Abstract
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disorder that affects approximately 15% of children in the United States. A complex disorder, AD is characterized by both skin barrier impairment and immunologic abnormalities, including decreased innate immune function and a polarized adaptive immune response. Mouse models have demonstrated the complex interdependence of immune cell–keratinocyte interactions and teased apart gene–environment relationships in a controlled setting. In this issue, Nagelkerken et al. present a mouse model with transgenic expression of apolipoprotein C1 that disrupts the skin lipid barrier and manifests many hallmark features of AD.
Atopic dermatitis (AD) is characterized by an erythematous and intensely pruritic rash. It is popularly referred to by the common term “eczema,” derived from the Greek eczeo, meaning “to boil over or effervesce,” because of its tendency to form epidermal microvesicles through a pathologic process known as spongiosis. The condition typically manifests during the first year of life, with progression from an extensor to a flexural distribution later in childhood. AD resolves in about 70% of cases by the age of 3, but its incidence has increased significantly over the past few decades. Medical management of AD is associated with $1 billion in annual direct costs in the United States, as well as significant indirect costs and emotional burden in affected families (Bickers et al., 2006). More than half of children with moderate to severe AD progress to develop allergic rhinitis (hay fever) or asthma.
AD is characterized by both skin barrier impairment and immunologic abnormalities, including decreased innate immune function and a polarized type 2 helper T-cell (Th2)-dominant immune response, which is more predominant in the early stage of the disease. Although increased serum IgE levels and specific Th2 cytokines have been found to correlate directly with AD severity, it has yet to be established whether these immune alterations represent the primary cause of AD or a secondary response to barrier impairment. The skin barrier, which is formed in the exterior layers of the epidermis, is composed of cornified envelopes—enucleated keratinocytes—held together by a lipid matrix. Compromising the integrity of either of these barrier components could potentially lead to increased entry across the skin surface of allergens, irritants, microbes, or toxins, with subsequent activation of cutaneous immune responses. This long-proposed theory of an “outside-to-inside” pathogenesis of AD recently gained broader acceptance with the discovery that mutations in the gene encoding a skin barrier protein, filaggrin, are a major factor underlying AD susceptibility (Elias and Feingold, 2001; Sandilands et al., 2007).
In this commentary, we examine the salient clinical features of AD and ask whether they can be modeled successfully in mice. This leads to an inquiry into the role of epicutaneous sensitization and how this phenomenon can inform gene–environment interactions. Finally, we challenge the community to use mouse models to ask basic questions that have been difficult to address in patients, for example:
What pathophysiology underlies the typical physical distribution of AD?
Why do patients frequently clear their dermatitis but progress along an “atopic march” to asthma or hay fever?
Why does barrier-impaired AD skin mount an insufficient innate immune response but an exaggerated Th2 response?
What are the primary defect(s) in AD and how can more effective therapeutics against AD be targeted?
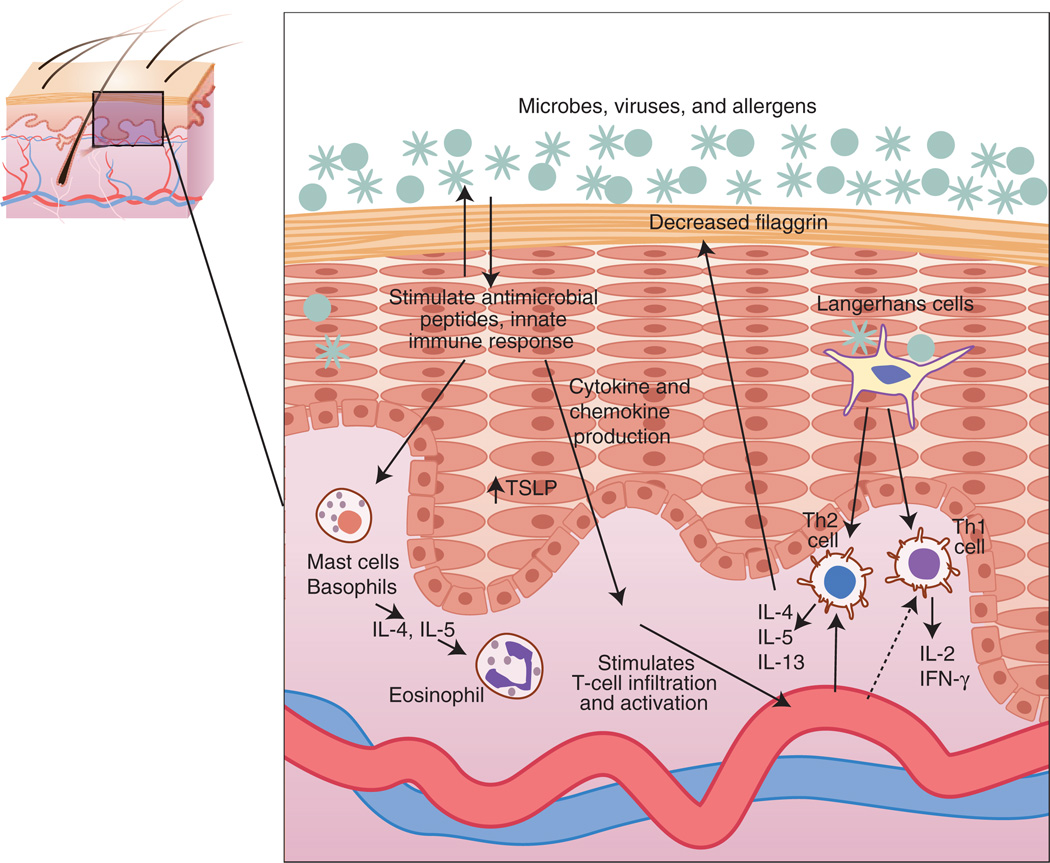
One puzzling feature of atopic patients is their robust Th2 response to commonly encountered environmental antigens (Figure 1). Animal models have been used successfully to study both environmental triggers and the underlying immune pathways that regulate this polarized response. Experiments involving allergen challenge have yielded a major mechanistic insight pertaining to the atopic march, namely, that epicutaneous (i.e., through the skin) sensitization is a potent vehicle for systemic Th2 sensitization that can readily progress to allergic airway hyperresponsivity. Epicutaneous sensitization models have traditionally utilized chicken-egg albumin—ovalbumin—as a protein allergen because of the many immunologic resources available for characterizing the specific type of immune response elicited. Hapten-induced AD models typically use trinitrochloro-benzene to elicit certain clinical and immunologic features of human AD. A recent publication from Elias’s group presented a new model in which topical treatment with the hapten oxazolone generated both the immunologic and the structural/functional epidermal features of AD (Man et al., 2008).
Figure 1.
Immune response to impaired skin barrier in atopic dermatitis.
NC/NgA mice, which remain dermatitis-free in specific pathogen-free conditions but develop a spontaneous AD-like eruption when conventionally housed, have historically been viewed as one of the best animal models of the condition. One of the major advantages of this model is the varied response to environmental factors (e.g., exposure to dust mite or Staphylococcus aureus antigens or increased psychological stress), which provides insight into the heterogeneity of AD pathophysiology. Experiments in NC/NgA mice have also helped to dissect further the role of Th1 and Th2 cytokines and the immunopathologic basis of AD. Although these mice provide an advantageous model for studying triggers and treatments for AD, their undefined genetic structure limits their utility, either as a standalone model or via crosses with transgenic mice, in dissecting the role of genetic susceptibility (Terada et al., 2006).
To date, animal models have yielded informative results concerning the role of genes whose expression is correlated with but not known to be causative for AD in human patients. For example, thymic stromal lymphopoietin (TSLP), a proinflammatory cytokine that activates dendritic cells to generate Th2 inflammatory responses, is highly upregulated in the keratinocytes of AD patients (Soumelis et al., 2002). Skin-specific (keratin (K)5 promoter) transgenic expression of TSLP (K5-TSLP) results in an AD-like phenotype, with infiltration of Th2-dominant CD4+ lymphocytes, mast cells, and eosinophils, as well as elevated serum IgE (Yoo et al., 2005). To address the question of whether Th2 cells are required for the development of these AD-like features and to determine their hierarchy in the AD inflammatory response, these investigators generated T-cell-deficient K5-TSLP transgenic mice, which, aside from normal serum IgE, demonstrate many features of the inflammatory skin phenotype. These experiments have begun to tease apart the roles of lymphocyte and myeloid lineage cells in AD. An important future approach would involve epicutaneously challenging these mice to determine whether T cells are necessary to proceed with the atopic march.
In this issue, Nagelkerken et al. (2008) present an exciting new animal model with transgenic expression of apolipoprotein C1 (APOC1) disrupting the lipid component of the skin barrier and resulting in a mouse with many of the clinical features of atopic dermatitis. (Table 1). The model displays both gross and histologic features of AD, including pruritus and spongiosis, respectively, which are hallmark features of the condition in humans. Importantly, this model also responds to topical treatment with corticosteroids, suggesting its potential utility for studying the efficacy of novel therapies. Finally, the work with APOC1 transgenic mice not only suggests that components of the lipid metabolism pathway are good candidates for identifying additional AD-susceptibility genes but also represents an opportunity to manage and perhaps even prevent subsequent atopic disorders with barrier restoration.
Table 1.
Salient clinical features of AD modeled in APOC1 transgenic animals
| Gross morphology | Scaling, lichenification, excoriations, and pruritus |
| Histology | Acanthosis and spongiosis, immune-cell infiltration (CD4+ T cells, eosinophils, mast cells, and macrophages) |
| Antibody titers | Increased serum IgE parallels disease progression |
| Treatment | Corticosteroids suppress development of AD-like phenotype |
Animal models have demonstrated how gene–environment and immune cell–keratinocyte interactions can be studied in controlled settings. Experiments with human cells and reconstructed human epidermis have also contributed greatly to our understanding of these complex relationships, with many new models having been published recently. These models have collectively challenged prior hypotheses about the role of barrier function and inflammation in AD. The lipid and protein components of the stratum corneum have often been considered independent modulators of barrier function. However, recent characterization of mice deficient in 12R-lipoxygenase, which converts arachidonic acid to barrier lipid components, revealed not only defects in ceramide composition but also increased cornified envelope fragility and impaired filaggrin processing (Epp et al., 2007). Animal and in vitro models have also been used to re-examine the relationship between barrier function and the innate and adaptive immune response. For example, mice deficient in the antimicrobial peptide CRAMP demonstrate impaired barrier function (Aberg et al., 2007), and human keratinocytes were shown to express lower levels of filaggrin when differentiated with the Th2 cytokines interleukin 4 and interleukin 13 vs. interferon-γ (Howell et al., 2007). The latter results are intriguing because they suggest that filaggrin deficiency in AD patients may result from a combination of genetic mutations and Th2 polarization (Figure 1).
To date, animal models for AD have provided great insight into the disease, but they have also generated many new questions. Topics to be addressed include the effect of underlying genetic variation, the relationship between the skin barrier and the innate and adaptive immunity, and the role of epicutaneous sensitization by environmental allergens. A reproducible, accessible, and relevant animal model of AD would be immensely valuable for all these investigations. However, the complexity and variability of AD as a clinical entity make creating such a model particularly challenging. Because there are multiple genetic and environmental factors underlying AD, the notion of developing a single comprehensive animal model is unrealistic. However, many of these disease components can be modeled both alone and in combination to address questions of pathophysiology and treatment. When working in another biologic system we must be aware that not all factors, either genetic or environmental, will necessarily have the same effect on mice that they do in humans; conversely, we must remain open to fortuitous surprises such as mouse models that unexpectedly manifest AD-like features, lending new insight into possible human genetic predisposition.
Given these caveats, we challenge ourselves and the scientific community to develop clinically relevant animal models that take their cue from observations in human disease. For example, it will be fascinating to examine the extent to which mice with a targeted deletion of filaggrin are predisposed to developing AD-like features. Although the flaky tail mouse has defects in filaggrin processing, the underlying genetic defect has not yet been determined (Presland et al., 2000). Our ability to model genetic predisposition to AD in mice will allow us to understand how selective skin barrier impairment modulates adaptive and innate immune responses, discover how AD initiates atopic progression, and, finally, test novel therapies.
This is a commentary on article Nagelkerken L, Verzaal P, Lagerweij T, Persoon-Deen C, Berbee JF, Prens EP, Havekes LM, Oranje AP. Development of atopic dermatitis in mice transgenic for human apolipoprotein C1. J Invest Dermatol. 2008;128(5):1165-72.
Footnotes
CONFLICT OF INTEREST
The author states no conflict of interest.
REFERENCES
- Aberg KM, Man M-Q, Gallo RL, Ganz T, Crumrine D, Brown BE, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701099. published online 18 October 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases, 2004: a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079–1081. [PubMed] [Google Scholar]
- Epp N, Furstenberger G, Muller K, de Juanes S, Leitges M, Hausser I, et al. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J Cell Biol. 2007;177:173–182. doi: 10.1083/jcb.200612116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man M-Q, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008;128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerken L, Verzaal P, Lagerweij T, Persoon-Deen C, Berbee JF, Prens EP, et al. Development of atopic dermatitis in mice transgenic for human apolipoprotein C1. J Invest Dermatol. 2008;128:1165–1172. doi: 10.1038/sj.jid.5701182. [DOI] [PubMed] [Google Scholar]
- Presland RB, Boggess D, Lewis SP, Hull C, Fleckman P, Sundberg JP. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115:1072–1081. doi: 10.1046/j.1523-1747.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–654. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Terada M, Tsutsui H, Imai Y, Yasuda K, Mizutani H, Yamanishi K, et al. Contribution of IL-18 to atopic-dermatitis-like skin inflammation induced by Staphylococcus aureus product in mice. Proc Natl Acad Sci USA. 2006;103:8816–8821. doi: 10.1073/pnas.0602900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]