Abstract
The N-terminal tail domains (NTDs) of histones play important roles in the formation of higher-order structures of chromatin and the regulation of gene functions. Although the structure of the nucleosome core particle has been determined by X-ray crystallography at near-atomic resolution, the histone tails are not observed in this structure. Here, we demonstrate that large quantities of nucleosome arrays with well-defined DNA positioning can be reconstituted using specific DNA sequences and recombinant isotope-labeled histones, allowing for the investigation of NTD conformations by amide hydrogen exchange and multi-dimensional nuclear magnetic resonance (NMR) methods. We examined the NTD of Drosophila melanogaster histones H3 in condensed nucleosome arrays. The results reveal that the majority of the amide protons in the NTD of H3 are protected from exchange, consistent with the NTDs having formed folded structures. Our study demonstrates hydrogen exchange coupled with NMR can provide residue-by-residue characterization of NTDs of histones in condensed nucleosome arrays, a technique that may be used to study NTDs of other histones and those with post-translational modifications.
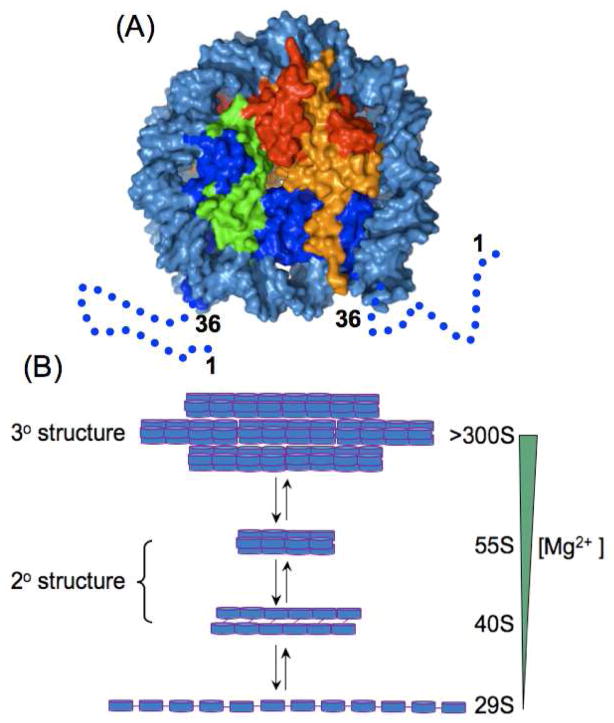
The N-terminal tail domains (NTDs) of histones, which are targets of many post-translational modifications, play important roles in the regulation of higher-order structures of chromatin and in the regulation of gene functions. To understand the functional roles of NTDs, it is essential to characterize their conformations at various chromatin states. The crystal structure of the fundamental unit of chromatin, the nucleosome core particle,1 has been determined at near-atomic resolution,2 in which 146 base pairs (bps) of DNA are wrapped around an octameric core of histone proteins composed of two copies of each of H2A, H2B, H3 and H4 (Fig. 1A). However, the NTDs are not defined in this structure, suggesting that they have flexible conformations.
Figure 1.
Nucleosome core particle structure and models of nucleosome arrays. (A) Surface representation of the nucleosome core particle structure (PDB_ID: 2PYO). Histones and DNA are color coded as follows: H2A (yellow); H2B (red); H3 (blue); H4 (green); and DNA (light blue). The dashed lines and numbers illustrate the regions of the H3 NTDs that are absent in the crystal structure. (B) Models of nucleosome arrays.13 Disks represent nucleosomes and lines represent linker DNAs.
To study higher-order structures of chromatin, nucleosome arrays have been developed as model systems, and the structure of a highly packed tetranucleosome has been determined by X-ray crystallography at 9 Å resolution.3 Because of the low resolution, no definitive conclusion could be reached regarding the conformation of the NTDs in this structure. Longer nucleosome arrays adopt increasingly more compact conformations as the Mg2+ concentration is raised (Fig. 1B),4 commencing at an extended 10-nm beads-on-a-string structure at < 0.2 mM Mg2+, through a folded 30-nm chromatin fiber at ~1 mM Mg2+, to an insoluble highly condensed form at a high Mg2+ concentration (> 2 mM). For example, these conformations sediment at 29S, (40S, 55S), and >300S for a 12-mer nucleosome array, respectively. The 10-nm fiber is not known to exist under physiological conditions and often serves as a reference state for describing the interactions in the higher-order structures of chromatin. The 30-nm chromatin fiber and the highly condensed nucleosome arrays were observed for native chromatin in interphase in yeast5 and CHO cells.6 To date, the conformation of NTDs in these arrays has been addressed only by sedimentation and chemical cross-linking studies coupled with protein engineering or mutagenesis.7,8 Here we demonstrate that amide hydrogen exchange coupled with NMR can be used to characterize the conformational states of NTDs in highly condensed nucleosome arrays by applying the technique to the NTD of recombinant Drosophila melanogaster histone H3.
Protein amide protons can chemically exchange with their isotopes in D2O (NH + D2O → ND + HOD) and the H/D exchange has been widely used to study protein folding, structure, and dynamics.9 The exchange rate for an amide proton is determined by the side chains of its nearest neighboring residues in the protein sequence10 and by its surroundings in the folded structure. For example, the exchange rate may be slowed if the amide proton participates in the formation of hydrogen bonds or is blocked by amino acid side chains in a folded structure. Amide H/D exchange has also been used to explore the conformation of peptides in amyloid fibers.11 In such a study, DMSO is used to dissolve protein aggregates for NMR detection and for quenching the H/D exchange reaction.12 To date, however, no H/D exchange studies have been performed for aggregates involving protein-DNA complexes.
In this study, nucleosome arrays were reconstituted with recombinant 15N-labeled H3 and nonlabeled H2A, H2B, H4, and the “12_601_167” DNA of Dorito et al.,7 which includes 12 equally spaced and well-positioned nucleosomes. The saturation of all 12 nucleosome positions was confirmed by digesting the array with restriction enzyme SacI and by running native APAGE gel, which yields the expected ladders of shorter nucleosome arrays (Fig. S1). Condensed nucleosome arrays were produced by adding 10 mM Mg2+ to the nucleosome array solution (20 mM sodium phosphate at pH 6) and by centrifugation (Fig. S2). To perform the hydrogen exchange experiment, the precipitated nucleosome arrays were then incubated for 1 hour in 99.9% D2O with the same buffer and 10 mM Mg2+. To quench the H/D exchange reaction, the nucleosome arrays were precipitated again by centrifugation and by dissolving the precipitates in 95% (v/v) d6-DMSO, 4.5% (v/v) D2O and 0.5% d2-dichloroacetic acid.12 Under such conditions, the nucleosome arrays dissociate such that the histone proteins remain dissolved in DMSO and the DNA molecules precipitate. A 1H-15N HSQC spectrum of the solution was then taken. To measure the H/D exchange quantitatively, a reference spectrum was taken for a sample prepared similarly except that H2O was substituted for D2O.
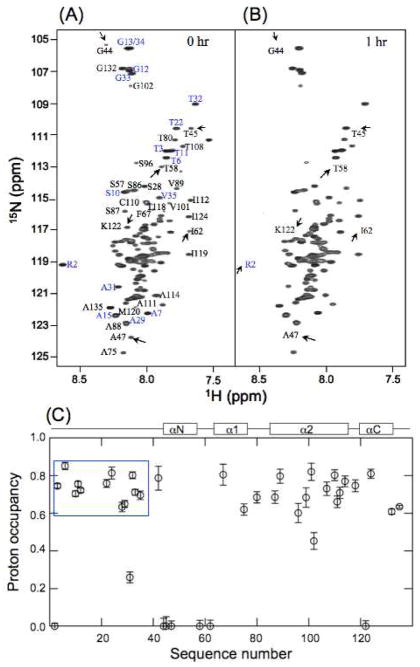
Fig 2A shows the reference HSQC spectrum with peaks assigned using {15N, 13C}-labeled H3 and triple-resonance methods (Supporting methods). Fig 2B shows the HSQC spectrum of the sample that has exchanged in D2O for 1 h. Clearly, some peaks in Fig. 2B have weaker intensities than those in Fig. 2A whereas others retain high intensities. The proton occupancy, which is the ratio of the peak intensities in Fig. 2B to those of the corresponding peaks in Fig. 2A, is shown in Fig. 2C.
Figure 2.
Hydrogen exchange of H3 in the condensed nucleosome array. 1H-15N HSQC spectra of H3 of (A) reference and (B) sample that stayed in D2O for 1 h in the presence of 10 mM Mg2+ at pDread 6 and 20°C in D2O. Arrows in (A) and (B) illustrate the amide protons whose peak intensities have decreased significantly after exchange. (C) Occupancy of amide protons in H3 after exchanging for 1 h. The spectra of (A) and (B) are measured with the same NMR parameters and the peak intensities are normalized to the same protein concentration.
Many amide protons in the NTD of H3 show high proton occupancy, indicating that they are well protected in the condensed nucleosome arrays; amide protons of the NTD in an unfolded conformation would have exchanged in less than 0.3 min under the above exchange condition, according to the calculated intrinsic exchange rates.9 Indeed, the amide protons in the NTD of free H3, which forms soluble aggregates, exchange rapidly in D2O at pD 6.0 and 20 °C in the presence of 10 mM Mg2+ (Fig. S3). Some of the amide protons of H3 show zero proton occupancy, indicating that they have completely exchanged to deuterons. These protons are located at the N-terminus, a loop region, and the N-terminal regions of helices in the structure of the nucleosome core particle (Fig. 2C), which do not form stable hydrogen bonds.
The above results show that the NTD of H3 forms stable structures in the condensed nucleosome array. They also demonstrate that amide H/D exchange coupled with NMR can be a powerful technique for studying the conformation of the NTDs of histones at the resolution of residues; this technique should be applicable to the NTDs of H2A, H2B and H4, and those with post-translational modifications.
We note that an earlier chemical cross-linking experiment showed that the NTD of H3 engaged in inter-nucleosome interactions in condensed nucleosome arrays.8 However, it was not clear from that study whether the NTD has a flexible conformation that interacts only transiently with other nucleosomes or forms a stable folded structure. Our results unequivocally show that the NTD of H3 forms stable structures in the condensed nucleosome array.
Supplementary Material
Acknowledgments
We thank Drs. Carl Wu and Karolin Luger for the expression vectors of histones and DNA. This work is supported by the intramural research programs of the National Cancer Institute, the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung and Blood Institute, National Institutes of Health.
Footnotes
Supporting Information Available: Detailed experimental procedures; and figures confirming the saturation of 12 nucleosome positions and precipitation profiles of nucleosome array by Mg2+. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kornberg RD, Lorch Y. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 2.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 3.Schalch T, Duda S, Sargent DF, Richmond TJ. Nature. 2005;436:138–141. doi: 10.1038/nature03686. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JC. Ann Rev Biophys Biomol Str. 2002;31:361–392. doi: 10.1146/annurev.biophys.31.101101.140858. [DOI] [PubMed] [Google Scholar]
- 5.Bystricky K, Heun P, Gehlen L, Langowski J, Gasser SM. Proc Natl Acad Sci U S A. 2004;101:16495–16500. doi: 10.1073/pnas.0402766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belmont AS, Bruce K. J Cell Biol. 1994;127:287–302. doi: 10.1083/jcb.127.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorigo B, Schalch T, Bystricky K, Richmond TJ. J Mol Biol. 2003;327:85–96. doi: 10.1016/s0022-2836(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 8.Kan PY, Lu X, Hansen JC, Hayes JJ. Mol Cell Biol. 2007;27:2084–2091. doi: 10.1128/MCB.02181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englander SW, Kallenbach NR. Q Rev Biophys. 1983;16:521–655. doi: 10.1017/s0033583500005217. [DOI] [PubMed] [Google Scholar]
- 10.Bai Y, Milne JS, Mayne L, Englander SW. Proteins. 1993;17:75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino M, Katou H, Hagihara Y, Hasegawa K, Naiki H, Goto Y. Nat Struct Biol. 2002;9:323–325. doi: 10.1038/nsb792. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YZ, Paterson Y, Roder H. Protein Sci. 1995;4:804–814. doi: 10.1002/pro.5560040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caterino TL, Hayes JJ. Nat Struct Mol Biol. 2007;14:1056–1058. doi: 10.1038/nsmb1107-1056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.