Synopsis
The saliva of blood-feeding parasites is a rich source of peptidase inhibitors that help overcome the host’s defense during host-parasite interactions. Using proteomic analysis, the cystatin OmC2 was demonstrated in the saliva of the soft tick Ornithodoros moubata, an important disease-vector transmitting African swine fever virus and the spirochaete Borrelia duttoni. A structural, biochemical and biological characterization of this peptidase inhibitor was undertaken. Recombinant OmC2 was screened against a panel of physiologically relevant peptidases and found to be an effective broad-specificity inhibitor of cysteine cathepsins, including endopeptidases (cathepsins L and S) and exopeptidases (cathepsins B, C and H). The crystal structure of OmC2 was determined at a resolution of 2.45 Å and used to describe the structure-inhibitory activity relationship. The biological impact of OmC2 was demonstrated both in vitro and in vivo. OmC2 affected the function of antigen-presenting mouse dendritic cells by reducing the production of the proinflammatory cytokines TNF-α and IL-12, and proliferation of antigen-specific CD4+ T cells. This suggests that OmC2 may suppress the host’s adaptive immune response. Immunization of mice with OmC2 significantly suppressed the survival of O. moubata in infestation experiments. We conclude that OmC2 is a promising target for the development of a novel anti-tick vaccine to control O. moubata populations and combat the spread of associated diseases.
Keywords: cathepsin, cystatin, immune cells, structure-activity relationship, parasite, peptidase inhibitor
INTRODUCTION
Ticks (Ixodida) are blood-feeding parasites that transmit many pathogens of medical and veterinary importance. The order Ixodida has two main families, the Ixodidae (hard ticks) and Argasidae (soft ticks), which differ in many aspects of their biology. In the hard ticks, the adult female engorges only once (for several days), lays a batch of eggs and then dies. In the soft ticks, the female lays a batch of eggs after each feeding episode which takes minutes, and typically repeats the cycle several times. The soft tick Ornithodoros moubata is widely distributed throughout drier parts of south and east Africa. It is an important disease vector, since it transmits the spirochaete Borrelia duttoni, causing African tick-borne relapsing fever in man, and African swine fever virus, which causes a highly lethal hemorrhagic disease of domestic swine [1,2].
Successful feeding of ticks depends on a cocktail of salivary proteins with antihaemostatic, antiinflammatory and immunomodulatory properties that are injected into the host [3]. The tick feeding site, which is highly modified by the pharmacologically active molecules, is the place where transmission of tick-borne pathogens occurs (for a recent review, see [4]). Much of the current vector-host research focuses on the discovery of novel components of tick saliva and on the description of their physiological functions, as this may aid the development of vaccines to control tick populations and the diseases they transmit. Moreover, compounds of tick saliva have clear therapeutic potential, which have, in some cases, been evaluated in animal models [5].
The saliva of ticks and other blood-feeding parasites contains a wide variety of peptidase inhibitors belonging to several structural families. Inhibitors from the cystatin family are small proteins that interact and block the active site of cysteine peptidases. Cystatins are found in all living organisms and regulate various biological processes, including the immune response (for a review, see [6]). They have been shown to play an important role in parasite-host interactions. Immunomodulatory effects were ascribed to cystatins of parasitic nematodes, e.g. Onchocerca volvulus [7], which inhibit antigen processing associated with class II MHC antigen presentation. Such interference reduces the proliferation of human peripheral blood mononuclear cells stimulated with a specific antigen [8].
Cystatins were found in the transcriptome of the salivary glands (sialome) of several hard tick species [9,10]. Silencing of a cystatin gene in the hard tick Amblyomma americanum by means of RNA interference significantly reduced the tick’s ability to feed successfully [11]. Recently, two salivary cystatins (Sialosatin L and L2) have been characterized in the hard tick Ixodes scapularis [12]. They displayed high affinity for cathepsins L and S, which are cysteine peptidases that play an important role in the processing of antigens by dendritic cells (DCs). Subsequent studies showed that maturation of DCs is significantly impaired in the presence of Sialostatin L [13], and that vaccination against Sialostatin L2 can lead to decreased feeding success of I. scapularis [14].
In this study, we focus on OmC2, a cystatin expressed in the salivary glands of the soft tick O. moubata [15]. First, we showed that OmC2 is secreted by the glands to form part of the saliva, using a proteomic approach. Second, we determined the crystal structure of OmC2, providing a structural basis for understanding its inhibitory specificity. Third, we demonstrated the immunomodulatory effect of OmC2 on antigen presenting cells, and evaluated the protein’s vaccine potential using an animal model.
EXPERIMENTAL
Animals
Mice
C57BL/6 and C3H/HeN female mice were from Charles River Laboratories, and transgenic female mice OTII were from the Jackson Laboratory. Mice aged 7 to 8 weeks were used. Animals were housed at 22 °C and a relative humidity of 65%. Experimental procedures complied with the rules of European Union and institutional guidelines on the use of experimental animals.
Ticks
O. moubata ticks were maintained in an established laboratory colony at the Institute of Parasitology using natural feeding on mice.
Tick saliva preparation
Saliva was obtained from fasting O. moubata females after injecting 1 μl of a 1% solution of pilocarpine hydrochloride in PBS into their genital pores. After stimulation, saliva was collected from the mouthpart using capillary tubes and stored at −80 °C.
Mass spectrometry proteomic analysis
LC-MS/MS analysis was performed on a hybrid mass spectrometer LTQ Orbitrap XL (Thermo) coupled to a 2D capillary LC system Rheos 2000 (Flux instruments). The first dimension column was a monolithic PS-DVB (200 μm × 10 mm, Dionex) and the second dimension column was a C18 PepMap 100 (75 μm × 150 mm × 3 μm, Dionex) with gradient elution in a 0.1% formic acid/acetonitrile system. The identification of OmC2 in tick saliva was accomplished in two steps. In the first step, the LC-MS/MS profile was obtained from a tryptic digest of recombinant OmC2. The DDA experiment consisted of one full MS scan (a resolution of 60000), and three MS/MS CID experiments of the most intensive ions from the full scan spectra. In the second step, a tryptic digest of the protein fraction of tick saliva was searched for the presence of OmC2-derived peptides. This analysis was limited to the major indicative peptides detected in step one. The applied method consisted of one full MS scan (a resolution of 60000), and five MS/MS CID experiments with HR/MS analysis of fragments in the Orbitrap (a resolution of 7500). The following parameters were used for the MS/MS experiments: collision energy 35 eV, isolation width 1, activation Q 250 and activation time 250 ms. Identification of the selected peptides (Supplementary Table S1) was confirmed by the analysis of retention time, mass accuracy and CID fragmentation spectra. The mass data were processed by the Bioworks (Thermo) and Peaks (Bioinformatics Solutions) software.
Expression and purification of OmC2
OmC2 was expressed in Sf9 insect cells (Gibco) using the flashBAC™ one-step baculovirus protein expression system (Oxford Expression Technologies). Briefly, OmC2 cDNA (GenBank accession no. AY547735) prepared as described [15] was cloned in the transfer vector pBacPAK9. Primers (forward: 5′-GGA GGA TCC ATG TCA AGT TTT AAG GTG G - 3′, reverse: 5′-GGA GCG GCC GCC TAA TGG TGA TGG TGA TGG TGT CCC TCA CAT CTG TAT GAC GTG) were designed to include the tick signal sequence for export from the cell and to add a carboxyterminal oligohistidine tag (-Gly-His6) to aid purification. The NotI and BamHI restriction sites were used for cloning into the transfer vector. The construct was amplified in XL1-Blue cells (Stratagene). Baculovirus containing the OmC2 gene was prepared by homologous recombination between the flashBAC and transfer vector DNA, following the manufacturer’s instructions. For expression, Sf9 cells were infected with high titre baculovirus construct virus stock, in a shaker incubator at 28°C. The recombinant protein was purified from the expression media by heparin (HiTrap™ Heparin HP, GE Healthcare) and Co2+ (TALON® Superflow™ Metal Affinity Resin, Clontech) affinity chromatography using the manufacturer’s protocol. This was followed by size-exclusion chromatography, using a Superdex 75 10/300 GL column (GE Healthcare) and a 15 mM HEPES, pH 7.2, 200 mM NaCl running buffer; OmC2 eluted close to the cytochrome c standard (12.4 kDa) indicating it is monomeric. The product was concentrated with a 5 kDa cut-off ultrafiltration unit (Sartorius). Purified protein was tested for endotoxin contamination by means of Limulus Amobecyte Lysate QCL-1000® (Lonza), following the manufacturer’s instructions. Endotoxin contamination did not exceed 0.1 EU/ml in any of the samples used for immunological assays used. The purified protein was stored at −80 °C.
Enzymatic assays
Inhibitory activity of recombinant cystatins, OmC2 and Sialostatin L (prepared according to [16]), was determined by continuous peptidase activity assays using specific fluorogenic substrates. The tested cysteine peptidases were as follows: human cathepsins H, S and L and human legumain (Calbiochem); papain and bovine cathepsins B and C (Sigma-Aldrich). The applied peptidase substrates were the following: N-carbobenzyloxy-Leu-Arg-7-amino-4-methylcoumarin (R&D Systems) for cathepsin B, L, and papain; N-carbobenzyloxy-Val-Val-Arg-7-amino-4-methylcoumarin, H-Gly-Arg-7-amino-4-methylcoumarin and N-carbobenzyloxy-Ala-Ala-Asn-7-amino-4-methylcoumarin (Bachem Bioscience) for cathepsins S and C and legumain, respectively; H-Arg-7-amino-4-methylcoumarin (Calbiochem) for cathepsin H. The assay system contained 0.25 mM substrate; cysteine peptidases were applied in the following concentrations: 0.04 nM papain, 1.0 nM legumain, 0.025 nM cathepsin L, 0.05 nM cathepsin S, 0.04 nM cathepsin C, 1.5 nM cathepsin H, and 1.3 nM cathepsin B. The assay buffer used was 0.1 M sodium acetate buffer, pH 5.5 (or 5.0 for legumain), 0.1 M NaCl, 1 mM EDTA, 1 mg/ml cysteine, and 0.005% Triton X-100. For aspartic and serine peptidases, these assay conditions were modified according to [16]. Apparent inhibition constants were determined essentially as described [16] by measuring the loss of enzymatic activity at increasing concentrations of inhibitor in the presence of a substrate in large excess. Briefly, each enzyme was preincubated for 10 min with the inhibitor, and subsequently the assay was initiated and developed by the addition of the corresponding substrate at 30°C. The linear hydrolysis rate of the substrate was followed for 20 min in a Spectramax Gemini XPS 96 well plate fluorescence reader (Molecular Devices) using 365 nm excitation and 450 nm emission wavelength with a cutoff at 435 nm. All experiments were performed in triplicate. Inhibition constants were calculated by non-linear regression.
Crystallization and data collection
Screening for crystallization conditions was performed using the Crystallization Basic and Extension Kits (Sigma-Aldrich) by hanging drop vapor diffusion technique. Preliminary crystals were obtained in 0.1 M HEPES buffer, pH 7.5, 1.5 M lithium sulfate. Optimal crystals of OmC2 were prepared at 20 °C using the hanging drop vapor diffusion technique at 24-well Nextal plates (Qiagen). The crystallization drop consisted of 1.25 μl of the OmC2 protein solution (3.5 mg/ml in 20 mM HEPES buffer, pH 7.2) and 0.75 μl of the reservoir solution (0.1 M HEPES buffer, pH 7.5, 1.5 M lithium sulfate). Crystals shaped as bi-pyramids reached their final size of 0.35 × 0.15 × 0.15 mm within 4 days.
For data collection, crystals were soaked gradually in reservoir solution supplemented with 10, 15 and 20 % (v/v) glycerol and flash cooled in liquid nitrogen. Diffraction data were collected at 100 K using the X12 EMBL beamline at DESY, Hamburg, Germany, and processed using the HKL-2000 suite of programs [17]. Crystals exhibited the symmetry of space group P3121 and contained two molecules in the asymmetric unit. Crystal parameters and data collection statistics are given in Supplementary Table S2.
Structure determination
The phase problem was solved by molecular replacement using program Molrep [20]. The search model was derived from the structure of Sialostatin L2 from I. scapularis (PDB code 3LH4, to be released) sharing 37 % sequence identity with OmC2. Model refinement was carried out using the program REFMAC 5.3 [21] from the CCP4 package [22]. Manual building was done using Coot [23]. Tight non-crystallographic symmetry (NCS) restraints were applied during initial refinement, and in later stages, NCS restraints were loosened as guided by the behavior of Rfree. The final refinement steps included TLS refinement [24]. The quality of the final models was validated with Molprobity [25]. Final refinement statistics is given in Supplementary Table S2. Figures showing structural representations were prepared with the program PyMOL [26]. The following services were used to analyze the crystal structure: DALI [27], and PISA server [28].
Isolation of immune cells
Dendritic cells (DCs): The spleen of a C57BL/6 mouse was dissected, minced with scissors, digested in RPMI 1640 medium containing 1 mg/ml collagenase-D (Roche) at 37 °C for 30 min, and passed through a 70 μm nylon cell strainer (BD Falcon). DCs were isolated using magnetic beads conjugated with anti-CD11c (N418) Ab and MACS Column (Miltenyi Biotec) separation following the manufacturer’s instructions. The purity of isolated DCs (~90% CD11c+ cells) was evaluated by flow cytometry. CD4+ T cells: Whole splenocytes from OTII mice were obtained by mechanical disruption of the spleen and washed three times in RPMI 1640 medium. Thereafter, CD4+ T cells were isolated by immunomagnetic separation using the Dynal Mouse CD4 Negative Isolation Kit (Invitrogen) following the instructions of the manufacturer. The purity of isolated CD4+ T cells (~90% CD4+ cells) was evaluated by flow cytometry (see below).
Quantification of cytokine production
Purified spleen DCs were cultured in 96-well plates, at a density of 1 × 105 cells per well, in 200 μl of culture media (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 50 μM 2-mercaptoethanol, 100 μg/ml penicillin, and 100 U/ml streptomycin). OmC2 was added to wells in 1–30 μg/ml final concentrations. After a 2 h incubation (37 °C, 5% CO2) DCs were activated with LPS (50 ng/ml). Cell free supernatant samples were collected 3 and 48 h later and analysed for the presence of TNF-α and IL-12, respectively, using Ready-SET-Go! ELISA kits (eBiosciences) according to the manufacturer’s instructions. Samples were tested in triplicate.
Antigen specific CD4+ T cell proliferation
DCs (3 × 104/well) isolated from the spleen of C57BL/6 mouse were preincubated (4 h) with culture medium (see above) in the presence or absence of OmC2 (1–30 μg/ml). After the preincubation period, CD4+ T cells (2 × 105/well) from OTII mouse were added to the culture, thereafter medium with or without EndoGrade Ovalbumin (Profos AG) (10 μg/ml) was added. The culture was incubated for 72 h at 37 °C, 5% CO2. Proliferation was assessed by adding Cell Counting Kit-8 reagent (Fluka) at 5% of the incubation volume in the last 4 h of the cultivation and evaluated by reading the supernatant absorbance at 490 nm. Samples were tested in triplicate.
Flow cytometry
Samples (0.5 × 106 cells) were incubated with CD4-specific MAb (conjugated with fluorescein isothiocyanate) and CD11c-specific MAb (conjugated with Alexa Fluor® 488) for CD4 T cells and DCs, respectively. Isotype control antibodies were used for appropriate controls. All reagents were obtained from eBiosciences. Labeled cell samples were analyzed on an Epics XL flow cytometer (Coulter) equipped with a 15-mW argon-ion laser with excitation capabilities at 488 nm using System II software (Coulter).
Vaccination with OmC2 and tick feeding success
A group of six C3H/HeN mice was vaccinated by two intraperitoneal injections of OmC2 supplemented with Freund’s adjuvant (Sigma-Aldrich). Briefly, 20 μg of OmC2 was administered with complete Freund’s adjuvant. The same dose of OmC2 was delivered with incomplete Freund’s adjuvant two weeks later. Samples of blood were taken from vaccinated mice ten days later and the presence of specific anti-OmC2 antibodies was assessed by ELISA. A control group of six C3H/HeN mice was vaccinated with ovalbumin (Sigma-Aldrich) using the same vaccination protocol. Thereafter, the ability of ticks to feed on these animals was tested. The mice were placed into small cages to reduce possibility of movement, and a plastic tube containing 6 to 7 O. moubata nymphs (of the first nymphal stage) was fixed to the tail of each mouse using Parafilm® M (Sigma-Aldrich). Whether engorgement occurred or not was assessed after 1 h, and monitored as a percentage of nymphs that were able to feed. Nymphs that fed were weighed. Survival of engorged nymphs to the next nymphal stage was assessed after 14 days.
Determination of specific anti-OmC2 antibody titer
ELISA based systems were used to detect specific anti-OmC2 antibodies in the sera of immunized and control mice. A flat bottom plate (Nunc) was coated with OmC2. 100 μl of coating buffer containing OmC2 (10 μg/ml) was added to each well and incubated overnight at 4°C. After washing two times with 0.05% Tween 20 in PBS (T-PBS), unoccupied sites were blocked with sample buffer (10% newborn calf serum in PBS) for 30 min at 37 °C. The plate was washed three times with T-PBS and wells were incubated with serial dilutions of the sera in sample buffer (100 μl final volumes), for 60 min at 37 °C. Subsequently the plate was washed three times with T-PBS and incubated with peroxidase-labeled goat anti-mouse antibodies (Sigma-Aldrich) diluted 1:1000 in sample buffer (100 μl/well) for 45 min at 37 °C. After washing three times with T-PBS, an enzymatic color reaction was generated using o-phenylenediamine (Sigma-Aldrich), following the manufacturer’s instructions. Enzymatic reactions were stopped after 10 min by adding 50 μl of 2 M H2SO4. Absorbance was measured at 490 nm with an ELISA spectrophotometer Multiskan MCC340 (Labsystem). Specific anti-OmC2 antibody titers were determined as the reciprocal value of the serum dilution that gave an absorbance read twice that of the negative control.
Statistical analysis
Differences in cytokine production and proliferation of T cells were analyzed by the Student t test. Homogeneity of variances assumption was tested by the Levene test. Data of OmC2 affected groups were compared to corresponding controls and P values of 0.05 or less were considered significant. Data of tick feeding success and survival were compared by means of the Fisher exact test. The link between the ability of nymphs to transform to the next stage and the anti-OmC2 antibody titer of infested mice was tested by the Spearman rank correlation (percentage data were arcsine transformed). In addition to conventional statistical significance, the effect size is reported. The effect size measures the magnitude of a treatment effect independent of sample size. Cohen’s d, which represents the difference between means divided by SD was used. The Cohen’s d was manually computed from the t value of the t test and r value of Spearman rank correlation. Small, medium and large effects for d are defined as 0.2, 0.5 and 0.8 [29]. All statistical analyses were conducted using Statistica 8.0 (StatSoft, Inc.).
RESULTS
Proteomic identification of OmC2 in tick saliva
Saliva collected from O. moubata adult females was subjected to proteomic analysis to directly determine presence or absence of OmC2. The applied LC-MS/MS strategy is based on enzymatic digestion of a complex protein mixture and MS/MS peptide sequencing (see Experimental). This analysis provided ~53% peptide coverage of the OmC2 sequence and mass accuracy <5 ppm (Supplementary Table S1) allowing us to conclude that OmC2 is secreted in the saliva of O. moubata.
Preparation of recombinant OmC2
The complete cDNA of OmC2 (GenBank accession no. AY547735) contains one ORF coding for 129 amino acids (including a 19-residue signal sequence), followed by a 1071-bp-long, 3′-untranslated region. OmC2 was prepared in insect cells as a recombinant protein with an oligohistidine-tag added to its C-terminus, using the baculovirus expression system. Predicted pI and MW values of the protein including the tag were 6.2 and 13115 Da, respectively. The protein was purified to homogeneity from the expression media using a three step chromatographic procedure (see Experimental). Purified OmC2 migrated as a single band of 13 kDa on reducing SDS-PAGE. The identity of the purified protein was confirmed by LC-MS/MS analysis (peptide coverage 100%).
Inhibitory specificity of OmC2
The purified recombinant OmC2 was screened in vitro for its inhibitory activity against a panel of cysteine peptidases. The inhibitory profile of OmC2 is summarized in Table 1, where it is compared with that of Sialostatin L, a salivary cystatin from the hard tick I. scapularis.
Table 1. Inhibitory effect of OmC2 on the activity of cysteine peptidases.
The inhibitory potency of OmC2 from the soft tick O. moubata is compared with that of sialostatin L (SialoL) from the hard tick I. scapularis. The IC50 values (mean values ±SE) were determined by the peptidase activity assays using specific fluorogenic substrates. The MEROPS database classification (http://merops.sanger.ac.uk) of the cysteine peptidases tested (clan) and their specificity (activity mode) are given. NI, no significant inhibition at 10 μM inhibitor concentration.
| Enzyme | Enzyme specificity, classification | OmC2 | SialoL |
|---|---|---|---|
| Inhibition IC50(nM) | |||
| papain | endopeptidase, CA | 0.164±0.003 | 0.548±0.037 |
| cathepsin L | endopeptidase, CA | 0.146±0.016 | 0.178±0.005 |
| cathepsin S | endopeptidase, CA | 0.149±0.015 | 0.559±0.026 |
| cathepsin C | dipeptidyl peptidase, CA | 1.07±0.12 | 314±5 |
| cathepsin H | aminopeptidase, CA | 1.21±0.01 | NI |
| cathepsin B | peptidyl dipeptidase/endopeptidase, CA | 8.81±0.52 | NI |
| legumain | endopeptidase, CD | NI | NI |
In a first step, we demonstrated that both cystatins interact with peptidases of the CA but not the CD clan of the cysteine peptidase class. This was tested with papain and legumain, which are representative members of the CA and CD clan, respectively, and archetypes in cystatin research (Table 1) [30]. The activity of aspartic (cathepsin D) or serine peptidases (cathepsin G and trypsin) was not affected by either tick cystatin (data not shown).
In a second step, a set of papain-type peptidases (family C1, clan CA) of mammalian origin was screened, including: cathepsins L and S (endopeptidases), cathepsin B (a peptidyl dipeptidase and endopeptidase), cathepsin C (dipeptidyl peptidase I) and cathepsin H (an aminopeptidase). These enzymes were selected to cover a wide range of endo- and exopeptidase cleavage specificities. OmC2 inhibited all these peptidases, with IC50 values ranging from about 0.15 to 8.9 nM. Inhibition of endopeptidase cathepsins L and S by OmC2 was similar to that of Sialostatin L (subnanomolar IC50 values), whereas, OmC2 was a far more potent inhibitor than Sialostatin L of the exopeptidases cathepsin B, C and H (Table 1). Three hundred times more Sialostatin L than OmC2 was needed to inhibit Cathepsin C based on IC50 ~314 and ~1.07 nM, respectively. Moreover, even in high molar excess, Sialostatin L did not block cathepsins B and H, while nanomolar IC50 values characterized the interaction of OmC2 with these peptidases.
Three dimensional structure of OmC2
The crystal structure of OmC2 was determined by molecular replacement using the structure of Sialostatin L2 as a search model, and refined using data to 2.45 Å resolution (Supplementary Table S2). The hexagonal crystal form contains two molecules in the asymmetric unit with a relatively high solvent content of 65.4%. All protein residues could be modeled into a well defined electron density map (Figure S1) with the exception of the first two N-terminal residues (Thr1 and Ser2). Due to its inherent disorder, the C-terminal oligohistidine tag (except the proximal residue) is also missing in the crystallographic model. The final OmC2 model consists of two molecules of OmC2, each containing 108 protein residues. The root mean square deviation (RMSD) for superposition of the main-chain atoms of the two molecules is 0.62 Å, a value within the range observed for different crystal structures of identical proteins [31]. Minor structural changes are localized in the loop regions, which are exposed to solvent and/or involved in crystal contacts (residues 68–72, 48–52 and 95–99).
Figure 1a shows the overall structure of OmC2. The molecule adopts a typical cystatin fold similar to that of vertebrate homologues, characterized by a five-stranded twisted antiparallel β-sheet, which wraps around an α-helix. OmC2 contains two conserved disulfide bridges connecting Cys65 with Cys77, and Cys88 with Cys108 (Figure 2a). The sequence alignment together with a structural comparison with known cystatin structures clearly demonstrated that OmC2 belongs to family 2 of the cystatin superfamily (Figure 2a and b). The closest structural homolog of OmC2 is Sialostatin L2 from the hard tick I. scapularis, the structure of which was recently determined (PDB code 3LH4, to be released): the RMSD is 1.25 Å (for 99 aligned Cα atoms), and the sequence identity is 37%. A lower structural similarity was found to family 2 cystatins from vertebrates, namely human cystatin F (PDB code 2CH9, RMSD ~2.0 Å), chicken egg white cystatin (PDB code 1YVB, RMSD ~2.6 Å), and human cystatin D (PDB code 1RN7, RMSD ~2.8 Å). The sequence identity of OmC2 to representative members of this group is in the range of 13–24% (Figure 2a), with the highest values found for salivary S-type cystatins (represented by human cystatin SN in Figure 2a).
Figure 1. Crystal structure of OmC2.
(a) A cartoon representation of the protein main-chain colored by secondary structure elements (α1 cyan, β1–5 magenta). The N- and C-termini, and two disulfide bridges Cys65-Cys77 and Cys88-Cys108 (yellow sticks) are indicated. The hairpin loops L1 and L2, and the N-terminus of cystatins are involved in binding of papain-type peptidases. (b) Top view on the solvent accessible surface of the OmC2 molecule showing the binding site for papain-type peptidases. In the left figure, the N-terminus and L1 and L2 loops are colored green, yellow and orange, respectively. In the right figure, the surface is colored by its electrostatic potential displayed at a scale from − 2 kT (red) to + 2 kT (blue).
Figure 2. Comparison of the structure of OmC2 with other family 2 cystatins.
(a) Sequence alignment of OmC2 (from the soft tick O. moubata) with Sialostatins (SialoL and SialoL2 from the hard tick I. scapularis), chicken egg white cystatin (CEW) and representative human members of family 2 cystatins (cystatins SN, D, C, E/M and F). An initial alignment was made using ClustalW and then adjusted using structural alignments where available. Residues identical to those of OmC2 are shaded in gray or black (fully conserved). The secondary structure elements of OmC2 are depicted as in Fig. 1a (magenta for strands, cyan for helices); the L1 and L2 loops are labeled. The conserved disulfide bridges are indicated by the connecting lines. Three conserved regions involved in the interaction with papain-type peptidases are boxed in green [32], and the putative legumain binding site in four cystatins is highlighted in red [30]. The top line gives residue numbers for mature OmC2. Residue numbering at right gives mature protein sequences used for the alignment (the activation processing of cystatin F is included [33]). (b) Stereo image showing a superposition of Cα traces of OmC2 with four other cystatin structures. The tick salivary cystatins OmC2 and SialoL2 (PDB code 3LH4, to be released) are colored magenta and orange, respectively. Chicken egg white cystatin (PDB code 1CEW) and human cystatins F (2CH9) and D (1RN7) are shown in green, grey and cyan, respectively. Positions of the binding sites for papain-type peptidases and legumains are indicated.
Interaction of the family 2 cystatins with papain-type peptidases is mediated by three regions, the N-terminal segment and two hairpin loops L1 and L2 (Figure 2a), which form a tripartite wedge-shaped edge that binds to the enzyme active-site cleft [32] (Figure 1b). The first part of the binding site is formed by the N-terminal segment extending to a totally conserved Gly5 residue (Figure 2a). Orientation of this region in OmC2 suggests conformational flexibility, as seen in vertebrate cystatins (Figure 2b). Thus Gly5 can function as a hinge that allows the flexible N-terminal segment to adopt a conformation that is optimal for target enzyme binding. The loop L1 of OmC2 (between β2 and β3) is quite similar in conformation to other cystatin structures and contains the conserved sequence motif Gln-Xaa-Val-Xaa-Gly (Figure 2a). The loop L2 (between β4 and β5) is characterized in OmC2 and vertebrate cystatins by the presence of a conservative Pro-Trp residues; this motif is, however, missing in sialostatins, where the L2 loop is shortened by a one-residue deletion (Figure 2a). Furthermore, in contrast to the other cystatins depicted in Figure 2b, the N-terminal segment of Sialostatin L2 is tightly packed against the β-sheet and stabilized there by a net of interactions.
Several members of the cystatin family 2 are able to inhibit legumain, and the legumain binding site was localized at critical residue Asn31 [30]. This functional motif is absent in the OmC2 structure (Figure 2a), which is in line with the fact that OmC2 does not suppress legumain activity (Table 1).
Effects of OmC2 on host immune cells
OmC2 decreases levels of TNF-α and IL-12 produced by lipopolysaccharide (LPS) activated DCs
The influence of OmC2 on cytokine production of LPS-activated DCs was tested. DCs are an important subset of immunocompetent cells that typically initiate the development of the innate and adaptive immune response by producing cytokines and presenting foreign antigen to T cells. LPS (a component of the outer membrane of Gram-negative bacteria) activates DCs by binding to Toll-like receptor 4 of these cells. TNF-α and IL-12 are pro-inflammatory cytokines produced by DCs upon LPS-activation [34]. The levels of these cytokines in culture supernatants were measured by capture ELISA. The presence of OmC2 (10 μg/ml) suppressed (by 20%) the production of TNF-α in the culture (t-test, p=0.043; d=2.9) after a 3 h incubation (Figure 3a). Lower concentrations of OmC2 did not cause a significant reduction of TNF-α. A similar suppressive effect of OmC2 was observed on the production of IL-12 (Figure 3b). At the highest concentration tested, OmC2 (30 μg/ml) was able to partially inhibit (by 25%) the production of this cytokine by LPS-activated DCs following a 48 h incubation period (t-test, p= 0.051; d=2.8). OmC2 had no suppressive effect on IL-6 production by LPS-activated DCs (data not shown).
Figure 3. Effect of OmC2 on TNF-α (a) and IL-12 (b) production by LPS-stimulated dendritic cells.
Enriched CD11+ cells (105/well) were preincubated with OmC2 for 2 h and subsequently stimulated with LPS (50 ng/ml). Supernatants were collected 3 h and 48 h later for TNF-α and IL-12 quantification, respectively. Data are shown as mean ±SD, * indicates statistical significance (p<0.05) versus the LPS-activated control group.
OmC2 reduces DC-mediated proliferation of naive CD4+ T cells
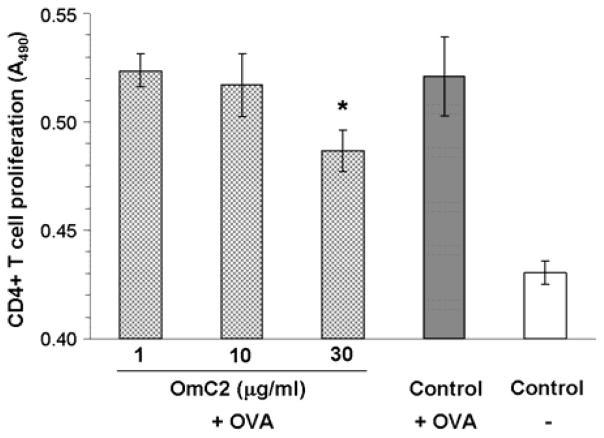
The effect of OmC2 on the development of adaptive immunity was assessed in this experiment. The antigen-dependent T cell proliferation induced by DCs presenting a specific antigen was tested using CD4+ T lymphocytes from OTII mice. The T cell receptor of these transgenic mice specifically recognizes the ovalbumin (OVA) peptide 323–339 presented by their DCs. DC presentation of the OVA peptide in complex with the MHC II protein leads to the proliferation of the transgenic CD4+ T cells which was measured using a chromogenic substrate. Partial, statistically significant, inhibition of CD4+ T cell proliferation (by 38%) was observed in culture wells containing OmC2 (30μg/ml) at 72 h of incubation (t-test, p=0.03; d=2.18). Lower concentrations of OmC2 did not cause a significant reduction of CD4+ T cell proliferation (Figure 4).
Figure 4. Effect of OmC2 on antigen specific CD4+ T cell proliferation.
Enriched CD11c+ cells (3 × 104/well) were preincubated with OmC2 (concentrations as indicated) for 4 h, before adding CD4+ T cells (2 × 105/well) from transgenic OTII mice together with ovalbumin (OVA) (10 μg/ml). The culture was incubated for 72 h and the cell proliferation was assessed using a chromogenic assay (A490). Data are shown as mean ±SD, * indicates statistical significance (p<0.05) versus the OVA activated controls.
OmC2 vaccination decreases tick feeding success
The effect of vaccinating mice with recombinant OmC2 on the feeding success of O. moubata ticks was assessed. The first nympal feeding stage of O. moubata and an inbred mouse strain (C3H/HeN) were used. Feeding success on OmC2 immunized and control mice was evaluated using three criteria: (i) the ability of nymphs to engorge, (ii) the weight of engorged nymphs, (iii) the survival of engorged nymphs to the next developmental stage. Six mice were immunized with OmC2 and six mice (the control group) with ovalbumin. Ten days after the last injection, sera were collected and tested by ELISA for anti-OmC2 antibodies. Anti-OmC2 reciprocal titers ranged from 1× 103 to 512 × 103 with an average of 203 × 103. No anti-OmC2 antibodies could be detected in the sera of the control mice. Subsequently 6 to 7 O. moubata nymphs were allowed to feed on each of these mice. The analysis of their ability to engorge showed that 62.5% of ticks (25 out of 40 ticks) were able to feed on OmC2-immunized mice compared to 78% of ticks (32 out of 41 ticks) fed on control mice (Fisher exact test, p=0.149). Similarly, there was no significant difference in the weight of engorged nymphs (the second criterion for feeding success) fed on OmC2-immunized mice (1.7±0.5 mg) or control mice (1.8±0.3 mg) (t-test, p=0.55; d=0.24). The last criterion for assessment of feeding success was the survival of engorged nymphs to the next developmental stage. All ticks fed on control animals were able to moult to the second nymphal stage. By contrast, only 76% of ticks fed on OmC2-immunized animals survived and successfully developed to the next stage. Thus, the mortality rate in the group of ticks fed on OmC2-immunized animals (24 %) was significantly higher than in the control group (no mortality) (Fisher exact test, p=0.005). The results from the vaccination experiment are summarized in Figure 5a. Only 47.5% of the total ticks initially allowed to feed on OmC2-immunized animals were able to successfully reach the second nymphal stage, compared to 78% of the ticks feeding on control animals, illustrating a significant decrease in the feeding success of OmC2-immunized animals (p=0.006). Detailed statistical analysis of tick survival showed that the ability of nymphs to survive and develop into the next stage was negatively correlated with the anti-OmC2 titer in the blood of the host mice (Spearman rank correlation, N=12, rs=−0.66, p=0.019; d=1.8). Ticks fed on the mouse with the highest level of anti-OmC2 antibodies (titer 512 × 103) had the highest mortality (83.3%); only 1 of 6 engorged ticks was able to survive and transform into the next stage (16.7 % success rate) (Figure 5b).
Figure 5. Effect of vaccination with OmC2 on tick feeding success.
(a) Summary of the results of the vaccination experiment with OmC2. Mice and the first nymphal stage of O. moubata ticks were used as a host-parasite infestation model. The ability of ticks to engorge and survive to the next developmental stage is shown as percentages. OmC2: group of ticks fed on OmC2-immunized mice; OVA: group of ticks fed on control (ovalbumin-immunized) mice. (b) Relationship between the survival of O. moubata ticks and anti-OmC2 antibody titers in infested mice. Column height indicates the percentage of engorged nymphs that survived and were able to develop from the first to the second nymphal stage (left y axis). The ratio of surviving to total number of nymphs that engorged is given above the columns (grey, OmC2; open, OVA). The anti-OmC2 reciprocal antibody titer (×1000, right y axis) in each mouse is indicated by the numbers above the black squares.
DISCUSSION
The present study characterizes OmC2 present in the saliva of the soft tick O. moubata as a cystatin with an unusually broad specificity. The high affinity for cysteine cathepsins with endopeptidase and exopeptidase activities clearly distinguishes the soft-tick derived OmC2 from the hard-tick derived Sialostatins, which display weak or no inhibition of these exopeptidases [12]. The highest overall similarity of OmC2, on the level of tertiary (as well as primary) structure, is to Sialostatin L2. However, local structural differences occur at the N-terminus and the L2 loop, segments that determine the cystatin’s affinity and selectivity [35,36], and these are very likely the reason why OmC2 targets both endo- and exopeptidases, whilst Sialostatins preferentially target endopeptidases. Interestingly, the structures of the N-terminus and the L2 loop in OmC2 resemble the corresponding segments in vertebrate cystatins. In accordance with this, the broad specificity of OmC2 is similar to that of some vertebrate cystatins, e.g. human cystatin C (for review, see [37]). We hypothesize that the soft-tick derived OmC2 mimics specific host-derived cystatin(s) to interfere with its/their in vivo functions in controlling cathepsin-mediated proteolysis.
Mammalian papain-type cathepsins play an essential role in a variety of immunological mechanisms. The biochemical properties of OmC2 that we have investigated help us to suggest aspects of the host’s immune system that the inhibitor may affect. Cathepsins S and L are critically important for the processing of antigens by antigen presenting cells and for their presentation in complex with MHC II proteins, mechanisms that are required for the development of an adaptive immune response. In addition, these peptidases interact with the invariant chain of MHC II and are responsible for its degradation [38]. We studied the ex vivo effect of OmC2 on DCs, professional antigen presenting cells, as they are an important resident cell type in the skin, where the tick feeding takes place. The observed inhibition of antigen presentation and suppression of TNF-α and IL-12 production agrees with the recent finding that Sialostatin L suppresses DCs through the inhibition of cathepsin S [13]. TNF-α and IL-12 are important messengers between immunocompetent cells and reducing their production may impair a normal host immune response. Thus, by suppressing DC activation and antigen presentation, OmC2 can suppress the recognition of salivary antigens secreted into the host by O. moubata ticks. Manipulating the adaptive immunity may greatly facilitate repeated feeding by ticks and their offspring on the same host.
OmC2 was also a potent inhibitor of cathepsins B and H, which are lysosomal exopeptidases involved in intracellular protein degradation. They contribute to the variable peptide length of MHC II-bound peptides by trimming their N- and C-termini [39]. Thus, by blocking these cathepsins, OmC2 may further suppress the recognition of tick saliva antigens. OmC2 also strongly inhibits cathepsin C (dipeptidyl peptidase I) which is a key component of the innate immunity where it activates serine proteases expressed by mature neutrophils [40]. Neutrophils are a major population of immunocompetent cells in the blood that are part of the first line of defence in cellular immunity. By inhibiting cathepsin C OmC2 may combat an immediate, neutrophil-driven reaction against feeding ticks. It should be mentioned that cathepsin C is also involved in the activation of granzymes of cytotoxic T-lymphocytes and natural killer cells [41]. These immune mechanisms take some time to develop and soft ticks feed for a short period only. However, they may be relevant when ticks feed on a host whose immune system has been primed by the feeding of other ticks.
The vaccination experiments indicated a role of OmC2 in blood feeding, as the presence of anti-OmC2 antibodies in the blood of immunized animals reduced the tick feeding success. Interestingly, the weight of O. moubata nymphs was not lower when they were fed on immunized animals rather than on control animals. Immunization of guinea pigs with Sialostatin L2, on the other hand, not only promoted rejection of I. scapularis nymphs but also led to a reduction in their weight [14]. This difference could be explained by a different uptake of, and exposure to host antibodies reflecting the specific feeding patterns of soft and hard ticks.
The principal result of our vaccination study was the finding that O. moubata nymphs feeding on the OmC2-immunized host showed significantly increased post engorgement mortality that was linked to the amount of specific anti-OmC2 antibodies in the host serum. We previously reported that OmC2 is expressed not only in the salivary glands, but also in the gut of the ticks, where it is secreted into the lumen [15]. Ingested blood is stored in the tick gut lumen, while digestion takes place in the gut epithelium [42]. We speculated that OmC2 can protect the stored blood meal against undesired proteolysis by endogenous peptidases that are released upon lysis of digestive cells [15]. However, exogenous peptidases may also be present in the ingested blood (including cathepsins derived from immunocompetent cells) and could be targeted by OmC2. Anti-OmC2 antibodies in the blood of sensitized hosts may block the physiological function of OmC2 in the tick gut, thereby contributing to the observed mortality of engorged O. moubata nymphs.
Since OmC2 occurs both in the saliva and the gut of the tick, a vaccine based on this protein may have dual activity, and may therefore be more efficient. Specific anti-OmC2 antibodies could neutralize OmC2 that is secreted into the host (interfering with its immunomodulatory function), as well as OmC2 that is expressed in the gut (interfering with a putative role in the regulation of digestion). A ‘dual vaccine’, which recognizes both exposed and concealed antigens, has been developed, on the basis of a tick cement protein. This vaccine, reportedly, protects the host against the transmission of tick-borne encephalitis virus from the hard tick I. ricinus [43].
In summary, this study provides a comprehensive structure-function analysis of the salivary cystatin OmC2 from the soft tick O. moubata. This cystatin differs in its inhibitory profile and in its three-dimensional structure from the other salivary cystatins described in ticks. We demonstrate that OmC2 acts upon immunocompetent cells, and that it has potential as an anti-tick vaccine that could be used to control O. moubata populations and combat the spread of associated diseases.
Supplementary Material
Acknowledgments
We are grateful to Markéta Ondračková for help with statistical analysis. Diffraction data were collected at beam line X12, EMBL-Hamburg outstation (at DESY, Hamburg, Germany).
FUNDING
This work was supported by the Grant Agency of the Academy of Sciences of the Czech Republic [grant numbers KJB500960702, IAA600960811] and the Grant Agency of the Czech. Republic [grant number P207/10/2183], by Research Center LC06009 and research projects Z60220518, Z40550506 and MSM-123100003, and by the UK Natural Environment Research Council.
Abbreviations used
- DCs
dendritic cells
- IL-12
interleukin 12
- TNF-α
tumor necrosis factor alpha
Footnotes
AUTHOR CONTRIBUTION
Jiří Salát was responsible for protein expression and biological experiments. Guido C. Paesen, Miles A. Nunn, Juraj Majtán, Lenka Grunclová and Petr Kopáček participated in protein production. Helena Horká and Jan Kopecký participated in the biological studies. Michalis Kotsyfakis, Zuzana Kovářová and Martin Horn performed the biochemical characterization; Miloslav Šanda performed the proteomic analysis. Zuzana Kovářová, Pavlína Řezáčová, Jiří Brynda and John F. Andersen were involved in the crystallization and structure determination. Jiří Salát and Michael Mareš designed the project and wrote the manuscript.
Atomic coordinates and experimental structure factors have been deposited in the Protein Data Bank with the accession code 3L0R.
References
- 1.Varma MG. Infections of Ornithodororos tick with relapsing fever spirochaetes, and the mechanisms of their transmission. Ann Trop Med Parasitol. 1956;50:18–31. doi: 10.1080/00034983.1956.11685735. [DOI] [PubMed] [Google Scholar]
- 2.Dixon LK, Abrams CC, Bowick G, Goatley LC, Kay-Jackosn PC, Chapman D, Liverani E, Nix R, Silk R, Zhang FQ. African swine fever virus proteins involved in evading host defence systems. Immunopathology. 2004;100:117–134. doi: 10.1016/j.vetimm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Bowman AS, Coons LB, Needham GR, Sauer JR. Tick saliva: recent advances and implications for vector competence. Med Vet Entomol. 1997;11:277–285. doi: 10.1111/j.1365-2915.1997.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 4.Francischetti IMB, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coullin I, Maillet I, Vargaftig BB, Jacobs M, Paesen GC, Nuttall PA, Lefort J, Moser R, Weston-Davies W, Ryffel B. Arthropod-derived histamine binding protein prevents murine allergic asthma. J Immunol. 2004;173:3281–3286. doi: 10.4049/jimmunol.173.5.3281. [DOI] [PubMed] [Google Scholar]
- 6.Turk V, Stoka V, Turk D. Cystatins: Biochemical and structural properties, and medical relevance. Front Biosci. 2008;13:5406–5420. doi: 10.2741/3089. [DOI] [PubMed] [Google Scholar]
- 7.Schonemeyer A, Lucius R, Sonnenburg B, Brattig N, Sabat R, Schilling K, Bradley J, Hartmann S. Modulation of human T cell responses and macrophage functions by onchocystatin, a secreted protein of the filarial nematode Onchocerca volvulus. J Immunol. 2001;167:3207–3215. doi: 10.4049/jimmunol.167.6.3207. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann S, Lucius R. Modulation of host immune response by nematode cystatins. Int J Parastitol. 2003;33:1291–1302. doi: 10.1016/s0020-7519(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 9.Valenzuela JG, Francishetti IMB, Pham VM, Garfield MK, Mather TN, Ribeiro JMC. Exploring the sialome of the tick Ixodes scapularis. J Exp Biol. 2002;205:2843–2864. doi: 10.1242/jeb.205.18.2843. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro JM, Alarcon-Chaidez FB, Francishetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Karim S, Miller NJ, Valenzuela J, Sauer JR, Mather TN. RNAi-mediated gene silencing to assess the role of synaptobrevin and cystatin in tick blood feeding. Biochem Biophys Res Commun. 2005;334:1336–1342. doi: 10.1016/j.bbrc.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 12.Kotsyfakis M, Karim S, Andersen JF, Mather TN, Ribeiro JMC. Selective cysteine protease inhibition contributes to blood-feeding success of the tick Ixodes scapularis. J Biol Chem. 2007;282:29256–29263. doi: 10.1074/jbc.M703143200. [DOI] [PubMed] [Google Scholar]
- 13.Sa-Nunes A, Bafica A, Antonelli LR, Choi EY, Francischetti IMB, Andersen JF, Shi G, Chavakis T, Ribeiro JMC, Kotsyfakis M. The immunomodulatory action of Sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J Immunol. 2009;182:7422–7429. doi: 10.4049/jimmunol.0900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotsyfakis M, Anderson JM, Andersen JF, Calvo E, Francischetti IMB, Mather TN, Valenzuela JG, Ribeiro JMC. Cutting edge: Immunity against a “Silent” salivary antigen of the Lyme vector Ixodes scapularis impairs its ability to feed. J Immunol. 2008;181:5209–5212. doi: 10.4049/jimmunol.181.8.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grunclova L, Horn M, Vancova M, Sojka D, Franta Z, Mares M, Kopacek P. Two secreted cystatins of the soft tick Ornithodoros moubata: differential expression pattern and inhibitory specificity. Biol Chem. 2006;387:1635–1644. doi: 10.1515/BC.2006.204. [DOI] [PubMed] [Google Scholar]
- 16.Kotsyfakis M, Sa-Nunes A, Francischetti IM, Mather TN, Andersen JF, Ribeiro JM. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem. 2006;281:26298–26307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- 17.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution--from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 18.Brunger AT. Free R-value - a novel statistical quantity for assessing the accuracy of crystal-structures. Nature. 1992;355:472–475. doi: 10.1038/355472a0. [DOI] [PubMed] [Google Scholar]
- 19.Laskowski RA, Macarthur MW, Moss DS, Thornton JM. Procheck - a program to check the stereochemical quality of protein structures. J Appl Crystal. 1993;26:283–291. [Google Scholar]
- 20.Vagin A, Teplyakov A. An approach to multi-copy search in molecular replacement. Acta Crystallogr D Biol Crystallogr. 2000;56:1622–1624. doi: 10.1107/s0907444900013780. [DOI] [PubMed] [Google Scholar]
- 21.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 22.CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 23.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 24.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr D Biol Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 25.Lovell SC, Davis IW, Arendall WB, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 26.DeLano WL. The PyMOL User’s Manual. DeLano Scientific; Palo Alto: 2002. [Google Scholar]
- 27.Holm L, Sander C. Searching protein structure databases has come of age. Proteins. 1994;19:165–173. doi: 10.1002/prot.340190302. [DOI] [PubMed] [Google Scholar]
- 28.Krissinel E, Henrick K. Detection of protein assemblies in crystals. In: Berthold MR, Glen R, Diederichs K, Kohlbacher O, Fischer I, editors. CompLife. Springer-Verlag; Berlin: 2005. pp. 163–174. [Google Scholar]
- 29.Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Earlbaum Associates; Hillsdale: 1988. pp. 8–108. [Google Scholar]
- 30.Alvarez-Fernandez M, Barrett AJ, Gerhartz B, Dando PM, Ni J, Abrahamson M. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem. 1999;274:19195–203. doi: 10.1074/jbc.274.27.19195. [DOI] [PubMed] [Google Scholar]
- 31.Betts MJ, Sternberg MJ. An analysis of conformational changes on protein protein association: implications for predictive docking. Protein Eng. 1999;12:271–283. doi: 10.1093/protein/12.4.271. [DOI] [PubMed] [Google Scholar]
- 32.Stubbs MT, Laber B, Bode W, Huber R, Jerala R, Lenarcic B, Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990;9:1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton G, Colbert JD, Schuettelkopf AW, Watts C. Cystatin F is a cathepsin C-directed protease inhibitor regulated by proteolysis. EMBO J. 2008;27:499–508. doi: 10.1038/sj.emboj.7601979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaisho T, Akira S. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 2001;22:78–83. doi: 10.1016/s1471-4906(00)01811-1. [DOI] [PubMed] [Google Scholar]
- 35.Hall A, Håkansson K, Mason RW, Grubb A, Abrahamson M. Structural basis for the biological specificity of cystatin C. Identification of leucine 9 in the N-terminal binding region as a selectivity-conferring residue in the inhibition of mammalian cysteine peptidases. J Biol Chem. 1995;270:5115–5121. doi: 10.1074/jbc.270.10.5115. [DOI] [PubMed] [Google Scholar]
- 36.Hall A, Ekiel I, Mason RW, Kasprzykowski F, Grubb A, Abrahamson M. Structural basis for different inhibitory specificities of human cystatins C and D. Biochemistry. 1998;37:4071–4079. doi: 10.1021/bi971197j. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson DP. Salivary (SD-type) cystatins: over one billion years in the making--but to what purpose? Crit Rev Oral Biol Med. 2002;13:485–508. doi: 10.1177/154411130201300606. [DOI] [PubMed] [Google Scholar]
- 38.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 39.Chapman HA. Endosomal proteases in antigen presentation. Curr Opin Immunol. 2006;18:78–84. doi: 10.1016/j.coi.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Adkison AM, Raptis SZ, Kelley DG, Pham CT. Dipeptidyl peptidase activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J Clin Invest. 2002;109:363–371. doi: 10.1172/JCI13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lara FA, Lins U, Paiva-Silva G, Almeida IC, Braga CM, Miguens FC, Oliveira PL, Dansa-Petretski M. A new intracellular pathway of haem detoxification in the midgut of the cattle tick Boophilus microplus: aggregation inside a specialized organelle, the hemosome. J Exp Biol. 2003;206:1707–1715. doi: 10.1242/jeb.00334. [DOI] [PubMed] [Google Scholar]
- 43.Labuda M, Trimnell AR, Lickova M, Kazimirova M, Davies GM, Lissina O, Hails RS, Nuttall PA. An antivector vaccine protects against a lethal vector-borne pathogen. Plos Pathog. 2006;2:251–259. doi: 10.1371/journal.ppat.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.