Abstract
Purpose
This phase II study (S0341) evaluated the efficacy and tolerability of single-agent erlotinib in unselected chemotherapy-naïve patients with advanced non-small cell lung cancer (NSCLC) and a performance status (PS) of 2. Exploratory analyses of a number of biomarkers relating to EGFR pathway activation were also performed.
Patients and Methods
Patients with stage IIIB (pleural effusion) or stage IV NSCLC with a PS of 2 and no prior chemotherapy or biologic treatment for NSCLC received erlotinib 150 mg daily.
Results
A total of 81 patients entered the study; 76 were assessable. One complete and 5 partial responses were noted for an overall response rate of 8% (95% CI 3%–16%).Stable disease (SD) was seen in 26 patients (34 %) resulting in a disease control rate (DCR=CR/PR/SD) of 42%. Progression free and median survival were 2.1 months (95% CI 1.5–3.1) and 5 months (95% CI 3.6–7.2) respectively. One-year survival was 24% (95% CI 15%–34%). Although treatment was generally well tolerated, grade 3–4 toxicity was reported in 30 patients (40%), including fatigue (16%), rash (9%), diarrhea (7%) and anorexia (7%). There was one possible treatment related death (pneumonitis).
Conclusion
In chemotherapy-naïve patients with advanced NSCLC and a PS of 2, single agent erlotinib resulted in an acceptable but significant level of treatment-related side effects. With an overall DCR of 42% and median survival of 5 months, results are comparable to those achieved with chemotherapy in this population. Development of an EGFR-directed biomarker selection strategy may optimize use of erlotinib in PS 2 patients.
INTRODUCTION
The overall prognosis for patients with non small cell lung (NSCLC) cancer that involves the pleural space or has spread beyond the thorax is poor. A number of treatment approaches including the use of frontline platinum based-chemotherapy with or without bevacizumab have modestly extended survival and improved quality of life.(1–3) The benefits of systemic treatment have been most clearly defined for patients with a good performance status (Zubrod 0–1). The potential benefits and risks of treatment are much less well characterized in patients with a poor performance status (PS). Patients with a performance status of two represent a particularly noteworthy dilemma. PS 2 patients constitute at least 35–40% of newly diagnosed patients with advanced NSCLC and have a markedly shorter survival compared to good PS patients.(4) They experience enhanced adverse effects with systemic therapies and as a consequence have to a great extent been excluded from clinical trials. Available information on treatment outcomes in this population are confined to a limited number of prospective trials and retrospective subset analyses from several phase III trials.(4–7)
A number of new molecularly-directed drugs are currently being evaluated in the treatment of advanced NSCLC. The epidermal growth factor receptor (EGFR) is the target of several agents, including the small molecule tyrosine kinase inhibitors (TKI) gefitinib and erlotinib and the monoclonal antibody cetuximab. The EGFR TKI’s have demonstrated value primarily when used as second-line therapy in patients progressing after platinum-based chemotherapy.(8–10) Their role as primary therapy in patients with advanced NSCLC is less well defined.(11–13) Given their favorable toxicity profile compared to antineoplastic chemotherapy, they offer a potentially attractive treatment option in PS 2 patients, where fear of treatment-related adverse effects is a significant limitation to the administration of systemic treatment. This phase II trial was conducted to obtain preliminary information on the efficacy and tolerability of erlotinib in unselected chemotherapy-naïve patients with advanced NSCLC and a PS of 2. A number of correlative studies were also incorporated into the trial in an attempt to gain insights into EGFR biology in this patient population.
PATIENTS AND METHODS
Eligibility
Patients were required to have histologically or cytologically documented NSCLC, Stage IIIB with malignant pleural effusion or Stage IV, new or recurrent after previous surgery and/irradiation. Patients had to have a SWOG performance status of 2. All patients were required to have measurable disease, be ≥ 18 years of age and have acceptable hepatic, renal and hematologic function. Patients with brain metastases, prior hormonal, chemotherapy or biologic therapy for NSCLC or active pregnancy were ineligible. The study was approved by the Institutional Review Board’s of the respective institutions, and all patients gave written informed consent.
Treatment Plan
Patients received erlotinib 150 mg orally daily on a continuous basis. One cycle of therapy was considered 21 consecutive calendar days. Treatment was continued until any of the following criteria were met: 1) progression of disease or symptomatic deterioration; 2) unacceptable toxicity; 3) treatment delay > 3 weeks; 4) patient election to withdraw from the study.
Dose Modifications
Patients were evaluated at week 2 and then once every three weeks to determine toxicities and/or dose modification. Erlotinib dose level reductions were as follows: starting dose 150mg/day; first reduction 100 mg/day; second reduction 50 mg/day. If a third dose level reduction was required or a treatment delay greater than three weeks occurred, the patient was removed from the study. Patients developing a rash ≤ grade 2 were managed at the discretion of the treating physician. Rash ≥ grade 3 required a dose reduction. Patients with ≥ grade 2 diarrhea occurring despite the optimal use of loperamide required a dose reduction. Patients developing grade 2 keratitis required a dose interruption until resolution or amelioration of findings to ≤ grade 1 and then could be retreated at the discretion of the physician with a dose reduction. For Grade 2 non-hematological toxicity that was medically concerning (e.g prolonged cardiac, pulmonary, or neurotoxicity), treatment was held until resolution to ≤ Grade 1 and then re-instituted with a dose reduction. For other forms of toxicities ≥ grade 3 (with the exception of alopecia), treatment was held until the toxicity resolved to grade 1 or less and then treatment was resumed with a dose reduction. No dose re-escalations were permitted.
Response and Toxicity Criteria
Patients were evaluated for disease response prior to every third cycle of treatment. Response was assessed using RECIST criteria (14) and toxicities were assessed using NCI Common Toxicity Criteria Version 3.0. (15)
Ancillary Treatment
Patients received supportive care including transfusions of blood products, antibiotics and medications with utility in managing the common adverse effects of erlotinib. These included topical antibiotics, corticosteroids or short course prednisone or minocycline for cutaneous toxicities, loperamide for diarrhea, and preservative-free artificial tears and ophthalmic ointments and ointments for keratitis.
Patient Report Measures
The Medical Conditions Questionnaire developed by Katz was administered once at study entry for use as a covariate in analyses. (16) The scoring algorithm based on the Katz system incorporates severity of medical conditions; higher scores reflect more severe co-morbid conditions. A count of organ systems affected by a medical condition was also calculated.
Laboratory Correlative Studies
At time of study entry, patients were offered the opportunity to participate in a companion protocol S9925 (Lung Master Correlative Science trial) through which blood, plasma and tumor tissue for correlative science studies was collected in consenting patients. Biomarkers relating to EGFR pathway activation were performed: immunohistochemistry was performed for EGFR protein expression (DAKO, Glostrup, Denmark # K1494), E-cadherin (DAKO, Glostrup, Denmark #M3612) and Vimentin (DAKO, Glostrup, Denmark #M0725). EGFR gene copy number was assessed using fluorescence in-situ hybridization (FISH) using LSI EGFR SpectrumOrange/CEP 7 SpectrumGreen probes (Vysis, Abbott Laboratories, Abbott Park, IL) as described.(17) In brief, tumors with 4 or more copies of the EGFR gene in ≥ 40% of the cells (high polysomy) or tumors with EGFR gene amplification (gene/chromosome ratio > 2, or presence of gene cluster or ≥ 15 gene copies in > 10% of the cells) were considered FISH positive, while all others were considered FISH negative.
Statistical Considerations
Considering that prior studies have shown that erlotinib results in a relatively low response rate, and that clinical benefit of erlotinib therapy has largely been defined by non-progression, including the category of stable disease, the primary endpoint of the trial was overall survival. The regimen would be considered promising if the true median survival from registration was 4 months or longer in this PS 2 patient population, in conjunction with acceptable toxicity. It would be considered of no further interest if the true median survival were 2.5 months or shorter. Assuming exponential survival and 65 patients accrued over 18 months, with an additional 6 months of follow-up, the study would have 81% power to rule out the null hypothesis of a 2.5 month median survival at the one-sided 0.05 alpha level versus an alternative of a 4-month median survival, using a Brookmeyer Crowley-like test.(18) Response rates and toxicity rates were assessed as secondary endpoints. Sixty-five patients would be sufficient to estimate response rates and rates of specific toxicities to within ± 12%.
The subset of patients who consented to participate in S9925, and from whom analyzable specimens were collected are included in the molecular correlative studies. The results for FISH score, ECAD index, EGFR index, and Vimentin index were dichotomized into two groups (high versus low) based on the observed median values. Cox regression models were fit to investigate the relationships between each biomarker and overall survival and progression-free survival. The relationship between each biomarker and response rate and disease control rate were explored using logistic regression methods and exact tests. This analysis was considered exploratory in nature, with the objective of generating hypotheses for future Phase III studies. Therefore no adjustments for multiple comparisons were made. Due to the limited sample size, there would only be sufficient power to detect very strong relationships. Exploratory analyses also examined the prognostic value of the extent of baseline comorbidities.
RESULTS
Patient Characteristics
Between September 15, 2004 and October 1, 2005, 81 patients were registered onto the study. Five patients were ineligible for the following reasons: incorrect timing of pre-study assessments (2); insufficient documentation of correct stage (1); prior chemotherapy (1); no measurable disease (1). Characteristics of the 76 eligible patients assessable for response, survival and toxicity are displayed in Table 1. Most patients (87%) had Stage IV disease and the median age was 74 years (range 47–89). Males and females were nearly evenly divided and approximately one-half of patients (51%) had an adenocarcinoma pathologic subtype. Eight-nine percent of patients were current or former smokers.
Table 1.
Patient Characteristics (N=76)
| Variable | No. of Patients | % |
|---|---|---|
|
| ||
| Median age, years (range) | 74 (47–89) | - |
|
| ||
| Male: Female | 36:40 | 47:53 |
|
| ||
| Cell Type | ||
| Adenocarcinoma | 39 | 51 |
| Squamous cell | 18 | 24 |
| Large cell | 1 | 1 |
| Other | 18 | 24 |
|
| ||
| Stage | ||
| IIIB | 10 | 13 |
| IV | 66 | 87 |
|
| ||
| Smoking History | ||
| Current | 26 | 34 |
| Former | 42 | 55 |
| Never | 6 | 8 |
| Missing | 2 | 3 |
Treatment Received
Median time on treatment was 49 days (range 4–866). One patient remains on treatment. Forty-four patients (58%) and 18 patients (24%) discontinued treatment secondary to progressive disease and toxicities, respectively.
Toxicity
Treatment related toxicity (≥ grade 3) is displayed in Table 2. Grade 4 toxicities were uncommon. A total of six grade 4 events were noted in 5 (7%) patients. They included: fatigue (3 patients), dyspnea (2 patients) and renal failure (1 patient). One-third of patients had a grade 3 toxicity. The most common grade 3 toxicities included: fatigue (9 patients), rash (7 patients), diarrhea (5 patients) and anorexia (5 patients). There was one probable treatment-related death in a patient who developed pneumonitis.
Table 2.
Toxicity (≥Grade 3) (N=76)
| Adverse Effect | Grade 3 No. of pts (%) |
Grade 4 No. of pts (%) |
Grade 5 No. of pts (%) |
|---|---|---|---|
|
| |||
| Constitutional | |||
| Dehydration | 3 (4) | 0 | 0 |
| Fatigue | 9 (12) | 3 (4) | 0 |
| Weight loss | 2 (3) | 0 | 0 |
|
| |||
| Gastrointestinal | |||
| Anorexia | 5 (7) | 0 | 0 |
| Diarrhea | 5 (7) | 0 | 0 |
| Nausea | 1 (1) | 0 | 0 |
| Vomiting | 1 (1) | 0 | 0 |
|
| |||
| Hematologic | |||
| Anemia | 2 (3) | 0 | 0 |
| Lymphopenia | 2 (3) | 0 | 0 |
|
| |||
| Pulmonary | |||
| Dyspnea | 2 (3) | 2 (3) | 0 |
| Pneumonitis | 0 | 0 | 1 (1) |
|
| |||
| Renal | |||
| Renal Failure | 0 | 1 (1) | 0 |
|
| |||
| Skin | |||
| Rash | 7 (9) | 0 | 0 |
|
| |||
| Maximum Grade Any Toxicity | 25 (33) | 5 (7) | 1 (1) |
Response and Survival
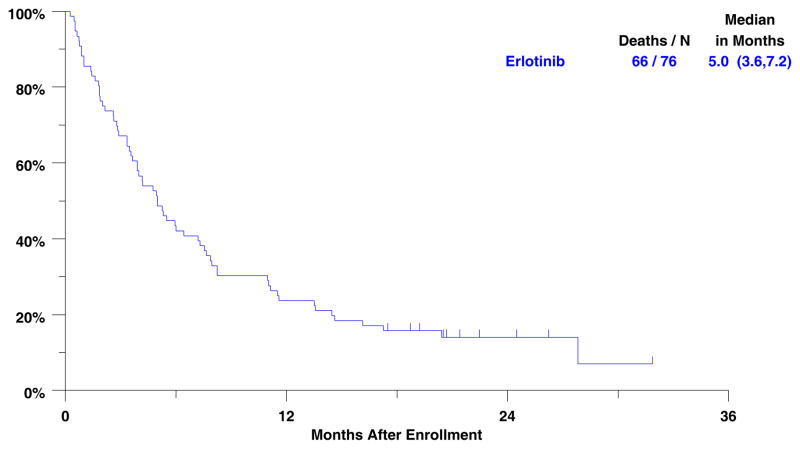
Objective response to treatment is displayed in Table 3. One patient (1%) achieved a complete response and five patients (7%) partial responses. Stable disease was seen in 26 patients for an overall Disease Control Rate (complete + partial response + stable disease) of 42% (32 patients). Ten patients had inadequate assessments of response and are presumed non-responders. Reasons for inadequate assessment included: no follow-up assessment performed (7 patients); inconsistent assessment methods (2 patients); delinquent data (1 patient). Median follow-up for surviving patients is 21 months (minimum 17/maximum 32 months). Median progression free survival is 2.1 months (95% CI 1.5 – 3.1 months). Median overall survival is 5 months (95% CI 3.6 – 7.2 months). (Figure 1) Survival at one and two years is 24% (95% CI 15% – 34%) and 14% (95% CI 7% –23%), respectively.
Table 3.
| Treatment Outcome (N=76) | ||
|---|---|---|
| Response | No. of Patients | % |
| Overall | 6 | 8 (3–16*) |
| Complete | 1 | 1 |
| Partial | 5 | 7 |
| Stable | 26 | 34 |
| Progressing Disease | 28 | 37 |
| Early Death | 1 | 1 |
| Assessment Inadequate | 10 | 13 |
95% Confidence Intervals
Figure 1.
Overall survival for 76 eligible patients.
Patients with Katz scores of 0 (N=18), 1 (N=14) and ≥ 2 (N=42) had median survivals of 4, 6, and 5 months respectively. Patients with organ counts (number of organ systems involved by significant co-morbid conditions) of 0 (N=18), 1 (N= 22) and ≥ 2 (N= 34) had median survivals of 4, 5 and 6 months respectively.
Molecular Correlative Studies
Although sixty-six patients consented to submission of biologic specimens, assessable specimens were received from only 38 clinically eligible patients, primarily due to scant cytologic specimens from fine needle aspiration. Additionally, the number of assessable results obtained varied by marker (range: 19 – 37). Exploratory analyses were conducted evaluating the relationship between the biologic markers and a number of clinical outcomes including response rate, disease control rate (complete + partial response + stable disease), progression-free survival (PFS) and overall survival (OS). Realizing the small sample size for statistical correlations, no significant relationships were noted with any of the clinical outcomes and EGFR gene copy by FISH (N= 34), EGFR protein expression (N= 37) or E-cadherin expression (N= 35). Vimentin index (N= 19) > 0 was associated with an improved PFS (p = 0.02). When EGFR FISH scores were analyzed by a dichotomous grouping into FISH positive (N= 15) vs. FISH negative (N= 19), all clinical outcomes were numerically more favorable in the FISH positive group, but none of these comparisons were statistically significant. These included disease control rate: 53% versus 32% (p=0.3); PFS: 4 versus 1 month (p=0.44); and overall survival: 8 versus 5 months (p=0.84) in the FISH positive and FISH negative groups, respectively.
DISCUSSION
Patients with a performance status of 2 constitute a large proportion of patients with advanced NSCLC and have a clearly inferior survival when compared with good performance status (0–1) patients. A trial reported by the Eastern Cooperative Oncology Group more than 20 years ago illustrates the dramatic impact of performance status on survival and toxicity from treatment.(19) Whereas patients with a PS of 0 and 1 had median survivals of 8.3 and 6 months respectively, median survival for PS 2 patients was only 2.3 months. Similarly, toxic deaths were seen in 3% of good PS patients compared with a toxic death rate of 10% in PS 2 patients. As a consequence of the clinical outcomes from this and similar trials, participation of PS 2 patients in frontline advanced stage NSCLC studies has often been restricted, limiting the body of clinical data available to guide treatment decisions by practicing oncologists.
To date, our understanding of the value of chemotherapy in patients with advanced NSCLC and a PS of 2 is derived primarily from retrospective subset analyses from therapeutic trials composed predominantly of good PS patients, and a few recent prospective trials exclusively targeting PS 2 patients. An analysis of the small cohort of patients with a PS of 2 from the ELVIS trial comparing single agent vinorelbine with best supportive care in patients ≥ 70 years of age suggests a benefit for chemotherapy. Median survival was 6 versus 2 months in the vinorelbine and supportive care arms, respectively.(20) Whether standard platinum-based doublets are superior to single agents is unclear, as no prospective Phase III trial has been reported to date addressing this question. A preplanned subset analysis of CALGB 9730 comparing carboplatin/paclitaxel with paclitaxel alone in the PS 2 subset suggested improved outcome with the combination, with median survivals of 4.7 and 2.2 months, respectively.(21) Conversely, in a trial evaluating platinum/vinca alkaloid combination therapy versus vinorelbine alone, Le Chevalier and colleagues noted no survival difference between single versus combination therapy in the PS 2 cohort. (22)
In recent years, a number of prospective chemotherapy trials targeting PS 2 patients with advanced NSCLC have been completed, including two trials conducted in the U.S. by National Cancer Institute sponsored cooperative groups. (23,24) Eastern Cooperative Oncology Group trial E1599, a randomized phase II trial, compared two platinum-based combination regimens (carboplatin/paclitaxel and gemcitabine/cisplatin) and noted median survivals of 6.7 and 6.1 months, respectively.(23) The Southwest Oncology Group evaluated a sequential single agent chemotherapy approach employing vinorelbine followed by docetaxel in patients ≥ age 70 with PS of 0–1 or PS 2 patients any age, S0027. (24) Median survivals were 5 months in the PS 2 cohort and 9 months in patients ≥ 70 PS 0–1.
Although the results reported with chemotherapy in advanced NSCLC patients with PS 2 would suggest somewhat improved outcomes compared to historical results with best supportive care alone, the persistently disappointing survival rates and concerns about excessive toxicity with chemotherapy in this impaired population make a compelling case for new treatment approaches. The recent availability of a number of relatively well tolerated new therapeutic agents directed against molecular targets relevant to NSCLC offers an attractive therapeutic option. Agents in this category include those that target the epidermal growth factor receptor (EGFR), expressed in a substantial proportion of patients with NSCLC.( 25,26)
In the current trial, we demonstrate that single agent erlotinib resulted in an acceptable but significant level of treatment-related side effects for a substantial minority of chemotherapy-naïve patients with advanced NSCLC and PS 2, including one probable treatment-related death. The rates of objective response and overall disease control appear similar to the experience noted in other trials treating unselected populations of patients with single agent EGRF-TKI’s. In addition, the median survival of 5 months is comparable to that reported in prior trials employing chemotherapy alone in the PS 2 population.(23,24,27) A recently reported randomized phase II trial comparing erlotinib with the combination of carboplatin and paclitaxel in PS 2 patients with advanced NSCLC also noted a similar median survival (6.5 months) in the erlotinib monotherapy arm. (28) Of note, survival was numerically higher (9.7 months) in patients randomized to the chemotherapy arm in this trial.
The results of our study suggest that erlotinib does not offer a significant treatment advance over chemotherapy in unselected PS 2 patients, and either selection of patients by clinical criteria or an EGFR biomarker-driven strategy could prove more effective. A number of clinical factors such as adenocarcinoma histology, female gender, never-smoking status, and Asian ancestry have been identified as predictors of benefit for EGFR-TKI’s.(29,30) In addition, a number of molecular factors such as EGFR protein expression assessed by IHC, EGFR gene copy number determined by FISH, and activating EGFR mutations in exons 19 and 21 have also been found to have potential predictive benefit.(31–35) In regard to survival outcomes, most consistent results have been demonstrated for EGFR gene copy number detected by FISH. (32,36)
An important component of the current study was to perform exploratory correlative science studies. Unfortunately, the small specimen sample size in our study and the relatively modest yield of biologic specimens (approximately 50% of evaluable patients), limit interpretation of our results. Regardless, a vimentin index > 0 was associated with improved PFS, a finding meriting further discussion. Gene expression studies in NSCLC cell lines have indicated that transition from an epithelial to mesenchymal phenotype may confer insensitivity to erlotinib.(37) Vimentin is a classic marker for mesenchymal expression; preclinical studies predict reduced sensitivity to EGFR-TKI’s in cells over expressing this marker. The counter-intuitive improvement in PFS noted in our study in patients with higher vimentin expression may be explained by mere chance alone due to the small sample size (19 patients), the multiple comparisons made in this analysis, or other factors not yet clarified in EGFR-related biology.
A growing body of data suggests that high EGFR gene copy number as determined by FISH may be a useful predictive marker in patients receiving EGFR TKI’s. Both single-arm studies (17,38) and phase III trials comparing gefitinib and erlotinib to placebo (32,36) have demonstrated increased survival in patients with tumors demonstrating high EGFR gene copy number. In our trial, patients with high tumor EGFR gene copy number did numerically better in all outcome parameters, when compared with patients with low EGFR gene copy number. For the reasons noted above, interpretation of these data are limited.
Efforts to optimize use of the EGFR-targeted therapies in patients with advanced NSCLC through biomarker selection strategies are continuing. An Intergroup trial (N0723) will utilize a biomarker validation clinical trial design for EGFR FISH in second line therapy of patients with advanced NSCLC randomized to receive erlotinib versus pemetrexed. In SWOG, efforts to further define the respective roles of erlotinib and chemotherapy in PS 2 patients will employ a recently reported serum proteonomic predictor based on matrix-assisted laser desorption ionization (MALDI) mass spectrometry to identify patients likely to benefit from EGFR-TKI’s. (39) Chemonaive advanced NSCLC patients with PS 2 who test positive by the proteonomic profiler will be randomized to either erlotinib alone or a combination of intermittent erlotinib plus chemotherapy, testing the concept of pharmacodynamic separation. (40) These studies and other planned biomarker-driven strategies designed to personalize therapy in patients with advanced stage NSCLC offer promise for improving the risk/benefit ratio in the large PS 2 population which is poorly served by currently available therapies.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA-32102, CA-38926, CA-14028, CA-42777, CA-46441, CA-67575, CA-45808, CA-35119, CA-11083, CA-46282, CA-12644, CA-46368, CA-45560, CA-27057, CA-04919, CA-35178, CA-63850, CA-35128, CA-58861, CA-35431, CA-45461, CA-58416, CA-20319, CA-68183 and supported in part by OSI Pharmaceuticals
References
- 1.Raez LE, Lilenbaum R. New developments in chemotherapy for advanced non-small cell lung cancer. Curr Opin Oncol. 2006;18:156–61. doi: 10.1097/01.cco.0000208789.37689.6b. [DOI] [PubMed] [Google Scholar]
- 2.Socinski MA, Crowell R, Hensing TE, et al. Treatment of non-small cell lung cancer, stage IV: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:277s–289s. doi: 10.1378/chest.07-1381. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2452–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Blackhall FH, Bhosie J, Thatcher N. Chemotherapy for advanced non-small cell lung cancer patients with performance status 2. Curr Opin Oncol. 2005;17:135–9. doi: 10.1097/01.cco.0000152629.20996.9e. [DOI] [PubMed] [Google Scholar]
- 5.Devlin JG, Langer CJ. The 800-lb gorilla we all ignore: treatment of NSCLC in elderly and PS 2 patients. Clin Adv Hematol Oncol. 2007;5:216–33. [PubMed] [Google Scholar]
- 6.Lilenbaum RC. Treatment of patients with advanced lung cancer and poor performance status. Clin Lung Cancer. 2004;2:571–4. doi: 10.3816/clc.2004.s.017. [DOI] [PubMed] [Google Scholar]
- 7.Gridelli C, Ardizzoni A, Le Chevaller T, et al. Treatment of advanced non-small-cell lung cancer patients with ECOG performance status 2: results of an European Experts Panel. Ann Oncol. 2004;15:419–26. doi: 10.1093/annonc/mdh087. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd FA, Rodrigues Pereira J, Cluleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;14:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 9.Feld R, Sridhar SS, Shepherd FA, et al. Use of the epidermal growth factor receptor inhibitors gefitinib and erlotinib in the treatment of non-small cell lung cancer: a systemic review. J Thorac Oncol. 2006;1:367–76. [PubMed] [Google Scholar]
- 10.Gridelli C, Rossi A, Maione P, et al. Erlotinib in non-small-cell lung cancer. Expert Opin Pharmacother. 2007;8:2579–92. doi: 10.1517/14656566.8.15.2579. [DOI] [PubMed] [Google Scholar]
- 11.Giaccone G, Gallegos Ruiz M, Le Chevalier T, et al. Erlotinib for frontline treatment of advanced non-small cell lung cancer: a phase II study. Clin Cancer Res. 2006;15:6049–55. doi: 10.1158/1078-0432.CCR-06-0260. [DOI] [PubMed] [Google Scholar]
- 12.Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naïve patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–6. doi: 10.1200/JCO.2006.07.5754. [DOI] [PubMed] [Google Scholar]
- 13.Spigel DR, Hainsworth JD, Burkett ER, et al. Single-agent gefitinib in patients with untreated advanced non-small-cell lung cancer and poor performance status: a Minnie Pearl Cancer Research Network Phase II trial. Clin Lung Cancer. 2005;7:127–32. doi: 10.3816/CLC.2005.n.028. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;2:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.NCI Common Toxicity Criteria. https//ctep.cancer.gov.
- 16.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;4:643–55. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 18.Brookmeyer R, Crowley JJ. 4 confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 19.Ruckdeschel JC, Finkelstein DM, Ettinger DS, et al. A randomized trial of the four most active regimens for metastatic non-small-cell lung cancer. J Clin Oncol. 1986;4:14–22. doi: 10.1200/JCO.1986.4.1.14. [DOI] [PubMed] [Google Scholar]
- 20.Perrone F. Personal Communications. Nov, 1999.
- 21.Lilenbaum RC, Herndon JE, 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the center and leukemia group B (study 9730) J Clin Oncol. 2005;1:190–6. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 22.Le Chevalier T, Brisgand D, Soria JC, et al. Long term analysis of survival in the European randomized trial comparing vinorelbine/cisplatin to vindesine/cisplatin and vinorelbine alone in advanced non-small cell lung cancer. Oncologists. 2001;6:8–11. doi: 10.1634/theoncologist.6-suppl_1-8. [DOI] [PubMed] [Google Scholar]
- 23.Langer C, Li S, Schiller J, et al. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in eastern cooperative oncology group performance status 2 non-small-cell lung cancer patients: ECOG 1599. J Clin Oncol. 2007;25:418–23. doi: 10.1200/JCO.2005.04.9452. [DOI] [PubMed] [Google Scholar]
- 24.Hesketh PJ, Chansky K, Lau DH, et al. Sequential vinorelbine and docetaxel in advanced non-small cell lung cancer patients age 70 and older and/or with a performance status of 2: a phase II trial of the Southwest Oncology Group Mukohara (S0027) J Thorac Oncol. 2006;1:537–44. [PubMed] [Google Scholar]
- 25.Kudho TS, Yamauchi S, et al. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC) Lung Cancer. 2003;41:123–30. doi: 10.1016/s0169-5002(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki S, Dobashi Y, Sakurai H, et al. Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas. An immunohistochemical and fluorescence in site hybridization study. Cancer. 2005;15:1265–73. doi: 10.1002/cncr.20909. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney C, Zhu J, Sandler A, et al. Outcome of patients with a performance status of 2 in eastern cooperative oncology group study E1594. Amer Can Soc. 2001:2639–40. doi: 10.1002/1097-0142(20011115)92:10<2639::aid-cncr1617>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small cell lung cancer and a performance status of 2. J Clin Oncol. 2008;26:863–869. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 29.Clark GM, Zborowski DM, Santabarbara P, et al. Smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group study BR.21. Clin Lung Cancer. 2006;7:389–94. doi: 10.3816/clc.2006.n.022. [DOI] [PubMed] [Google Scholar]
- 30.Ramalingam S, Sandler AB. Salvage therapy for advanced non-small cell lung cancer: factors influencing treatment selection. Oncologist. 2006;11:655–65. doi: 10.1634/theoncologist.11-6-655. [DOI] [PubMed] [Google Scholar]
- 31.Dziadziuszko R, Hirsch FR, Varella-Garcia M, et al. Selecting lung cancer patients for treatment with epidermal growth factor receptor tyrosine kinase inhibitor by immunohistochemistry and fluorescence in situ hybridization—why, when and how? Clin Cancer Res. 2006;15:4409s–4415s. doi: 10.1158/1078-0432.CCR-06-0087. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch FR, Varella-Garcia M, Bunn PA, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol. 2006;1:5034–42. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 33.Uramoto H, Mitsudomi T. Which biomarker predicts benefit from EGFR-TK1 treatment for patients with lung cancer? BR J Cancer. 2007;26:857–63. doi: 10.1038/sj.bjc.6603665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98:1817–24. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappuzzo F, Toschi L, Finopcchiaro G, et al. Surrogate predictive biomarkers for response to anti-EGFR agents: state of the art and challenges. Int J Biol Markers. 2007;22:510–23. [PubMed] [Google Scholar]
- 36.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer-molecular and clinical predictors of outcome. N Engl J Med. 2005;14:133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 37.Yauch RL, Januario T, Eberhad DA, et al. Epithelial versus mesenchymal phenotypes determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;15:8686–98. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 38.Hirsch FR, Varella-Garcia M, McCoy J, et al. Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increase sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol. 2005;1:6838–45. doi: 10.1200/JCO.2005.01.2823. [DOI] [PubMed] [Google Scholar]
- 39.Taguchi F, Solomon B, Gregorc V, et al. Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. J Natl Cancer Inst. 2007;6:838–46. doi: 10.1093/jnci/djk195. [DOI] [PubMed] [Google Scholar]
- 40.Davies AM, Ho C, Lara PN, Mack P, Gumerlock PH, Gandara DR. Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small cell lung cancer. Clin Lung Cancer. 2006;7(6):385–388. doi: 10.3816/CLC.2006.n.021. [DOI] [PubMed] [Google Scholar]