Abstract
The objective of our study was to determine the effects of two antihypertensive drug dose schedules (‘PM dose’ and ‘Add on dose’) on nocturnal blood pressure (BP) in comparison to usual therapy (‘AM dose’) in African Americans with hypertensive chronic kidney disease (CKD) and controlled office BP. In a three period, cross-over trial, former participants of the African American Study of Kidney Disease were assigned to receive the following three regimens, each lasting 6 weeks, presented in random order: AM dose (once daily antihypertensive medications taken in the morning), PM dose (once daily antihypertensives taken at bedtime) and ‘Add on dose’ (once daily antihypertensives taken in the morning and an additional antihypertensive medication before bedtime [diltiazem 60–120 mg, hydralazine 25 mg, or additional ramipril 5 mg]). Ambulatory BP monitoring was performed at the end of each period. The primary outcome was nocturnal systolic BP. Mean age of the study population (n=147) was 65.4 years, 64% were male, mean estimated GFR was 44.9 ml/min/1.73 m2. At the end of each period, mean (SE) nocturnal systolic BP was 125.6 (1.2) mm Hg in the AM dose, 123.9 (1.2) mm Hg in the PM dose, and 123.5(1.2) mm Hg in the Add-on dose. None of the pairwise differences in nocturnal, 24-hour and daytime systolic BP were statistically significant. Among African Americans with hypertensive CKD, neither PM (bedtime) dosing of once daily antihypertensive nor the addition of drugs taken at bedtime significantly reduced nocturnal BP compared to morning dosing of anti-hypertensive medications.
Keywords: Nocturnal blood pressure, chronic kidney disease, hypertension
Introduction
Chronic kidney disease (CKD) is a global public health problem.1 The burden of end-stage renal disease (ESRD) is substantial in many industrialized countries despite the widespread use of interventions that might slow the progression of CKD, including control of blood pressure and the use of antihypertensive drugs that inhibit the renin angiotensin system.2 The limitations of these two treatment strategies was recently described among African Americans with hypertension-related CKD who participated in the trial and cohort phases of the African American Study of Kidney Disease (AASK). In these long-term studies, a majority of participants experienced a doubling of serum creatinine, end-stage renal disease or death despite good office blood pressure control and use of renin angiotensin system blockers.3 This finding suggests that there are other risk factors that influence loss of kidney function.4;5
One potential risk factor that has received increasing attention is the lack of diurnal variation in level of blood pressure often observed in persons with CKD.6 Among AASK Cohort Study participants, 80% either did not show a nocturnal decline in blood pressure (“non-dipper”) or had a higher blood pressure at night than during the day (“reverse dipper”).7 Prior observational studies have found an association between elevated nocturnal blood pressure and “hard outcomes” such as death and cardiovascular disease among persons with high blood pressure in the general population,8–10 Studies of the effect of nocturnal blood pressure among persons with CKD although less common and smaller in size, have also shown a direct relationship with progression to end-stage renal disease.11–13 These findings suggest that lowering nocturnal blood pressure may reduce morbidity and mortality. An important issue that must be addressed before embarking on a major trial of nocturnal BP reduction on clinical outcomes in CKD patients is whether nocturnal blood pressure can be lowered.14–18 In this context, we tested two approaches to lower nocturnal BP in African Americans with hypertensive CKD and controlled office blood pressure.
Methods
We recruited former participants of the AASK Cohort Study.19 The protocol for this study was approved by the institutional review board at each site, and all participants provided written informed consent. AASK Cohort Study participants were eligible for the nocturnal blood pressure lowering study if during the course of the cohort study they had completed a technically acceptable session of ambulatory blood pressure monitoring (ABPM) at the last study visit, attended at least two in-clinic visits within the last 12 months, and had average office blood pressure ≤140/90 mm Hg based on the most recent two BP values measured at least one week apart. In addition, in order to facilitate evaluation of the effect of timing of once daily medications on level of nocturnal blood pressure, candidates for this study had to be prescribed antihypertensive drug therapy at the baseline visit as follows: i) one antihypertensive medication had to be a once daily medication, ii) for those prescribed 2 antihypertensive medications, at least one had to be a once daily medication, and iii) for persons prescribed 3 or more antihypertensive medications, at least two had to be once a once daily medication. A 4-week period was established for investigators to change the participants’ antihypertensive medications to meet the above eligibility requirements.
AASK Cohort Study participants were excluded from the nocturnal blood pressure study if they a) had an arm circumference > 50 cm, b) had reached ESRD and required renal replacement therapy or received a kidney transplant, c) were currently institutionalized, d) were shift workers and worked at night e) reported a myocardial infarction or cerebrovascular accident which occurred within 3 months of AASK Cohort close-out visit, f) had a known ejection fraction < 40%, g) were pregnant or lactating, or h) in the opinion of the investigators, were likely to reach ESRD within the next six months.
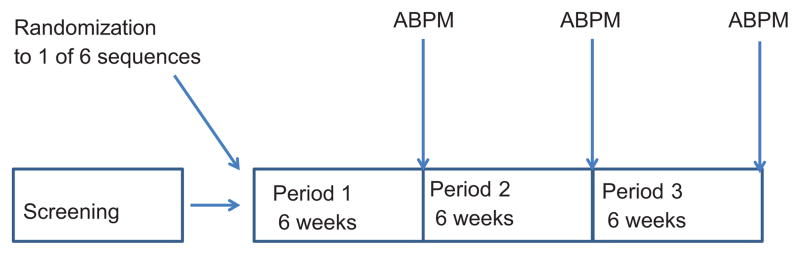
We conducted an open label randomized, three period, cross-over trial (Figure 1) of 3 antihypertensive dosing schedules, each lasting 6 weeks. The sequence of the dosing schedules was randomized. Two strategies of dosing (referred to as PM, and Add-On as described below) were studied in comparison to the standard dose schedule (AM Dose). In all dosing schedules, the time of day a diuretic was taken was not changed from that at time of study entry.
Fig 1.
Study schema; 3 period randomized, cross over design
AM Dose Schedule: – The participant’s antihypertensive regimen at the baseline visit was used as the comparison (or control) regimen. Study participants were instructed to take all of their once a day medications in the morning.
PM Dose Schedule: Study participants were advised to take all of their once-a-day antihypertensive drugs at bedtime.
Add-On Dose schedule: Participants were instructed to take their once daily antihypertensive medications in the morning. Study investigators prescribed an additional dose of either ramipril, diltiazem, or hydralazine to be taken at bedtime based on the following guidelines: A) participants prescribed ramipril 5 mg or less received an additional bed time dose of ramipril (5 mg), b) Participants prescribed beta-blockers, or resting pulse rate less than 60 /min received hydralazine 25 mg at bed time, c) Participants not meeting (a) or (b) criteria received diltiazem 60–120 mg at bed time.
In the last week of each 6-week treatment period, a 24-hour session of ABPM was performed; detailed methodology is available in the online supplement. Office BP measurements were performed by trained, certified staff using a Tycos classic handheld aneroid device (Tycos Instruments, Inc, Arden, NC) using recommended techniques.20
Statistical analyses
Baseline characteristics of the study population were described using mean +/− standard deviation (SD) for quantitative variables and frequencies and percents for categorical variables. Antihypertensive medication use at both baseline and follow-up in the study were summarized. Nocturnal systolic blood pressure was the pre-specified primary outcome. Secondary outcomes were nocturnal diastolic BP, daytime systolic minus nocturnal systolic BP, daytime diastolic minus nocturnal diastolic BP, night systolic/day systolic ratio, night diastolic/day diastolic ratio, daytime systolic and diastolic BP, 24 hours systolic and diastolic BP. In this cross-over trial, each study participant served as his/her control. A linear mixed effects model with a random subject effect, and fixed effects for period, treatment, and latest nocturnal systolic blood pressure prior to study entry was used to relate treatment to nocturnal systolic blood pressure. Results were expressed as adjusted mean difference (95% confidence interval) between PM dose schedule versus AM dose schedule, and Add-on dose schedule compared to AM dose schedule. The possibility of a carry-over effect of treatment on nocturnal systolic BP was examined by testing whether treatment comparisons differed between the three periods. Although we did not observe a significant carry-over effect, data were analyzed by each period in exploratory analyses. Exploratory subgroup analyses were performed by stratifying study participant according to baseline dipping status, presence of masked hypertension, level of urinary sodium excretion, level of nocturnal blood pressure prior to study entry, and number of antihypertensive medications. Treatment effects was estimated separately for each subgroup, and treatment by subgroup interactions were tested. We present analyses based on the order of treatment actually administered; two study participants were treated in different order than their randomized assignment and were included in the “per protocol” analysis. Sensitivity analyses based on the randomly assigned treatment (intention to treat) order provided similar results. The primary analysis and secondary analyses are reported, without adjustment for multiple comparisons. Given the number of participants enrolled in the AASK Cohort Study, we estimated that the number of available participants would be ~180 patients. At this sample size, assuming hypothesis tests are performed at a 2-sided significance level of 5% without adjustment for multiple comparisons, the minimum detectable treatment effect with 80% power was 4.3 mm Hg for nocturnal systolic BP and 3.0 for the difference between nocturnal and daytime systolic BP.
Results
Of the 430 potentially eligible participants, 151 were randomized. Four study participants did not have any ABPM measurements and were not included in the analyses. Participants enrolled in the nocturnal blood pressure study were compared to others in the AASK cohort study who did not participate in the nocturnal blood pressure study; participants who enrolled were similar in age and gender, but had lower mean clinic blood pressure (123/71 mm Hg vs. 131/76 mm Hg, p<0.05) and lower mean serum creatinine (2.03 vs. 2.28 mg/dl p= 0.009) compared to AASK Cohort study participants persons who did not enroll in the nocturnal blood pressure study.
The mean age of the study population was 65.4 years (Table 1). All participants were African-American, 64% were male, with longstanding hypertension (mean of 29.9 years), mean serum creatinine was 2.03 mg/dl, and the mean estimated GFR was 44.9 mL/min/1.73m2. At baseline about one-half (45%) of the study participants had a non-dipper blood pressure pattern, and twenty nine percent had a reverse dipper pattern diurnal profile.
Table 1.
Baseline characteristics of study population (n=147) * Lab and 24 hour ambulatory blood pressure values from data obtained at the last visit of the Cohort Study
| Baseline characteristics | n (%) or mean ± s.d. |
|---|---|
| Age (years) | 65.4 ± 9.77 |
| Gender (Male) | 94 (63.9%) |
| Years with Hypertension | 29.9 ± 9.85 |
| History of cardiovascular disease (CVD)* | 46 (31.3%) |
| Tobacco use -Never smoked tobacco | 58 (39.5%) |
| Current tobacco smoking | 29 (19.7%) |
| Past tobacco smoking | 60 (40.8%) |
| Baseline Systolic Blood Pressure (SBP mm Hg) | |
| Clinic | 127 ± 13.8 |
| 24 hour mean | 127 ±15.2 |
| Daytime | 129 ± 14.7 |
| Nighttime | 124 ± 18.1 |
| Baseline Diastolic Blood Pressure (DBP mm Hg) | |
| Clinic | 74.9 ± 9.54 |
| 24 hour mean | 74.3 ± 9.6 |
| Daytime | 77.0 ± 10.1 |
| Night time | 71.5 ± 10.5 |
| Dipping Status SBP Dipped at Night 10–20+% | 36 (24.5%) |
| SBP Dipped at Night 0–10% | 67 (45.6%) |
| SBP higher at Night than day | 44 (29.9%) |
| Body Mass Index (kg/m2) > 30 | 87 (60.0%) |
| Total Cholesterol (mg/dL) | 188 ± 45.0 |
| Estimated GFR (mL/min/1.73m2) | 44.9 ± 14.6 |
| Serum Creatinine (mg/dL) | 2.03 ± 0.75 |
| Urine protein/creatinine ratio (g/g) >. 22 | 24 (16.3%) |
The mean number of antihypertensive medications prescribed at baseline was 4.06 ± 1.43; most of the study participants (83.7%) were prescribed three or more drugs. In the PM Dose Schedule, one antihypertensive medication was changed to bedtime dosing in 48.8% of participants, and 2 or more antihypertensive medications drugs changed to bedtime dosing in 47.6% of participants. In participants where one antihypertensive medication was changed to bedtime dosing, the medication was an ACE inhibitor in 72.5%; angiotensin receptor blocker in 15%, and a calcium channel blocker in 12.5%. In the add-on dose schedule, the most common medication added at bedtime was hydralazine in 49.5%, diltiazem in 18.1%, ramipril in 7.6% and another medication in 24.8% of participants.
Eleven participants did not complete the study; the reasons for not completing the study were low blood pressure based on the clinical determination of the site investigator (n=4), hospitalization not related to the study (n=3), hospitalization for a fall during the usual dosing phase (n=1, reported as possibly study related), participant request (n=1), high blood pressure (n=1), and medical condition other than hypertension (n=1).
Mean (SE) nocturnal systolic blood pressure was 125.5 (1.2) mm Hg in the AM dose schedule, 123.8 (1.2) mm Hg in the PM dose schedule, and 123.5 (1.2) mm Hg during the Add-on dose schedule. Although nocturnal systolic blood pressure was lower with the PM dose schedule compared to the AM dose schedule (mean night time SBP in the PM phase minus mean night time SBP in the AM phase was −1.7 mm Hg 95% confidence intervals (CI) −4.05, 0.65), this difference was not statistically significant (p=0.15) (Table 2). Nocturnal systolic blood pressure was non-significantly lower with Add-on dosing compared to AM dosing; mean night time SBP in the Add-On phase minus mean night time SBP in the AM phase was −2.0 mm Hg 95% CI −4.40, 0.26 (p=0.08). Compared with the AM dose schedule, twenty four hour and daytime systolic blood pressure, and 24 hour, day time and night time diastolic blood pressure were not statistically significantly reduced with PM dose schedule or the Add-on dose schedule. The difference between daytime and night time systolic blood pressure was 3.25 mm Hg (95% CI 1.42, 5.08, p=<0.001) higher, and night/day systolic blood pressure ratio was 0.02 (95% CI −0.04, −0.01)lower in the PM compared to the AM dosing.
Table 2.
Primary analyses based on cross-over design, each patient used as their own control. AM Dose Schedule: Study participants were instructed to take all of their once a day medications in the morning. PM Dose Schedule: Study participants were advised to take all of their once-a-day antihypertensive drugs at bedtime. Add-On Dose schedule: Participants were instructed to take their once daily antihypertensive medications in the morning. Study investigators prescribed an additional dose of either ramipril, diltiazem, or hydralazine to be taken at bedtime
| Mean (SE) | PM minus AM | Add-on minus AM | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Blood Pressure parameter | AM | PM | Add-on | mean (95% CI) | p-value | mean (95% CI) | p-value |
| Nocturnal Systolic BP | 125.6 (1.17) | 123.9(1.17) | 123.5(1.16) | −1.70 (−4.05,0.65) | 0.16 | −2.07 (−4.40,0.26) | 0.08 |
| Daytime-Nocturnal SBP | 4.6 (0.80) | 7.8(0.80) | 5.8(0.79) | 3.25 (1.42,5.08) | <0.001 | 1.28 (−0.54,3.09) | 0.17 |
| Night/Day SBP Ratio | 0.97 (0.01) | 0.94(0.01) | 0.96(0.01) | −0.02 (−0.04,0.01) | <0.001 | −0.01 (−0.02,0.01) | 0.21 |
| Daytime SBP | 130.1 (1.10) | 131.7(1.10) | 129.4(1.09) | 1.55 (−0.52,3.63) | 0.14 | −0.74 (−2.79,1.32) | 0.48 |
| 24 Hour SBP | 129.0 (1.05) | 129.7(1.05) | 128.0(1.04) | 0.72 (−1.28,2.73) | 0.47 | −1.03 (−3.01,0.95) | 0.31 |
| Nocturnal Diastolic BP | 71.7 (0.72) | 71.7(0.72) | 70.8(0.72) | −0.03 (−1.53,1.47) | 0.97 | −0.90 (−2.39,0.59) | 0.24 |
| Daytime-Nocturnal DBP | 5.6(0.55) | 6.4(0.54) | 5.9(0.54) | 0.84 (−0.46,2.14) | 0.21 | 0.32 (−0.98,1.61) | 0.63 |
| Night/Day DBP Ratio | 0.97(0.01) | 0.92(0.01) | 0.93(0.01) | −0.01 (−0.03,0.01) | 0.24 | −0.00 (−0.02,0.01) | 0.63 |
| Daytime DBP | 77.2(0.68) | 78.1(0.68) | 76.7(0.68) | 0.88 (−0.39,2.16) | 0.17 | −0.52 (−1.78,0.74) | 0.41 |
| 24 Hour DBP | 75.9(0.65) | 76.5(0.65) | 75.3(0.64) | 0.64 (−0.57,1.86) | 0.3 | −0.59 (−1.79,0.61) | 0.34 |
In participants receiving hydralazine as the add-on agent, the mean nocturnal systolic blood pressure was lower in the PM add-on schedule compared to the AM schedule; mean night time SBP in the Add-On phase minus mean night time SBP in the AM phase was −5.32 mm Hg (95% CI −9.37,−1.28, p(=0.01). In participants on medications other than hydralazine as the add on agent, mean night time SBP in the Add-On phase minus mean night time SBP in the AM phase was 0.80 (95% CI −2.92,4.53, p=0.67) mm Hg. However, a test for interaction between treatment effect and choice of add-on medication was not statistically significant.
Tests for interaction with dose schedule were not statistically significant for subgroups stratified by dipping status, presence of masked hypertension, urinary sodium excretion, nocturnal blood pressure prior to study entry, and number of antihypertensive medications at baseline (table 3). In participants with lower urinary sodium excretion, mean nocturnal systolic blood pressure in the add-on dosing was −4.13 mm Hg (95% CI −7.68,−0.58, p=0.02) lower than AM dosing. However, given the multiple statistical tests performed and post-hoc nature of these analyses, these findings should be interpreted with caution.
Table 3.
Post hoc subgroup analyses of effects of the two regimens on nocturnal systolic blood pressure. AM Dose Schedule: Study participants were instructed to take all of their once a day medications in the morning. PM Dose Schedule: Study participants were advised to take all of their once-a-day antihypertensive drugs at bedtime. Add-On Dose schedule: Participants were instructed to take their once daily antihypertensive medications in the morning. Study investigators prescribed an additional dose of either ramipril, diltiazem, or hydralazine to be taken at bedtime
| Subgroup | Mean Nocturnal Systolic BP (SE) | PM minus AM | Add-on minus AM | Interaction p value | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| AM | PM | Add-on | mean (95% CI) | p-value | mean (95% CI) | p-value | ||
| Dipping status* | ||||||||
| Dipper | 120.3(2.5) | 118.5(2.58) | 119.2(2.53) | −1.79 (−7.34, 3.76) | 0.52 | −1.14 (−6.54, 4.25) | 0.67 | 0.96 |
| Non-Dipper | 124.2(1.63) | 121.5(1.60) | 121.3(1.58) | −2.74 (−6.22,0.75) | 0.12 | −2.99 (−6.40,0.42) | 0.09 | |
| Reverse Dipper | 132.6(2.19) | 131.4(2.19) | 130.5(2.17) | −1.23 (−5.57,3.10) | 0.57 | −2.10 (−6.30,2.09) | 0.32 | |
| Nocturnal SBP prior to study entry (median 123 mm Hg) | ||||||||
| Below median | 118.3(1.56) | 116.9(1.55) | 116.5(1.56) | −1.50 (−4.54,1.55) | 0.33 | −1.87 (−4.92,1.18) | 0.23 | 0.95 |
| Above median | 133.0(1.73) | 130.9(1.73) | 130.2(1.70) | −2.11 (−5.73,1.52) | 0.25 | −2.79 (−6.37,0.79) | 0.13 | |
| Masked hypertension prior to study entry | ||||||||
| Masked hypertension present | 131.6(1.73) | 129.4(1.71) | 129.1(1.70) | −2.24 (−6.20,1.71) | 0.26 | −2.56 (−6.49,1.37) | 0.20 | 0.95 |
| Masked hypertension not present | 121.0(1.58) | 119.7(1.59) | 119.0(1.58) | −1.27 (−4.14,1.60) | 0.38 | −2.03 (−4.87,0.82) | 0.16 | |
| Number of antihypertensive medications at baseline | ||||||||
| One or two | 121.3(2.80) | 120.5(2.81) | 120.0(2.71) | −0.82 (−6.60,4.96) | 0.78 | −1.34 (−6.36,3.68) | 0.59 | 0.99 |
| 3+ | 126.3(1.29) | 124.6(1.29) | 124.2(1.28) | −1.76 (−4.42,0.89) | 0.19 | −2.14 (−4.77,0.49) | 0.11 | |
| 24 hour urinary sodium excretion (median Na/K ratio 2.14) | ||||||||
| Above median | 124.23(1.89) | 123.44(1.86) | 122.70(1.86) | −0.79 (−4.19,2.60) | .64 | −1.53 (−4.90,1.84) | .37 | 0.51 |
| Below median | 128.89(1.70) | 125.49(1.70) | 124.75(1.70) | −3.40 (−6.99,0.19) | .063 | −4.13 (−7.68,−0.58) | .02 | |
Although comparisons between randomized treatment groups did not significantly differ for lowering of nocturnal blood pressure between the three periods, data were analyzed by period in exploratory analyses. Within period one, nocturnal systolic blood pressure was −5.73 mm Hg lower in the PM compared to the AM phase (95% CI −10.6,−0.89, p=0.02). There were no statistically differences in nocturnal systolic blood pressure between the PM and AM dosing in periods 2 (−2.7 mm Hg, p=0.36) and 3 (2.8 mm Hg, p=0.36). For period 1, there were no statistically significant differences in baseline characteristics between participants assigned to AM, PM or add-on dosing (data not shown).
Discussion
Among African Americans with hypertensive CKD and controlled clinic BP, administration of antihypertensives in the evening (PM dose schedule) or by an additional bedtime dose (Add-on dosing) resulted in a modest, nonsignificant lowering of nocturnal BP compared to the AM dose schedule. Nocturnal blood pressure is a robust predictor of increased risk of cardiovascular events and mortality.9,10;21–24 Patients with chronic kidney disease often have elevated nocturnal blood pressure which is associated with more microalbuminuria and progression of kidney disease.12;13;25 In the AASK cohort study, 80% of patients had a nondipping or reverse dipping blood pressure profile; elevated nocturnal blood pressure was associated with increased left ventricular hypertrophy and albuminuria and higher risk of CKD progression.7;26,27 Other studies have also documented that nondipping of blood pressure is an independent predictor for ESRD.11;28
Given these observational data, some have hypothesized a benefit of modifying the timing of antihypertensive drug therapy to lower nocturnal blood pressure and restore “normal” circadian rhythm of blood pressure. In patients with primary hypertension, several studies have reported that altering timing of administration of antihypertensive medication is associated with reduction in nocturnal blood pressure and change in the diurnal blood pressure profile. 14–18 The Ambulatory Blood Pressure Monitoring in the Prediction of Cardiovascular Events and Effects of Chronotherapy (MAPEC) study demonstrated that patients taking ≥1 BP-lowering medications at bedtime had a lower risk of CVD events than those ingesting all medications upon awakening. 29 Data in patients with chronic kidney disease are more limited. In an uncontrolled eight week study, Minutolo etal demonstrated that with administration of antihypertensive medications at night, night-day ratio of mean ABP decreased in 93.7% of patients, with normal circadian rhythm restored in 87.5%.30 Interestingly, proteinuria was also reduced with evening administration of antihypertensive drugs. The reduction of cardiovascular risk seen in the MAPEC study was consistent in the CKD subgroup; in 661 patients with CKD, patients who took at least one BP-lowering medication at bedtime had lower sleep time blood pressure and a lower adjusted risk for total cardiovascular events. (adjusted HR 0.31, p< 0.001).31 However, information about drug doses was not provided and the racial/ethnic composition was different compared to this study.31
Our study evaluated the effects of two treatment strategies in lowering nocturnal blood pressure in the setting of chronic kidney disease. One approach was to administer antihypertensive medications at bed time (PM dosing) and the other was to continue AM administration of medications but to add an antihypertensive medication at bed time (Add-on dosing). Both strategies showed only modest effects on nocturnal blood pressure, effects that were not statistically significant compared to morning dosing. Secondary outcomes such as the night/day ratio was lower, and the daytime-night blood pressure was higher in the PM compared to the standard group reflecting modification in the diurnal variation in blood pressure. In exploratory analyses, these findings were consistent in subgroups defined by dipping status, nocturnal blood pressure, urinary sodium excretion and number of antihypertensive medications at baseline. In the PM add-on dose schedule, hydralazine administration was associated with reduction in nocturnal blood pressure. However, this was a post-hoc analysis, and the test of interaction was not significant; therefore this finding should be interpreted with caution. In comparing these results with the study by Minutolo,30 the patient populations between the two studies were quite different with regard to ethnicity and duration of hypertension. In addition, in the Minutolo study there was no control group to fully evaluate the effect of the intervention.
Our findings suggest that approaches to reduce the level of nocturnal blood pressure and restore diurnal rhythm of blood pressure in persons with chronic kidney disease require further study. Several factors may be responsible for the lack of significant nocturnal blood pressure lowering we observed. The dose of the “add-on” agents used in this study was relatively low; increasing the dose used in the add-on strategy may be one area to explore in future trials, particularly in patients with CKD or populations with more resistant hypertension. In addition, our interventions did not address sleep abnormalities that are commonly present in patients with chronic kidney disease and that may contribute to persistent elevation of blood pressure at night.32 There are also data to suggest that elevated nocturnal blood pressure may relate to salt sensitivity of blood pressure, with higher blood pressure at night reflecting a “pressure natriuresis” due to decreased sodium excretion during the day.33 This suggests that perhaps optimization of salt and water balance with appropriately timed diuretic therapy may be helpful in maintaining the diurnal profile of blood pressure. In any case, interventions that consistently lower nocturnal blood pressure need to be developed and tested prior to the implementation of a prospective trial that evaluates the impact of nocturnal pressure reduction in chronic kidney disease on clinical outcomes.
This study has a number of strengths making an important contribution to this field. The patient population was well defined, and interventions were practical to implement in daily practice. Measurement of blood pressure both in the clinic and ambulatory setting was done by trained staff using standard protocols. However, there also some important limitations to be considered in the interpretation and extrapolation of these results. All patients were African Americans with hypertensive chronic kidney disease and had relatively well controlled blood pressure; therefore whether similar results would be seen in patients of other race/ethnicity, etiology of chronic kidney disease or higher blood pressure remains uncertain. The projected sample size was not achieved with some loss of power to detect a difference between the interventions. Accordingly, as reflected in the lower limits of the 95% confidence intervals for the treatment effects on nocturnal blood pressure (−4.05 mmHg for PM vs. AM dosing and −4.40 mmHg for Add-On vs. AM dosing), we are unable to rule out undetected moderate reductions of night time systolic BP of up to approximately 4 mmHg by the two nocturnal dosing strategies. The smaller sample size in this study (n=147), compared the larger MAPEC study (n=2156 overall,29 and 661 with CKD31) may limit our ability to detect significant differences. Finally, there was no washout period between the interventions, though the six week duration makes it unlikely that there would be significant residual effect.
In summary, among African Americans with hypertensive chronic kidney disease, PM dosing of once daily antihypertensive medications, and the administration of an add on drug at bed time after AM administration of once daily medications was safe and had modest, nonsignificant effects on nocturnal blood pressure.
Supplementary Material
Perspective.
In observational studies, elevated night time blood pressure is associated with increased risk of adverse kidney and cardiovascular outcomes in the general population and in patients with chronic kidney disease. Antihypertensive drug therapy lowers clinic blood pressure, but little is known about how to reduce night time blood pressure. We tested two practical strategies, each designed to lower nocturnal blood pressure. One strategy switched anti-hypertensive medications from morning to bedtime dosing. A second strategy added a bedtime dose of an anti-hypertensive medication. We tested these strategies in African-Americans with hypertension-related chronic kidney disease, a condition associated with high levels of nighttime blood pressure. However, neither of the strategies lowered night time blood pressure. Additional research is needed to develop and test strategies that lower night time blood pressure.
Novelty and Significance.
What is new?
This study shows that in African Americans with hypertensive CKD, neither PM (bedtime) dosing of once daily antihypertensive nor the addition of drugs taken at bedtime significantly reduced nocturnal BP compared to morning dosing of anti-hypertensive medications.
What is relevant?
There is increasing interest in a night time blood pressure as a risk factor for long term outcomes
Summary
Nocturnal blood pressure is well known as risk factor for renal and cardiovascular outcomes. This is one of the first studies to evaluate whether nocturnal blood pressure can be lowered by simple changes in antihypertensive drug therapy in patients with chronic kidney disease.
Acknowledgments
5. Sources of funding: This study was supported by the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) and an unrestricted grant from King Pharmaceuticals
Footnotes
Clinical trials.gov registration number NCT00582777
6. Conflict of interest/disclosures
Dr. Rahman has research support from NIH, and has received honoraria from Boehringer Ingelheim. Dr. Bakris has served as a consultant/ advisory board for Takeda, Servier, Abbott, CVRx, Johnson and Johnson, Eli Lilly and Medtronic. Dr. Jamerson has research grant support from NIH, NIDDK and NHLBI, serves on the speakers bureau for Daichi-Sankyo pharmaceuticals, has received honoraria from Boehringer-Ingelheim, Daichi-Sankyo, Forest, Novartis and Xuma Pharm, served as a consultant advisory board for Boehringer-Ingelheim, Daichi Sankyo, Forest, Pfizer, Novartis, Xoma pharm, and Invasc Therapeutics. Dr. Rostand holds shares of common stock in Merck. Dr. Toto has served on the speakers bureau for Amgen and Merck, has served as consultant/advisory board for Boehringer-Ingelheim and Amgen. Dr. Wright has served as consultant/advisory board Medtronics, Takeda and Medical Letter.
References
- 1.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. J Intern Med. 2010;268:456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Wright JT, Jr, Greene T, et al. Long-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med. 2008;168:832–839. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg F. Emerging risk factors and markers of chronic kidney disease progression. Nat Rev Nephrol. 2009;5:677–689. doi: 10.1038/nrneph.2009.173. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R. Home and ambulatory blood pressure monitoring in chronic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:507–512. doi: 10.1097/MNH.0b013e3283319b9d. [DOI] [PubMed] [Google Scholar]
- 7.Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–27. doi: 10.1161/HYPERTENSIONAHA.108.115154. [DOI] [PubMed] [Google Scholar]
- 8.Verdecchia P, Schillaci G, Gatteschi C, Zampi I, Battistelli M, Bartoccini C, Porcellati C. Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation. 1993;88:986–992. doi: 10.1161/01.cir.88.3.986. [DOI] [PubMed] [Google Scholar]
- 9.Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- 10.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–1180. doi: 10.1038/sj.ki.5000247. [DOI] [PubMed] [Google Scholar]
- 12.Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 13.Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboli P, Conte G, Gabbai FB, De Nicola L. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171:1090–1098. doi: 10.1001/archinternmed.2011.230. [DOI] [PubMed] [Google Scholar]
- 14.Hermida RC, Calvo C, Ayala DE, Dominguez MJ, Covelo M, Fernandez JR, Mojon A, Lopez JE. Administration time-dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension. 2003;42:283–290. doi: 10.1161/01.HYP.0000084855.32823.DA. [DOI] [PubMed] [Google Scholar]
- 15.Hermida RC, Calvo C, Ayala DE, Dominguez MJ, Covelo M, Fernandez JR, Fontao MJ, Lopez JE. Administration-time-dependent effects of doxazosin GITS on ambulatory blood pressure of hypertensive subjects. Chronobiol Int. 2004;21:277–296. doi: 10.1081/cbi-120037772. [DOI] [PubMed] [Google Scholar]
- 16.Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alpha-adrenergic blocker, doxazosin: results from the HALT study. Hypertension. 2000;35:787–794. doi: 10.1161/01.hyp.35.3.787. [DOI] [PubMed] [Google Scholar]
- 17.White WB, Black HR, Weber MA, Elliott WJ, Bryzinski B, Fakouhi TD. Comparison of effects of controlled onset extended release verapamil at bedtime and nifedipine gastrointestinal therapeutic system on arising on early morning blood pressure, heart rate, and the heart rate-blood pressure product. Am J Cardiol. 1998;81:424–431. doi: 10.1016/s0002-9149(97)00935-1. [DOI] [PubMed] [Google Scholar]
- 18.White WB, Lacourciere Y, Gana T, Pascual MG, Smith DH, Albert KS. Effects of graded-release diltiazem versus ramipril, dosed at bedtime, on early morning blood pressure, heart rate, and the rate-pressure product. Am Heart J. 2004;148:628–634. doi: 10.1016/j.ahj.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Appel LJ, Middleton J, Miller ER, III, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14:S166–S172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 20.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Boggia J, Thijs L, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O’Brien E, Staessen JA. Is blood pressure during the night more predictive of cardiovascular outcome than during the day? Blood Press Monit. 2008;13:145–147. doi: 10.1097/MBP.0b013e3282fd16cc. [DOI] [PubMed] [Google Scholar]
- 22.Fan HQ, Li Y, Thijs L, Hansen TW, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036–2045. doi: 10.1097/HJH.0b013e32833b49fe. [DOI] [PubMed] [Google Scholar]
- 23.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 24.Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000;17:360–364. doi: 10.1046/j.1464-5491.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 25.Townsend RR, Ford V. Ambulatory blood pressure monitoring: coming of age in nephrology. J Am Soc Nephrol. 1996;7:2279–2287. doi: 10.1681/ASN.V7112279. [DOI] [PubMed] [Google Scholar]
- 26.Peterson GE, De Backer T, Gabriel A, Ilic V, Vagaonescu T, Appel LJ, Contreras G, Kendrick C, Rostand S, Phillips RA. Prevalence and correlates of left ventricular hypertrophy in the African American Study of Kidney Disease Cohort Study. Hypertension. 2007;50:1033–1039. doi: 10.1161/HYPERTENSIONAHA.107.090613. [DOI] [PubMed] [Google Scholar]
- 27.Gabbai FB, Rahman M, Hu B, Appel LJ, Charleston J, Pogue V, Wright JT, Lipkowitz M, Phillips RA AASK Study Group. Ambulatory Blood Pressure (ABP) as a Predictor of Renal (R) and Cardiovascular (CV) Outcomes in CKD Patients with Masked and Sustained Hypertension. Results from the Cohort Phase of the African American Study of Kidney Disease and Hypertension (AASK) Clin J Am Soc Nephrol. 2012 Aug 30; [Epub ahead of print] [Google Scholar]
- 28.Ishikawa J, Shimizu M, Hoshide S, Eguchi K, Pickering TG, Shimada K, Kario K. Cardiovascular risks of dipping status and chronic kidney disease in elderly Japanese hypertensive patients. J Clin Hypertens(Greenwich) 2008;10:787–794. doi: 10.1111/j.1751-7176.2008.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk results of the MAPEC study. Chronobiol Int. 2010;27:1629–1651. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 30.Minutolo R, Gabbai FB, Borrelli S, Scigliano R, Trucillo P, Baldanza D, Laurino S, Mascia S, Conte G, De Nicola L. Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis. 2007;50:908–917. doi: 10.1053/j.ajkd.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. doi: 10.1681/ASN.2011040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adeseun GA, Rosas SE. The impact of obstructive sleep apnea on chronic kidney disease Curr. Hypertens Rep. 2010;12:378–383. doi: 10.1007/s11906-010-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura G, Dohi Y, Fukuda M. Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertens Res. 2010;33:515–520. doi: 10.1038/hr.2010.47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.