Abstract
Vascular calcification, prevalent in diabetes and chronic kidney disease, contributes to morbidity and mortality. To investigate the effect of receptor activator of NF-kB ligand (RANKL) on vascular calcification in vivo, transgenic mice, where RANKL expression was targeted to vascular smooth muscle cells using SM22alpha promoter (SM22α-Rankltg), was created. Sixteen-month-old male SM22α-Rankltg mice had higher body weight and higher serum calcium levels but lower lumbar bone mineral density (BMD) compared with age- and gender-matched WT littermates. BMD of long bones, body fat (% of weight) of the leg, and serum levels of phosphate and RANKL were not significantly different. No significant differences in these parameters were observed in the female mice. Histological analysis did not reveal calcium deposits in the aortic roots of the SM22α-Rankltg mice. To analyze the osteoblastic differentiation and mineralization potentials of vascular cells, aortic smooth muscle cells (SMCs) were isolated and cultured. Results showed that SM22α-Rankltg SMCs had higher baseline alkaline phosphatase (ALP) activity, but not the baseline matrix calcification. When induced by the PKA agonist, forskolin, ALP activity was greater in SM22α-Rankltg than in WT SMCs. Realtime RT-qPCR revealed higher baseline expression of ALP and ankylosis genes, but lower osteoprotegerin gene, in SM22α-Rankltg SMCs. Matrix mineralization induced by inorganic phosphate or forskolin was greater in SM22α-Rankltg than in WT SMCs. Treatment of these cells with the ALP inhibitor, levamisole, abolished forskolin-induced matrix mineralization but not Pi-induced matrix mineralization. These findings suggest that RANKL overexpression in the vasculature may promote mineralization potential.
Keywords: RANKL, smooth muscle cells, vascular, calcification, SM22 alpha
INTRODUCTION
Vascular calcification is present in half of middle-aged individuals and in 90% of those over age 70 [1]. It particularly affects patients with atherosclerosis, diabetes, end-stage renal disease, and other metabolic disorders [2]. It dramatically alters vascular physiology, and key molecular regulators need to be identified to develop therapeutic approaches. We previously developed an in vitro model in which aortic cells undergo osteogenic differentiation with matrix mineral formation [3]. Using both in vitro cell culture and in vivo studies, we and others have demonstrated that many of the same factors that modulate bone formation and resorption, including cytokines and hormones, also modulate vascular calcification [4–9].
Two cytokines, receptor activator of NF-kB ligand (RANKL) and its soluble decoy receptor, osteoprotegerin (OPG), have a central role in controlling differentiation of osteoclasts [10]. Their potential role in vascular calcification is evidenced by the unexpected finding of extensive aortic mineralization in mice deficient in OPG [11]. Since the absence of OPG results in unopposed activity of RANKL, it suggests that RANKL may promote vascular calcification. In support of this, calcified arteries are known to have higher levels of RANKL expression and lower levels of OPG [12, 13]. However, other findings suggest a more complex picture. Epidemiological studies show vascular calcification is inversely related to serum RANKL and positively related to serum OPG [14–18]. Moreover, some in vitro studies showed that RANKL treatment induced vascular calcification [19, 20], whereas we and others found no effect [21, 22], albeit in different species and culture conditions.
In previous studies with RANKL transgenic mice, the overexpression was global or the distribution systemic. Mice with ubiquitous RANKL overexpression die at the late fetal stage [23]. Mice with overexpression of soluble RANKL, released from the liver, have osteoporosis [23]. These models would not be suitable for studying the role of RANKL on vascular calcification, since bone and vascular mineralization share many endocrinological mechanisms, and since excess resorption in bone may cause spontaneous mineral deposition in other tissues, including arteries. Thus, in the present study, we targeted the expression of RANKL to vascular smooth muscle cells (SM22α-Rankltg). Results showed no effect of vascular-specific RANKL overexpression on basal levels of calcification but enhancement of osteoblastic differentiation and matrix mineralization in vascular cells treated with inorganic phosphate and a PKA agonist, forskolin.
METHODS
Generation of Rankltg mouse
The promoter sequence for the SM22alpha gene was PCR amplified from pSM1343-luciferase vector (1344 bps of the 5′ promoter and 62 base pairs of intron 1; kindly provided by Dr. Eric Olson, UT Southwestern) into pCR-Script vector, kindly provided by Dr. Scott Simonet (Amgen Inc.). The first coding sequence (CDS1) of the full-length (membrane-bound) murine RANKL cDNA (951 bp) was then sub-cloned into the newly constructed SM22alpha promoter transgenic expression vector [24]. The sequences of the sub-cloned SM22α and Rankl gene were confirmed by direct and reverse strand sequencing and compared with the published GeneBank entries (SM22α promoter U36589; RANKL AF053713). Gel-purified linearized transgenic constructs were microinjected into the male pronucleus of fertilized C57BL/6 one-cell oocytes. Surviving oocytes were subsequently transplanted into the oviducts of pseudopregnant foster mothers. Transgenic founder mice (on a C57BL/6 background) were identified by PCR analysis of genomic DNA isolated from tail biopsies. To minimize variability, we used a line with high penetrance for subsequent studies. The relative copy number of the transgene was determined to be approximately 14–16 (Charles River, New York). Animals were fed a normal chow diet ad lib. At necropsy, blood, heart, aorta, and long bones were isolated. The experimental protocols were reviewed and approved by the Office of Animal Research Oversight of the University of California at Los Angeles and the Institutional Animal Care and Use Committee at Amgen Inc.
Bone mineral density
Bone mineral density (BMD) was determined in vivo using dual energy x-ray absorptiometry (DXA; PIXImus II, GE Lunar, Madison, WI, USA), with lumbar vertebrae (LV1–LV5) and whole leg subregion analyses.
Histological analysis
Aortic root specimens were embedded in OCT and cryosectioned at 10 μm. The sections were stained for mineralization by the von Kossa silver nitrate method and counterstained with eosin. Immunohistochemical staining was performed using anti-RANKL antibody (R&D Systems).
Serum biochemical assays
Serum RANKL was measured by ELISA (R&D Systems) following the manufacturer’s protocol. Serum calcium and phosphate levels were determined using a Hitachi 717 auto-analyzer (Roche Diagnostics, Indianapolis IN).
Cell culture
Murine aortic cells (passages 6–10) were isolated from the aortas of C57BL6 (WT) and SM22α-Rankltg mice as described previously [25]. Cells were maintained in DMEM containing 20% fetal bovine serum (FBS). One day after plating, cells were treated with forskolin (25 μM) or inorganic phosphate (3 mM) in MEM media supplemented with 10% FBS.β-glycerophosphate (5 mM) was supplemented in control media and in media with forskolin.
Matrix calcium quantitation
After the indicated periods, matrix calcium levels were analyzed by the o-cresolphthalein complexone method (Teco Diagnostics, Anaheim, CA). Each condition was assayed in quintuplicate and normalized to total protein using the Bradford method [25].
Alkaline phosphatase activity
After the indicated periods, alkaline phosphatase activity was assessed colorimetrically using Sigma 104 Phosphatase Substrate (Sigma St. Louis, MO). Each condition was assayed in quintuplicate and normalized to total protein using the Bradford method [25].
Gene expression
Total RNA was isolated from cells using TRIzol reagent (Invitrogen Carlsbad, CA). Realtime RT-qPCR was performed using 1-step qRT-PCR (Biochain Institute, Inc. Hayward, CA) in the Mx3005P (Strategene La Jolla, CA). β-actin was used for normalization.
Data analysis
Each experiment was performed in at least quadruplicate wells and repeated at least three times (n ≥ 3). Data are expressed as mean ± SEM. Student’s t-test was used for comparison between two groups. For more than two groups, mean values were compared using one-way ANOVA, with comparison of different groups by Fisher’s protected least significant difference test. A value of p≤ 0.05 was considered significant.
RESULTS
SM22α-Rankltg mice
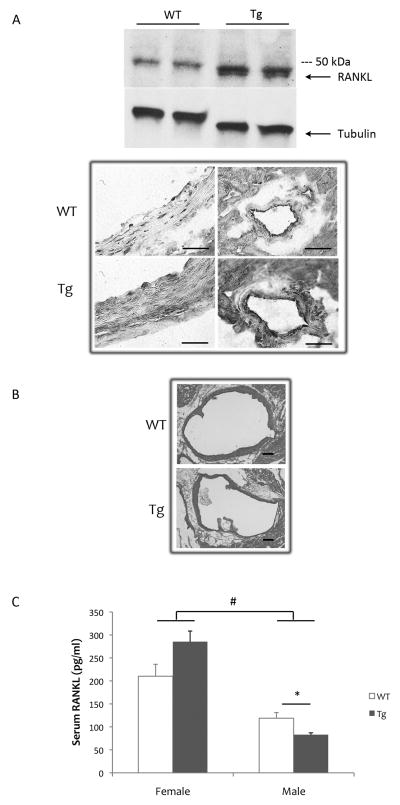
To test the effects of RANKL on vascular calcification specifically, we created RANKL transgenic mice under the control of the SM22α promoter (SM22α-Rankltg). Li et al., showed that SM22 expression in vivo is highly restricted to vascular SMCs in adult tissues [24], and Lepore et al. showed that expression via this promoter is in arterial SMCs as well as in cardiac, but not in skeletal, myocytes [26]. Western blot analysis showed a band with the expected molecular weight of 35 kDa only in the aortic lysates from the SM22α-Rankltg mice (lower band, Fig 1A). Consistent with the findings of Lepore et al. [26], transgene expression was observed in SMCs and cardiomyocytes (Fig. 1A). There were no obvious phenotypes in the founders, and, in the male and female mice of the F1 generation, no vascular calcification was detected by DXA analysis (data not shown). Histological analyses by von Kossa staining of aortic sections from 16-month-old SM22α-Rankltg mice also showed no calcium deposits (Fig 1B).
Figure 1. RANKL expression in SM22α-Rankltg mice.
(A) Upper panel: Western analysis of aortas from 2.5-month-old male mice with tubulin as a loading control. Lower panel: RANKL immunohistochemistry of aorta (left) and intramyocardial coronary arteries (right) of WT and Tg mice. As expected for this promoter, cardiomyocytes also express the transgene. Scale bar 50 μ. (B) Von Kossa histochemistry staining of aortic root sections for calcium mineral. Scale bar: 200 μ (C) Levels of serum RANKL assessed by ELISA (n = 6/group). *p < 0.05; #p < 0.001.
To test whether vascular-specific overexpression of RANKL affected circulating levels, serum levels of RANKL were assessed. Results showed that serum RANKL levels were not increased in the SM22α-Rankltg compared with WT mice (Fig. 1C). As observed in humans, female mice had higher serum RANKL levels than male mice [27]. In male SM22α-Rankltg mice, serum RANKL was found to be lower than in WT littermates (Fig. 1C).
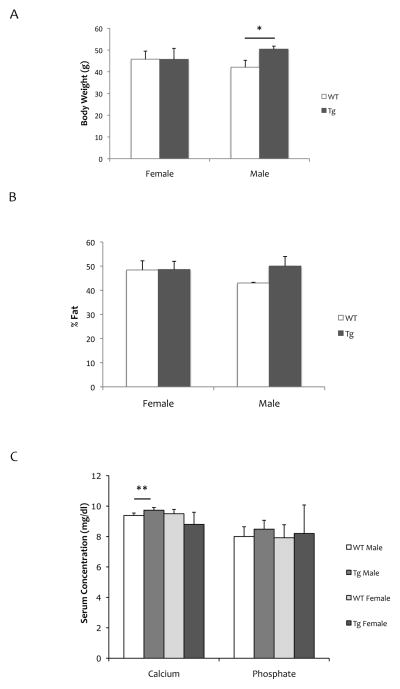
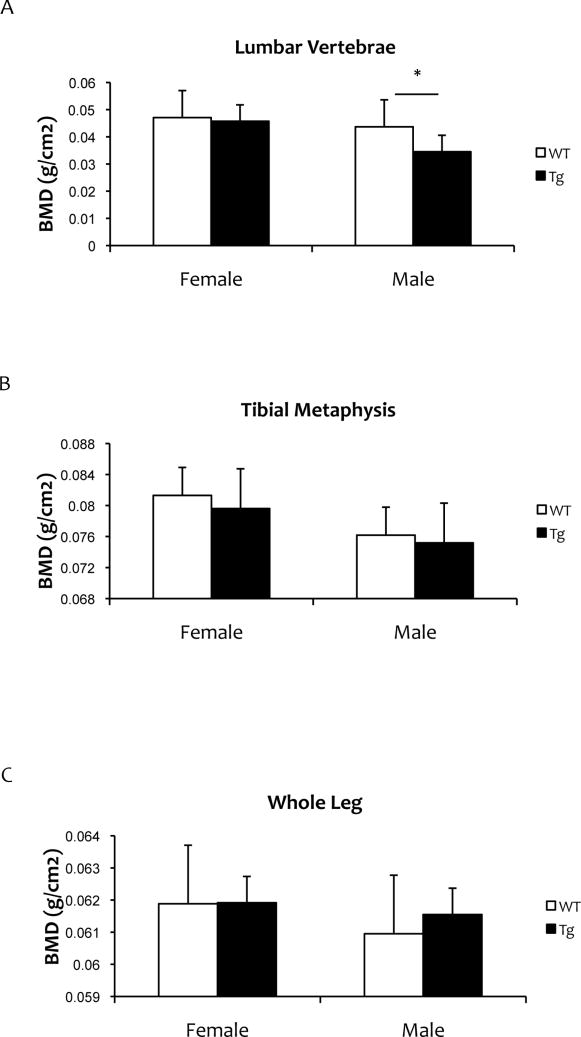
We also assessed the effects of vascular-specific RANKL expression on body weight, percent fat, and bone mineral density of long bones and vertebrae. Although no weight difference was detected in female mice, male SM22α-Rankltg weighed more than WT mice at 16 months of age (Fig 2A). Body fat of the leg was not changed in either male or female mice (Fig 2B). In female mice, serum calcium, phosphorus levels and BMD were not altered compared with WT mice (Fig 2C, Fig 3). In male SM22α-Rankltg mice, although serum RANKL levels were lower, serum calcium levels were higher than in WT mice (Fig. 2C). BMD of lumbar vertebrae, but not of long bones or tibial metaphyses, were less in male SM22α-Rankltg than in WT mice (Fig 3).
Figure 2. Physical and biochemical properties of SM22α-Rankltg mice.
(A) Body weight, (B) percent body fat of the whole leg, and (C) serum calcium and phosphate levels in 16-month old mice (n = 6/group). *p = 0.05; **p < 0.01.
Figure 3. Bone mineral density of SM22α-Rankltg mice.
Dual energy x-ray absorptiometry analysis of 12-month-old mice (n = 6/group). (A) Lumbar vertebrae, (B) tibial metaphyses, and (C) whole leg. *p < 0.05.
SM22α-Rankltg SMCs
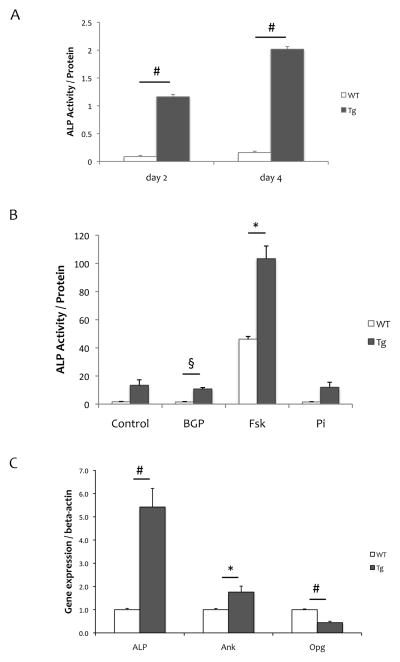
We further evaluated osteogenic activity of aortic SMCs from SM22α-Rankltg mice. Results showed that aortic SMCs from SM22α-Rankltg mice had substantially higher baseline alkaline phosphatase activity than WT cells at 2 and 4 days of culture (1.2 ± 0.04 vs. 0.09 ± 0.02, p < 0.001 on day 2; 2.02 ± 0.05 vs. 0.16 ± 0.03, p < 0.001 on day 4; Fig 4A). When induced using the PKA agonist, forskolin, ALP activity was induced in both SM22α-Rankltg and WT SMCs, with a much greater induction in SM22α-Rankltg than in WT SMCs (14 ± 4 to 103 ± 9 vs. 2 ± 0.3 to 46 ± 2, p < 0.05; Fig 4B). Realtime RT-qPCR revealed that baseline expression levels of ALP and ankylosis genes were approximately 5-fold and 2-fold greater in SM22α-Rankltg compared with WT SMCs; whereas that of OPG was lower (0.4-fold; Fig 4C). Expression levels of Enpp1, SM22α, and Pit-1 were not significantly different between the groups (data not shown).
Figure 4. Osteoblastic differentiation of SM22α-Rankltg SMCs.
(A) Alkaline phosphatase activity, normalized for total protein, in cell lysates from Rankltg and WT SMCs cultured for 2 and 4 days. (B) ALP activity, normalized for total protein, in Rankltg and WT SMCs treated with control vehicle, beta-glycerophosphate (BGP, 5 mM), forskolin (Fsk, 25 μM), or inorganic phosphate (Pi, 3 mM) for 4 days. (C) Realtime RT-qPCR analysis of RNA isolated from Rankltg and WT SMCs cultured for 7 days. *p < 0.05; §p < 0.01; #p < 0.001.
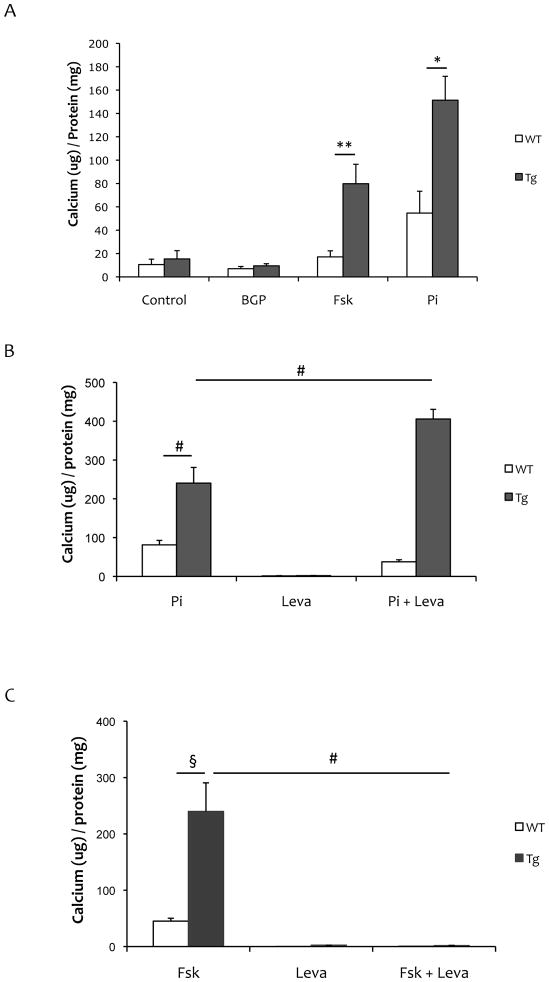
When induced by inorganic phosphate or forskolin, matrix calcium levels were greater in SM22α-Rankltg than in WT SMCs (Fig 5A). Treatment with an alkaline phosphatase inhibitor, levamisole, abolished forskolin-induced matrix mineralization but not Pi-induced matrix mineralization (Fig 5B–C).
Figure 5. Matrix mineralization of SM22α-Rankltg SMCs.
(A) Matrix calcium, normalized for total protein, in Rankltg and WT SMCs treated with control vehicle, beta-glycerophosphate (BGP, 5 mM), forskolin (Fsk, 25μM) or inorganic phosphate (Pi, 3 mM) for 7 days. (B) Matrix calcium, normalized for total protein, in Rankltg and WT SMCs treated with inorganic phosphate (Pi, 3 mM), and/or levamisole (Leva, 100 μM) for 10 days. (C) Matrix calcium, normalized for total protein, in Rankltg and WT SMCs treated with forskolin (Fsk, 25 μM) and/or levamisole (Lev, 100 μM) for 13 days. **p = 0.05; *p < 0.05; §p < 0.01; #p < 0.001.
DISCUSSION
The RANKL/RANK/OPG axis regulates differentiation of resorptive osteoclasts in bone. RANKL has been implicated in vascular calcification in some studies [19, 20, 28] but not others [21, 22, 29]. These differences may relate to different model systems and cell types. Previous efforts to elucidate its role in vascular calcification in vivo used a transgenic mouse model overexpressing soluble RANKL released from the liver [24]. However, this model was confounded by RANKL induction of skeletal resorption, which led to levels of serum calcium and phosphate capable of inducing spontaneous soft tissue calcification. To overcome this confounding effect, in the present study, we generated transgenic mice with vascular-specific expression of RANKL driven by the SM22 promoter. As evidence that this model provided adequate specificity, there was no significant difference in serum RANKL levels, suggesting that vascular-specific overexpression of the membrane-bound RANKL overcame the confounding effect. We found that these SM22α-Rankltg mice did not develop spontaneous vascular calcification.
In cell culture, SM22α-Rankltg SMCs showed significantly greater baseline alkaline phosphatase gene expression and activity than WT SMCs. However, this increase was not sufficient to cause matrix calcification. Possible explanations include that the levels of ALP activity were below the threshold required for calcification, and/or that the increase in ALP activity was overcome by even greater increase in pyrophosphate, an inhibitor of mineralization which is 1,000-fold more potent an inhibitor than Pi is an activator. The latter possibility is supported by our finding of a simultaneous increase in expression of Ank, the membrane-bound channel responsible for pyrophosphate transport, which may compensate for the increase in ALP. SM22α-Rankltg SMCs did produce more matrix mineral in response to the procalcific factors, inorganic phosphate and forskolin, and these findings probably have distinct mechanisms. The response to inorganic phosphate is considered independent of ALP activity, whereas the response to forskolin is considered dependent on ALP activity (Fig 6). The mechanism for reduced expression of OPG is not clear. However, given that Opg−/− mice develop substantial vascular calcification, this fall in OPG may have a role in our observed potentiation of forskolin- and Pi-induced mineralization.
Since, in our previous studies, exogenous (soluble) RANKL showed no induction of mineralization in cultured SMCs, there may be a different mechanism for the action of endogenously expressed RANKL in SM22α-Rankltg SMCs. Another possible explanation for the different effects of exogenous vs. transgenic RANKL is that intracellular RANKL may have a novel function in osteoblastic differentiation. Such a phenomenon was reported for intracellular Enpp1, which when altered by overexpression or shRNA, regulated osteoblastic differentiation of calvarial osteoblastic cell line, in a manner independent of its catalytic activity [30].
Interestingly, we found effects on serum RANKL levels, body weight, and bone density of lumbar vertebrae that appeared to be gender specific. The gender differences may be due to effects of estrogen, which has been shown to inhibit RANKL signaling [28]. Gender differences in serum RANKL in our mice are also consistent with the gender differences reported in human studies, with levels almost twice as high in women as in men [27, 31]. Serum RANKL levels are also influenced by female hormonal cycling, with levels higher in the follicular vs. luteal phases [32]. Estrogen is also known to regulate RANKL expression in osteoblasts [33] and in peripheral blood mononuclear cells in human [34]. The increased body weight observed in male mice does not appear to be due to skeletal effects, since BMD was decreased rather than increased. It also does not appear to be due to circulating RANKL levels since the levels were reduced. However, the body fat showed a non-significant trend toward an increase, which suggests a role of RANKL interactions with neuro-endocrine activation [35].
Although SM22α-Rankltg mice do not develop spontaneous vascular calcification, it is possible that these mice may develop greater vascular calcification than WT mice when exposed to pro-calcific interventions, such as uremia, renal insufficiency, and/or hyperlipidemia, especially in light of our in vitro finding that transgenic SMCs produce more calcium mineral than WT SMCs in the presence of pro-calcific factors.
Acknowledgments
Sources of Funding: This research was supported by funding from the National Heart, Lung, and Blood Institute as well as the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (HL081202 and DK081346).
Footnotes
Disclosure:
APS, JL, YT, LLD – None
S.M and T.C are employees of Amgen, Inc.
S Morony and T Corbin have received remuneration from and have stock ownership in Amgen. All other authors have stated that they have no conflict of interest.
References
- 1.Newman AB, Naydeck BL, Sutton-Tyrrell K, Feldman A, Edmundowicz D, Kuller LH. Coronary artery calcification in older adults to age 99: prevalence and risk factors. Circulation. 2001;104:2679–2684. doi: 10.1161/hc4601.099464. [DOI] [PubMed] [Google Scholar]
- 2.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 3.Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 4.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 5.Cheng SL, Shao JS, Halstead LR, Distelhorst K, Sierra O, Towler DA. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 2010;107:271–282. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol. 2005;25:686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 8.Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M, Neumann M, Shanahan CM, Proudfoot D. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008;103:e28–34. doi: 10.1161/CIRCRESAHA.108.181305. [DOI] [PubMed] [Google Scholar]
- 9.Chen NX, O’Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62:1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 10.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 11.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoppet M, Al-Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, Preissner KT, Hofbauer LC. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Monckeberg’s sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004;89:4104–4112. doi: 10.1210/jc.2003-031432. [DOI] [PubMed] [Google Scholar]
- 14.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–637. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 15.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–1194. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 16.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 17.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 18.Schoppet M, Schaefer JR, Hofbauer LC. Low serum levels of soluble RANK ligand are associated with the presence of coronary artery disease in men. Circulation. 2003;107:e76. doi: 10.1161/01.cir.0000060815.25798.02. author reply e76. [DOI] [PubMed] [Google Scholar]
- 19.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 21.Tseng W, Graham LS, Geng Y, Reddy A, Lu J, Effros RB, Demer L, Tintut Y. PKA-induced receptor activator of NF-kappaB ligand (RANKL) expression in vascular cells mediates osteoclastogenesis but not matrix calcification. J Biol Chem. 2010;285:29925–29931. doi: 10.1074/jbc.M110.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 31:1387–1396. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno A, Kanno T, Hoshi M, Shibata O, Yano K, Fujise N, Kinosaki M, Yamaguchi K, Tsuda E, Murakami A, Yasuda H, Higashio K. Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J Bone Miner Metab. 2002;20:337–344. doi: 10.1007/s007740200049. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang MS, Sage AP, Lu J, Demer LL, Tintut Y. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem Biophys Res Commun. 2008;374:553–558. doi: 10.1016/j.bbrc.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepore JJ, Cheng L, Min Lu M, Mericko PA, Morrisey EE, Parmacek MS. High-efficiency somatic mutagenesis in smooth muscle cells and cardiac myocytes in SM22alpha-Cre transgenic mice. Genesis. 2005;41:179–184. doi: 10.1002/gene.20112. [DOI] [PubMed] [Google Scholar]
- 27.Jung K, Lein M, Hosslin K, Grosse A, Roth S, Possinger K, Luftner D. Osteoprotegerin and receptor activator of nuclear factor-kappaB ligand (RANKL) in the serum of healthy adults. Int J Biol Markers. 2002;17:177–181. doi: 10.1177/172460080201700306. [DOI] [PubMed] [Google Scholar]
- 28.Osako MK, Nakagami H, Koibuchi N, Shimizu H, Nakagami F, Koriyama H, Shimamura M, Miyake T, Rakugi H, Morishita R. Estrogen inhibits vascular calcification via vascular RANKL system: common mechanism of osteoporosis and vascular calcification. Circ Res. 2010;107:466–475. doi: 10.1161/CIRCRESAHA.110.216846. [DOI] [PubMed] [Google Scholar]
- 29.Olesen M, Skov V, Mechta M, Mumm BH, Rasmussen LM. No influence of OPG and its ligands, RANKL and TRAIL, on proliferation and regulation of the calcification process in primary human vascular smooth muscle cells. Mol Cell Endocrinol. doi: 10.1016/j.mce.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Nam HK, Liu J, Li Y, Kragor A, Hatch NE. Ectonucleotide pyrophosphatase/phosphodiesterase-1 (ENPP1) protein regulates osteoblast differentiation. J Biol Chem. 286:39059–39071. doi: 10.1074/jbc.M111.221689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerschan-Schindl K, Wendlova J, Kudlacek S, Gleiss A, Woloszczuk W, Pietschmann P. Serum levels of receptor activator of nuclear factor kappaB ligand (RANKL) in healthy women and men. Exp Clin Endocrinol Diabetes. 2008;116:491–495. doi: 10.1055/s-2007-993142. [DOI] [PubMed] [Google Scholar]
- 32.Hofbauer LC, Schoppet M, Schuller P, Viereck V, Christ M. Effects of oral contraceptives on circulating osteoprotegerin and soluble RANK ligand serum levels in healthy young women. Clin Endocrinol (Oxf) 2004;60:214–219. doi: 10.1046/j.1365-2265.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 33.Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32:136–141. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 34.Bashir A, Mak YT, Sankaralingam S, Cheung J, McGowan NW, Grigoriadis AE, Fogelman I, Hampson G. Changes in RANKL/OPG/RANK gene expression in peripheral mononuclear cells following treatment with estrogen or raloxifene. Steroids. 2005;70:847–855. doi: 10.1016/j.steroids.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Loncar G, Bozic B, Cvorovic V, Radojicic Z, Dimkovic S, Markovic N, Prodanovic N, Lepic T, Putnikovic B, Popovic-Brkic V. Relationship between RANKL and neuroendocrine activation in elderly males with heart failure. Endocrine. 2010;37:148–156. doi: 10.1007/s12020-009-9282-z. [DOI] [PubMed] [Google Scholar]