Abstract
Ethylene gas is essential for many developmental processes and stress responses in plants. ETHYLENE INSENSITIVE2 (EIN2), an NRAMP-like integral membrane protein, plays an essential role in ethylene signaling, but its function remains enigmatic. Here we report that phosphorylation-regulated proteolytic processing of EIN2 triggers its endoplasmic reticulum (ER)–to–nucleus translocation. ER-tethered EIN2 shows CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) kinase–dependent phosphorylation. Ethylene triggers dephosphorylation at several sites and proteolytic cleavage at one of these sites, resulting in nuclear translocation of a carboxyl-terminal EIN2 fragment (EIN2-C′). Mutations that mimic EIN2 dephosphorylation, or inactivate CTR1, show constitutive cleavage and nuclear localization of EIN2-C′ and EIN3 and EIN3-LIKE1–dependent activation of ethylene responses. These findings uncover a mechanism of subcellular communication whereby ethylene stimulates phosphorylation-dependent cleavage and nuclear movement of the EIN2-C′ peptide, linking hormone perception and signaling components in the ER with nuclear-localized transcriptional regulators.
The plant hormone ethylene (C2H4) is essential for a myriad of physiological and developmental processes. Molecular genetic dissection has revealed that ethylene is perceived by a family of the endoplasmic reticulum (ER)–membrane—bound receptors that are similar in sequence and structure to bacterial two-component histidine kinases (1–4). Each receptor has an N-terminal transmembrane domain that binds ethylene via a copper cofactor, most likely provided by the copper transporter RESPONSIVE TO ANTAGONIST1 (5). Signaling from one of the receptors, ETR1 (ETHYLENE RESPONSE1), is promoted by interacting with another ER-localized protein REVERSION TO ETHYLENE SENSITIVITY1 (6). The ethylene receptors function redundantly to negatively regulate ethylene responses (2) via CTR1 (CONSTITUTIVE TRIPLE RESPONSE1), a downstream Raf-like protein kinase (7, 8). CTR1 is also associated with the ER membrane, where it directly interacts with ETR1 (8, 9). Downstream of CTR1 is EIN2 (ETHYLENE INSENSITIVE2) (10, 11), an essential positive regulator of ethylene signaling, which shares sequence identity at its N terminus with the 12-transmembrane domain of the NRAMP family of metal transporters and contains a large ~800–amino acid C-terminal domain (CEND) (11). Previous studies using heterologous expression of Arabidopsis EIN2 in Nicotiana benthamiana suggested that EIN2 might be localized to the ER, where it can interact with ETR1 (12). Furthermore, EIN2 is targeted by F-box proteins EIN2-INTERACTING PROTEIN1 and EIN2-INTERACTING PROTEIN2, which mediates protein degradation of EIN2 via the ubiquitin-proteasome pathway in the absence of ethylene (13). In an unknown fashion, EIN2 transduces signals to the transcription factors EIN3/EIL1 (EIL1, ETHYLENE INSENSITIVE LIKE1), which are sufficient and necessary for activation of all ethylene-response genes (14). A model for hormone signaling has emerged in which the perception of ethylene by the receptors alters the activity of CTR1, which in turn, by an unknown mechanism, functions to relieve repression of EIN2, resulting in activation of EIN3/EIL1-dependent transcription and the activation of an ethylene response.
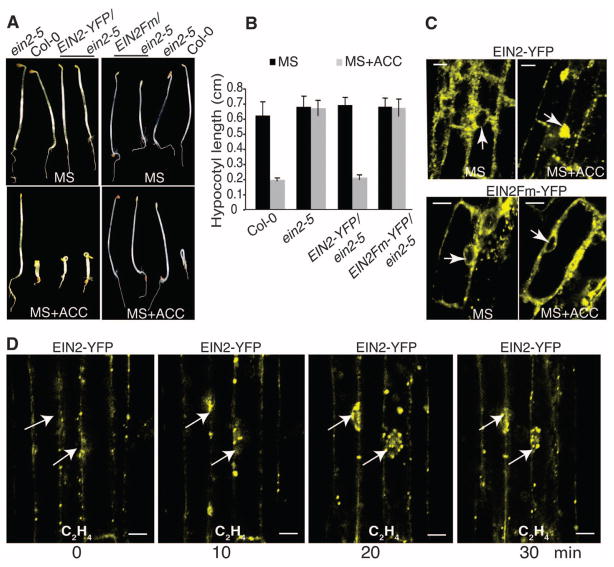
To explore the mechanism of EIN2 function, we identified and tested the requirement for a putative nuclear localization signal (NLS) (15) in the evolutionarily conserved EIN2 C terminus (fig. S1, A to E) and found that a wild-type EIN2-YFP (YFP, yellow fluorescent protein) fusion protein maintained its normal function(s), because its expression was able to rescue the ein2-5 mutant phenotype (Fig. 1, A and B, and fig. S1F); whereas an NLS-mutated EIN2Fm-YFP protein was unable to complement the ein2-5 mutant phenotype (Fig. 1, A and B). In the absence of the ethylene precursor ACC (1-aminocyclo-propane-1-carboxylate), the EIN2-YFP protein was localized in the ER (Fig. 1C) (12) and accumulated in the nucleus upon exposure to ethylene (Fig. 1C and fig. S1G). However, nuclear localization of the EIN2Fm-YFP protein was not observed in the presence of ACC (Fig. 1C and fig. S1H). Therefore, we conclude that the NLS is necessary for EIN2 to function in the ethylene response.
Fig. 1.
The NLS in EIN2 is essential for nuclear localization and the response to ethylene. (A) Wild-type EIN2, but not EIN2 NLS mutations, fully rescue ein2-5. Seedlings were grown for 3 days in the dark with or without ACC. MS, Murashige and Skoog medium. (B) Hypocotyl measurements of 3-day-old etiolated seedlings. Each bar is the average length of at least 15 hypocotyls (error bars indicate mean ±SD). (C) Confocal images of root cells from 3-day-old etiolated transgenic seedlings treated with or without ACC. (D) Time-lapsed confocal images of a series of root cells expressing EIN2-YFP in 3-day-old etiolated seedlings exposed to 10 ppm ethylene gas. Arrows track specific cell nuclei, showing the accumulation of EIN2-YFP in response to ethylene.
Plants respond to ethylene rapidly, and this process is dependent on the function of EIN2 (16). Time-lapsed imaging was used to monitor the dynamics of EIN2 protein movement upon ethylene treatment. In the absence of ethylene (at time 0), EIN2 protein was absent from the nucleus (Fig. 1D). Nuclear accumulation of EIN2 protein was observed within 10 min of exposure to ethylene, and protein levels further increased in the nuclei of the same cells during the subsequent 30 min (Fig. 1D). A greater amount of EIN2 protein in the nucleus was observed with longer ethylene treatments (fig. S1I), demonstrating that the ER-nucleus translocation of EIN2 is important in response to ethylene.
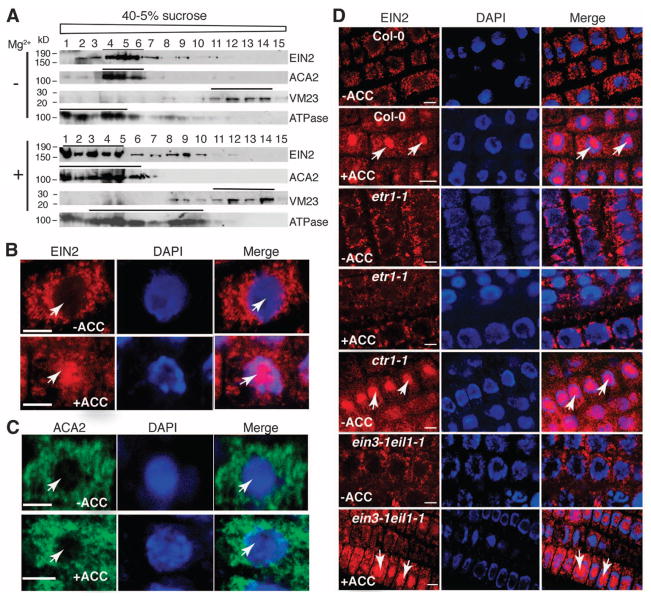
EIN2 protein specifically localized to the ER in the absence of ethylene (Fig. 2, A and B, and fig. S2, A to C) and amassed in the nucleus upon ethylene treatment (Fig. 2B), whereas a known ER-localized protein was unaffected by ethylene (Fig. 2C). Additionally, in wild-type Col-0, EIN2 protein was localized to the ER membrane in the absence of ACC, whereas upon growth on ACC, nuclear translocation of EIN2 protein was observed (Fig. 2D). However, in etr1-1, nuclear accumulation of EIN2 protein was abolished. In contrast, in ctr1-1, a constitutive nuclear accumulation of EIN2 protein was observed, even in the absence of ACC (Fig. 2D). An ein3-1/eil1-1 double mutant had no effect on the nuclear translocation of EIN2 protein (Fig. 2D). Therefore, we conclude that ETR1 and CTR1 are important in the ER-nucleus translocation of EIN2, whereas EIN3/EIL1 are not required for this process.
Fig. 2.
Ethylene-stimulated nuclear accumulation of the ER-localized EIN2 requires ETR1 and CTR1 but not EIN3/EIL1. (A) Sucrose density-gradient centrifugation was performed by fractionation of microsomal membranes containing Mg2+ or without Mg2+. ACA2 is an ER marker protein; VM23 is a vacuole membrane marker protein; adenosine triphosphatase (ATPase) is a plasma membrane marker protein. (B) In Arabidopsis root cells, ER localization of EIN2 in the absence of ACC contrasts with nuclear accumulation in the presence of ACC. (C) Immunofluorescence staining of the subcellular location of ACA2 in Arabidopsis root cells from 3-day-old etiolated seedlings grown with or without ACC. (D) Representative images were acquired from root cells using the same exposure times in all panels. Red staining indicates EIN2 immunofluorescence using a polyclonal antibody to the EIN2 C terminus. Green staining indicates ACA2 immunofluorescence. Blue staining indicates DAPI. Arrows indicate nuclei. Scale bar, 5 μm.
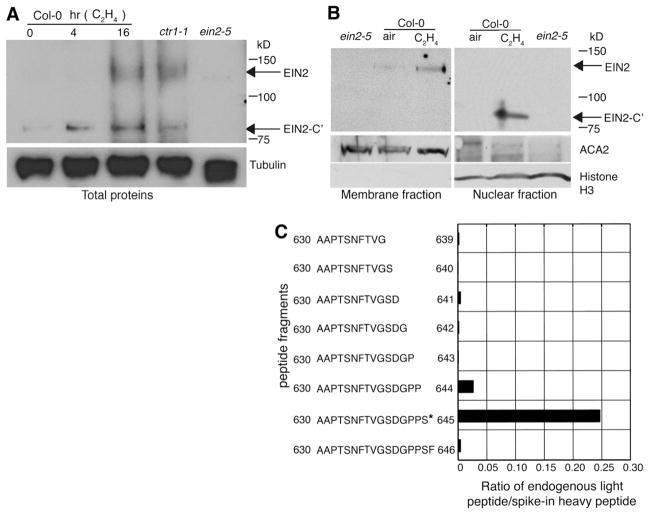
EIN2 is a bifunctional protein (11), and positioning the EIN2-CEND polypeptide in the nucleus was sufficient to mimic both ethylene responses (fig. S3, A to E). We hypothesized that EIN2 may be proteolytically processed and its C-terminal fragment translocated to the nucleus upon exposure to ethylene. To test this hypothesis, we first examined the presence of a cleaved form of the native EIN2 protein by Western blotting. Although full-length EIN2 was not detectable at 0 and 4 hours of ethylene treatment, it was detected in situations that mimic a constitutive ethylene exposure, such as in the ctr1-1 mutant and in Col-0 plants treated with 16 hours of ethylene. Additionally, we observed the presence of an ~75-kD C-terminal EIN2 fragment (called EIN2-C′) whose abundance correlated with the duration of ethylene treatment (Fig. 3A and fig. S4, A and B). Although full-length EIN2 was easily detected in the membrane protein fraction [Fig. 3B and (13)], EIN2-C′ was barely detected in the nuclear fraction without ethylene treatment (Fig. 3B). In contrast, the EIN2-C′ polypeptide was readily detected in the nuclear protein fraction treated with ethylene (Fig. 3B). We generated transgenic lines expressing EIN2-C1-YFP, which contained a YFP protein fused to a fragment of EIN2 estimated from the observed EIN2-C′ polypeptide size (638 to 1294 amino acids), and found that EIN2-C1-YFP protein was localized to the nucleus exclusively, and its expression was sufficient to cause a severe constitutive ethylene response phenotype, reminiscent of ctr1-1 mutants (fig. S4, C and D).
Fig. 3.
EIN2 is cleaved and a C-terminal polypeptide fragment is translocated to the nucleus in response to ethylene. (A) Total proteins were subjected to Western blotting with antibodies to EIN2 and tubulin as loading controls. (B) Total cell membrane and nuclear fractions were prepared and subjected to Western blotting with antibodies to EIN2, ACA2, or the histone H3 as loading controls. (C) Absolute amounts of each endogenous peptide were obtained by calculating the ratios of light to heavy peptide signals. An asterisk indicates the cleavage site.
We next used mass spectrometry (17) to map the cleavage site of the EIN2 protein. Three EIN2-C′ peptides [630 to 647 amino acids (630-647aa), 648-662aa, and 754-766aa; table S1A] were detected at similar levels in samples treated with air. In the presence of ethylene, the abundance of the peptide 630-647aa was ~20 times less, suggesting that cleavage of the EIN2 protein may occur within this peptide. Pseudo-multiple reaction monitoring (18) was used to identify the EIN2 protein cleavage site. Contiguous peptides designated 630-647aa and 648-662aa behaved differently after ethylene treatment; only the former decreased in abundance, suggesting that it contained an ethylene-dependent protease cleavage site (table S1A). We tested a set of overlapping peptides that differed by the progressive loss of single amino acids from the C terminus of the peptide 630-647aa. These represent 18 possible cleavage products derived from cleaving within residues 630 to 647 (plus the trypsin cleavage site corresponding to the N terminus of the peptide). After ethylene treatment, none of the 10 deletions from the N terminus corresponded to a detectable peptide (table S1B). However, among the eight deletions from the C terminus, one peptide was significantly enriched (AAPTSNFTVGSDGPPS, 630-645aa; Fig. 3C, fig. S4E, and table S1B), indicating that ethylene-induced cleavage occurs between 645aa and 646aa.
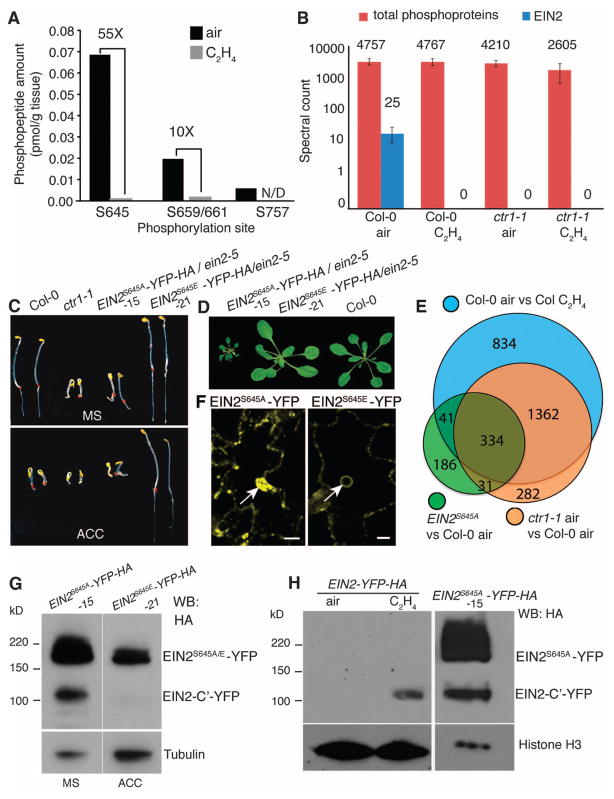
Although several phosphoproteomic studies have identified ethylene-responsive phosphopeptides (19, 20), the function of phosphorylation regulation in the ethylene signaling pathway is still unknown. We carried out a phosphoproteomic survey using proteins purified from etiolated seedlings treated with hydrocarbon-free air or ethylene gas (10 ppm) and identified three sites in EIN2 where phosphorylation was highly enriched in air-treated samples (Fig. 4A, table S1A, and fig. S5A). Ser645 (S645) is conserved in all plant species examined (fig. S5B) and this position coincided with the experimentally determined cleavage site. We examined the phosphorylation status of EIN2 S645 using ctr1-1 and wild-type plants treated with or without ethylene. In wild-type plants treated with air, residue S645 of EIN2 was phosphorylated, whereas in wild-type plants treated with ethylene, S645 was not phosphorylated (Fig. 4B). In ctr1-1 mutants, however, phosphorylation of S645 was undetectable (Fig. 4B) indicating that CTR1 is required for EIN2 S645 phosphorylation.
Fig. 4.
CTR1-dependent ethylene-regulated phosphorylation of EIN2 S645 regulates proteolysis and nuclear translocation of EIN2-C′. (A) Absolute amounts of three EIN2-C′ phosphopeptides before and after treatment with 10 ppm ethylene gas. N/D, not detectable. (B) Relative phosphorylation levels of EIN2 peptides in wild-type or ctr1-1 plants treated for 4 hours of air or 10 ppm ethylene gas. Spectral counts were computed by averaging three biological replicates. The total spectral counts from all phosphorylated proteins in each sample are indicated as an internal control. (C) EIN2-C′ S645A results in constitutive ethylene response phenotypes in dark-grown seedlings. (D) EIN2 S645A results in constitutive ethylene response phenotypes in 7-week-old plants. (E) EIN2 S645A plants show transcriptional activation of ethylene responses. (F) EIN2 S645A plants show constitutive nuclear localization without ethylene from leaf cells. Arrows indicate nuclei. (G) S645A leads to constitutive cleavage of EIN2. Total proteins from 3-day-old etiolated seedlings were subjected to Western blotting using antibodies to HA and to tubulin as loading controls. EIN2S645A/E represents either full-length EIN2S645A or EIN2S645E. (H) Purified nuclear proteins prepared from EIN2-YFP-HA plants treated with or without ethylene. Total protein from EIN2S645A-YFP-HA–overexpressing plants was prepared and subjected to Western blotting using antibodies to HA and to histone H3 as loading controls. Scale bar, 5 μm.
To test whether phosphorylation of S645 regulates ethylene-dependent EIN2 cleavage, constructs carrying point mutations in EIN2 that convert serine to alanine (S645A) (EIN2S645A-YFP-HA) or serine to glutamic acid (S645E) (EIN2S645E-YFP-HA) were introduced into both wild-type and ein2-5 plants. EIN2S645A did not alter the function of the EIN2 protein, because it fully rescued ein2-5 in contrast to EIN2S645E (Fig. 4C). In fact, etiolated seedlings and adult EIN2S645A plants exhibited constitutive ethylene-response phenotypes in the absence of ethylene (Fig. 4, C and D, and fig. S5, C to E).
Transcriptome analysis revealed that >60% of genes with significant changes in expression in EIN2S645A-YFP-HA transgenic lines significantly overlapped with genes differentially expressed in wild-type ethylene-treated plants (P < 1 × 10−200, using Fisher’s exact test) or with genes differentially expressed in ctr1-1 mutant plants treated with hydrocarbon-free air (Fig. 4E), suggesting that the EIN2 S645A mutation affects numerous ethylene-responsive genes at the transcriptional level. Moreover, EIN2S645A-YFP-HA transgenic plants showed both constitutive cleavage of EIN2 at residue S645 and constitutive nuclear translocation of the EIN2-C′ protein (Fig. 4, F and G). The predicted length of the EIN2-derived polypeptide released from EIN2S645A-YFP-HA matched with that observed in the nucleus of EIN2-YFP-HA transgenic plants after exposure to ethylene (Fig. 4H). In contrast, in transgenic plants containing the S645E mutant, both cleavage and nuclear translocation of EIN2-C′ protein were abolished, even in the presence of ACC (Fig. 4, F and G).
We have uncovered a mechanism whereby EIN2 protein processing and subcellular nuclear translocation are required for response to ethylene (fig. S6). Recent studies in animals have also demonstrated the importance of dephosphorylation-dependent nuclear translocation of transcription factor EB in regulating homeostasis of the lysosome (21), and of nuclear translocation of ATFS-1 (activating transcription factor associated with stress–1) in response to mitochondrial stress (22). Further studies to determine the biochemical mechanisms that are needed for the cleavage of EIN2 in response to ethylene will be of great importance. In addition, identification of the kinase(s) and phosphatase(s) that target the EIN2 protein directly, as well as the enzymes that promote processing of this key regulatory molecule, are future challenges that will further our understanding of this highly conserved and agriculturally important plant stress and growth-controlling signaling pathway.
Supplementary Material
Acknowledgments
We thank L. Song, N. Benson, and R. O’Malley for comments; J. Lin for plant maintenance; J. Chory for access to, and N. Geldner for assistance with, confocal microscopy; and J. Harper for the antibody to ACA2. We thank J. Nery for assistance with next-generation sequencing. R.J.S. was supported by an NIH National Research Service Award postdoctoral fellowship (F32-HG004830). This work was supported by grants from NSF (MCB-0924871 and MCB-1024999 to J.R.E. and DBI-0924023 to S.P.B.). J.R.E is an Investigator of the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation. Sequence data can be downloaded from the National Center for Biotechnology Information’s Sequence Read Archive (accession no. SRA052247).
Footnotes
References and Notes
- 1.Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Science. 1993;262:539. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 2.Hua J, Meyerowitz EM. Cell. 1998;94:261. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- 3.Sakai H, et al. Proc Natl Acad Sci USA. 1998;95:5812. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YF, et al. PLoS ONE. 2010;5:e8640. doi: 10.1371/journal.pone.0008640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirayama T, et al. Cell. 1999;97:383. doi: 10.1016/s0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 6.Dong CH, et al. J Biol Chem. 2010;285:40706. doi: 10.1074/jbc.M110.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. Cell. 1993;72:427. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ. Plant J. 2003;33:221. doi: 10.1046/j.1365-313x.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 9.Kendrick MD, Chang C. Curr Opin Plant Biol. 2008;11:479. doi: 10.1016/j.pbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetics. 1995;139:1393. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. Science. 1999;284:2148. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 12.Bisson MM, Bleckmann A, Allekotte S, Groth G. Biochem J. 2009;424:1. doi: 10.1042/BJ20091102. [DOI] [PubMed] [Google Scholar]
- 13.Qiao H, Chang KN, Yazaki J, Ecker JR. Genes Dev. 2009;23:512. doi: 10.1101/gad.1765709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao Q, et al. Cell. 1997;89:1133. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 15.Bisson MM, Groth G. Plant Signal Behav. 2011;6:164. doi: 10.4161/psb.6.1.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB. Plant Physiol. 2004;136:2921. doi: 10.1104/pp.104.050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cravatt BF, Simon GM, Yates JR., 3rd Nature. 2007;450:991. doi: 10.1038/nature06525. [DOI] [PubMed] [Google Scholar]
- 18.Lange V, Picotti P, Domon B, Aebersold R. Mol Syst Biol. 2008;4:222. doi: 10.1038/msb.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, et al. Mol Biosyst. 2011;7:2637. doi: 10.1039/c1mb05159h. [DOI] [PubMed] [Google Scholar]
- 20.Li H, et al. Proteomics. 2009;9:1646. doi: 10.1002/pmic.200800420. [DOI] [PubMed] [Google Scholar]
- 21.Roczniak-Ferguson A, et al. Sci Signal. 2012;5:ra42. doi: 10.1126/scisignal.2002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Science. 2012;337:587. doi: 10.1126/science.1223560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.