Introduction
This review considers the presentation of β-cell-derived antigens during the initial stages of autoimmune diabetes, focusing on the islet of Langerhans as well as on the lymph nodes (LN) that drain the pancreas. The islets contain distinct antigen presenting cells (APC). Recent evidence points to these APC as central cells in early diabetogenesis.
Islets normally have APC
The presence of APC within islets was documented in the late 1970's in the context of allogeneic transplantation studies performed by the groups of Paul Lacy and Kevin Lafferty (1, 2). They identified in donor-isolated islets “passenger leukocytes” expressing Major Histocompatibility Complex Class II (MHC II) molecules with ultrastructural characteristics resembling splenic dendritic cells (DC) (3, 4). The passenger leukocyte theory had been proposed by George Snell (5) and championed by several transplantation biologists, among them Kevin Lafferty (6). It stated that leukocytes carried in the transplant were a major stimulus for the allogeneic reaction (6, 7). Indeed, depletion of the “passenger leukocytes” of the islets delayed their rejection (1, 8). The initial findings were followed by the identification of the islet leukocytes in animals (9-13) and in humans (11, 14-17). The first report that directly identified the phenotype of the passenger leukocyte came from Lacy's group: they demonstrated that the MHC II+ cells within the islets stained for a DC marker (10). HLA-DR+ cells with characteristic of APC have been identified in human islets in limited evaluations (11, 14-17).
Features of the mouse islet APC
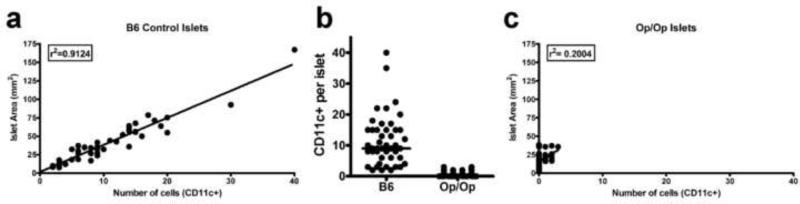
Observations have been made on the features, turnover time and function of islet APC (18-24). Islets from several non-diabetic mouse strains, including the diabetic-prone NOD mouse on the Rag-1-/- background, showed ~10 APC per islet (19, 20). There was a broad distribution of APC, and a relationship between the size of islet and content of APC. Small islets contained the fewest APC while the bigger islets (mega-islets), contained the largest number, (Figure 1a). No T cells or NK cells were found in islets of normal non-diabetic mouse strains.
Figure 1. Islet size and DC content in normal and in CSF-1-/- mice (Op/Op).
(a) Distribution of islet area vs. CD11c+ cells in islets of 6 week old C57BL/6 mice. (b) Comparison of islet CD11c+ content in islets of C57BL/6 and Op/Op mice. (c) Distribution of islet area vs. CD11c+ cells in islets of 6 week old Op/Op mice. From reference 19.
As previously described in earlier studies (3, 9, 10, 12), the islet APC contained features of DC; we will refer to them as such here, although their phenotype overlaps with those of other APC. From our published data (19), all CD45+ cells in islets were identified as MHC II+ and CD11c+ cells. Islet endocrine cells and the endothelial cells did not express MHC II.
Most islet DC were positive for CD11b, CD11c and F4/80. Islet DC were also positive for ICAM-1, integrin α4, and CX3CR1 (21). Most were negative or weakly reactive for CD4, DEC205, Ly-6C, CD8α, CD101, 33D1, B7-H3, B7-H4, ICOS-L, PD-L1, PD-L2, CD40, CD40L, B220, 440c (plasmacytoid DC), and langerin). B7-1 expression was evident, but there was weak expression of B7-2, suggesting an immature state. The chemokine receptor profile showed about half of the islet DC to be positive for CCR5, CCR6, CXCR3 and CXCR4 (19).
From our unpublished data and reports by Merad's and Tang's group, (20, 21), two APC subsets are found within islets. The major population (~85% of the CD11c+) is the one detailed above in isolated islets, expressing CD11b, F4/80, CX3CR1 and SIRPα. The remaining ~15% expressed CD103 but lacked CD11b, F4/80 and CX3CR1.
Islet DC were relatively stable in numbers, decreased by 30%, three to seven days after whole body irradiation (19). Inflammation in the islets promoted either by administering low doses of streptozotocin (STZ) or by infiltrating diabetogenic T cells increased the number of DC in islets (19, 20, 24). The incoming DC during islet inflammation were phenotypically different from the resident DC expressing high levels of B7–2 (19, 20), as well as CD40, CD11b and ICAM-1 (24).
Localization of DC within the islet
Most DC were in tight association with the blood vessels. Electron microscopy analysis showed a stretched DC always next to blood vessels and often showing dendrites in close apposition to a thin basement membrane below the endothelium (19). Islet capillaries were positive for endoglin, ICAM-1, and PECAM-1, and negative for VCAM-1 (19).
Imaging studies disclosed a highly dynamic dendrite activity in situ, with extensions and retractions of long dendrites between the blood vessels and β-cells (19). Most DC had a point of attachment to the blood vessels with very little displacement. About one-third of the DC extensions penetrated the lumen of the vessel (a “periscope” function) (23). The periscoping of DC dendrites has been documented in the intestine (25-27), airways epithelium (28), and aorta (29). About 10% of the islet DC were highly motile, with less dynamic dendrite activity, and in some cases escaped from the islets (19).
Trophic function of the islet DC
Phagocytes may have a role in the development of the pancreas and islets (30). As first reported by Jeffrey W. Pollard's laboratory, the CSF-1 mutant mice “op/op mice” showed small islets and reduced β-cell mass, about half the size of wild type islets (31). The islets of such mice showed a conspicuous reduction in the number of DC, (Figure 1b and 1c) (19). Thus the development of islet DC requires expression and signaling by CSF-1 receptor. These findings indicate a trophic role of the APC in islet physiology, be it by maintaining vascularization and/or by fostering β-cells viability. The nature of this supportive role has not been determined.
Antigen presentation in the islet and in the pancreatic lymph nodes (PLN)
Presentation of β-cell antigens by either MHC class I (MHC I) or MHC II molecules was examined using two different approaches. In one islet cells were tested for their relevant peptide-MHC (pMHC) complex ex vivo using diabetogenic T cells as probes. The second approach tested diabetogenic T cells interactions with APC in vivo. Diabetogenic T cells were injected and their localization and site of activation examined in islets and PLN.
Presentation by islet DC to CD4 T cells
Under normal conditions, β-cells have low levels of MHC I and do not express MHC II. Presentation of β-cell antigens to autoreactive CD4 T cells involves the transfer of antigenic material from the β-cells to the APC. This situation was evident in the first reports by Haskins group examining the response of diabetogenic CD4 T cells to islet antigens (32, 33). In culture, β-cells (even allogeneic) activated CD4 T cells but only in the presence of syngeneic APC (34). In vivo experiments also pointed to direct lack of presentation by β-cells to CD4 T cells (35).
Are APC from resting islets (i.e., in non-inflammatory conditions), presenting to T cells or is β-cell damage or death a requirement for charging the APC with β-cell-derived antigens? These important questions are relevant for our understanding of how autoimmune diabetes develops. The answer is that islet DC are normally presenting peptides derived from β-cell proteins in high amounts, in a constitutive process unrelated to β-cell death. Islet DC from resting islets of non-diabetic NOD mice presented to and activated diabetogenic CD4 T cells, indicating that they contained the β-cell-derived pMHC complex (18, 19). DC isolated from islets of NOD mice activated a number of CD4 T cells of unknown specificity (34), as well as the BDC-2.5 transgenic CD4 T cell (19): this T cell was isolated by the Haskins laboratory (32) and its T cell receptor (TCR) genes cloned by Mathis and Benoist (36). A number of insulin-reactive T cells also were activated when cultured with islet DC (22). The presentation took place equally well by islet DC isolated from pre-diabetic NOD as well as NOD.scid mice where no inflammation or apparent cell death was evident (19, 22).
Islet APC from mice expressing the small protein hen egg white lysozyme (HEL) under the insulin promoter (37) likewise stimulated HEL-reactive CD4 T cells (19, 38, 39). In our studies most of the islet DC were positive for the HEL-pMHC complex testing with a monoclonal antibody (mAb) specific to the dominant pMHC complex (19). As in the NOD mouse, there was no apparent cell death when examined for terminal deoxynucleotidyl transferase dUTP nick end-labeling positive (TUNEL+) β-cells. We estimated that at a given time the islet DC contained about 2% of the HEL expressed by the islets. Beta cell-derived HEL was about 20-fold more effective than soluble HEL in charging APC (38). In brief, the capacity of the islet DC to present self-proteins is high (Figure 2).
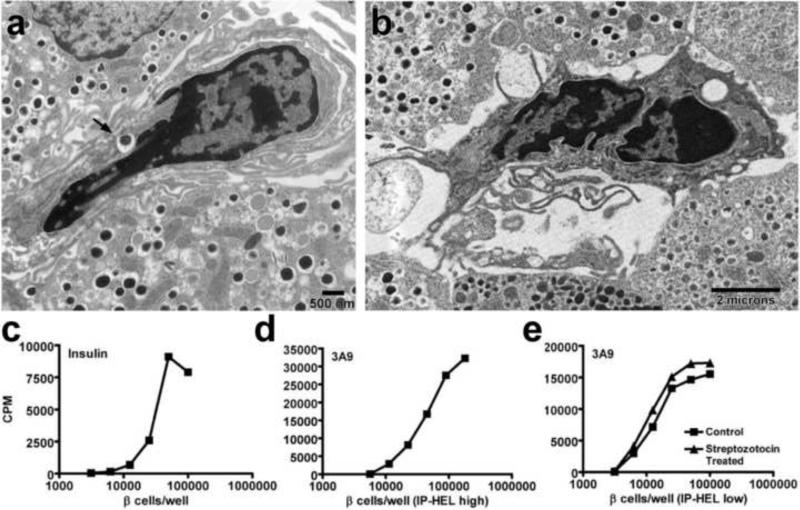
Figure 2. Islet DC present β-cell derived antigen to specific T cell hybridomas.
(a and b) Electron microscopy analysis of NOD.Rag-1-/- islets showing islet DC with an insulin granule inside a vacuole (arrow) (a) and islet DC with dendrites extending to adjacent β-cells (b). (c to e) Dispersed islet T cell assays showing: insulin-specific T cell hybridomas cultured with titrating amounts of NOD.Rag-1-/- dispersed islets (c), 3A9 T cell hybridomas cultured with dispersed islets from high producer IP-HEL mice (ILK3 strain) (d) or cultured with dispersed islets from low producer IP-HEL mice (117 strain) with or without low dose STZ treatment 4 days before islet isolation. Panel (a), (c), (d) and (e) published as Figure 3 and 6 from reference 19, with permission from PNAS. Panel (b) is from unpublished data.
Cytological examinations, including electron microscopy, confirmed the presence of secretory granules or their products inside most islet DC (19). Importantly, a mAb to the immunodominant peptide derived from the β-chain of insulin, residues 9-23, showed that most APC contained insulin granules (22). Considering that each granule has about 106 insulin molecules, it well explains the very high presenting activity of the islet DC. Insulin is a dominant autoantigen in autoimmune diabetes (40, 41). In agreement, examination of islets from NOD mice in which the green fluorescent protein (GFP) was expressed under the insulin promoter (42) showed most of the DC bearing GFP+ granules (20).
The role of the PLN
A major consideration in presentation of β-cell antigens is the role of the pancreatic lymph nodes (PLN). The PLN drain the acinar component of pancreas except for the islets, which lack lymphatic circulation. PLN also drain segments of the intestine (43, 44), a relevant issue regarding the influence of the microbiome in diabetes (45). All indications at present are that the PLN are essential in the initial activation of diabetogenic T cells, prior to their islet migration. This issue has been mostly explored with CD8 T cells. However, the number of T cells examined with specificities to different β-cell antigens has been limited and generalizations need to be made with caution.
The importance of the PLN in the development of diabetes was shown in two experimental settings. First, surgical excision from NOD mice resulted in the absence of diabetes without apparent priming of T cells (46). Second, offspring of pregnant NOD mothers injected with lymphotoxin-β receptor fused to human Ig Fc lacked LN and did not develop diabetes (47). The level of autoreactivity was limited in these mice lacking PLN. In both situations, transfer of activated diabetogenic T cells resulted in diabetes.
Proliferation of diabetogenic CD4 T cells in the PLN was evident from experiments transferring labeled T cells. Transfer of un-activated BDC-2.5 CD4 T cells resulted in strong selective proliferation in the PLN (48). Direct examination showed the presence of DC containing antigen (19). In the PLN from insulin-promoter-HEL, APC presented to anti-HEL CD4 T cells (38, 39): 56% expressed the pMHC complex from the dominant peptide of HEL (19). In the study from Krummel, Tang and associates, nodes examined from insulin promoter-GFP mice showed DC containing GFP (49).
Diabetogenic CD8 T cells likewise proliferated strongly in the PLN. These observations were first made by Bill Heath's group examining the ovalbumin specific OT-1 T cells in mice displaying the protein under the insulin promoter (50-52). In NOD mice, two TCR transgenic CD8 T cells proliferated strongly in the PLN (53).
The process by which the PLN APC receives and presents β-cell antigens is not entirely known. Beta cell antigens released from β-cells, or some of the islet DC, or both, may move to the stroma to reach the lymphatic vessels that will drain their content into the PLN. Clearly, injections into the pancreas of cells or labeled proteins reach the PLN (54), although these are non-physiological conditions. It is not feasible to posit that the proteins or the APC leave the islet via blood, in which case there would not be PLN selectivity presentation.
We favor the hypothesis that the sensitization of the PLN is a constitutive process by way of the islet DCs, which at some point migrate out into the stroma to then enter the lymphatic network. This flow from islet antigen-presentation to the node could well be a normal biological process resulting from islet DC turnover or be influenced by biological changes of the islets (i.e., during inflammation). We consider the former to be the most feasible; however, inflammation may accelerate this process.
It is important to note that the APC network that surrounds the islets may not be presenting islet antigens (19). We examined the stromal APC in the insulin-promoter-HEL transgenic mice: while the islet DC were rich in HEL-pMHC complexes, the stromal DC were negative, and thus there was no apparent transport of β-cell HEL to the peri-islet areas. If this finding is applicable to all β-cell antigens, it has implications concerning the development of the peri-insulitic lesion prior to islet injury.
PLN from neonatal mice, up to about the third week of life, presented poorly to T cells (48, 53, 56, 57). Subsequently, the node became receptive to the activation of transferred T cells. There were no major differences in DC subsets of the PLN between young or old mice (48, 57). This was evaluated in B6 mice using the insulin promoter-ovalbumin system by Heath's group (57) and in the NOD mouse by Mathis and Benoist (48). The PLN from young mice developed antigen presenting capabilities following β-cell injury, either by transfer of activated CD8 T cells (57) or by administering STZ (the β-cell poison) (53). One interpretation is that sensitization of the node develops subsequent to reorganization or reconstruction of the islets which takes place a few weeks post-birth (57). A discrete wave of β-cell apoptosis takes place shortly post-birth in rats (58-61), mice (61, 62) and humans (63). A relationship has been argued between this discrete wave and the acquisition of presenting capabilities by the nodes (57, 61, 64). This relationship has been questioned: manipulations that reduce or favor β-cell death have not affected the development of diabetes (65).
Presentation in vivo to CD4 T cells
An important issue to consider is the role of the islet DC in the migration and localization of either diabetogenic CD4 or CD8 T cells into islets bearing their cognate antigens. Specific T cells localized into islets where there was presentation of the pMHC complex. In all instances tested, the CD4 T cells required activation in order to directly enter islets.
Our laboratory examined the entry of diabetogenic CD4 T cells in B10.BR mice expressing HEL under the insulin promoter or in the NOD mouse (Table 1) (23, 24). T cells from TCR transgenic mice directed to the major segment of HEL presented by I-Ak molecules localized only to islets of mice expressing HEL under the insulin promoter. This specific localization was found to the same extent whether the mice were normal (untreated) or were lightly x-irradiated. For localization to take place, T cells had to be activated first by a short incubation with APC presenting the relevant peptide. Non-activated T cells did not localize to any extent. Neither did non-specifically activated CD4 T cells localize to islets of mice bearing HEL. Thus, the localization was a specific event that depended on the state of activation of the CD4 T cells and the expression of HEL by the islets. As noted before, most of the islet DC presented HEL peptides.
Table 1.
Early islet cell entry evaluation performed with adoptive transferred specific and non-specific CD4 T cells
| CD4 T cell evaluated | Permutation | Recipient strain | Islet entry | |
|---|---|---|---|---|
| Manipulation | ||||
| 3A9* | ||||
| Non-activated | None | IP-HEL | No | |
| Activated | None | IP-HEL | Yes | |
| Activated | None | Non-IP-HEL | No | |
| Activated | Anti-class II | IP-HEL | Reduced | |
| Activated | Anti-ICAM-1 | IP-HEL | Reduced | |
| Activated | Anti-class II + Anti-ICAM-1 | IP-HEL | Reduced | |
| Activated | Anti-VCAM-1 | IP-HEL | Yes | |
| Activated | Anti-PECAM-1 | IP-HEL | Yes | |
| Activated + PTX | None | IP-HEL | Yes | |
| Activated | Anti-IFN-γ | IP-HEL | Yes | |
| B10.BR‡ | ||||
| Non-activated | None | IP-HEL | No | |
| Non-activated | None | Non-IP-HEL | No | |
| Activated | None | IP-HEL | No | |
| Activated | None | Non-IP-HEL | No | |
| Activated | STZ | IP-HEL | No | |
| Non-activated | Co-transfer with 3A9 | IP-HEL | Reduced | |
| Activated | Co-transfer specific | IP-HEL | Yes | |
| Activated + PTX | Co-transfer specific | IP-HEL | No | |
| Activated | Co-transfer specific + anti-VCAM-1 | IP-HEL | No | |
| Activated | Co-transfer specific + anti-IFN-γ | IP-HEL | Reduced | |
| BDC2.5* | ||||
| Non-activated | None | NOD.Rag-1-/- | Reduced | |
| Activated | None | NOD.Rag-1-/- | Yes | |
| Activated | None | B6.g7 | Yes | |
| Activated | None | BALB/c | No | |
| Activated | None | NOD.Class II-/- | No | |
| Activated | Anti-Class II | NOD.Rag-1-/- | Reduced | |
| Activated | None | NOD.ICAM-1-/- | Reduced | |
| Activated | Anti-Class II | NOD.ICAM-1-/- | Reduced | |
| Activated | Anti-CD44 | NOD.Rag-1-/- | Yes | |
| Activated + PTX | None | NOD.Rag-1-/- | Yes | |
| Activated | None | NOD. IFN-γR-/- | Reduced | |
| B6.g7‡ | ||||
| Non-activated | None | NOD.Rag-1-/- | No | |
| Non-activated | None | B6.g7 | No | |
| Activated | None | NOD.Rag-1-/- | No | |
| Activated | None | B6.g7 | No | |
| Activated | Co-transfer specific | NOD.Rag-1-/- | Yes | |
| Activated + PTX | Co-transfer specific | NOD.Rag-1-/- | No | |
| Activated | Co-transfer specific + anti-VCAM-1 | NOD.Rag-1-/- | No | |
| Activated | Co-transfer specific | NOD. IFN-γR-/- | No | |
3A9 is a CD4 T cell that recognizes the 48-62 peptide of HEL bound to I-Ak molecules. Cells were isolated from a T cell receptor transgenic mice and transferred directly (non-activated), or following a short period of activation with the peptide (activated). Recipients were mice that express HEL under the insulin promoter (IP-HEL) (37, 39). Results were identical in lightly irradiated and non irradiated mice. PTX refers to Pertussis toxin. STX refers to streptozotocin.
B10.BR refers to CD4 T cells from normal mice, incubated or not incubated with concanavalin A (ie activated and non-activated, respectively).
BDC2.5 refers to the diabetogenic CD4 T cells isolated from T cell receptor transgenic mice (36). Cells were activated by incubation with a mimotope peptide.
B6.g7 refers to CD4 T cells isolated from non-diabetic B6.g7 mice.
Localization started within a few minutes after injection peaking by about 24 hrs. We could not localize T cells in the DC network surrounding peri-islet areas. Examination of live islets by two-photon microscopy showed that about half of the CD4 T cells that crossed into the islet were in close contact with the DC. A visible immunological synapse was evident in many of the T cell-DC clusters (23, 24). The conclusion was that T cells entered directly from blood into islets crossing the intra-islet vessels. As noted before (23), islet DC protruded dendrites into the lumen, which could be the initial site of interaction with the circulating T cells. In support of this interpretation, injection of 0.5u beads coated with anti-MHC II mAb, led to specific localization in islet vessels always next to a DC (24). Islet blood circulation is slow and intermittent (66) which could foster the contact of the two cell types. An important consideration is whether T cells enter islets normally, at random, to then rapidly exit unless there is interaction with their cognate antigen. Although we could not eliminate this possibility, attempts to localize T cells in normal islets through this putative migratory stage failed. Even under circumstances where non-specifically activated T cells were abundant in blood, no localization was evident (23).
Similar findings were made in the NOD mice. The BDC-2.5 TCR transgenic CD4 T cells localized to the islets of NOD mice following a brief activation. BDC-2.5 T cells taken directly from the transgenic mice showed some localization (about 20% of the cells had activation markers). BDC-2.5 T cells did not localize to islets of NOD mice lacking MHC II or to NOD.H2b mice. Thus localization depended in the correct presentation of the I-Ag7 pMHC (23).
The presence of cloned T cells in islets was examined by the Vignali laboratory, using “retrogenic” technology in which stem cells were transfected with retrovirus containing TCR genes from diabetogenic T cells (67). Some T cells directed to β-cell antigens were identified weeks later and correlated with the development of diabetes.
In our experiments, the entry of diabetogenic CD4 T cells was partially inhibited by administering blocking mAb to MHC II, prior to the transfer of the T cells. Partial inhibition was also found by blocking ICAM-1 or by transferring T cells into NOD.ICAM-1-/- mice (23). We concluded that the two molecules cooperated in the entry of T cells. Of interest, treatment of diabetogenic CD4 T cells with pertussis toxin (PTx) had no influence in islet localization, indicating that signaling via G protein coupled receptors was not involved (23). The results suggest that diabetogenic T cells enter islets by contacting the islet DC, perhaps by their dendrites exposed inside the blood vessels.
Diabetogenic T cell entry caused rapid and profound changes in the islets (24, 55). Within a few hours post-entry, islet vessels expressed VCAM-1 and increased their expression of ICAM-1. Of note, β-cells also reacted by expressing ICAM-1 one or two days after the localization of T cells (23). Following the entry of diabetogenic CD4 T cells, islets developed profound amplification gene changes: transcriptome analysis disclosed a rapid induction of interferon genes within a short time after T cell entry (23). The strongest transcriptional changes were identified in the non-leukocyte-component of the islets (β-cells and endothelial cells).
Importantly, following localization of diabetogenic CD4 T cells, islets were receptive to the entry of non-specific T cells. Their entry mechanisms were far different from the diabetogenic CD4 T cells: entry was inhibited by blocking VCAM-1 which was not the situation with diabetogenic CD4 T cells. Importantly, PTx treatment inhibited the localization of the non-specific CD4 T cells (23) (Table 1 and Figure 3).
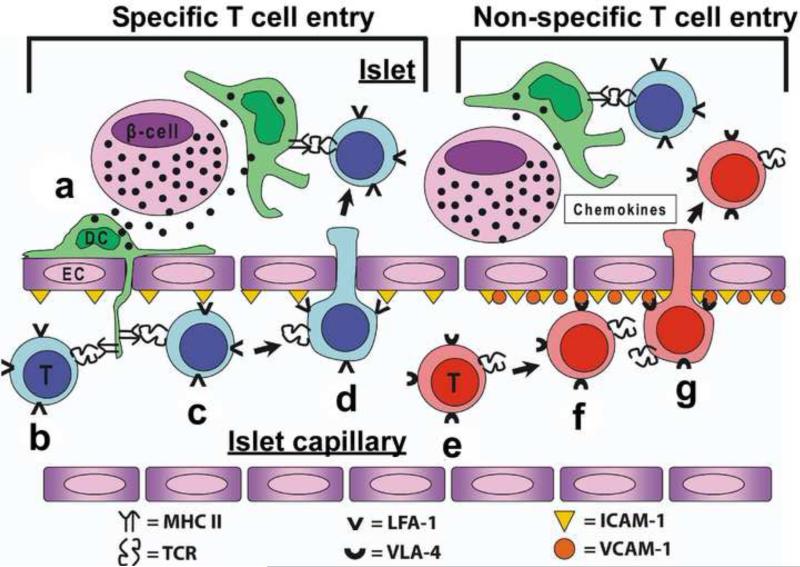
Figure 3. Proposed model of CD4 T cell entry into the islets of Langerhans Specific T cell entry.
(a) Secretory granules are taken up by the islet DC (shown in green), which are then processed and its peptides presented by MHC II. (b) Specific CD4 T cells (shown in blue) encounter their antigen presented by islet DC protruding through the fenestrated endothelium of the vessel. (c) MHC II recognition and the interaction of LFA-1/ICAM-1 favors the retention and adhesion to the endothelium of the specific T cell in the vessel. (d) The retention allows the entry of the T cell into the islet. Once inside the islet, T cell interacts with islet DC and become activated. These steps will trigger inflammatory signals and gene changes in the islet increasing ICAM-1, VCAM-1 and inflammatory chemokines. Non-specific T cell entry: (e) Non-specific T cells in circulation (shown in red) will encounter the inflammatory signals (chemokines and VCAM-1) that will allow its retention and adhesion to the islet endothelium. (f and g) Attachment to VCAM-1 and the inflammatory chemokine gradient will lead to non-specific T cell entry into the islet.
These findings using transfer of T cells need to be placed in the context of the normal diabetic process in which T cells start localizing early but diabetes does not take place until much later. Clearly a number of control events take place subsequent to T cell entry which modulate the effector reaction, an issue beyond the scope of this review. Another important issue is the status of the islet DC during the diabetogenic process. Regardless of the DC content of β-cell-pMHC complexes, the presenting capabilities via costimulatory molecules or cytokine expression are modulated and influence interactions with T cells. Such changes are to be expected (24).
Presentation in vivo to CD8 T cells
Most findings agree that cross presentation of β-cell antigens to unactivated diabetogenic CD8 T cells takes place in the PLN (51-53, 56). Direct entry of unactivated CD8 T cells into islets has not been identified. [A recent claim to this effect needs confirmation and further evaluation (68).]
Santamaria's group made a detailed analysis of a CD8 T cell to the islet-specific glucose-6-phospatase catalytic subunit protein (IGRP) (69, 70). IGRP contains a strong MHC I-epitope presented by Kd molecules (71), and a TCR transgenic mouse (the 8.3 CD8 T cell) was generated to it. Unactivated 8.3 T cells proliferated in the PLN and apparently did not enter islets until after a period of activation. In contrast, activated 8.3 T cells entered islets readily, agreeing with the findings made in CD4 diabetogenic T cells. One important issue is the cellular site of MHC I expression required for the localization of activated diabetogenic CD8 T cells. The response to IGRP was completely abolished by having the adenovirus E19 protein expressed in β-cells (69). Transfer of 8.3 T cells into these mice resulted in PLN proliferation but without localization in islets. NOD mice selectively lacking MHC I on the β-cells showed insulitis and low diabetes incidence. Although this experiment indicated that expression of MHC I in islets favored diabetes penetrance, it did not address CD8 T cell localization in such islets (72). Selective absence of MHC I on the APC lacked presentation in the PLN, indicating, a lack of cross-presentation and activation of the CD8 T cell (73).
Chervonsky's laboratory examined the migration of the CD8 T cell reactive to an insulin peptide, the IS-CD8 clone (74). T cells migrated specifically into islets bearing the appropriate MHC I (Kd) (75, 76). Their findings suggested that the initial localization of CD8 T cells was to the islet endothelium. Chemokine signaling was essential for specific CD8 T cell migration into the islet. Additional work showed the requirement of VCAM-1 expression in the islet vasculature for diabetogenic CD8 T cell migration to occur (55). The differences in localization between CD8 and CD4 T cells, point to distinct differences in local antigen presentation.
Concluding Remarks
Evidence is growing that the islet DC is a central cell in diabetogenesis. The islet DC is heavily charged with class II pMHC complexes due to constitutive uptake of β-cell granules. As a result of this uptake, the islet DC is instrumental in the initial sensitization, most likely by moving to the PLN. How this movement takes place and how generalizable it is for priming of all diabetogenic T cells needs further analysis. Islet DC are also the central cells in the localization of activated CD4 T cells. The extent to which changes in islet biology and of the β-cell alters these processes needs to be examined, but is a likely possibility. One issue that remains to be evaluated is the capacity of the islet DC to cross present MHC I-epitopes. Most of the islet DC have monocyte /macrophage features but a small percentage are CD103+; which of the two if any cross presents β-cell granules needs to be examined. Moreover the mechanism of localization of the diabetogenic CD8 T cells appears to be different from CD4 T cells, involving cells other than the islet DC.
Acknowledgments
Our research was supported by NIH Grants AI024742, DK058177, and P60DK20579; JDRF Grant 1-2007-731; and the Kilo Diabetes and Vascular Research Foundation. The recent work quoted from our laboratory involved Craig Byersdorfer, Javier Carrero, Richard DiPaolo, Katherine Fredericks, Jeremy Herzog, Matteo Levisetti, Mark Miller, James Mohan, Shirley Petzold, and Anish Suri.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lacy PE, Davie JM, Finke EH. Prolongation of islet allograft survival following in vitro culture (24 degrees C) and a single injection of ALS. Science. 1979;204:312–313. doi: 10.1126/science.107588. [DOI] [PubMed] [Google Scholar]
- 2.Bowen KM, Lafferty KJ. Reversal of diabetes by allogenic islet transplantation without immunosuppression. Aust J Exp Biol Med Sci. 1980;58:441–447. doi: 10.1038/icb.1980.45. [DOI] [PubMed] [Google Scholar]
- 3.Parr EL, Lafferty KJ, Bowen KM, McKenzie IF. H-2 complex and Ia antigens on cells dissociated from mouse thyroid glands and islets of Langerhans. Transplantation. 1980;30:142–148. doi: 10.1097/00007890-198008000-00013. [These three studies are the first reporting on APC in the islets of Langerhans.] [DOI] [PubMed] [Google Scholar]
- 4.Lacy PE. Transplantation of islet cells—isografts and allografts. Monogr Pathol. 1980;21:156–165. [PubMed] [Google Scholar]
- 5.Snell GD. The homograft reaction. Ann Rev Microbiol. 1957;11:439–458. doi: 10.1146/annurev.mi.11.100157.002255. [DOI] [PubMed] [Google Scholar]
- 6.Lafferty KJ, Prowse SJ, Simeonovic CJ. Immunobiology of tissue transplantation: A return to the passenger leukocyte concept. Ann Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- 7.Billingham RE. The passenger cell concept in transplantation immunology. Cell Immunol. 1971;2:1–12. doi: 10.1016/0008-8749(71)90022-0. [DOI] [PubMed] [Google Scholar]
- 8.Faustman D, Lacy PE, Davie JM. Transplantation without immuno-suppression. Diabetes. 1982;31:11–16. doi: 10.2337/diab.31.4.s11. [DOI] [PubMed] [Google Scholar]
- 9.Hart DN, Newton MR, Reece-Smith H, Fabre JW, Morris PJ. Major histocompatibiliy complex antigens in the rat pancreas, isolated pancreatic islets, thyroid, and adrenal. Transplantation. 1983;36:431–435. doi: 10.1097/00007890-198310000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci USA. 1984;81:3864–3868. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shienvold FL, Alejandro R, Mintz DH. Identification of Ia-bearing cells in rat, dog, pig, and human islets of Langerhans. Transplantation. 1986;41:364–372. doi: 10.1097/00007890-198603000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Von Gaudecker B, Ulrichs K, Müller-Ruchholtz W. Immunoelectron microscopic localization of MHC structures in isolated pancreatic rat islets. Diabetes. 1989;38:150–153. doi: 10.2337/diab.38.1.s150. [DOI] [PubMed] [Google Scholar]
- 13.McInerney MF, Rath S, Janeway CA., Jr Exclusive expression of MHC class II protein on CD45+ cells in pancreatic islets of NOD mice. Diabetes. 1991;40:648–651. doi: 10.2337/diab.40.5.648. [DOI] [PubMed] [Google Scholar]
- 14.Danilovs JA, Hofman FM, Taylor CR, Brown J. Expression of HLA-DR antigens in human fetal pancreas tissue. Diabetes. 1982;31:23–9. doi: 10.2337/diab.31.4.s23. [DOI] [PubMed] [Google Scholar]
- 15.Leprini A, Valente U, Celada F, Fontana I, Barocci S, Nocera A. Morphology, cytochemical features, and membrane phenotype of HLA-DR+ interstitial cells in the human pancreas. Pancreas. 1987;2:127–135. doi: 10.1097/00006676-198703000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Lautenschlager I, Inkinen K, Taskinen E, Charles MA, Hayry P. Major histocompatibility complex protein expression on pancreas and pancreatic islet endocrine cell subsets. Am J Pathol. 1989;135:1129–1137. [PMC free article] [PubMed] [Google Scholar]
- 17.Lu W, Pipeleers DG, Klöppel G, Bouwens L. Comparative immunocytochemical study of MHC class II expression in human donor pancreas and isolated islets. Virchows Arch. 1996;429:205–211. doi: 10.1007/BF00198335. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu J, Carrasco-Marin E, Kanagawa O, Unanue ER. Relationship between beta cell injury and antigen presentation in NOD mice. J Immunol. 1995;155:4095–4099. [PubMed] [Google Scholar]
- 19.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present beta cell derived peptides bound to their class II MHC molecules. Proc Natl Acad Sci USA. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [This report identified the phenotype, localization and steady state of islet DC. More importantly, this work showed direct visualization of the β-cell-derived antigen presented by the MHC II of the islet DC.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melli K, Friedman RS, Martin AE, Finger EB, Miao G, Szot GL, Krummel MF, Tang Q. Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J Immunol. 2009;182:2590–2600. doi: 10.4049/jimmunol.0803543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux F, Liu K, Heift J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [This paper describes the role of islet DC in selecting T cells with unique specificity to insulin peptides.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calderon B, Carrero JA, Miller MJ, Unanue ER. Cellular and molecular events in the localization of diabetogenic T cells to islets of Langerhans. Proc Natl Acad Sci USA. 2011;108:1561–1566. doi: 10.1073/pnas.1018973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon B, Carrero JA, Miller MJ, Unanue ER. Entry of diabetogenic T cells into islets induces changes that lead to amplification of the cellular response. Proc Natl Acad Sci USA. 2011;108:1567–1572. doi: 10.1073/pnas.1018975108. [These two papers from our laboratory describe the localization mechanisms of diabetogenic CD4 T cells and how islets become receptive to the entry of non-specific CD4 T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 26.Chieppa M, rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hapfelmeier S, Muller AJ, Stecher B, Kaiser P, Barthel M, Endt K, Eberhard M, Robbiani R, Jacobi CA, Heikenwalder M, Kirschning C, Jung S, Stallmach T, Kremer M, Hardt WD. Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med. 2008;205:437–450. doi: 10.1084/jem.20070633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahnsen FL, Strickland DH, Thomas JA, Tobagus IT, Napoli S, Zosky GR, Turner DJ, Sly PD, Stumbles PA, Holt PG. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177:5861–5867. doi: 10.4049/jimmunol.177.9.5861. [DOI] [PubMed] [Google Scholar]
- 29.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med. 2009;206:497–505. doi: 10.1084/jem.20082129. [References 23 and 25-29 show DC dendrites periscoping between endocrine, vascular and epithelial cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Dreshage HA. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994;43:667–675. doi: 10.2337/diab.43.5.667. [DOI] [PubMed] [Google Scholar]
- 31.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf10p macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. doi: 10.1189/jlb.1103591. [Op/op mice having a nonfunctional CSF-1 molecule have small islets. Reference 19 confirmed this finding and showed the absence of islet DC.] [DOI] [PubMed] [Google Scholar]
- 32.Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci USA. 1989;86:8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [References 32 and 33 are major contributions in which diabetogenic T cells are identified and isolated.] [DOI] [PubMed] [Google Scholar]
- 34.Shimizu J, Kanagawa O, Unanue ER. Presentation of beta-cell antigens to CD4+ and CD8+ cells of non-obese diabetic mice. J Immunol. 1993;151:1723–1730. [PubMed] [Google Scholar]
- 35.Sarukhan A, Lechner O, von Boehmer H. Autoimmune insulitis and diabetes in the absence of antigen-specific contact between T cells and islet β cells. Eur J Immunol. 1999;29:3410–3416. doi: 10.1002/(SICI)1521-4141(199910)29:10<3410::AID-IMMU3410>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T-cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [A major contribution examining the biology of a TCR transgenic diabetogenic T cells.] [DOI] [PubMed] [Google Scholar]
- 37.Akkaraju S, Ho WY, Leong D, Canaan K, Davis MM, Goodnow CC. A range of CD4 T cell tolerance: partial inactivation to organ-specific antigen allows nondestructive thyroiditis or insulitis. Immunity. 1997;7:255–271. doi: 10.1016/s1074-7613(00)80528-2. [DOI] [PubMed] [Google Scholar]
- 38.DiPaolo RJ, Unanue ER. The level of peptide-MHC complex determines the susceptibility to autoimmune diabetes: Studies in HEL transgenic mice. Eur J Immunol. 2001;31:3453–3459. doi: 10.1002/1521-4141(200112)31:12<3453::aid-immu3453>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Byersdorfer CA, Schweitzer GG, Unanue ER. Diabetes is predicted by the beta cell level of autoantigen. J Immunol. 2005;175:4347–4354. doi: 10.4049/jimmunol.175.7.4347. [DOI] [PubMed] [Google Scholar]
- 40.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in prediabetic NOD mice. Eur. J. Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [The first identification of insulin-reactive CD4 T cells in NOD diabetes.] [DOI] [PubMed] [Google Scholar]
- 41.Wegmann DR. Eisenbarth GS: It's insulin. J Autoimmun. 2000;15:286–291. doi: 10.1006/jaut.2000.0444. [DOI] [PubMed] [Google Scholar]
- 42.Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am J Physiol. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 43.Carter PB. Collins FM: The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci USA. 2005;102:17729–17733. doi: 10.1073/pnas.0509006102. [Refs 43 and 44 show that the PLN drains part of the colon and small intestine and is subject to influences from intestinal content.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of β cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levisetti MG, Suri A, Frederick K, Unanue ER. Absence of lymph nodes in NOD mice treated with lymphotoxin-β receptor immunoglobulin protects from diabetes. Diabetes. 2004;53:3115–3119. doi: 10.2337/diabetes.53.12.3115. [References 46 and 47 show the requirements of PLN in the development of diabetes in the NOD mouse.] [DOI] [PubMed] [Google Scholar]
- 48.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [The CD4 BDC-2.5 T cells do not proliferate in PLN of 10 day old mice. This paper and references 53, 56 and 57 point to an early period where the PLN is inactive in priming CD4 and CD8 diabetogenic T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurts C, Miller JFAP, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurts C, Sutherland RM, Davey G, Li M, Lew AM, Blanas E, Carbone FR, Miller JF, Heath WR. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc Natl Acad Sci USA. 1999;96:12703–12707. doi: 10.1073/pnas.96.22.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, O'Brien B, Trudeau J, Tan R, Santamaria P, Dutz JP. In situ beta cell death promotes priming of diabetogenc CD8 T lymphocytes. J Immunol. 2002;168:1466–1472. doi: 10.4049/jimmunol.168.3.1466. [References 50-53 show the proliferation in the PLN of CD8 T cells to β-cell antigens.] [DOI] [PubMed] [Google Scholar]
- 54.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological β cell death triggers priming of self-reactive T cells by dendritic in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hänninen A, Nurmela R, Maksimow M, Heino J, Jalkanen S, Kurts C. Islet beta-cell-specific T cells can use different homing mechanisms to infiltrate and destroy pancreatic islets. Am J Pathol. 2007 Jan;170(1):240–50. doi: 10.2353/ajpath.2007.060142. Erratum in: Am J Pathol. 2008,172:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan DJ, Kurts C, Kreuwel HT, Holst KL, Heath WR, Sherman LA. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc Natl Acad Sci USA. 1999;96:3854–3858. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mintern JD, Sutherland RM, Lew AM, Shortman K, Carbone FR, Heath WR. Constitutive, but not inflammatory, cross-presentation is disabled in the pancreas of young mice. Eur J Immunol. 2002;32:1044–1051. doi: 10.1002/1521-4141(200204)32:4<1044::AID-IMMU1044>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 58.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- 59.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinol. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- 60.Petrik J, Arany E, McDonald TJ, Hill DJ. Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology. 1998;139:2994–3004. doi: 10.1210/endo.139.6.6042. [DOI] [PubMed] [Google Scholar]
- 61.Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes. 2000;49:1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- 62.Hill DJ, Strutt B, Arany E, Zaina S, Coukell S, Graham CF. Increased and persistent circulating insulin-like growth factor II in neonatal transgenic mice suppresses developmental apoptosis in the pancreatic islets. Endocrinology. 2000;141:1151–1157. doi: 10.1210/endo.141.3.7354. [DOI] [PubMed] [Google Scholar]
- 63.Kassem SA, Ariel L, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apopotosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49:1325–1333. doi: 10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- 64.Mathis D, Vence L, Beniost C. β-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [This study makes the case for β-cell death in diabetes development.] [DOI] [PubMed] [Google Scholar]
- 65.Carrington EM, Kos C, Zhan Y, Krishnamurthy B, Allison J. Reducing or increasing β-cell apoptosis without inflammation does not affect diabetes initiation in neonatal NOD mice. Eur J Immunol. 1980;41:2238–2247. doi: 10.1002/eji.201141476. [DOI] [PubMed] [Google Scholar]
- 66.Wayland H. Microcirculation in pancreatic function. Microsc Res Tech. 1997;37:418–43. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<418::AID-JEMT6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 67.Lennon GP, Bettini M, Burton AR, Vincent E, Arnold PY, Santamaria P, Vignali DA. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31:643–653. doi: 10.1016/j.immuni.2009.07.008. [An important study using TCR retrogenic mice to explore the specificity of T cell migration into the islets. With this approach the localization of diabetogenic T cells depended much on their antigenic specificity. Nondiabetogenic T cells did not localize to the islets.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pang S, Zhang L, Wang H, Yi Z, Li L, Gao L, Zhao J, Tisch R, Katz JD, Wang B. CD8(+) T cells specific for beta cells encounter their cognate antigens in the islets of NOD mice. Eur J Immunol. 2009;39:2716–2724. doi: 10.1002/eji.200939408. [DOI] [PubMed] [Google Scholar]
- 69.Yamanouchi J, Verdaguer J, Han B, Amrani A, Serra P, Santamaria P. Cross-priming of diabetogenic T cells dissociated from CTL-induced shedding of beta cell autoantigens. J Immunol. 2003;171:6900–6909.24. doi: 10.4049/jimmunol.171.12.6900. [Beta cells expressing the adenovirus E19 protein failed to present the IGRP epitope to the CD8 8.3 T cell, but the PLN could present the epitope.] [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Tsai S, Shameli A, Yamanouchi J, Alkemade G, Santamaria P. In situ recognition of autoantigen as an essential gatekeeper in autoimmune CD8+ T cell inflammation. Proc Natl Acad Sci USA. 2010;107:9317–9322. doi: 10.1073/pnas.0913835107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, Santamaria P, DiLorenzo TP. Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton-Williams EE, Palmer SE, Charlton B, Slattery RM. Beta cell MHC class I is a late requirement for diabetes. Proc Natl Acad Sci USA. 2003;100:6688–6693. doi: 10.1073/pnas.1131954100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Jersey J, Snelgrove SL, Palmer SE, Teteris SA, Mullbacher A, Miller JFAP, Slattery RM. β cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice. Proc Natl Acad Sci. 2007;104:1295–1300. doi: 10.1073/pnas.0610057104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong FS, Moustaka AK, Wen L, Papadopoulos GK, Janeway CA., Jr Analysis of structure and function relationships of an autoantienic peptide of insulin bound to H-2K(d) that stimulates CD8 T cells in insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 2002;99:5551–5556. doi: 10.1073/pnas.072037299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Savinov AY, Wong FS, Chervonsky AV. IFN-γ affects homing of diabetogenic T cells. J Immunol. 2001;167:6637–6643. doi: 10.4049/jimmunol.167.11.6637. [DOI] [PubMed] [Google Scholar]
- 76.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med. 2003;197:643–656. doi: 10.1084/jem.20021378. [Evidence is presented on an important role of endothelial cells in presenting MHC I-epitopes.] [DOI] [PMC free article] [PubMed] [Google Scholar]