Abstract
Ticks developed a multitude of different immune evasion strategies in order to obtain a blood meal. Sialostatin L is an immunosuppressive cysteine protease inhibitor present in the saliva of the hard tick Ixodes scapularis. Herein we demonstrate that sialostatin L strongly inhibits the production of IL-9 by Th9 cells. Since we could show recently that Th9-derived IL-9 is essentially involved in the induction of asthma symptoms, sialostatin L was used for the treatment of experimental asthma. Application of sialostatin L in a model of experimental asthma almost completely abrogated airway hyperresponsiveness and eosinophilia. Our data suggest that sialostatin L can prevent experimental asthma, most likely by inhibiting the IL-9 production of Th9 cells. Thus, alternative to IL-9 neutralization sialostatin L provides the basis for the development of innovative therapeutic strategies to treat asthma.
Keywords: Th9 cells, Lung, Allergy, Parasites, Rodents
Introduction
When feeding on their hosts, hard ticks face the problem of host inflammation and immunity. Therefore, the feeding success of hard ticks depends on an array of immune suppressive substances in tick saliva, among others cysteine protease inhibitors (cystatins), which have been demonstrated to have strong immunomodulatory activity (1, 2). Cystatins from various ectoparasites interfere with antigen processing and presentation, phagocytosis, modulate cytokine expression, and nitric oxide production, and thereby impair the immune response (3). The salivary cystatin sialostatin L (sialoL) from the tick Ixodes scapularis inhibited the proliferation of the mouse T cell line CTLL-2, and reduced an inflammatory reaction (footpad swelling) induced by carrageenan (1). Furthermore, it prevented maturation of dendritic cells and inhibited proliferation of antigen-specific CD4+ T lymphocytes due to inhibition of cathepsin S that results in prevention of invariant chain degradation in dendritic cells (2). Recently, a cystatin from parasitic nematodes has been shown to reduce allergic and inflammatory responses (4). This filarial cystatin suppressed Th2-related inflammation and the Th2-mediated asthmatic disease in a murine model of OVA-induced allergic airway hyperresponsiveness (AHR). In addition, eosinophil recruitment was inhibited, the level of IgE was reduced, IL-4 production downregulated and allergic airway hyperreactivity suppressed.
Experimental allergic asthma was shown to rely on a combination of cytokines (IL-4, IL-5, IL-9, IL-13) that were initially thought to be of Th2 cell origin. Concerning IL-9, it has meanwhile been shown that this cytokine can also be produced by mast cells, eosinophils and, very recently, by Th9 cells (5–7). Obviously, IL-9 can be produced by cell types closely associated with induction and maintenance of allergic diseases. Additionally, IL-9 was termed a candidate gene for asthma since it was found by linkage analyses to reside within a cytokine gene cluster together with IL-3, IL-4, and IL-5 on human chromosome 5 (8). In line with these findings, IL-9 as a pleiotropic cytokine, has several activities that favor the development of allergic asthma (9, 10). IL-9 enhances the IL-4-mediated production of IgE in human and murine B cells (11), promotes eosinophil maturation in synergy with IL-5 (12), and stimulates mucin transcription in respiratory epithelial cells (13). Studies on IL-9 transgenic mice (14) and on recombinant IL-9 instilled into airways (15) showed that IL-9 is sufficient to produce a classical Th2-like response in vivo, and upregulates airway hyperresponsiveness (AHR), lung eosinophilia, and serum total IgE. Administration of IL-9-neutralizing antibody ablated an OVA-induced asthmatic response (16) and phase I clinical trials have been started using anti-IL-9 antibodies (17). Recently, we have shown that IL-9 from Th9 cells induces and maintains asthmatic symptoms (7).
Herein we describe a novel mechanism of immunomodulation in which tick cystatin (sialostatin L) inhibits host hypersensitivity by suppression of Th9 cell-derived IL-9 production. Accordingly, sialoL inhibits asthmatic symptoms in a model of experimental asthma.
Materials and Methods
Mice
BALB/c RAG1−/− mice were obtained from Jackson Lab (Bar Harbor). BALB/c and DO11.10 mice, transgenic for the OVA323–339-specific TCR-αβ (18), were bred in our animal facility (Mainz). Males and females were used at the age of 6–12 weeks. Animal procedures were performed in accordance with the national convention for the use and care of animals.
Cytokines, antibodies and reagents
Mouse rIL-4 was affinity purified using a column with IL-4 mAb 11B11. Proleukin (Chiron Cooperation) served as a source of human IL-2. Porcine TGF-beta1 was obtained from R&D Systems. Murine rIL-9 and hamster IL-9 mAb C12 were given to us by Dr. J. Van Snick (Ludwig Institute, Brussels, Belgium). Rat IL-9 mAb 229.4 was generated as described (19). In addition we used the following antibodies: CD28 mAb 37.51, IFN-γ mAb XMG 1.2, IL-4 mAb 11B11, IL-2 mAb S4B6.1, JES6-1A12, biotinylated JES6-5H4, CD3 mAb 145-2C11, CD4 mAb GK1.5, APC-conjugated IL-9 mAb RM9A4 (BioLegend).
SialoL preparation and LPS decontamination
SialostatinL was expressed in Escherichia coli, and the corresponding active protein was purified as previously described (1). Any potential LPS contamination in the stock solution was removed by Arvys Proteins using detergent extraction; endotoxin presence by the end of the procedure was estimated as <4×10−5 endotoxin U/μg protein (roughly, <3×10−14 g of endotoxin per microgram of protein) with a sensitive fluorescent-based endotoxin assay (PyroGene recombinant factor C endotoxin detection system; LonzaBiologics).
CD4+ T cell isolation
Murine naïve CD4+CD25− T cells were isolated as described previously (20, 21).
Stimulation and differentiation of murine T cells
Murine naïve CD4+ T cells were cultured in IMDM (Sigma), supplemented with 5% FCS, 1% Penicillin/Streptomycin, 1% L-Glutamin and Na-Pyruvat and 50μM β-Mercaptoethanol. For Th9 differentiation cells were stimulated with plate bound CD3 mAb (3μg/ml) and CD28 mAb (5μg/ml) in the presence of 300 U/ml IL-4, 20 μg/ml IFN-γ mAb, and 4 ng/ml porcine TGF-ß1.
Supernatants were used on day three to determine primary IL-9 secretion by IL-9-specific ELISA and activated T cells were used on day two or three for intracellular FACS staining and qRT-PCR analyses. For secondary stimulation Th9 cells were harvested on day five and restimulated with CD3 mAb (5μg/ml) for two days to detect IL-9 by ELISA, intracellular FACS and qRT-PCR analyses.
Lymphokine assays (ELISA)
Mouse IL-2 was detected using mAb JES6-1A12 (1μg/ml) and biotinylated mAb JES6-5H4 (1μg/ml). IL-9 was detected by mAb 229.4 (1μg/ml) and biotinylated mAb C12 (1μg/ml). ELISAs were evaluated according to reference standard curves by using known amounts of the specific cytokine.
mRNA detection
RNA was isolated using Trizol (Invitrogen) and cDNA was synthesized with RevertAid M-MuLV reverse transcriptase following the recommendations of the supplier (MBI Fermentas). Quantitative qRT-PCRs were performed using the following oligonucleotides:
Murine HGPRT forward: 5′-GTT GGA TAC AGG CCA GAC TTT GTT G-3′
Murine HGPRT reverse: 5′GAG GGT AGG CTG GCC TAT AGG CT-3′
Murine IL-9 forward: 5′-CTG ATG ATT GTA CCA CAC CGT GC-3′
Murine IL-9 reverse: 5′-GCC TTT GCA TCT CTG TCT TCT GG-3′
Murine IL-13 forward: 5'-GGA GCT GAG CAA CAT CAC ACA-3'
Murine IL-13 reverse: 5'-GGT CCT GTA GAT GGC ATT GCA-3'
Oligonucleotides were chosen to span at least one intron at the level of genomic DNA. qRT-PCR analyses were performed in triplicates on an iCycler (Bio-Rad,) using the SYBR GreenER qPCR Supermix (Invitrogen). After normalization of the data according to the expression of HGPRT mRNA, the relative expression level of IL-9 mRNA was calculated.
Intracellular staining (FACS analyses)
For intracellular staining of IL-9 murine naïve CD4+ T cells were stimulated for 48–72 h as outlined in Stimulation and differentiation of murine T cells chapter of this section. BrefeldinA (Sigma) was added to the cells 4 h before harvest and washed with PBS. Fixation and permeabilization was performed with buffers from a FoxP3 staining kit (eBiosciences). Cells were stained for IL-9 (RM9A4-APC and rat IgG isotype control).
Asthma experimental protocols
OVA-induced asthma model
10- to 12-wk-old BALB/c mice were sensitized by i.p. injection of 20 μg OVA (Grade V; Sigma) in a total volume of 100 μl on days 1 and 14. Mice were challenged (20 min) via the airways with OVA (1% in saline) alone or together with sialoL (i.v. 10 μg/challenge) for 3 days (days 28, 29, and 30), using ultrasonic nebulization (NEU17, Omron). Control mice groups received OVA challenge without prior sensitization. Assays were performed 24 h after the last challenge on day 31 (see Fig. 4A).
Figure 4. OVA-induced AHR and eosinophilia are strongly alleviated by treatment with sialoL.
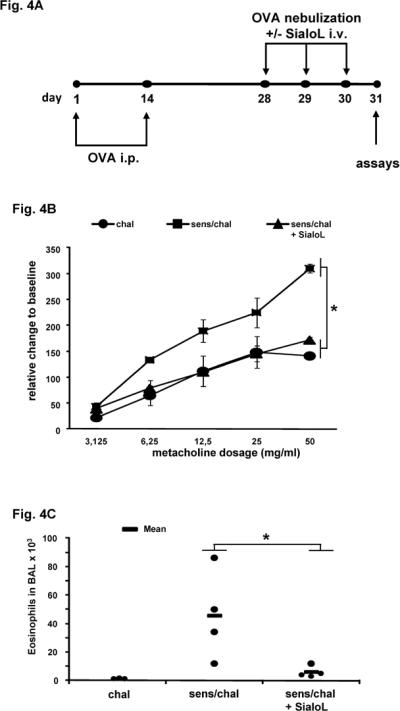
BALB/c mice were sensitized by two injections (i.p.) of OVA (20 μg) and subsequently challenged with nebulized OVA in the absence or presence of sialoL (10 μg, i.v.) as outlined in (A). Airway resistance (B) and numbers of eosinophils in BAL fluid (C) were measured in mice 24h after a third airway challenge on day 30. In (B) bars represent mean ±SEM AHR and in (C) every dot represents the number of eosinophils from an individual mouse. In (B) and (C) * p<0.05 compared to chal and sens/chal + SialoL.
Th9-induced asthma model
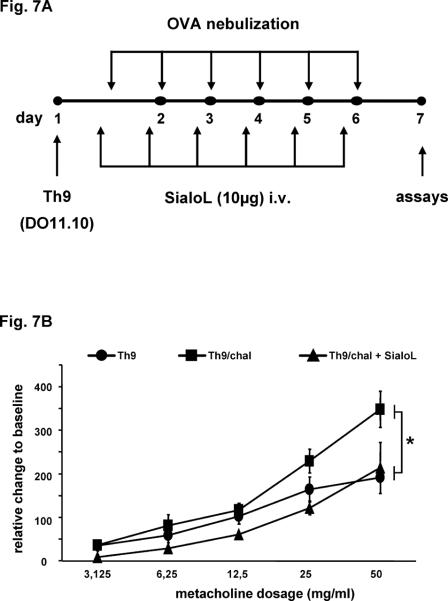
BALB/c RAG1−/− mice received 2×106 Th9 cells derived from naïve CD4+ T cells from DO11.10 BALB/c mice on day 1 by i.v. injection. Mice were then left unchallenged or challenged via the airways using nebulized OVA from day 1 to 6. Thirty minutes before each challenge mice received sialoL (i.v. 10μg/challenge). On day 7 airway reactivity was assessed and bronchoalveolar lavage (BAL) fluid was collected on the same day (see Fig. 7A).
Figure 7. Th9-induced AHR is significantly reduced by treatment with sialoL.
Rag1−/− mice were adoptively transferred with Th9 cells developed from DO11.10 mice and challenged with nebulized OVA in the absence or presence of sialoL as outlined in Materials and Methods and (A). Airway resistance (B) was measured in mice 24h after a final airway challenge on day 7. Bars represent mean ±SEM AHR; * p<0.05 compared to Th9 and Th9/chall + SialoL.
Measurement of airway reactivity
Measurements of the airway resistance (RL) were performed on anesthetized, intubated and mechanically ventilated mice (FlexiVent, Scireq, Montreal, QC) in response to increasing doses of inhaled methacholine (MCh: 3.125, 6.25, 12.5, 25, and 50 mg/ml). Measurements of RL were performed every 15 seconds following each nebulization step until a plateau phase was reached (22).
Bronchoalveolar lavage
After assessment of airway function, cells were isolated by lavage of the lungs via a tracheal tube with PBS (1 ml). Numbers of living cells were counted by using trypan blue dye exclusion. Differential cell counts were made from cytocentrifuged preparations fixed and stained with the Microscopy Hemacolor ®-Set (Merck).
Histology
Lungs were fixed by inflation (1ml) and immersion in 4% formalin and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS).
Lung single cell preparation and OVA peptide-specific activation
Isolated lungs (day 31) from BALB/c mice were minced and enzymatically digested with 280 units/ml collagenase type IA (Sigma) in PBS in a 37°C water bath. After 1h of incubation, a single-cell suspension was achieved by pushing the digested lung tissue fragments through a 0.9 × 40-mm canula (BD Microlance) and a 70-μm nylon cell strainer (BD Falcon). Red blood cells were removed using EDTA containing Gey's lysis buffer.
Isolated lung cells (4×106/ml in a 24 well plate) were stimulated with 2.5μg/ml OVA peptide 323–339 for 48h (mRNA expression) and for 72h (protein production), respectively.
Statistical analysis
ANOVA was used to determine the levels of difference between all groups. Differences in responsiveness to MCh were assessed by repeated measures ANOVA. Numbers of eosinophils, were initially analyzed by non-parametric ANOVA (Kruskal-Wallis Test) for overall differences. In case of significant results Mann-Whitney-U-Test was used to elucidate which specific differences were statistically significant. P values for significance were set at 0.05. Values for all measurements are expressed as the mean ± SEM.
Statistical evaluations of cytokine production and qRT-PCR were performed by Graph Pad Prism software (Version 5.0) using the Unpaired Student's t-test. Values of p < 0.05 were considered statistically significant.
Results
IL-9 production of Th9 cells is impaired in the presence of recombinant sialoL
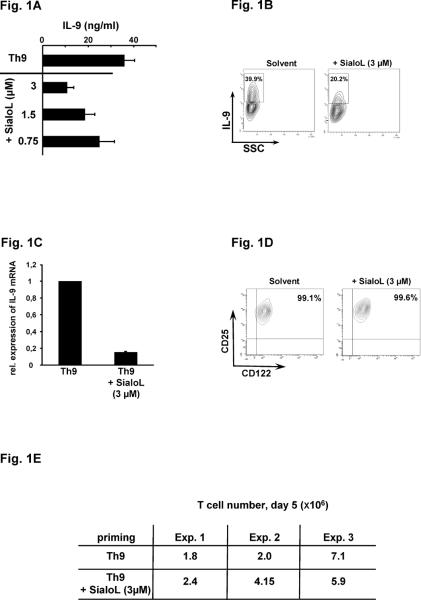
We have previously shown that polyclonal activation of naïve CD4+ T cells in the presence of IL-4 combined with TGF-β induces within 3 days a strong production of IL-9 and leads to the development of Th9 cells (19). The addition of recombinant sialoL strongly reduced primary IL-9 production in a concentration-dependent manner (Fig. 1A). This was confirmed by intracellular staining of IL-9 as well by quantifying mRNA-expression via qRT-PCR (Fig. 1B/C). Admittedly, it is possible that sialoL inhibits the activation of CD4+ T cells in general. However, this was ruled out because neither the expression of CD25 and CD122 nor the proliferation of CD4+ T cells was reduced by sialoL (Fig. 1D/E).
Figure 1. SialoL impairs primary T cell-derived production of IL-9 but not T cell activation and proliferation.
Naïve CD4+ T cells from BALB/c mice were stimulated (anti-CD3/CD28) in the presence and absence of different concentrations of sialoL (0.75 μM, 1.5 μM, 3 μM) under Th9-skewing conditions. Production of IL-9 was determined by (A) ELISA, (B) FACS, and (C) qRT-PCR. (D) T cell activation was determined by FACS analyses of CD25 and CD122 cell surface expression. Shown is each time one representative from three independent experiments ± SD. (E) T cell proliferation was determined by counting the cell numbers on day five after primary activation.
We have previously shown that endogenous IL-2 is essentially involved in primary IL-9 production (19). Thus, it was conceivable that the inhibitory influence of sialoL on the primary production of IL-9 was based on an impaired production of IL-2. This assumption was supported by the measurement of IL-2, which revealed that sialoL inhibited primary IL-2 production similarly to primary IL-9 production (Fig. 2A). Neutralization of endogenous murine IL-2 by mAb strongly curtailed IL-9 production (Fig. 2B). This could be completely restored by the addition of human IL-2 because it was not neutralized by murine IL-2 mAb but perfectly active on murine T cells. However, when CD4+ T cells were activated in the presence of sialoL alone and together with human IL-2, this cytokine did not influence the inhibitory effect of sialoL indicating that the inhibitory mechanism of sialoL is not based on the reduction of endogenous murine IL-2 (Fig. 2C).
Figure 2. Exogenous human (h)IL-2 cannot compensate for the IL-9-suppressive effect of SialoL.
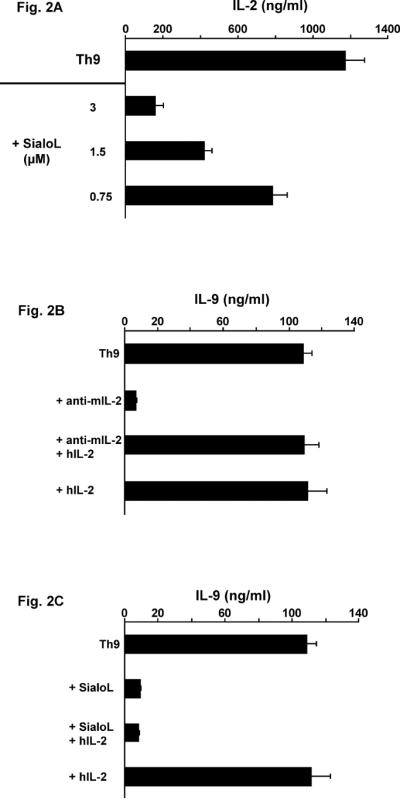
(A) Naïve CD4+ T cells from BALB/c mice were stimulated (anti-CD3/CD28) in the presence or absence of different concentrations of sialoL (0.75 μM, 1.5 μM, 3 μM) under Th9-skewing conditions. Production of IL-2 was determined by ELISA. (B) Naïve CD4+ T cells from BALB/c mice were stimulated (anti-CD3/CD28) solely under Th9-skewing conditions or in the presence of neutralizing murine (m)IL-2 mAb (S4B6.1: 20μg/ml) or human(h)IL-2 (100ng/ml) and a combination of both. (C) Naïve CD4+ T cells from BALB/c mice were stimulated (anti-CD3/CD28) solely under Th9-skewing conditions or in the presence of sialoL (3 μM) or hIL-2 (100ng/ml) and a combination of both. IL-9 was determined after 72h by ELISA. Shown is one representative from two independent experiments.
SialoL inhibits IL-9 production promoted by IL-1
We have previously shown that the production of IL-9 by Th2 cells was strongly enhanced in the presence of IL-1 (23). In addition, it was recently shown that IL-1 can replace IL-4 in the development of Th9 cells (24). Therefore, we analyzed whether IL-1 could also enhance the primary production of IL-9 after three days of culture in the presence of conditions that favor Th9-development and secondary production upon restimulation after five days. Primary IL-9 production was strongly enhanced by IL-1β (Fig. 3A) and further analyses revealed that the same held true for IL-1α (data not shown). The addition of sialoL however, strongly reduced this additional increase of IL-9 production promoted by IL-1. Secondary IL-9 production was also enhanced by IL-1β but sialoL could only modestly suppress this IL-9 production (Fig. 3B). The suppressive effect of sialoL on secondary IL-9 production was less pronounced as compared to its suppressive effect on primary IL-9 production. Therefore, the expression of IL-9 was assessed with the aid of qRT-PCR to confirm the results obtained by measuring IL-9 in the supernatants. Fig. 3C clearly demonstrates that sialoL could also significantly inhibit secondary IL-9 expression even if promoted by IL-1β. These data suggest that IL-1 can enhance the production of IL-9 by Th9 cells in a similar manner to its effect on IL-9-producing T cells that develop under Th2-promoting conditions. In addition, sialoL can strongly suppress this IL-9-promoting effect of IL-1 although to a lesser extent concerning secondary production of IL-9. IL-1β from alveolar macrophages was supposed to be a mediator of asthma (25). Thus it is tempting to speculate that a part of this activity is mediated through the upregulation of the asthma-promoting cytokine IL-9 and that sialoL can alleviate asthma symptoms by preventing this detrimental interaction of IL-1 and IL-9.
Figure 3. IL-1-mediated enhancement of secretion and expression of IL-9 is inhibited by sialoL.
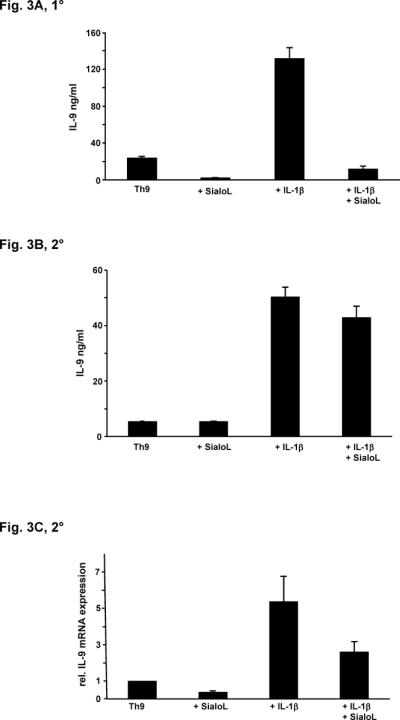
(A) Naïve CD4+ T cells from BALB/c mice were stimulated (anti-CD3/CD28) under Th9-skewing conditions in the absence or presence of sialoL (3 μM) or IL-1β (10ng/ml) and a combination of both. IL-9 was determined after 72h by ELISA. (B) Naïve CD4+ T cells from BALB/c mice were stimulated (anti-CD3/CD28) under Th9-skewing conditions. After five days T cells were restimulated in the absence or presence of sialoL (3 μM) or IL-1β (10ng/ml) and a combination of both. IL-9 was determined after 48h by ELISA. (C) Naïve CD4+ T cells from BALB/c mice were stimulated (anti-CD3/CD28) under Th9-skewing conditions. After five days T cells were restimulated in the absence or presence of sialoL (3 μM) or IL-1β (10ng/ml) and a combination of both. Expression of Il9 was quantified after 48h by qRT-PCR. Similar results were obtained in four independent experiments.
Features of experimental airway disease were strongly reduced upon the treatment with sialoL
IL-9 was found to be essentially involved in the development of airway inflammation and airway hyperresponsiveness (AHR) and we have recently shown that Th9 cells provoke AHR, eosinophilia and increased mucus production in a model of experimental asthma (7). By neutralizing IL-9, Th9-mediated symptoms were strongly reduced while Th2-mediated symptoms were only marginally affected. This indicated that the asthma-promoting activity of Th9 cells was based on the release of IL-9. In analogy to these data the inhibitory potency of sialoL for Th9 cell-derived production of IL-9 should lead to reduced asthma symptoms in an experimental asthma model. Thus, mice were sensitized and challenged with OVA in the absence and presence of sialoL according to the experimental procedure illustrated in Fig. 4A. Subsequently, AHR and eosinophilia were comparatively analyzed in the different groups of mice. OVA challenge in the absence of sensitization caused no symptoms while OVA challenge of sensitized mice resulted in profound AHR and severe eosinophilia (Fig. 4B/C). The treatment with sialoL almost completely prevented AHR and eosinophilia suggesting that sialoL could also inhibit Th9-derived IL-9 production under physiological conditions in vivo (Fig. 4B/C). These data were corroborated by staining of lung tissue sections with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS). Cellular infiltration (Fig. 5, H&E) as well as mucus production (Fig. 5, PAS) that could be observed after challenge of sensitized mice with nebulized OVA (Fig. 5, sens/chal) were strongly reduced after additional treatment (i.v.) with sialoL (Fig. 5, sens/chal + SialoL).
Figure 5. OVA-induced cellular infiltration and mucus production are significantly reduced by treatment with sialoL.
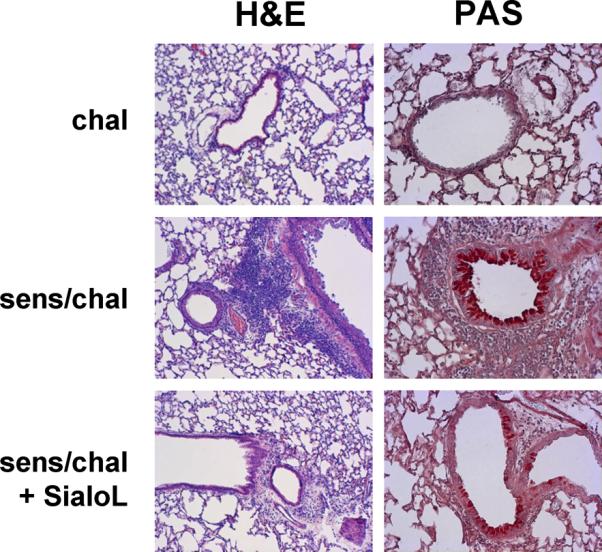
BALB/c mice were sensitized by two injections (i.p.) of OVA (20 μg) and subsequently challenged with nebulized OVA in the absence or presence of sialoL (10 μg, i.v.) as outlined in Fig. 4A. Tissue inflammation was evaluated 24h following the last challenge using hematoxylin and eosin staining (H&E). Periodic acid-Schiff (PAS) staining was applied to determine goblet cell metaplasia in mice which were only challenged with OVA (chal), sensitized and challenged (sens/chal) and treated in addition with sialoL (i.v.; sens/chal + SialoL).
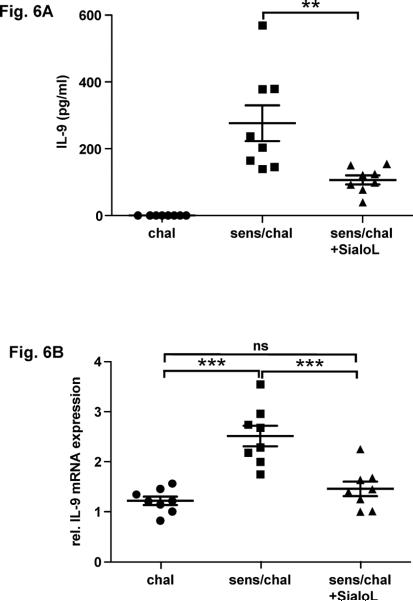
IL-9 could not be detected in the bronchoalveolar lavage (BAL) of such mice that had been treated as described in Fig. 4A. However, stimulating lung cells that had been prepared as described in Materials & Methods with OVA peptide 323–339 resulted in a significant production of IL-9 on the protein and mRNA level (Fig. 6A/B). This IL-9 production was strongly reduced when lung cells were analyzed that had been prepared from mice treated additionally with sialoL (Fig. 6A/B, sens/chal + SialoL). Expression of IL-13 that was described to be induced by IL-9 could not be detected. To further substantiate these findings Th9 cells were transferred to T cell-deficient RAG1−/− mice which were subsequently challenged by nebulized OVA as outlined in Fig. 7A. The challenge with OVA led to an increased AHR that was inhibited upon the treatment with sialoL (Fig. 7B). Since IL-9 was demonstrated to mediate the asthma promoting activity of Th9 cells (7, 26) this result strongly argues in favor of sialoL-mediated inhibition of AHR via suppression of IL-9 from transferred Th9 cells.
Figure 6. OVA-induced development of IL-9-producing T cells is strongly inhibited by treatment with sialoL.
BALB/c mice were sensitized by two injections (i.p.) of OVA (20 μg) and subsequently challenged with nebulized OVA in the absence or presence of sialoL (10 μg, i.v.) as outlined in Fig. 4A. Lung cells were prepared from mice 24h following the last challenge (day 31) and stimulated (4×106/ml, 24 well plate) with 2.5 μg/ml OVA peptide 323–339 for 48h (mRNA expression) and for 72h (protein production), respectively. (A) IL-9 production was determined in the supernatants by ELISA; n= 8 mice per group. **p = 0.0084. (B) IL-9 mRNA expression levels were analyzed by quantitative real time PCR; n= 8 mice per group. *** chal vs. sense/chal p < 0.0001; ***sense/chal vs. sense/chal + SialoL p=0.0009; ns: not significant.
Discussion
Hard ticks like Ixodes scapularis were found to be slow feeders that need up to 10 days for their blood meal. Principally, this long period of time for feeding is sufficient to provoke innate and adaptive immune reactions directed against these parasites. Therefore, they acquired immunosuppressive mechanisms in order to successfully feed from their hosts (27). This immunosuppression is mediated by an array of different substances in the saliva of ticks among others sialoL (1, 2, 28–33). Interestingly, several immunmodulatory substances seem to act specifically on distinct cell populations (34).
For example, PGE2 from saliva of I. scapularis has been shown to suppress DC-derived cytokine production (TNF-α, IL12p70) and OVA-induced IL-2 production of T cells as well (35). In addition, we have previously shown that sialoL in analogy to PGE2 can inhibit antigen-specific T cell proliferation while polyclonally (ConA-) induced proliferation was not affected (1). We concluded that sialoL preferentially suppressed the accessory function of DC rather than inhibited T cell functions directly. Data showing that sialoL impaired the expression of CD80/CD86 and the secretion of TNF-α and IL-12p70 by LPS-activated DC further supported this assumption (2). Herein, we can show that sialoL inhibits the production of IL-9 by Th9 cells that had been activated polyclonally by platebound CD3 mAb in combination with CD28 mAb indicating a direct inhibitory effect of sialoL on T cells. A general, nonspecific impairment of T cell activation could be excluded by the observation that proliferation and CD25/CD122 expression of these T cells were not affected by sialoL which is in agreement with our former finding that ConA-induced T cell proliferation was not inhibited by sialoL. A closely related cystatin from the saliva of I. scapularis is sialoL2. Notably, this cystatin showed only a marginal suppressive effect on IL-9 production of T cells (data not shown). This finding corresponds to the fact that sialoL could prevent the symptoms at the onset of EAE while sialoL2 was completely ineffective (2).
IL-9 was initially thought to be preferentially produced by Th2 cells and subsequently it has been shown that IL-9 induced asthmatic symptoms similar to those induced by IL-4 (9, 10). This asthma-promoting potency of IL-9 was found to be at least partially mediated by IL-13 that was produced from airway epithelial cells (36). However, we could not detect IL-13, presumably, because we used a comparatively mild asthma model applying OVA in the absence of alum. In addition, the studies showing that IL-13 was induced by IL-9 used IL-9 overexpressing mice, leading most likely to non-physiological and permanent high local concentrations of IL-9 (37) (38). Nevertheless, an array of distinct studies confirmed that IL-9 is one of the key players concerning the development of asthma. Recently, the reevaluation of findings which demonstrated that IL-9-producing T cells develop under the influence of TGF-β and IL-4, led to the definition of Th9 cells (5, 6). Furthermore, we found that Th9 development was dependent from IRF4 (7). IRF4-deficient mice were resistant to the induction of OVA-mediated asthma. The transfer of OVA-specific Th9 cells and consecutive challenge with nebulized OVA led to asthmatic symptoms that could be prevented when such animals were treated with neutralizing IL-9 mAb. Furthermore, it was shown that neutralizing IL-9 by IL-9 mAB i.v. not only inhibited airway inflammation and hyperreactivity, but also suppressed IL-4, IL-5, and IL-13 in the BAL indicating that IL-9 controls the expression of Th2 cytokines and possesses higher asthmatogenic potency than other asthma-promoting cytokines (39). These data clearly underscore that Th9-derived IL-9 is prominently involved in the development of allergic lung inflammations. Therefore, our finding that sialoL could substantially inhibit Th9 development and significantly reduce at least the expression of IL-9 by Th9 cells in the presence of the synergistic co-stimulator IL-1 prompted us to apply this cystatin in two preclinical asthma models. This approach clearly revealed that sialoL prevented OVA-induced AHR, cellular infiltration, mucus production as well as the development of IL-9-producing T cells (Fig. 4–6).
The finding that AHR caused by transfer of Th9 cells is strongly reduced by i.v. treatment with sialoL while secondary IL-9 expression of Th9 cells in vitro is only partially affected suggested that sialoL affects other cell types as well in vivo. Especially, antigen presentation by DC was described to be inhibited by sialoL (2). In fact, preliminary results obtained by restimulating Th9 cells with OVA in the presence of BMDC as accessory cells showed a comparatively strong inhibition of secondary IL-9 production (data not shown). Hence, sialoL represents a promising basis to develop a drug for asthma therapy.
The importance of the suppressive property of sialoL on IL-9 production is further emphasized by human studies showing that IL-9 is specifically upregulated after local allergen challenge in the lungs of asthmatic patients (40). Lymphocytes were identified as the major cellular source of IL-9. Furthermore, it was described that serum levels of IL-9 increased in rhinitis patients after allergen exposure (41). Finally, a phase 2b randomized study that will be completed in 2012 aims to evaluate the efficacy and safety of a humanized IL-9 mAb (MEDI-528) for the treatment of adults with uncontrolled asthma (42) (ClinicalTrials.gov Identifier: NCT00968669).
In conclusion, these data indicate that the inhibition of IL-9 is an auspicious approach for the treatment of asthma. Hence, the exploitation of the immunosuppressive potency of sialoL could be important for the development of new strategies -alternatively to humanized IL-9 mAb- for the treatment of allergic asthma.
Acknowledgements
We cordially thank Dr. J. Van Snick for providing us with IL-9 mAb and Dr. Josef Bodor for helpful discussions.
Footnotes
Grant support: This study was funded by Deutsche Forschungsgemeinschaft DFG, grants SFB TR52 TPA1 (M.K., T.B. and E.S.), SCHM 1014/5-1 (V.S., T.B. E.S.), the GRK 1043: International Graduate School of Immunotherapy (E.S.,T.B.), “Forschungszentrum Immunologie (FZI)” of the University medical center (E.S., T.B.) and the Asthma Core Facility (C.T.), JGU Mainz. Grant Agency of the Czech Republic billateral grant no.P302/11/J029 (H.H., M.K., J.K.), Research Center of the Ministry of Education, Youth and Sports of the Czech Republic grant no. LC06009 (H.H., J.K.).
References
- 1.Kotsyfakis M, Sa-Nunes A, Francischetti IM, Mather TN, Andersen JF, Ribeiro JM. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J Biol Chem. 2006;281:26298–26307. doi: 10.1074/jbc.M513010200. [DOI] [PubMed] [Google Scholar]
- 2.Sa-Nunes A, Bafica A, Antonelli LR, Choi EY, Francischetti IM, Andersen JF, Shi GP, Chavakis T, Ribeiro JM, Kotsyfakis M. The immunomodulatory action of sialostatin L on dendritic cells reveals its potential to interfere with autoimmunity. J Immunol. 2009;182:7422–7429. doi: 10.4049/jimmunol.0900075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavasnik-Bergant T. Cystatin protease inhibitors and immune functions. Front Biosci. 2008;13:4625–4637. doi: 10.2741/3028. [DOI] [PubMed] [Google Scholar]
- 4.Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, Hamann A, Hamelmann E, Lucius R, Hartmann S. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180:4265–4272. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- 5.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, Kuchroo VK. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhoen M, Uyttenhove C, Van Snick J, Helmby H, Westerndorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta `reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 7.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, Dehzad N, Becker M, Stassen M, Steinborn A, Lohoff M, Schild H, Schmitt E, Bopp T. Interferon-Regulatory Factor 4 Is Essential for the Developmental Program of T Helper 9 Cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, Zhou T, Kleeberger SR, Buetow KH, Levitt RC. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci U S A. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soussi-Gounni A, Kontolemos M, Hamid Q. Role of IL-9 in the pathophysiology of allergic diseases. J Allergy Clin Immunol. 2001;107:575–582. doi: 10.1067/mai.2001.114238. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, McLane M, Levitt RC. Th2 cytokines and asthma. Interleukin-9 as a therapeutic target for asthma. Respir Res. 2001;2:80–84. doi: 10.1186/rr42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dugas B, Renauld JC, Pene J, Bonnefoy JY, Peti-Frere C, Braquet P, Bousquet J, Van Snick J, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993;23:1687–1692. doi: 10.1002/eji.1830230743. [DOI] [PubMed] [Google Scholar]
- 12.Louahed J, Zhou Y, Maloy WL, Rani PU, Weiss C, Tomer Y, Vink A, Renauld J, Van Snick J, Nicolaides NC, Levitt RC, Haczku A. Interleukin 9 promotes influx and local maturation of eosinophils. Blood. 2001;97:1035–1042. doi: 10.1182/blood.v97.4.1035. [DOI] [PubMed] [Google Scholar]
- 13.Longphre M, Li D, Gallup M, Drori E, Ordonez CL, Redman T, Wenzel S, Bice DE, Fahy JV, Basbaum C. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest. 1999;104:1375–1382. doi: 10.1172/JCI6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitt RC, McLane MP, MacDonald D, Ferrante V, Weiss C, Zhou T, Holroyd KJ, Nicolaides NC. IL-9 pathway in asthma: new therapeutic targets for allergic inflammatory disorders. J Allergy Clin Immunol. 1999;103:S485–491. doi: 10.1016/s0091-6749(99)70165-x. [DOI] [PubMed] [Google Scholar]
- 16.Kung TT, Luo B, Crawley Y, Garlisi CG, Devito K, Minnicozzi M, Egan RW, Kreutner W, Chapman RW. Effect of anti-mIL-9 antibody on the development of pulmonary inflammation and airway hyperresponsiveness in allergic mice. Am J Respir Cell Mol Biol. 2001;25:600–605. doi: 10.1165/ajrcmb.25.5.4533. [DOI] [PubMed] [Google Scholar]
- 17.White B, Leon F, White W, Robbie G. Two first-in-human, open-label, phase I dose-escalation safety trials of MEDI-528, a monoclonal antibody against interleukin-9, in healthy adult volunteers. Clin Ther. 2009;31:728–740. doi: 10.1016/j.clinthera.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Murphy K, Heimberger A, Loh D. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kühn R, Müller W, Palm N, Rüde E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 20.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit H, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J.Exp.Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bopp T, Dehzad N, Reuter S, Klein M, Ullrich N, Stassen M, Schild H, Buhl R, Schmitt E, Taube C. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J Immunol. 2009;182:4017–4024. doi: 10.4049/jimmunol.0803310. [DOI] [PubMed] [Google Scholar]
- 22.Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand E, Stassen M, Buhl R, Taube C. Mast Cell-Derived tumour Necrosis Factor is Essential for Allergic Airway Disease. Eur Respir J. 2007;31:773–782. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt E, Beuscher HU, Huels C, Monteyne P, van Brandwijk R, van Snick J, Ruede E. IL-1 serves as a secondary signal for IL-9 expression. J Immunol. 1991;147:3848–3854. [PubMed] [Google Scholar]
- 24.Uyttenhove C, Brombacher F, Van Snick J. TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur J Immunol. 2010;40:2230–2235. doi: 10.1002/eji.200940281. [DOI] [PubMed] [Google Scholar]
- 25.Borish L, Mascali JJ, Dishuck J, Beam WR, Martin RJ, Rosenwasser LJ. Detection of alveolar macrophage-derived IL-1 beta in asthma. Inhibition with corticosteroids. J Immunol. 1992;149:3078–3082. [PubMed] [Google Scholar]
- 26.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brossard M, Wikel SK. Tick immunobiology. Parasitology. 2004;129(Suppl):S161–176. doi: 10.1017/s0031182004004834. [DOI] [PubMed] [Google Scholar]
- 28.Valenzuela JG, Charlab R, Mather TN, Ribeiro JM. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem. 2000;275:18717–18723. doi: 10.1074/jbc.M001486200. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie RD, Dolan MC, Piesman J, Titus RG. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J Immunol. 2001;166:4319–4326. doi: 10.4049/jimmunol.166.7.4319. [DOI] [PubMed] [Google Scholar]
- 30.Deruaz M, Frauenschuh A, Alessandri AL, Dias JM, Coelho FM, Russo RC, Ferreira BR, Graham GJ, Shaw JP, Wells TN, Teixeira MM, Power CA, Proudfoot AE. Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med. 2008;205:2019–2031. doi: 10.1084/jem.20072689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konnai S, Nakajima C, Imamura S, Yamada S, Nishikado H, Kodama M, Onuma M, Ohashi K. Suppression of cell proliferation and cytokine expression by HL-p36, a tick salivary gland-derived protein of Haemaphysalis longicornis. Immunology. 2009;126:209–219. doi: 10.1111/j.1365-2567.2008.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, Godfroid E. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J Biol Chem. 2002;277:10083–10089. doi: 10.1074/jbc.M111391200. [DOI] [PubMed] [Google Scholar]
- 34.Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sa-Nunes A, Bafica A, Lucas DA, Conrads TP, Veenstra TD, Andersen JF, Mather TN, Ribeiro JM, Francischetti IM. Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J Immunol. 2007;179:1497–1505. doi: 10.4049/jimmunol.179.3.1497. [DOI] [PubMed] [Google Scholar]
- 36.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int Immunol. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- 37.Steenwinckel V, Louahed J, Orabona C, Huaux F, Warnier G, McKenzie A, Lison D, Levitt R, Renauld J-C. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells. J Immunol. 2007:3244–3251. doi: 10.4049/jimmunol.178.5.3244. [DOI] [PubMed] [Google Scholar]
- 38.Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van Snick J, Renauld JC. IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182:4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 39.Cheng G. Anti-Interleukin-9 Antibody Treatment Inhibits Airway Inflammation and Hyperreactivity in Mouse Asthma Model. Am J Respir Crit Care Med. 2002:409–416. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- 40.Erpenbeck VJ, Hohlfeld JM, Discher M, Krentel H, Hagenberg A, Braun A, Krug N. Increased expression of interleukin-9 messenger RNA after segmental allergen challenge in allergic asthmatics. Chest. 2003;123:370S. [PubMed] [Google Scholar]
- 41.Ciprandi G, De Amici M, Castellazzi AM, Tosca MA, Marseglia G. Serum IL-9 Levels Depend on Allergen Exposure: Preliminary Study. Int Arch Allergy Immunol. 154:246–248. doi: 10.1159/000321111. [DOI] [PubMed] [Google Scholar]
- 42.Antoniu SA. MEDI-528, an anti-IL-9 humanized antibody for the treatment of asthma. Curr Opin Mol Ther. 2010;12:233–239. [PubMed] [Google Scholar]