SUMMARY
Genome-wide association studies have identified GALNT2 as a candidate gene in lipid metabolism, but it is not known how the encoded enzyme ppGal-NAc-T2, which contributes to the initiation of mucin-type O-linked glycosylation, mediates this effect. In two probands with elevated plasma high-density lipoprotein cholesterol and reduced triglycerides, we identified a mutation in GALNT2. It is shown that carriers have improved postprandial triglyceride clearance, which is likely attributable to attenuated glycosylation of apolipoprotein (apo) C-III, as observed in their plasma. This protein inhibits lipoprotein lipase (LPL), which hydrolyses plasma triglycerides. We show that an apoC-III-based peptide is a substrate for ppGalNAc-T2 while its glycosylation by the mutant enzyme is impaired. In addition, neuraminidase treatment of apoC-III which removes the sialic acids from its glycan chain decreases its potential to inhibit LPL. Combined, these data suggest that ppGalNAc-T2 can affect lipid metabolism through apoC-III glycosylation, thereby establishing GALNT2 as a lipid-modifying gene.
INTRODUCTION
Genome-wide association studies (GWAS) have identified loci that are associated with plasma lipids, but the unraveling of pathways through which these loci affect human metabolism is awaited (Ku et al., 2010). This also holds true for GALNT2 (locus NM_004481). In GWAS, SNPs in intron 1 of GALNT2 were found to be associated with plasma high-density lipoprotein cholesterol (HDL-c) and triglyceride levels (Kathiresan et al., 2008). It was subsequently shown that hepatic overexpression and silencing of GALNT2 in mice reduced and increased HDL-c levels, respectively (Teslovich et al., 2010). It is, however, not known through which mechanism the encoded enzyme UDPN-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalacto-saminyltransferase-2 (ppGalNAc-T2) mediates these effects. The enzyme belongs to a family of ppGalNAc transferases comprising 20 members in humans (Ten Hagen et al., 2003), all catalyzing the transfer of GalNAc residues onto proteins, and thereby initiating mucin-type O-glycan synthesis on threonine and/or serine residues. The size of this enzyme family, its level of evolutionary conservation, and the spatiotemporal changes in expression patterns point to important and isoform-specific functions for ppGalNAc-transferases in mammalian physiology (Ten Hagen et al., 2003), but these are largely unknown to date. Here, we provide evidence of a molecular pathway through which ppGalNAc-T2 affects plasma lipids.
RESULTS
A Rare GALNT2 Variant
GALNT2 was sequenced in 243 subjects referred to our lipid clinic for high plasma HDL-c levels (>95th percentile for age and gender). We identified two unrelated heterozygotes for the same point mutation at an evolutionary conserved position (c.941A > C, p.D314A; see Table S1 available online). The mutation was not found in 1,440 controls and 68 individuals with HDL-c levels <5th percentile. In one family, 7 carriers and 14 unaffected family members were identified. In a second family, 1 carrier and 3 unaffected family members were found.
Clinical Examination, Lipids, Lipoproteins
Carriers underwent physical examination; blood tests including protein spectrum, platelet aggregation, and plasma coagulation assays; and ultrasonography for carotid intima media thickness measurements. These investigations did not reveal any abnormalities. Measurements in fasting plasma showed that the probands have high HDL-c and low triglycerides (Table 1). Compared to 17 family controls, the 6 additional affected family members presented with a significant increase in total plasma cholesterol levels (p = 0.026) associated with nonsignificant increases in HDL-c and low-density lipoprotein cholesterol while a trend toward decreased triglyceride levels was observed (p = 0.064). See Table S2 for individual lipid profiles of all study subjects.
Table 1.
Demographic, Lifestyle, and Lipid Characteristics of Carriers of the GALNT2D314A Mutation and Controls
| Probands (n = 2) | Carriers (n = 6) | Noncarriers (n = 17) | P Probands versus Noncarriers |
P Carriers versus Noncarriers |
|
|---|---|---|---|---|---|
| Demographic and Lifestyle Characteristics | |||||
| Age (year) | 72.5 (15) | 39.5 (21) | 44.9 (13) | 0.010 | 0.46 |
| Gender (% male) | 50 | 17 | 53 | 0.94b | 0.12b |
| Body mass index (kg/m2) | 20.9 (1.0) | 25.5 (4.7) | 24.4 (2.4) | 0.063 | 0.49 |
| Smoking, n (%) | 0 | 1 (17) | 5 (29) | 0.37b | 0.37b |
| Alcohol use (U/week)a | 8 | 1 (0–9) | 4 (0–15) | 0.84 | 0.38 |
| Diabetes mellitus type 2 (n) | 0 | 1 | 1 | - | - |
| Hypertension (n) | 0 | 0 | 0 | - | - |
| Cardiovascular events (n) | 1 | 0 | 0 | - | - |
| Lipids and Lipoproteins | |||||
| Total cholesterol (mg/dl) | 278 (38) | 241 (48) | 195 (38) | 0.010 | 0.026 |
| LDL cholesterol (mg/dl) | 158 (14) | 156 (38) | 128 (37) | 0.27 | 0.12 |
| HDL cholesterol (mg/dl) | 106 (22) | 63 (11) | 56 (12) | <0.001 | 0.21 |
| Triglycerides (mg/dl)a | 82.3 | 80 (59–100) | 93 (72–114) | 0.35 | 0.064 |
| Apolipoproteins and LCAT Activity | |||||
| ApoB (mg/dl) | 105 (20) | 121 (26) | 110 (31) | 0.82 | 0.45 |
| Apo A-I (mg/dl) | 220 (46) | 147 (10) | 135 (36) | 0.007 | 0.44 |
| Apo A-II (mg/dl) | 23.6 (0.9) | 29.3 (5.2) | 29.4 (4.4) | 0.08 | 0.96 |
| ApoE (mg/dl) | 2.2 (0.3) | 2.3 (0.7) | 3.19 (1.1) | 0.23 | 0.09 |
| Apo C-II (mg/dl) | 3.8 (0.9) | 4.2 (4) | 4.05 (1.8) | 0.85 | 0.89 |
| Apo C-III (mg/dl) | 9.6 (0.3) | 10.1 (2.5) | 10.3 (2.5) | 0.72 | 0.89 |
| LCATc activity (cholesteryl ester/ml/hr) | 17.3 (0.42) | 17.7 (3.5) | 16.9 (1.3) | 0.73 | 0.26 |
Data are presented as mean (SD) unless otherwise specified.
Alcohol use and triglycerides are given as median (interquartile range) and were log-transformed prior to statistical analysis. P values were calculated using a t test unless indicated otherwise.
For gender and smoking, a chi-square test was used.
LCAT, lecithin cholesterol:acyltransferase.
Identification of ppGalNAc-T2 Substrates
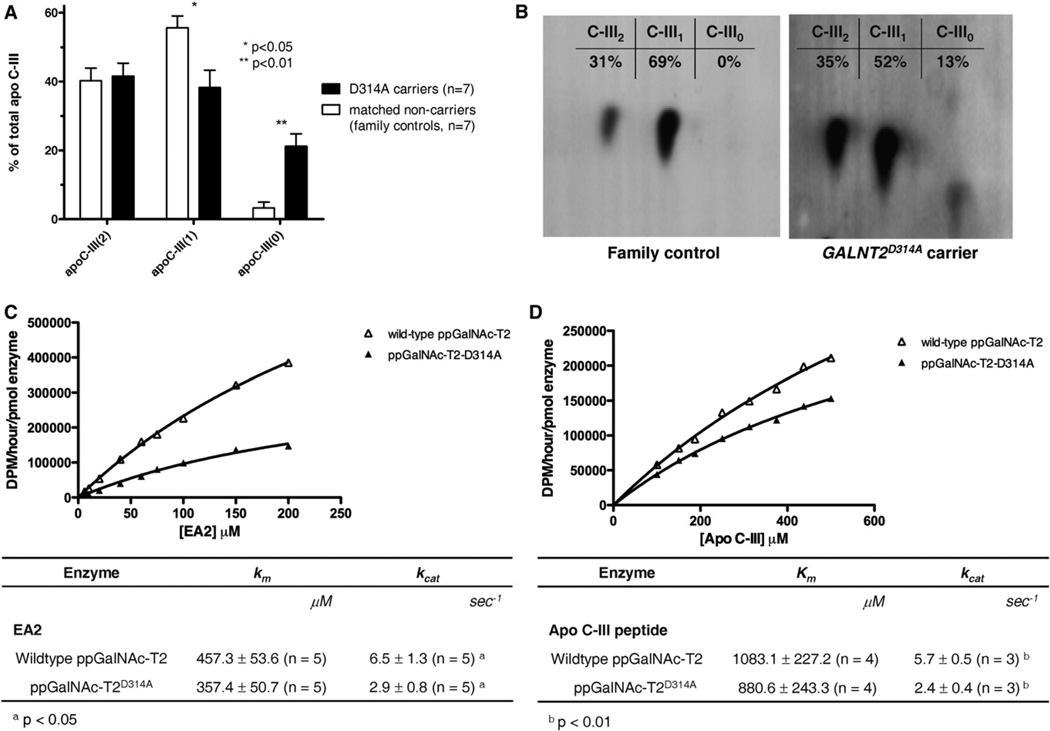
Plasma proteins were analyzed with 2D gel electrophoresis (2DE) to identify potential substrates of ppGalNAc-T2. Head-to-head comparisons of pairs of carriers and age-and-gender-matched family controls revealed differences in the relative distribution of apoC-III isoforms (Figure 1A). Compared to controls, carriers had 6.6-fold increased levels of nonsialylated apoC-III0 (p = 0.01) and decreased levels of monosialylated apoC-III (apoC-III1; p = 0.05), while levels of disialyated apoC-III (apoC-III2) were similar. MALDI-TOF mass spectrometry confirmed that the aberrant proteins were the denoted apoC-III isoforms (Table S3). Figure 1B illustrates increased levels of apoC-III0 isoforms in a carrier, while apoC-III of a noncarrier is only present as apoC-III1 or apoC-III2.
Figure 1. 2DE Analysis of ApoC-III Isoforms in Carriers of the GALNT2D314A Mutation Compared to Noncarriers and Enzyme Kinetics of Wild-Type and Mutant ppGalNAc-T2.
(A) Compared to controls, carriers have 6.6-fold increased levels of nonsialylated apoC-III0 (p = 0.01) and decreased levels of monosialylated apoC-III (apoC-III1; p = 0.05), while levels of disialyated apoC-III (apoC-III2) are similar. Data are expressed as means. Error bars are SEMs. P values are for Mann-Whitney U tests. (B) Western blot of 2DE gel showing a carrier with increased levels of apoC-III0 isoforms, while apoC-III of a family control is only present as apoC-III1 or apoC-III2. Note that apoC-III0 comprises nonsialylated apoC-III (apoC-III-GalNAc and apoC-III-GalNAc-Gal) and apoC-III that is not glycosylated (Bruneel et al., 2007). (C and D) Enzymes were overexpressed in COS7 cells. y axis indicates rate of transfer as dpm/hr/densitometric unit of enzyme. Figures represent kinetic plots and averages of replicate peptide glycosylation experiments catalyzed by wild-type or mutant ppGalNAc-T2. Mixtures of purified recombinant proteins with various concentrations of EA2 (C) or apoC-III (D) peptide substrates were used, and kinetic plots were fit to Michaelis-Menten equation. Kinetic parameters for peptide glycosylation by both enzymes are given in tables as means. Error bars are SD. N, number of replicate experiments.
Functional Characterization of ppGalNAc-T2D314A
To study whether the mutation was associated with changes at the mRNA and/or protein levels, we used skin fibroblast cultures from three carriers of the mutation and three unrelated controls. Figure S1 shows that GALNT2 mRNA and protein levels in lysates of these cells were similar in both groups (Figures S1A and S1B, respectively).
To study the effects of the mutation on enzyme function, mutant and wild-type ppGalNAc-T2 were expressed in COS7 cells. A study of enzyme kinetics showed that the maximum number of enzymatic reactions catalyzed per second (Kcat) by mutant ppGalNAc-T2 was more than 2-fold lower compared to wild-type ppGalNAc-T2 when using a standard ppGalNAc-T substrate (EA2) (p < 0.05, Figure 1C) (Ten Hagen et al., 2003). Since we found attenuated glycosylation of apoC-III in carriers, we also tested an 11-mer apoC-III peptide harboring the Thr74 residue which is normally glycosylated in native apoC-III (Vaith et al., 1978) as substrate. Figure 1D shows that for this peptide there was also a more than 2-fold lower Kcat (p < 0.01). The reductions in Kcat were observed without significant reductions in Km.
ppGalNAc-T1, the only other ppGalNAc-T reported to be highly expressed in human liver (Ten Hagen et al., 2003) and an enzyme with broad substrate specificity, had a high activity toward the EA2 substrate (Figure S1C) compared to wild-type ppGalNAc-T2 but was unable to use the apoC-III peptide as substrate (Figure S1D).
Oral Fat Challenge
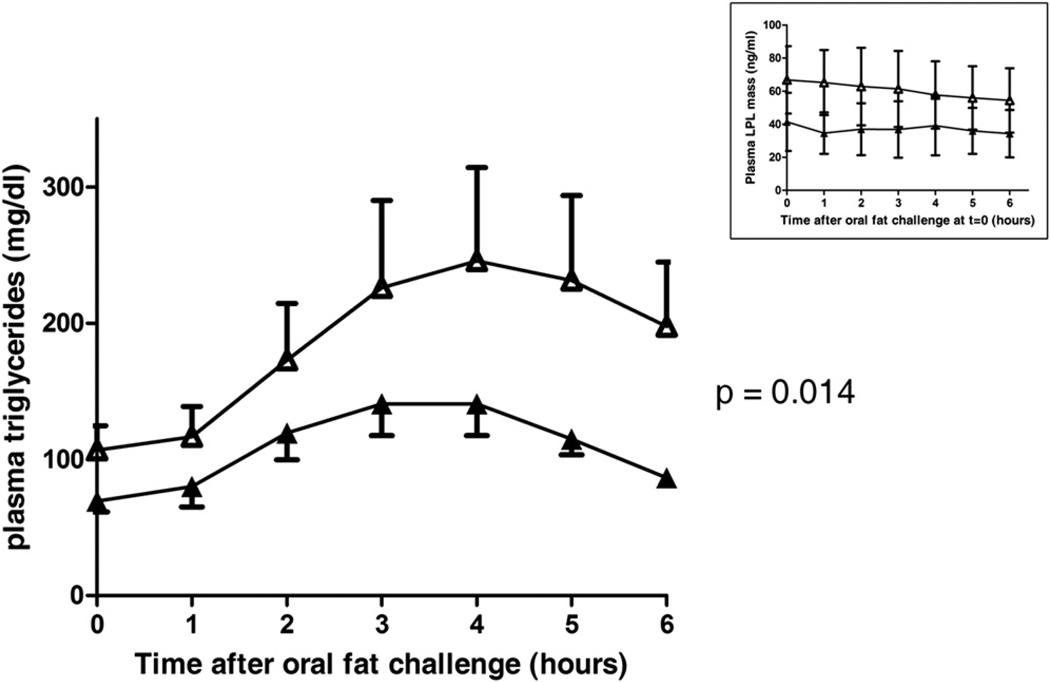
Since apoC-III is an inhibitor of LPL (Jong et al., 1999), the sole enzyme responsible for plasma triglyceride hydrolysis, we challenged carriers and noncarriers with an oral fat load. A significantly improved postprandial plasma triglyceride clearance was observed in four carriers compared to four noncarriers (p = 0.014; Figure 2). In addition, triglyceride levels peaked at 3 hr in carriers instead of 4 hr in noncarriers. The inset of Figure 2 shows that the lipid load did not affect the levels of plasma LPL in both groups at each time point.
Figure 2. Carriers of the GALNT2D314A Mutation Have Improved Postprandial Plasma Triglyceride Clearance.
Depicted are plasma triglyceride levels before (t = 0) and after an oral fat challenge (t = 1–6 hr) in four carriers and four noncarriers. Compared to noncarriers (open symbols), carriers (closed symbols) have lower baseline triglyceride levels, earlier maximum triglyceride levels (t = 3 versus t = 4 in controls), and a reduced overall triglyceride increase. The estimated difference in plasma triglycerides (averaged over time) between carriers and noncarriers using a mixed linear model was 15.4 mg/dl (95% confidence interval 3.3–27.5; p = 0.014). Data are expressed as means. Error bars are SEM. The inset shows that the oral fat challenge did not change plasma LPL levels in carriers and controls when using a commercially available ELISA. The LPL levels appear lower in carriers compared to controls, but these were not significantly different from controls at any time point as assessed by unpaired Student’s t tests. Data are given as means. Error bars are SD.
To investigate apoC-III distribution over lipoproteins, we fractionated plasma using fast protein liquid chromatography (FPLC) followed by apoC-III immunoblotting (Figure S2) and measured total apoC-III at t = 0 and t = 4 in all individuals. Average total apoC-III levels were not different between carriers and controls at these two time points. The top two panels of Figure S2 show that the distribution of apoC-III over lipoproteins was similar in carriers and noncarriers at baseline. Four hours postprandial, however, apoC-III was significantly increased in the HDL fraction in carriers compared to controls (p = 0.029). In addition, we used plasma to generate FPLC cholesterol profiles and triglyceride profiles (Figure S2, lower panels), but we did not identify significant quantitative differences between carriers and controls when comparing these profiles at t = 0 or at t = 4 hr.
Inhibition of Lipoprotein Lipase
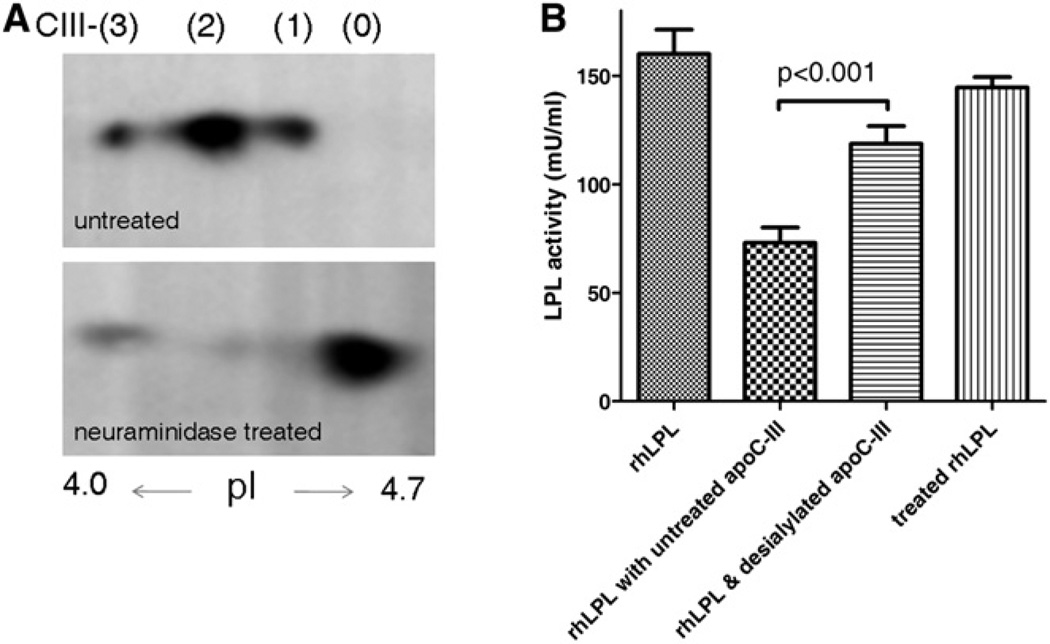
Having established that apoC-III is a specific substrate for ppGalNAc-T2, we studied whether sialylation of the sole O-linked glycan of apoC-III affects this property. To this end, we treated purified human apoC-III that was isolated from very low density lipoprotein (VLDL) of healthy controls (obtained from a commercial source) with neuraminidase. Figure 3A shows that neuraminidase treatment resulted in a shift from the acidic apoC-III isoforms to apoC-III0 due to the loss of sialic acids. This resulted in a significant reduction of the potential of apoC-III to inhibit human recombinant LPL activity. While untreated apoC-III inhibited LPL by 54%, neuraminidase-treated apoC-III inhibited LPL by 26% (p < 0.001; see Figure 3B).
Figure 3. Inhibition of Lipoprotein Lipase-Mediated Triglyceride Hydrolysis.
(A) 2DE analysis of untreated purified human apoC-III that was isolated from plasma VLDL (Academy Bio-Medical Company). In addition to the common apoC-III2 and apoC-III1 isoforms, apoC-III in VLDL is also present in a more acidic isoform(denoted apoC-III3), as has been described previously (Wopereis et al., 2003; Jabs and Assmann, 1987). The second panel shows that treatment of this apoC-III with neuraminidase results in desialylation of apoC-III, as evidenced by the appearance of the nonsialylated isoform, apoC-III0.
(B) Desialylation of this apoC-III with neuraminidase attenuates its potential to inhibit recombinant human LPL. LPL activity is inhibited by 54% after incubation with untreated apoC-III. Desialylation with neuraminidase reduces the inhibitory capacity of apoC-III (p < 0.001). Vertically shaded bar indicates that catalytic activity of LPL is not affected by neuraminidase treatment. Experiments were conducted in triplicate. Data are expressed as means. Error bars are SD.
DISCUSSION
This study identifies a missense mutation in GALNT2 causing a reduction of ppGalNAc-T2 catalytic activity which in carriers of the mutation is associated with improved postprandial triglyceride clearance. The data suggest that this enzyme mediates these effects through glycosylation of apoC-III, an established inhibitor of LPL. The decrease of ppGalNAc-T2 activity and increase in HDL-c in the probands of this study is in line with the finding that GALNT2 silencing increases HDL-c in mice (Teslovich et al., 2010).
The p.D314A mutation in ppGalNAc-T2 causes a reduction of glycosylation activity when using either a general ppGalNAc-T substrate or an apoC-III-based peptide. We also show that ppGalNAc-T2, but not ppGalNac-T1, can use the apoC-III peptide as substrate. Since ppGalNAc-T1 is the only other ppGalNAc-T expressed in human liver (Ten Hagen et al., 2003), this result indicates that apoC-III is preferentially glycosylated by ppGalNAc-T2, an intriguing finding, given the broad substrate specificity of ppGalNAc-T1. Molecular modeling studies suggest that the observed loss of catalytic activity may be due to a loss of enzyme stability (Figure S3 and Movie S1).
ppGalNAc-Ts catalyze coupling of GalNAc to Thr or Ser residues of protein substrates. Subsequently, the O-glycan chain may be elongated with a β1,3-linked galactose and negatively charged sialic acids to form mucin-core 1 structures (Ten Hagen et al., 2003). Failure of GalNAc addition can thus result in altered charge and/or mass of target proteins. 2DE and subsequent immunoblotting of plasma samples led to the discovery of marked changes in apoC-III isoforms in the carriers. This protein could indeed be a substrate for ppGalNAc-T2, since it has a single O-glycan chain at Thr74 (Vaith et al., 1978) that is preceded by Pro73 and Val71 (Gerken et al., 2011). In normal plasma, three main apoC-III isoforms are usually discriminated: apoC-III0, apoC-III1, and apoC-III2, with zero, one, or two sialic acid residues, respectively. Compared to apoC-III2 and apoC-III1, apoC-III0 is only present at very low levels (Wopereis et al., 2003). Comparing carriers and controls, we identified a marked 6.6-fold increased concentration of nonsialylated apoC-III0 and a 32% decrease of monosialylated apoC-III1. The absence of a change in apoC-III2 concentration when comparing carriers and controls is intriguing, especially when considering that our in vitro analyses show that the ppGalNAc-T1 enzyme cannot use the apoC-III peptide as substrate. However, we study a reduction in the initial step of the apoC-III glycosylation, which would lead to less apoC-III molecules available for sialylation. Besides rendering more unglycosylated apoC-III (apoC-III0), this could increase the probability of disialylation (rendering apoC-III2) rather than monosialylation (rendering apoC-III1) of the remaining apoC-III molecules, which would explain the distribution pattern observed in the heterozygotes.
ApoC-III is an inhibitor of LPL-mediated hydrolysis of plasma triglycerides in VLDL and chylomicrons (Jong et al., 1999), thereby affecting both HDL-c and triglyceride levels. On the other hand, apoC-III is used as a model molecule to study congenital disorders of mucin O-linked glycosylation (Wopereis et al., 2003). Combined, these findings suggest that altered glycosylation of apoC-III due to attenuated ppGalNAc-T2 activity causes an increased capacity to clear plasma triglycerides in carriers of the mutation. To test this, we challenged carriers and noncarriers with an oral fat load and observed a significantly improved postprandial plasma triglyceride clearance. The molecular details of this process are very difficult to unravel, due the complexity of in vivo plasma triglyceride lipolysis (Rip et al., 2006). From what is currently known, this process involves multiple interactions of apoC-II as cofactor of LPL (Jong et al., 1999), apoA-V as modulator of LPL function (van Dijk et al., 2004), and angiopoietin-related proteins 3 and 4 (Li, 2006) as LPL inhibitors. While most of these factors are present on HDL, VLDL, and chylomicrons, the apolipoproteins rapidly exchange between these lipoproteins (Eisenberg et al., 1972). In addition, the cocktail of activation and inhibitory processes at specific and differentially regulated sites of action (adipose tissue, skeletal muscle, heart muscle) (Zechner, 1997) occur with simultaneous effects on the hepatic uptake of VLDL and chylomicron remnants, which also reduces plasma triglyceride levels (LPL and apoC-III can, e.g., also act as ligands for hepatic receptors) (Beisiegel et al., 1991).
With this complexity in mind, we have used two straightforward approaches to try unraveling the molecular mechanism that may explain our findings. In one experiment, it is shown that under fasting conditions, plasma concentrations of apoC-III and its distribution over lipoproteins are similar in carriers and controls. At 4 hr after the oral fat load, however, total apoC-III levels remained similar in carriers and controls, but apoC-III was significantly increased in the HDL fraction of only carriers. Since lipolysis occurs at the surface of VLDL and chylomicrons, a change of apoC-III distribution over lipoproteins can be expected to affect plasma triglyceride hydrolysis. In a second experiment, we explored whether the glycan chain of apoC-III affects LPL activity. Using neuraminidase, we showed that desialylation of apoC-III indeed affects its potential to inhibit LPL. These data combined suggest that attenuated ppGalNac-T2-mediated glycosylation of apoC-III is involved in mediating the observed effects on plasma lipids. ApoC-III has, to our knowledge, not been studied in the context of attenuated ppGalNAc-T2 activity, but there are several aspects that merit careful discussion.
It has been reported that the N terminus of apoC-III is responsible for inhibiting LPL while its glycan chain is located in the C-terminal domain, and that synthetic apoC-III lacking the carbohydrate moiety can still inhibit LPL (McConathy et al., 1992). These and other investigators (Roghani and Zannis, 1988) have, however, not addressed the potential of the different apoC-III isoforms to inhibit LPL activity. Also, the effect of a loss of the carbohydrate moiety as a result of loss of ppGalNAc-T2 activity has not been studied in vivo. In addition, we also show that carriers have an altered distribution of apoC-III over lipoproteins in the postprandial phase. The notion that negative charge of apoC-III has effects on the distribution of apoC-III over lipoproteins was previously demonstrated (Luttmann et al., 1994), and it is likely that this effect also mediates part of the lipid changes that we observe.
Second, others have described a family with high apoC-III0 (Maeda et al., 1981) due to heterozygosity for an APOC3 mutation that changes the Thr74 to an alanine (Maeda et al., 1987). Thus, theoretically, half of the apoC-III in these individuals is not glycosylated. Although these investigators did not find marked effects on lipid levels, two out of three carriers had reduced triglyceride levels. This study of Maeda and the current study combined suggest that mutations in apoC-III as well as ppGalNAc-T2 can translate into similar fasting plasma lipid profiles. The current postprandial lipid data are moreover remarkably similar to those reported by Pollin, who studied the effects of an APOC3 non-sense mutation on postprandial lipid levels (Pollin et al., 2008).
Third, apoC-III isoforms have been extensively studied in man with various outcomes. Supporting our hypothesis, it has been shown that of the three apoC-III isoforms, the nonsialyated apoC-III0 is least associated with plasma triglyceride levels (Mauger et al., 2006). Others (Stocks et al., 1979) furthermore reported that neuraminidase treatment of hypersialylated apoC-III from VLDL of hypertriglyceridemic patients reduces its capacity to inhibit LPL. In addition, Kashyap showed decreased levels of the apoC-III0 isoform in 10 subjects with severely increased triglyceride levels (Kashyap et al., 1981). By contrast, van Barlingen showed an increase of apoC-III0 in 12 patients with severe familial hypertriglyceridemia (van Barlingen et al., 1996). The latter contrasting data show that apoC-III0 levels are not predictive of triglyceride levels, especially not in patients with hypertriglyceridemia of different molecular etiology. Many other parameters may affect apoC-III sialylation levels in plasma, such as the levels of sialyltransferase, sialidase activity, and the number of asialoglycoprotein receptors (Maeda et al., 1981), which may differ under pathophysiological conditions in patients and beneficial conditions observed in our study subjects.
Fourth, we did not study putative effects of the GALNT2 mutation on the hepatic uptake of lipoproteins (Shelburne et al., 1980; Windler et al., 1980), but Windler and Havel showed that neither the human apoC-III1 and apoCIII2 isoforms (Windler and Havel, 1985) nor the rat apoC-III0 and apoC-III3 isoforms (Windler et al., 1980) are different in this regard.
Finally, it has recently been reported that ppGalNAc-T2-mediated glycosylation of angiopoietin-related protein 3 affects proteolytic processing of the protein which is required to render angiopoietin-related protein 3 an active inhibitor of LPL (Schjoldager et al., 2010). Reduced ppGalNAc-T2 activity would cause improved processing of angiopoietin-related protein 3, thereby increasing LPL inhibition, which would result in increased plasma triglyceride and decreased HDL-c levels. However, the effects of the loss-of-function mutation described here, as well as the silencing studies of GALNT2 in mice (Teslovich et al., 2010), show the opposite. Figures S4A and S4B, moreover, show identical plasma concentrations of processed and unprocessed angiopoietin-related protein 3 in plasma of carriers and noncarriers, suggesting that in our study there is no direct evidence that changes in angiopoietin-related protein 3 cause the lipid phenotype that we observe. However, it is possible that ppGalNac-T2 affects lipid metabolism through other mucin-type O-linked glycosylated proteins. In this regard, studying apoA-II and apoE did not reveal differences in total plasma concentration (see Table 1) and isoforms (Figures S4C and S4D), while plasma activity of lecithin:cholesterol acyltransferase did not differ between carriers and controls (Table 1).
In conclusion, this study suggests that ppGalNAc-T2 can affect plasma lipids through posttranslational modification of apoC-III. This finding connects the glycosylation and cardiovascular research fields by showing how a mucin-type O-linked glycosylation defect can cause changes in lipid metabolism. As of yet, lipid metabolism in heterozygotes for congenital disorders of glycosylation has to our knowledge not been studied, which offers opportunities for future research, likely resulting in further insight in the relevance of glycosylation in lipid metabolism. Finally, this study also underscores the relevance of GWAS for the identification and unraveling of pathways in complex disorders.
EXPERIMENTAL PROCEDURES
Participants, DNA Analysis, Lipids
The participants with high and low HDL-c were recruited at the outpatient clinic in Amsterdam or at the community hospital in Hilversum, The Netherlands. Controls (n = 1440) were recruited at the collection sites of the Sanquin Blood Bank. Oral fat challenges were carried out as described (Nierman et al., 2005). The local institutional review board approved of all studies described and all participants gave written informed consent.
The GALNT2 mutation was identified through massive parallel sequencing (Herman et al., 2009) and was confirmed by Sanger sequencing. Genotyping of controls was performed on a LightCycler (Roche 480). Total cholesterol, LDL-c, HDL-c, triglycerides, and apoA-I, apoE, apoC-II, and apoC-III were measured on a COBAS MIRA analyzer using commercially available assays (Randox, Wako). FPLC to separate lipoproteins was carried out as described (Levels et al., 2003). Fractions were collected for apoC-III immunoblotting. Plasma LPL was measured using a commercial ELISA (Dainippon).
2D Gel Electrophoresis and Mass Spectrometry
2DE of plasma proteins was performed using IPGphor and Multiphor (Amersham Biosciences). Aliquots (200 µg) for western blots were applied on pH 4–7 IPGs and plasma (300 µg) for preparative gels on pH 3–10 IPGs (Karlsson et al., 2005). Separated proteins for quantification and identification were detected by silver staining (Shevchenko and Shevchenko, 2001). For protein identification, tryptic digests of proteins excised from the gels were analyzed by mass spectrometry (see the Supplemental Information for more detail). For western blots, proteins were transferred to a PVDF membrane. After blocking and incubation with primary antibodies against candidate proteins, the membranes were incubated with HRP-conjugated secondary antibodies. Proteins were visualized using an ECL plus western blotting detection system, exposed to X-ray film, and developed. Isoform intensities were determined as optical density per mm2 and expressed as percentage of the total protein level for each protein.
In Vitro Analyses
Generation of pIMFK4 constructs for wild-type human T1, wild-type T2, and mutant T2 is described in the Supplemental Information. COS7 cells were grown to 90% confluence and transfected with each of the vectors. Enzyme activities were measured against EA2 and an apoC-III peptide (PEVRPTSAVAA) using radioactively labeled UDP-GalNAc (Hagen et al., 1997). Wild-type and mutant T2 activities were measured as described (O’Connell and Tabak, 1993). Kinetic parameters were calculated using Hanes plot. To calculate kcat, enzyme concentrations were measured by western blots using a standard curve made with known concentrations of FLAG-BAP (Sigma-Aldrich).
ApoC-III isolated from human VLDL (Academy Bio-Medical Company) was treated with neuraminidase (NorthStar). A [3H]triolein substrate (Nilsson-Ehle and Schotz, 1976) was used to measure the potential of apoC-III to inhibit recombinant human LPL.
Statistical Analyses
Parameters were compared between carriers and noncarriers using Student’s t tests or Mann-Whitney U tests, where appropriate, for continuous variables. Chi-square tests were used for categorical variables. Fat challenge data were analyzed in a mixed linear model. Statistical analyses were performed using SPSS software (version 16.0, SPSS Inc., Chicago, IL). Error bars indicate standard deviation unless otherwise indicated. Probability values of <0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the study participants and thank C.A. Koch, A.W. Schimmel, J. Coelho Amado de Azevedo, J. Peter, J. Legemate, A. van der Made, and B. van den Bogaard for their help facilitating this family study. We thank the Sanquin Blood Bank for providing control DNA samples, M. Nieuwdorp for his help designing the desialylation experiments, and Xenon Genetics for financing the collection of DNA. This study was supported by the European Union (FP6-2005-LIFESCIHEALTH-6; STREP contract number 037631), Fondation Leducq Transatlantic Networks of Excellence (2010), NWO Medium Investment Grant (40-00506-98-9001 to D.J.L.), and the Intramural Research Program of the National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health. A.G.H. is supported by the Netherlands Organisation for Scientific Research (021.001.035). J.J.P.K. received the Lifetime Achievement Award of the Netherlands Heart Foundation (2010T082).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures, two tables, one movie, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.cmet.2011.11.005.
REFERENCES
- Beisiegel U, Weber W, Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA. 1991;88:8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneel A, Robert T, Lefeber DJ, Benard G, Loncle E, Djedour A, Durand G, Seta N. Two-dimensional gel electrophoresis of apolipoprotein C-III and other serum glycoproteins for the combined screening of human congenital disorders of O- and N-glycosylation. Proteomics Clin. App. 2007;1:321–324. [Google Scholar]
- Eisenberg S, Bilheimer DW, Levy RI. The metabolism of very low density lipoprotein proteins. II. Studies on the transfer of apoproteins between plasma lipoproteins. Biochim. Biophys. Acta. 1972;280:94–104. [PubMed] [Google Scholar]
- Gerken TA, Jamison O, Perrine CL, Collette JC, Moinova H, Ravi L, Markowitz SD, Shen H, Patel H, Tabak LA. Emerging paradigms for the initiation of mucin-type protein O-glycosylation by the polypeptide GalNAc transferase family of glycosyltransferases. J. Biol. Chem. 2011;286:14493–14507. doi: 10.1074/jbc.M111.218701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen FK, Ten Hagen KG, Beres TM, Balys MM, VanWuyckhuyse BC, Tabak LA. cDNA cloning and expression of a novel UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J. Biol. Chem. 1997;272:13843–13848. doi: 10.1074/jbc.272.21.13843. [DOI] [PubMed] [Google Scholar]
- Herman DS, Hovingh GK, Iartchouk O, Rehm HL, Kucherlapati R, Seidman JG, Seidman CE. Filter-based hybridization capture of subgenomes enables resequencing and copy-number detection. Nat. Methods. 2009;6:507–510. doi: 10.1038/nmeth.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs HU, Assmann G. Characterization of an apolipoprotein C-III mutant by high-performance liquid chromatography and time-of-flight secondary ion mass spectrometry. J. Chromatogr. A. 1987;414:323–333. doi: 10.1016/0378-4347(87)80057-9. [DOI] [PubMed] [Google Scholar]
- Jong MC, Hofker MH, Havekes LM. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 1999;19:472–484. doi: 10.1161/01.atv.19.3.472. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Leanderson P, Tagesson C, Lindahl M. Lipoproteomics II: mapping of proteins in high-density lipoprotein using two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:1431–1445. doi: 10.1002/pmic.200401010. [DOI] [PubMed] [Google Scholar]
- Kashyap ML, Hynd BA, Robinson K, Gartside PS. Abnormal preponderance of sialylated apolipoprotein CIII in triglyceride rich lipoproteins in type V hyperlipoproteinemia. Metabolism. 1981;30:111–118. doi: 10.1016/0026-0495(81)90158-x. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Melander O, Guiducci C, Surti A, Burtt NP, Rieder MJ, Cooper GM, Roos C, Voight BF, Havulinna AS, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CS, Loy EY, Pawitan Y, Chia KS. The pursuit of genomewide association studies: where are we now? J. Hum. Genet. 2010;55:195–206. doi: 10.1038/jhg.2010.19. [DOI] [PubMed] [Google Scholar]
- Levels JH, Lemaire LC, van den Ende AE, van Deventer SJ, van Lanschot JJ. Lipid composition and lipopolysaccharide binding capacity of lipoproteins in plasma and lymph of patients with systemic inflammatory response syndrome and multiple organ failure. Crit. Care Med. 2003;31:1647–1653. doi: 10.1097/01.CCM.0000063260.07222.76. [DOI] [PubMed] [Google Scholar]
- Li C. Genetics and regulation of angiopoietin-like proteins 3 and 4. Curr. Opin. Lipidol. 2006;17:152–156. doi: 10.1097/01.mol.0000217896.67444.05. [DOI] [PubMed] [Google Scholar]
- Luttmann S, von Eckardstein A, Wei W, Funke H, Kohler E, Mahley RW, Assmann G. Electrophoretic screening for genetic variation in apolipoprotein C-III: identification of a novel apoC-III variant, apoC-III (Asp45/Asn), in a Turkish patient. J. Lipid Res. 1994;35:1431–1440. [PubMed] [Google Scholar]
- Maeda H, Uzawa H, Kamei R. Unusual familial lipoprotein C-III associated with apolipoprotein C-III-O preponderance. Biochim. Biophys. Acta. 1981;665:578–585. doi: 10.1016/0005-2760(81)90273-3. [DOI] [PubMed] [Google Scholar]
- Maeda H, Hashimoto RK, Ogura T, Hiraga S, Uzawa H. Molecular cloning of a human apoC-III variant: Thr 74-Ala 74 mutation prevents O-glycosylation. J. Lipid Res. 1987;28:1405–1409. [PubMed] [Google Scholar]
- Mauger JF, Couture P, Bergeron N, Lamarche B. Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J. Lipid Res. 2006;47:1212–1218. doi: 10.1194/jlr.M500455-JLR200. [DOI] [PubMed] [Google Scholar]
- McConathy WJ, Gesquiere JC, Bass H, Tartar A, Fruchart JC, Wang CS. Inhibition of lipoprotein lipase activity by synthetic peptides of apolipoprotein C-III. J. Lipid Res. 1992;33:995–1003. [PubMed] [Google Scholar]
- Nierman MC, Rip J, Kuivenhoven JA, van Raalte DH, Hutten BA, Sakai N, Kastelein JJ, Stroes ES. Carriers of the frequent lipoprotein lipase S447X variant exhibit enhanced postprandial apoprotein B-48 clearance. Metabolism. 2005;54:1499–1503. doi: 10.1016/j.metabol.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle P, Schotz MC. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J. Lipid Res. 1976;17:536–541. [PubMed] [Google Scholar]
- O’Connell BC, Tabak LA. Separation of glycopeptides from in vitro O-glycosylation reactions using C18 cartridges. Anal. Biochem. 1993;210:423–425. doi: 10.1006/abio.1993.1219. [DOI] [PubMed] [Google Scholar]
- Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–1705. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rip J, Nierman MC, Ross CJ, Jukema JW, Hayden MR, Kastelein JJ, Stroes ES, Kuivenhoven JA. Lipoprotein lipase S447X: a naturally occurring gain-of-function mutation. Arterioscler. Thromb. Vasc. Biol. 2006;26:1236–1245. doi: 10.1161/01.ATV.0000219283.10832.43. [DOI] [PubMed] [Google Scholar]
- Roghani A, Zannis VI. Mutagenesis of the glycosylation site of human ApoCIII. O-linked glycosylation is not required for ApoCIII secretion and lipid binding. J. Biol. Chem. 1988;263:17925–17932. [PubMed] [Google Scholar]
- Schjoldager KT, Vester-Christensen MB, Bennett EP, Levery SB, Schwientek T, Yin W, Blixt O, Clausen H. O-glycosylation modulates proprotein convertase activation of angiopoietin-like protein 3: possible role of polypeptide GalNAc-transferase-2 in regulation of concentrations of plasma lipids. J. Biol. Chem. 2010;285:36293–36303. doi: 10.1074/jbc.M110.156950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne F, Hanks J, Meyers W, Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J. Clin. Invest. 1980;65:652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Shevchenko A. Evaluation of the efficiency of in-gel digestion of proteins by peptide isotopic labelling and MALDI mass spectrometry. Anal. Biochem. 2001;296:279–283. doi: 10.1006/abio.2001.5321. [DOI] [PubMed] [Google Scholar]
- Stocks J, Holdsworth G, Galton D. Hypertriglyceridaemia associated with an abnormal triglyceride-rich lipoprotein carrying excess apolipoprotein C-III-2. Lancet. 1979;2:667–671. doi: 10.1016/s0140-6736(79)92068-3. [DOI] [PubMed] [Google Scholar]
- Ten Hagen KG, Fritz TA, Tabak LA. All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology. 2003;13:1R–16R. doi: 10.1093/glycob/cwg007. [DOI] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaith P, Assmann G, Uhlenbruck G. Characterization of the oligosaccharide side chain of apolipoprotein C-III from human plasma very low density lipoproteins. Biochim. Biophys. Acta. 1978;541:234–240. doi: 10.1016/0304-4165(78)90396-3. [DOI] [PubMed] [Google Scholar]
- van Barlingen HH, Kock LA, de Man FH, Erkelens DW, de Bruin TW. In vitro lipolysis of human VLDL: effect of different VLDL compositions in normolipidemia, familial combined hyperlipidemia and familial hypertriglyceridemia. Atherosclerosis. 1996;121:75–84. doi: 10.1016/0021-9150(95)05703-x. [DOI] [PubMed] [Google Scholar]
- van Dijk KW, Rensen PC, Voshol PJ, Havekes LM. The role and mode of action of apolipoproteins CIII and AV: synergistic actors in triglyceride metabolism? Curr. Opin. Lipidol. 2004;15:239–246. doi: 10.1097/00041433-200406000-00002. [DOI] [PubMed] [Google Scholar]
- Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J. Lipid Res. 1985;26:556–565. [PubMed] [Google Scholar]
- Windler E, Chao Y, Havel RJ. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J. Biol. Chem. 1980;255:8303–8307. [PubMed] [Google Scholar]
- Wopereis S, Grunewald S, Morava E, Penzien JM, Briones P, Garcia-Silva MT, Demacker PN, Huijben KM, Wevers RA. Apolipoprotein C-III isofocusing in the diagnosis of genetic defects in O-glycan biosynthesis. Clin. Chem. 2003;49:1839–1845. doi: 10.1373/clinchem.2003.022541. [DOI] [PubMed] [Google Scholar]
- Zechner R. The tissue-specific expression of lipoprotein lipase: implications for energy and lipoprotein metabolism. Curr. Opin. Lipidol. 1997;8:77–88. doi: 10.1097/00041433-199704000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.