human adipocytes, Supplemental Table I for human and mouse adipocytes) and real-time PCR (expression was not detected, data not shown). In addition, the absence of macrophages in mouse adipocytes was supported by negative F4/80 staining (Supplemental Figure IA). To ensure that bi-nucleated adipocytes did not represent ECs or EC progenitor cells stuck to adipocytes, the adipocytes were stained with anti-CD31 or CD34 antibodies, and 1,500–2,000 adipocytes were inspected. The results showed no CD31 or CD34 expression in bi-nucleated adipocytes (Figure 1B), suggesting that the presence of a second nucleus is not due to an attached EC or EC progenitor cell. Moreover, some adipocytes appear to be in different stages of division (Supplemental Figure IB). We also ensured that ECs and macrophages in the ASC fraction did not normally stain for lipids using AdipoRed in combination with immunostaining for CD31, CD34 and F4/80 (Supplemental Figure II).
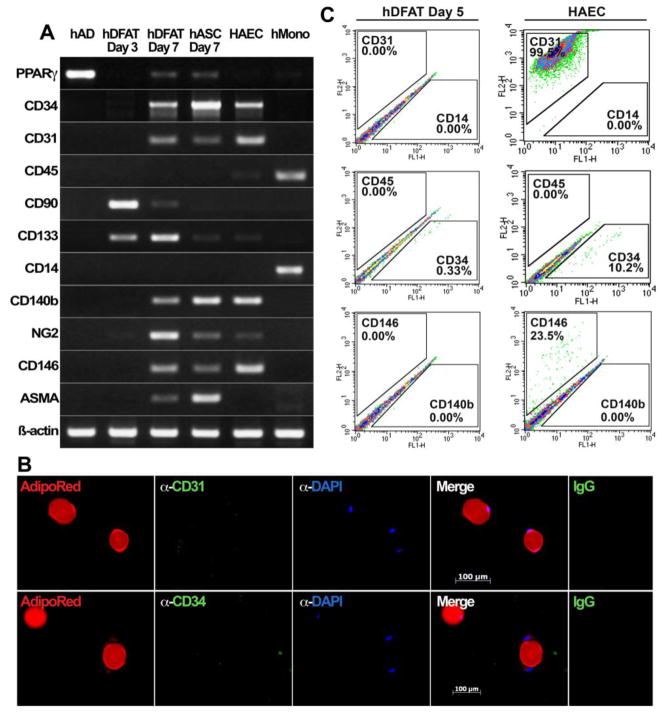
Figure 1. Expression of surface markers in human DFAT cells.
(A) Expression of cell surface markers in freshly isolated adipocytes, hDFAT cells (Day 3 and 7 days in culture), hASCs (Day 7), HAECs and human monocytes, as determined by RT-PCR. PPAR-γ is marker of adipogenic differentiation.
(B) Bi-nucleated adipocytes stained with AdipoRed™ and anti-CD31 or CD34 antibodies (green fluorescence). No CD31 or CD34 expression is detected in the adipocytes. DAPI (blue) is used to visualize nuclei. Non-specific IgG control showed no staining.
(C) Expression of surface markers CD31, CD14, CD45, CD34, CD146 and CD140b in hDFAT cells 5 days after culture start. HAECs are shown for comparison.
The expression in adipocytes was compared to that of unpassaged DFAT cells, 3 and 7 days after adipocyte isolation, and HAECs. The DFAT cells from day 3 expressed only CD90 and, weakly, CD133, whereas DFAT cells from day 7 also expressed CD34, CD31, CD140b, NG2, CD146 and ASMA (Figure 1A). Expression of CD45, and CD14 or CD11b (human or mouse DFAT cells, respectively) was not detected. The expression pattern in the hASCs from day 7 was similar to that of hDFAT cells from the same day except that they lacked expression of CD90 (Figure 1A). HAECs showed expression of CD34, CD31, CD140b, NG2, and CD146, and human monocytes showed expression of CD45 and CD14 (Figure 1A). β-Actin was used for cDNA control. Together, the results suggested that the adipocytes used for DFAT preparation was a pure adipocytes population without significant contamination of non-adipocytic cells. All adipocytes used for DFAT cell preparation in this study were similarly checked for contaminating cells.
To compare early DFAT cells with other types of progenitor cells that have previously been reported in stromal vascular fractions from adipose tissue [14], we determined expression of surface antigens CD34, CD31, CD146 and CD45 in unpassaged DFAT cells 5 days after adipocytes were placed in culture. We were unable to detect expression of any of CD34, CD31, CD146 and CD45, as determined by FACS (Figure 1C), which is consistent with the RT-PCR findings in DFAT cells from day 3. HAECs were included for comparison. This suggests that the initial DFAT cells are distinct from the previously identified subendothelial progenitor cells (CD34−, CD31−, CD146+), vascular wall resident endothelial progenitor cells (CD34+, CD31−, CD146+), vascular wall hematopoietic progenitor cells (CD45+), and white fat progenitor cells (CD34+) [14]. Because the DFAT cells do not express the pericyte markers (CD140b, NG2 and CD146) [14], they are also distinct from pericytes. However, expression of CD34, CD31, and CD146 is detected in DFAT cells from day 7 (Figure 1A), whereas expression of CD45 remained undetectable. Together, the results suggested that DFAT cells may constitute a transient precursor cell population that is an intermediary between the adipocytes and previously identified cells in the adipose stroma.
To monitor for early expression of CD34 and CD31 in individual DFAT cells derived from the adipocytes, we placed 25–50 floating adipocytes per well in 96-well plates. This ensured that a sufficient number of individual DFAT cells emerged from the adipocytes [1, 4], sunk to the bottom of the wells, divided and formed small clonal colonies (Figure 2A, left panels, Supplemental Movie). These clones were then stained for CD34 and CD31. Rare CD34+ cells could be detected as early as day 4, rare CD34+, CD31+ cells on day 6, and cells with predominantly CD31 expression on day 8, as determined by immunofluorescence (Figure 2A, right panels), consistent with CD34 preceding CD31 expression. The results supported that CD34 and CD31 were induced sequentially in cells derived from the adipocytes.
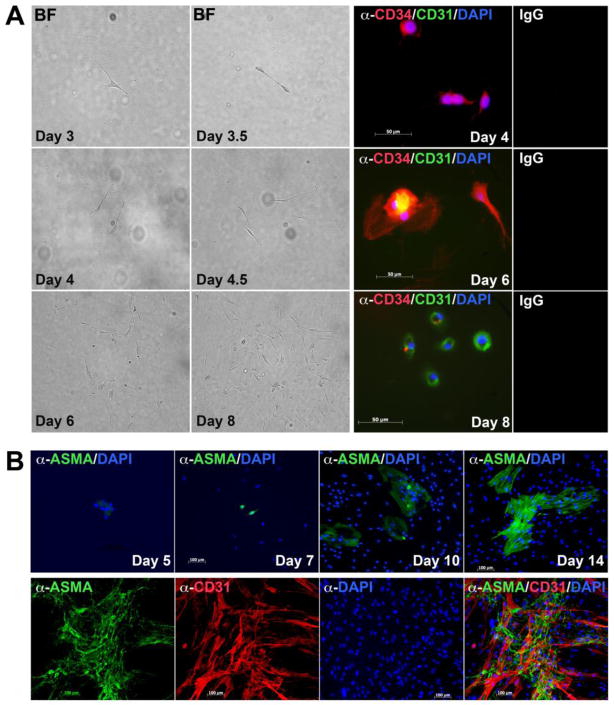
Figure 2. Early expression of CD34 and CD31 in mouse DFAT cells.
(A) (Left panels) Formation of cell colony formed from one mDFAT cell over the course of 8 days, as shown by phase contrast microscopy. (Right) Expression of CD34 (red fluorescence) is detected on Day 4, co-expression of CD34 and CD31 (red and green fluorescence, respectively) is detected on Day 6, and expression of CD31 (green fluorescence) is detected on Day 8. DAPI (blue) is used to visualize nuclei. Non-specific IgG control showed no staining in the experiments in panel A or B.
(B) (Upper panels) Expression of ASMA (green fluorescence) is detected in mDFAT cells on Day 5 in culture and later. (Lower panels) No co-expression of ASMA and CD31 (red fluorescence) was detected in mDFAT cells.
Because expression of ASMA was seen in DFAT cells by RT-PCR, we examined the time course of its appearance and whether it co-localized with CD31. The earliest ASMA expression was detected by immunofluorescence on day 5 and increased through day 14 (Figure 2B, top). We also co-stained the cells on day 14 for AMSA and CD31, but were unable to detect any co-staining (Figure 2B, bottom), which suggests that distinct smooth muscle cell populations arise in parallel with the ECs.
3.2. EC differentiation in mouse DFAT cells
To examine the potential of adipocyte-derived cells to give rise to ECs, we studied mouse adipocytes between day 0 (newly isolated adipocytes placed in culture) and day 15. EC differentiation occurred spontaneously between day 5 and 15 as determined by PCR and immunostaining for EC lineage markers, including CD31, the von Willebrand factor (vWF), Flk1, VE-cadherin, and the vascular endothelial growth factor (VEGF) (Figure 3A, B). Expression of the VEGF, CD31 and VE-cadherin increased up to 10–20 fold after 10–15 days as determined by real-time PCR (Figure 3A, B). After 15 days, the number of CD31+ cells had increased from 0.0% to 8.3% as determined by fluorescence-activated cell sorting (FACS) in 3 independent experiments (Figure 3C). The cells also formed multicellular tube structures if placed in Matrigel™/Collagen I gels as visualized by phase contrast microscopy (Figure 3D, top), and expressed CD31 and VE-cadherin as visualized by immunofluorescence and confocal microscopy (Figure 3D, bottom). 4′,6-Diamidino-2-phenylindole (DAPI) was used to visualize cell nuclei. These tube structures were stable for a month or longer, whereas tube structures formed by HAECs dissolved in less than 24 hours. Together the data supported a link between adipocytes, DFAT cells, and ECs in mice.
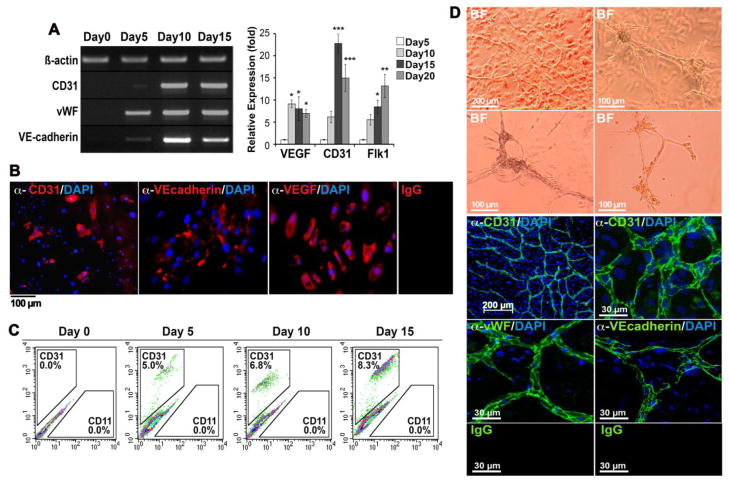
Figure 3. Spontaneous EC lineage differentiation in mouse DFAT cells.
(A) Expression of CD31, vWF, VE-cadherin and Flk1, markers of EC lineage, and VEGF increased in unpassaged mDFAT cells between day 5 and 20 as determined by RT-PCR and real-time PCR. β-Actin was used as RNA control. Asterisks indicate a statistically significant difference between compared to day 0. *<0.05, **<0.01, ***<0.001 Tukey’s test.
(B) MDFAT cells stained positive for CD31, VE-cadherin and VEGF. DAPI (blue) was used to visualize nuclei. Non-specific IgG control showed no staining.
(C) The number of CD31 positive mDFAT cells increased from 0% to 8.3% between day 0 and day 15 as determined by FACS.
(D) (Top) Phase contrast microscopy (BF; bright field) of tube formation in mDFAT cells cultured in Matrigel™/Collagen I gels. (Bottom) Multicellular tube structures stained positive for CD31, vWF and VE-cadherin as visualized by confocal microscopy.
3.3. Stimulation of EC differentiation in mouse DFAT cells by BMP4 and BMP9
BMP4 and BMP9 are known to have essential roles in the regulation of angiogenesis and EC differentiation [11, 12]. To test if BMP4 and BMP9 stimulate EC differentiation in adipocyte-derived cells, we treated isolated mouse adipocytes from day 3 with BMP4 (50 ng/ml) or BMP9 (10 ng/ml) for up to 15 days, and monitored expression of EC markers. Noggin (100 ng/ml) or neutralizing anti-BMP9 antibodies (100 ng/ml) were added as indicted in the figure to inhibit BMP4 or BMP9, respectively. Both BMP4 and BMP9 enhanced the expression of VE-cadherin, a marker for mature ECs, an effect that was in part limited by Noggin and anti-BMP9 antibodies, respectively, as determined by real-time PCR and immunoblotting (Figure 4A). The percentage of CD31+ cells increased between day 5 and 15 from 6.2% to 18.3% with BMP4 treatment, and from 4.8% to 10.0% with BMP9 treatment as determined by FACS (Figure 4B), as compared to Figure 3C. We also combined BMP4 and BMP9, which showed further enhancement of expression of VE-cadherin as determined by real-time PCR and immunoblotting (Figure 4C). The results suggest that BMP4, BMP9, or combinations of the two, may be used to enhance EC differentiation in DFAT cells.
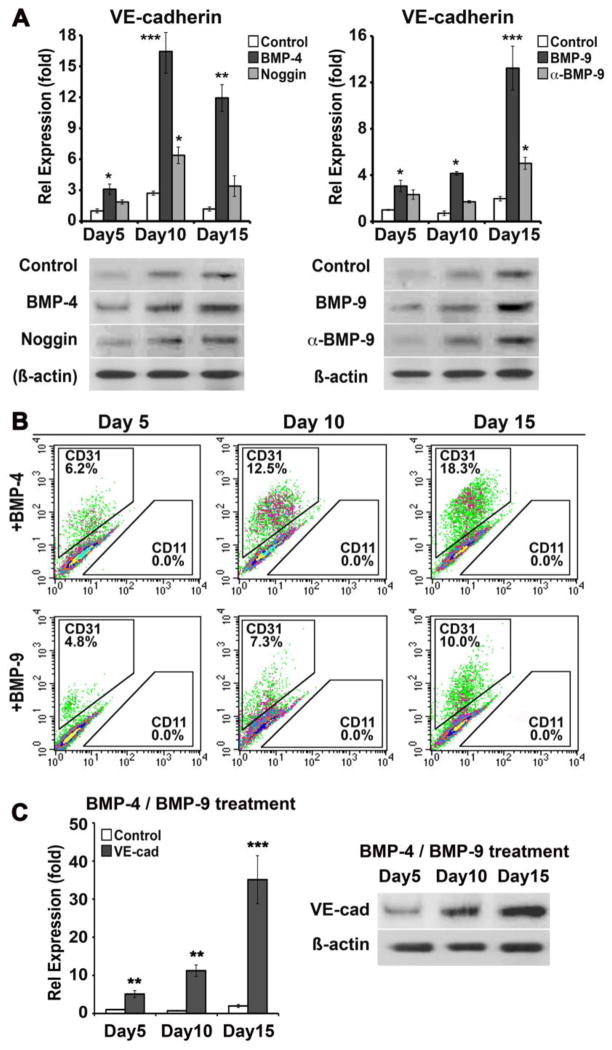
Figure 4. Stimulation of EC lineage differentiation in mouse DFAT cells.
(A) (Left) MDFAT cells were treated for 15 days with control vehicle, BMP4 (50 ng/ml) in absence or presence of Noggin (100 ng/ml) (left), or with BMP9 (10 ng/ml) in absence or presence of neutralizing anti-BMP9 antibodies (100 ng/ml) (right). Expression of VE-cadherin was determined by real-time PCR and immunoblotting. β-Actin was used as loading control.
(B) The percentage of CD31(+) cells was determined by FACS after 5, 10 and 15 days of BMP4 or BMP9 treatment.
(C) MDFAT cells were treated with BMP4 and BMP9 for 15 days and expression of VE-cadherin was determined by real-time PCR and immunoblotting. Asterisks indicate a statistically significant difference between compared to controls on day 0. *<0.05, **<0.01, ***<0.001 Tukey’s test.
The mDFAT cells express PPAR-γ at about the same time as the EC markers (Figure 1A). Therefore, we tested whether PPAR-γ agonists, known to have anti-angiogenic effects [22], modified the effects of BMP-4, which is also involved in adipogenic differentiation [23]. The DFAT cells were treated after the first passage with the PPAR-γ agonist GW1929 (100 ng/ml) for 7 days, and expression of EC markers (CD31 and VE-cadherin) and adipogenic markers (PPAR-γ and CCAAT/enhancer-binding protein (C/EBP) alpha) was determined by real-time PCR. The results showed that GW1929 suppressed the expression of the EC markers, instead promoting the adipogenic markers (Supplemental Figure III), suggesting a crosstalk between BMP- and PPAR-γ signaling in balancing EC differentiation.
Moreover, siRNA experiments in DFAT cells suggested that the ALK2, a BMP-4 receptor, was important for expression of both CD31 and VE-cadherin in BMP-treated DFAT cells (Supplemental Figure IV), further supporting the importance of BMP signaling in early EC differentiation.
3.4. Tube formation in β-Gal-expressing DFAT cells
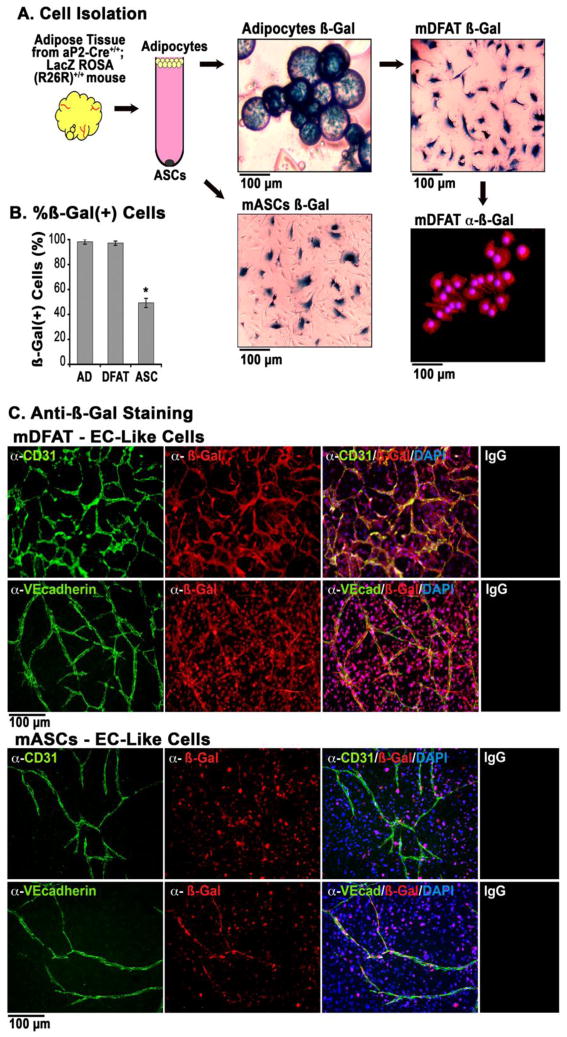
To validate the adipocytic origin of the ECs, we used adults aP2-Cre+/+;LacZ ROSA (R26R)+/+ double transgenic mice [15], where the aP2/FABP4 promoter directs adipocyte-specific expression of the LacZ gene [24–26] that is maintained even after redifferentiation. The adipocytes were checked for contamination as outlined above. We stained for β-Gal activity and determined the percentage of stained cells in adipocytes directly after isolation, and in mDFAT cells after one week in culture, and mASCs after two days in culture. The results revealed that 99.9% of both adipocytes and mDFAT cells stained positive for β-Gal activity (Figure 5A, B), supporting that the DFAT cells are derived from the adipocytes. Unexpectedly, about 45% of the mASCs also stained positive for β-Gal activity (Figure 5A, B), suggesting that the mASC population contains cells of adipocytic origin. Control cells from wild type mice were negative for β-Gal staining (Supplemental Figure V).
Figure 5. Tube formation in mouse DFAT cells and ASCs generated from aP2-Cre+/+;LacZ ROSA (R26R)+/+ double transgenic mice.
(A) Outline of generation of mDFAT cells and mASCs from adipocytes isolated from aP2-Cre+/+;LacZ ROSA (R26R)+/+ mice. β-Gal activity was detected by staining, and β-Gal protein was detected by immunofluorescence.
(B) Percentage of β-Gal positive mouse adipocytes (AD), DFAT cells, and ASCs.
(C) Tube forming cells from mDFAT cells and mASCs stained positive for CD31 and VE-cadherin (both green fluorescence), which co-localized with staining for β-Gal (red fluorescence). DAPI (blue) was used to visualize nuclei. Non-specific IgG control showed no staining.
To determine if β-Gal expressing mDFAT cells and mASCs differentiated into cardiovascular cells, unpassaged mDFAT cells were allowed to remain in culture for up to 14 days in regular culture medium. The cells formed colonies of cells resembling adipocytes, tube structures and networks of cardiomyocyte-like cells, all of which showed β-Gal activity (Supplemental Figure VI). Capillary-like tube structures with β-Gal activity formed from the mDFAT cells and the ASCs when cultured in Matrigel™/Collagen I gels (Figure 5C). Staining for CD31 and VE-cadherin co-localized with β-Gal in the tube structures as determined by immunofluorescence (Figure 5C). The mASCs formed fewer tubes, although all tubes we detected stained for β-Gal, suggesting that EC progenitors in this fraction at least in part derive from adipocytes, and that dedifferentiation of adipocytes might occur in vivo.
3.5. EC differentiation in human DFAT cells
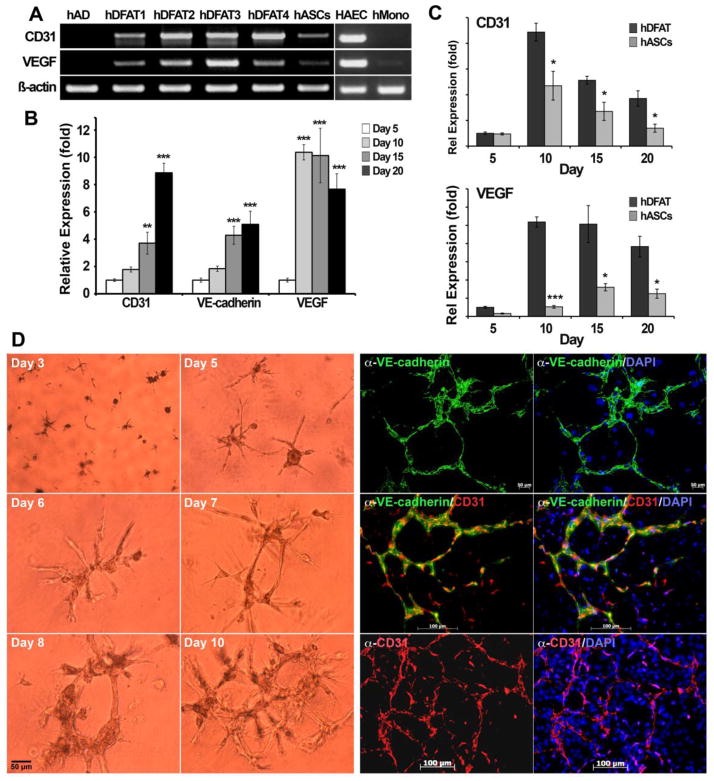
To examine endothelial lineage differentiation in human DFAT cells, we studied cells from four human lipoaspirates. We allowed unpassaged DFAT cells to remain in culture for up to 20 days, and determined expression of EC lineage markers by RT-PCR, real-time PCR and immunofluorescence. RT-PCR on day 10 revealed expression of CD31 and VEGF in all four hDFAT cell populations (Figure 6A), suggesting that the cells are able to undergo EC differentiation. HASCs usually showed less expression of CD31 and VEGF. No expression was detected in the original adipocyte isolates. Real-time PCR showed significant increases in expression of CD31, VEGF and VE-cadherin in the DFAT cells between day 7 and 20 (Figure 6B). This was significantly more than detected in the hASCs derived from the same adipocyte isolate (Figure 6C). Similar to the mDFAT cells, the hDAT cells also formed multicellular tube structures in Matrigel™/Collagen I gels as visualized by phase contrast microscopy (Figure 6D). These expressed CD31 and VE-cadherin after 10–15 days in culture, as visualized by immunofluorescence (Figure 6D). Together, the results supported that human adipocyte-derived cells are able to give rise to ECs similar to the mouse cells, possibly more efficiently than the ASCs.
Figure 6. Expression of lineage markers in human DFAT cells.
(A) Expression of CD31 (endothelial marker) and VEGF in 4 different preparations of hDFAT cells as determined by RT-PCR. Freshly prepared adipocytes (AD) and hASCs from the same lipoaspirate as hDFAT4 were included for comparison. HAECs and human monocytes were included as positive and negative controls, and β-Actin was used as RNA control.
(B) Expression of CD31, VE-cadherin, and VEGF in hDFAT cells between day 0–15, as determined by real-time PCR. Asterisks indicate a statistically significant difference compared to day 0. *<0.05, **<0.01, ***<0.001 Tukey’s test.
(C) Expression of CD31 and VEGF in hDFAT cells as compared to hASCs between day 5–20, as determined by real-time PCR. Asterisks indicate a statistically significant difference between hDFAT cells and hASCs. *<0.05, ***<0.001 Tukey’s test.
(D) Tube formation in hDFAT cells detected by phase contrast microscopy (left panels), and expression of VE-cadherin and CD31 after 10–15 days of culture, as visualized by immunofluorescence. DAPI (blue) was used to visualize nuclei. Non-specific IgG control showed no staining (not shown).
3.6. Injected DFAT cells detected in the vasculature of infarcted mouse myocardium
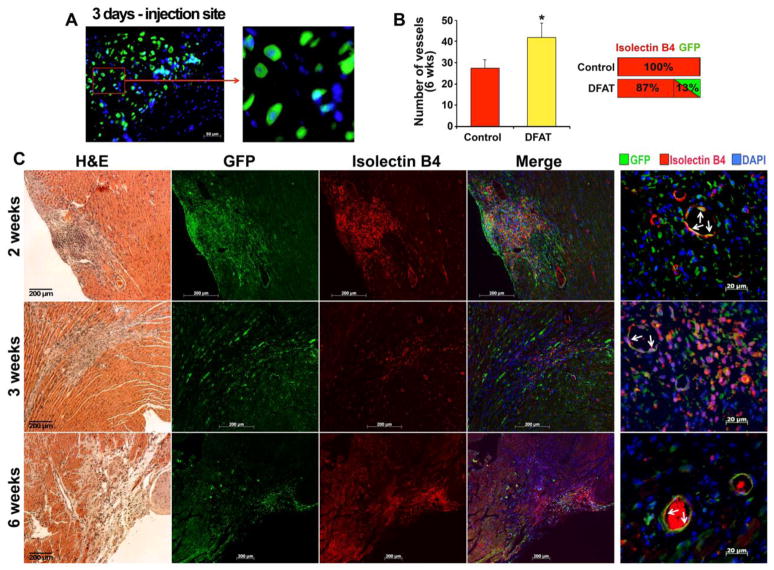
To determine if adipocyte-derived DFAT cells could be incorporated into actively forming vessels, we injected 500,000 previously unpassaged DFAT cells, approximately 10 days after adipocyte isolation from GFP-transgenic mice, into the infarct zone of C57BL6/J background right after LAD ligation. After 2, 3 and 6 weeks (3 mice for each time point), the mice were sacrificed, and the hearts were prepared for immunostaining for GFP, CD31, and isolectin B4, for visualization of myocardial microvasculature. The results showed that GFP-positive cells were easily detected at the injection site after 3–4 days (Figure 7A), and in a vascular pattern in the infarct zone, co-staining with isolectin B4, a vascular marker, after 2, 3 and 6 weeks (Figure 7B). Approximately 13% of the isolectin B4 positive cells were also positive for GFP after 6 weeks (Figure 7C). This suggests that adipocyte-derived cells can contribute cells directly to EC lineage and support new vessel formation.
Figure 7. Mouse GFP-positive adipocyte-derived cells in vessels of infarcted mouse myocardium.
(A) GFP-positive adipocyte-derived cells at injection site in myocardium.
(B) Number of vessels in infarcted area after 6 weeks, and percentage of cells that co-stained for with isolectin B4 and GFP.
(C) Localization of isolectin B4 and GFP in infarcted mouse myocardium 2, 3, and 6 weeks after LAD ligation and injection of GFP-positive DFAT cells. DAPI (blue) was used to visualize nuclei. Non-specific IgG control showed no staining (not shown).
4. DISCUSSION
In this study, we provide evidence that cells derived from white mature adipocytes spontaneously undergo EC differentiation without requiring the previously reported co-culture or specific treatments [27–29]. The EC differentiation was enhanced by addition of BMP4 and/or BMP9, which may be used in different combinations to optimize EC differentiation. Our findings support the close relationship between adipocytes and ECs previously suggested by Planat-Bernard et al. [5] who demonstrated that adipocytes and ECs have common progenitor cells, Tang et al. [6] who reported that adipocyte progenitor cells reside in the mural cell compartment of the adipose vasculature, and Gupta et al. [7] who reported that preadipocytes exist as a subset of both pericytes and capillary ECs. Indeed, our studies suggest that the DFAT cells constitute a link between adipocytes and ECs. In our experiments, the DFAT cells were initially devoid of expression of CD34, CD31, CD146, CD45 and pericyte markers, distinguishing them from other progenitor cells previously identified in adipose stroma [14]. We later detected expression of these markers, with the exception of CD45, suggesting that DFAT cells may serve as precursor cells to several types of stromal progenitor cell populations potentially also in vivo.
The use of adipocytes from the aP2-Cre+/+;LacZ ROSA (R26R)+/+ mice further supported the adipocytic origin of DFAT cells. These adipocytes were checked for contaminating cells just like the other adipocytes used in this study. Thus, even though selective expression of aP2/FABP4 has been described during embryonic development [15], and low levels (10,000-fold less) have been reported in macrophages [30], the β-Gal expression would signify adipogenic origin in these experiments. A large part of the ASCs also maintained expression of β-Gal, suggesting that at least part of the multipotent cells in the stromal fraction originate from adipocytes in a process similar to the generation of DFAT cells in vitro.
Adipocytes are widespread in tissues and in vascular adventitia, and could therefore serve as a widespread source of multipotent cells depending on the plasticity of the adipocytes in a particular location. We speculate that mesenchymal-endothelial transitions are important in catabolic states such as cancer cachexia and status post bariatric surgery, where adipose vascularity is increased [31, 32]. Interestingly, a recent study demonstrated that overexpression of VEGF in the adipose tissue, resulting in increased adipose vascularity, may even protect against diet-induced obesity and insulin resistance [33]. This would in part challenge previous studies where angiogenesis inhibitors have shown promising results in preventing obesity [34]. Preliminary data from our laboratory show that diabetic mouse models have significant alterations in adipose vascularity and expression of endothelial and adipogenic lineage markers (data not shown), supporting that adipose vascularity is affected by diabetes and obesity.
The stimulation of EC differentiation by BMP4 and BMP9 was consistent with our previous findings that showed that both BMPs are necessary for appropriate angiogenesis [13]. However, It is possible that BMP simply stimulates proliferation of EC progenitor cells or differentiated ECs. We know from our previous studies that BMP-4 and BMP-9 differentially affect proliferation of ECs depending on previous BMP-4 exposure and ALK1 expression [35]. To determine what proportion of the increase in ECs is due to proliferation of committed ECs at various stages, versus promotion of EC differentiation in progenitor cells, will require further studies and a better understanding of the different stages of EC differentiation. However, the suppressive effect of the PPAR-γ agonist 1929 on expression of CD31 and VE-cadherin, and the importance of the ALK2 receptor for the expression of these markers in BMP treated cells, suggest that the pro-angiogenic effect is at least in part due to an enhancement of differentiation.
The human adipocyte-derived cells used in this study were similar to mouse cells in acting as a source of cells undergoing EC differentiation. However, we did not have access to information about the location from which the fat was aspirated, or the characteristics of the human subjects such as age, gender, body mass index or presence of diabetes mellitus. Therefore, we were unable to assess if how the influence of such factors affected DFAT characteristics and EC differentiation, an area that will be important in future studies.
We examined the in vivo incorporation of GFP-positive cells in vasculature of infarcted myocardium up to 6 weeks after the LAD ligation and the injection of the such cells. At both time points, GFP-positive cells were detected in the endothelial layers of the microvasculature around the area of injection, further supporting the ability of adipocyte-derived cells to serve as a source of ECs. However, additional studies would be required to assess whether the inclusion of the adipocyte-derived cells had a beneficial effect on the myocardial function. Such studies would preferably include ASCs for comparison, and would require large number of animals to ensure sufficient statistical power.
In conclusion, our results support that mouse and human adipocytes are able to give rise to ECs through DFAT cells, which are distinct from previously identified progenitor cells in the adipose stroma, and that the process can be enhanced by BMP4 and BMP9. Our findings may be important for the understanding of cardiovascular regeneration, and provide a model system for further studies of EC differentiation in progenitor cells.
Supplementary Material
Highlights.
White adipocytes give rise to multipotent so-called dedifferentiated (DFAT) cells.
The DFAT cells are able to spontaneously undergo endothelial differentiation.
Bone morphogenetic proteins 4 and 9 have pro-angiogenic effects in the DFAT cells.
Acknowledgments
This work was funded in part by NIH grants HL30568, HL81397, and ZDK1 GRB-J, and the American Heart Association (Western Affiliate).
Footnotes
DISCLOSURES
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–22. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 2.Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780–5. doi: 10.1016/j.bbrc.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 3.Jumabay M, Zhang R, Yao Y, Goldhaber JI, Bostrom KI. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc Res. 2010;85:17–27. doi: 10.1093/cvr/cvp267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, et al. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565–75. doi: 10.1016/j.yjmcc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–63. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 6.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell metabolism. 2012;15:230–9. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–9. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 9.Boyd NL, Dhara SK, Rekaya R, Godbey EA, Hasneen K, Rao RR, et al. BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood, NJ) 2007;232:833–43. [PubMed] [Google Scholar]
- 10.Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–30. doi: 10.1074/jbc.M604239200. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Miralles I, Schisler JC, Patterson C. New insights into bone morphogenetic protein signaling: focus on angiogenesis. Curr Opin Hematol. 2009;16:195–201. doi: 10.1097/MOH.0b013e32832a07d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David L, Feige JJ, Bailly S. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev. 2009;20:203–12. doi: 10.1016/j.cytogfr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Jumabay M, Wang A, Bostrom KI. Matrix Gla protein deficiency causes arteriovenous malformations in mice. J Clin Invest. 2011;121:2993–3004. doi: 10.1172/JCI57567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallone T, Realini C, Bohmler A, Kornfeld C, Vassalli G, Moccetti T, et al. Adult human adipose tissue contains several types of multipotent cells. J Cardiovasc Transl Res. 2011;4:200–10. doi: 10.1007/s12265-011-9257-3. [DOI] [PubMed] [Google Scholar]
- 15.Urs S, Harrington A, Liaw L, Small D. Selective expression of an aP2/Fatty Acid Binding Protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–53. doi: 10.1007/s11248-006-9000-z. [DOI] [PubMed] [Google Scholar]
- 16.Kolk MV, Meyberg D, Deuse T, Tang-Quan KR, Robbins RC, Reichenspurner H, et al. LAD-ligation: a murine model of myocardial infarction. J Vis Exp. 2009;13:pii:1438. doi: 10.3791/1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–74. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 18.Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–52. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008;332:435–46. doi: 10.1007/s00441-008-0593-9. [DOI] [PubMed] [Google Scholar]
- 21.Gao Q, Zhao L, Song Z, Yang G. Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol Biol Rep. 2012;39:579–804. doi: 10.1007/s11033-011-1371-4. [DOI] [PubMed] [Google Scholar]
- 22.Bishop-Bailey D. PPARs and angiogenesis. Biochem Soc Trans. 2011;39:1601–5. doi: 10.1042/BST20110643. [DOI] [PubMed] [Google Scholar]
- 23.Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle. 2007;6:385–9. doi: 10.4161/cc.6.4.3804. [DOI] [PubMed] [Google Scholar]
- 24.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–7. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc Natl Acad Sci U S A. 2001;98:224–8. doi: 10.1073/pnas.011528898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 27.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 29.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 30.Shum BO, Mackay CR, Gorgun CZ, Frost MJ, Kumar RK, Hotamisligil GS, et al. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116:2183–92. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baptista LS, da Silva KR, da Pedrosa CS, Claudio-da-Silva C, Carneiro JR, Aniceto M, et al. Adipose tissue of control and ex-obese patients exhibit differences in blood vessel content and resident mesenchymal stem cell population. Obes Surg. 2009;19:1304–12. doi: 10.1007/s11695-009-9899-2. [DOI] [PubMed] [Google Scholar]
- 32.Ishiko O, Sumi T, Yoshida H, Hyun Y, Ogita S. Angiogenesis in the adipose tissue of tumor-bearing rabbits treated by cyclic plasma perfusion. Int J Oncol. 2001;19:785–90. doi: 10.3892/ijo.19.4.785. [DOI] [PubMed] [Google Scholar]
- 33.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–13. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–15. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- 35.Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, et al. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119:5037–47. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.