Abstract
The angiotensin II type 1 receptor (AT1R) mediates most hypertensive actions of angiotensin II. In order to understand the molecular regulation of the AT1 receptor in normal physiology and pathophysiology, methods for sensitive and specific detection of AT1R protein are required. Here, we examined the specificity of a panel of putative anti-AT1R antibodies that are commonly used by investigators in the field. For these studies, we carried out Western blotting and immunocytochemistry with kidney tissue from WT mice and genetically modified mice lacking the major murine AT1R isoform, AT1A (AT1AKO), or with combined deficiency of both the AT1A and AT1B isoforms (AT1ABKO). For the 3 antibodies tested, Western blots of protein homogenates from WT kidneys yielded distinct bands with the expected size range for AT1R. In addition, these bands appeared identical in samples from mice lacking one or both murine AT1R isoforms. Additionally, the pattern of immune histo-chemical staining in kidneys, liver and adrenal glands of WT mice was very similar to that of AT1ABKO mice completely lacking all AT1 receptors. We verified the absence of AT1R subtypes in each mouse line by: 1) quantitative PCR documenting the absence of mRNA species and, 2) functionally by assessing angiotensin II-dependent vasoconstriction, which was substantially blunted in both AT1AKOs and AT1ABKOs. Finally, these antibodies failed to detect epitope-tagged AT1AR protein over-expressed in HEK cells. We conclude that anti-AT1R antibodies available from commercial sources and commonly used in published studies exhibit non-specific binding in mouse tissue that may lead to erroneous results.
Keywords: angiotensin II type 1 receptor, Western blot, cross-reactivity, AT1A, AT1B
Introduction
The type 1 angiotensin receptor (AT1R) is a key component of the renin-angiotensin system (RAS). AT1 receptors mediate most of the classically recognized actions of angiotensin II. Activation of AT1Rs stimulates vascular contractility and renal sodium retention1-4 playing a crucial role in regulating blood pressure in health and disease. In addition to these hemodynamic effects, the AT1R also mediates cell proliferation, fibrosis and end organ damage.5-7 Due to the key role of these receptors in physiology and pathophysiology, accurate detection of the AT1R protein is paramount for investigations aimed at understanding molecular mechanisms of health and disease. Although several AT1R antibodies are available commercially we, and others8 had major concerns about their specificity.
Humans have only one AT1R isoform, however two isoforms exist in rodents: termed AT1A and AT1B. These receptor sub-types are products of two different genes: Agtr1a is located on mouse chromosome 13 (17 in rat); and Agtr1b on mouse chromosome 3 (2 in rats). They both encode a 375 amino acid protein with a predicted molecular weight of 42 kDa.9-11 They share 94% identity and are indistinguishable pharmacologically. Based on bioinformatics predictions (http://www.cbs.dtu.dk/services/NetNGlyc/) these receptors are presumed to undergo post-translational glycosylation. Accordingly, using a plasmid expressing the AT1R tagged to a myc epitope, Deslauriers and colleagues reported massive AT1R glycosylation in transfected COS-7 cells.12 In that study, the molecular size of the glycosylated AT1R form was estimated to be ~100-150kDa. Since the degree of glycosylation of proteins is a tissue-specific process, it is difficult to predict the molecular mass of these receptors under different tissues or experimental conditions and published data clarifying this issue are lacking.
During the last decade, anti-AT1R antibodies have been widely used in scientific reports related to AT1R signaling and functions. However, their specificity has not been thoroughly investigated in the medical literature. In preliminary studies using mice with targeted deletion of AT1R genes that were generated in our laboratory, we became concerned about the specificity of these antibodies. Accordingly, we carried out a systematic evaluation of a panel of anti-AT1R antibodies that were purchased from commercial vendors, focusing on their utility and specificity for Western blot analysis and immunohistochemistry.
Materials and Methods
Please see http://hyper.ahajournals.org
Results
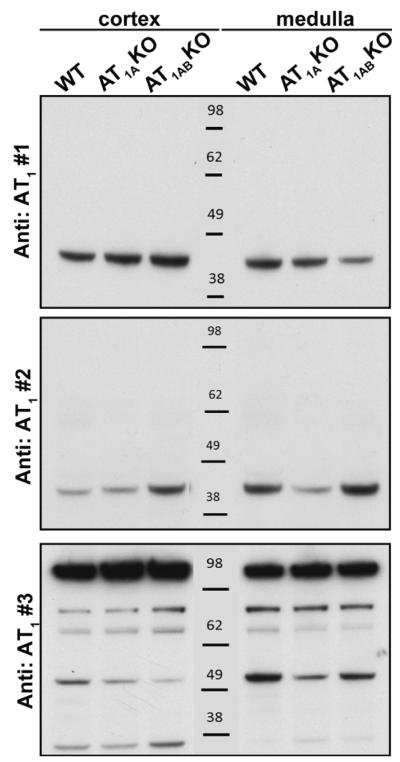
We first performed Western blot analysis using homogenates of cortex (C) and medulla (M) from kidneys of WT mice comparing band patterns produced by three anti-AT1R antibodies. As shown in Figure 1, antibodies #1 and #2 each generated a single band between 38-48 kDa, which is around the expected 41 kDa size of the AT1R 9-11. Although each of these antibodies identified a single band, the molecular weight of these bands was slightly different. In contrast, antibody #3 produced multiple bands of a broad range of sizes (Fig 1, n = 3). Between the 3 antibodies, the patterns of reactivity were very different with no common bands seen within the predicted molecular size range for the AT1R.
Figure 1.
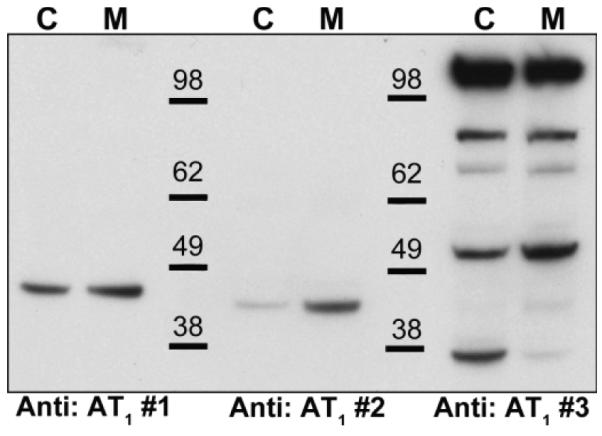
Western blot analysis of 40 μg homogenates of kidney cortex (C) and medulla (M) using three different commercially available angiotensin type 1 receptor (AT1R) antibodies. Antibody #1: Alomone AAR-011, antibody #2: Santa Cruz sc-1173 (n-10), antibody #3: Abcam 18801. X-ray films were exposed to the membranes for 2 min. Representative of n =2.
In order to test the specificity of each antibody for the AT1R, we performed additional Western blot analyses but now using protein homogenates from kidneys of mice genetically deficient in one or both AT1R subtypes. As shown in Figure 2, there were no apparent differences in the pattern of reactivity of each of the antibodies between protein extracts of kidneys from WT mice, compared to those from mice lacking the major AT1 receptor isoform, AT1AR (AT1AKO). Furthermore, the patterns of antibody reactivity were virtually identical in the kidneys from WT and AT1abKO (Fig 2, n = 3). To be certain that we were not missing a band corresponding to AT1R protein that was obscured or low abundance, we overexposed the x-ray film to the membrane for up to 60 minutes, but did not detect additional bands.
Figure 2.
Western blot analysis of kidney cortex and medulla from wild-type mice (WT), AT1AKO mice (A) and AT1ABKO mice (AB) using three different commercially available anti-AT1R antibodies. a) Antibody #1: Alomone AAR-011, b) antibody #2: Santa Cruz sc-1173 (n-10), c) antibody #3: AbCam 18801. Representative of n =3.
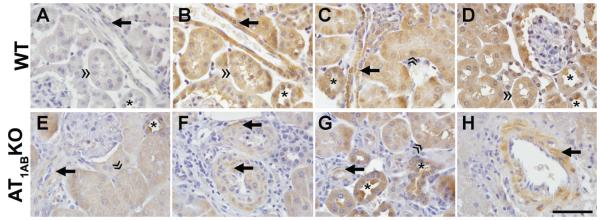
We also tested the polyclonal AT1R antibody #2 for immunohistochemical staining. As shown in Figure 3 (A-G), tissue sections from WT kidney in the absence (3A) of primary antibody demonstrate minimal background staining compared to sections incubated with the AT1R antibody (3B). Smooth muscle cells of the renal and arcuate arteries, interlobular (Figs 3B and 3F) and afferent arterioles (3C, 3E, 3F, 3G), liver (3H) and adrenal gland (not shown), stained positively with the AT1R antibody on WT and AT1ABKO tissues. Prominent anti-AT1R immunostaining was also visualized in the proximal tubule brush border and basolateral membranes (3B, 3C, 3D, 3E, 3G) of WT and AT1ABKO. Distal tubules, cortical and medullary collecting ducts (3B, 3C, 3D, 3E, 3G) also exhibited immunoreactivity. Thus, similar to the Western blotting data, patterns and localization of immunostaining with the AT1R antibody were identical in the kidneys of WT mice (3B-3D), and the AT1ABKO completely lacking AT1R (3E-3G).
Figure 3.
AT1R immunohistochemical localization using Santa Cruz N-10 rabbit polyclonal antibody (1:300) in kidney and liver of wild-type (WT) (A-D) mice and mice with combined deficiency of AT1A and AT1B receptors (AT1ABKO, E-H). Consecutive tissue sections from WT kidney in the absence (A) of primary antibody demonstrate minimal background immunostaining compared to incubation of the tissue section in the presence of the AT1R (B). AT1R was localized to vascular smooth muscle cells (arrow) of WT and AT1ABKO mice in renal cortical (A-G) and liver (H) tissues. Proximal tubule brush border and basolateral membrane (double arrowhead) and distal nephron segments (asterisk) demonstrated positive AT1R immunostaining in WT and AT1ABKO mice. Images were obtained using an X100 oil immersion lens. Bar = 50μm.
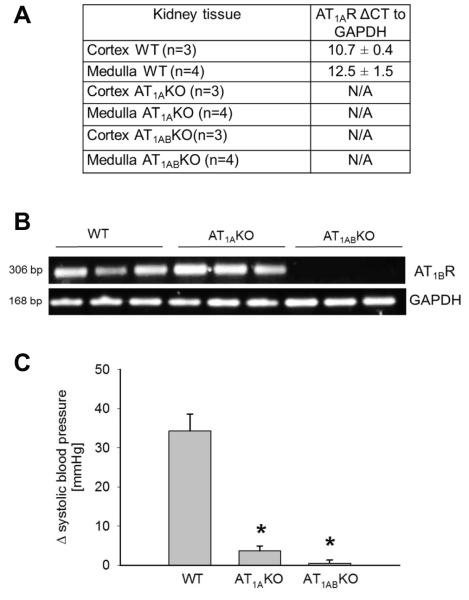
To verify that the AT1R knockout mice used in the studies were truly deficient in receptors we measured AT1R expression and functional responses to angiotensin II. By quantitative RT-PCR, AT1A mRNA receptor abundance in WT kidneys was similar in the cortex and medulla (10.7 ± 0.4 and 12.5 ± 1.5 arbitrary units for cortex and medulla, n = 4, n.s.). In contrast, AT1A receptor transcript was undetectable in either cortex or medulla of both AT1AKO and AT1ABKO kidneys (Fig 4a). Additionally, AT1B receptor mRNA was detected in adrenal glands from WTs and AT1AKO but undetectable in AT1ABKO (Fig 4b). Furthermore, to confirm physiological absence of functional AT1R in the knockouts, we tested the ability of angiotensin II to cause acute increases in blood pressure. Bolus infusions of 10 μg/kg angiotensin II increased blood pressure by 34 ± 4 mm Hg in WT mice. However this response was significantly blunted in AT1AKO and completely absent in AT1ABKO mice (change: 4 ± 1 and 0.5 ± 0.8 mmHg for AT1AKO and AT1ABKO, p < 0.001 vs. WTs, n = 3-4) (Fig 4c). These experiments confirm the lack of each AT1R sub-type expression and activity in our knock-out mouse lines and indicate that the anomalous results obtained by Western blotting and immunohistochemistry are likely due to antibodies cross-reacting with unknown proteins other than the AT1R.
Figure 4.
a) CT values for AT1A receptor relative to GAPDH obtained by Real-time PCR in kidney tissues from wild-type (WT), AT1AKO and AT1ABKO mice. b) Representative agarose gel to visualize AT1B receptor mRNA in WT, AT1AKO and AT1ABKO. c) Effect of acute angiotensin II infusion (10 μg/kg) on blood pressure in WT, AT1AKO and AT1ABKO mice. p < 0.005 vs. wild-type (WT); n= 3-4.
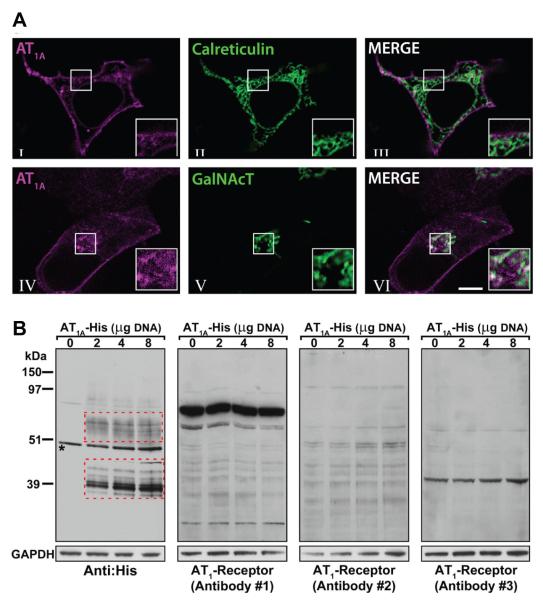
To explore the possibility that the lack of specificity of anti-AT1R antibodies was due to insufficient sensitivity, we over-expressed the AT1R in HEK cells by transfecting them with DNA encoding the mouse AT1AR. To verify appropriate targeting of the AT1R protein to the plasma membrane, we used a plasmid encoding the AT1A receptor fused with the mCherry fluorescence protein (AT1A-mCherry) and imaged the receptor by live cell-fluorescence confocal microscopy. We found that, after 24 hours of transfection, a significant amount of the total AT1AR pool was present at the plasma membrane (Fig 5a, I). In addition, we also observed that the non-plasma membrane associated AT1AR pool does not co-localize with the endoplasmic reticulum (ER)-associated protein Calreticulin (Fig 5a, II and III) but it partially co-localizes with the trans-Golgi network-associated protein GalNac-T (Fig 5a, IV-VI).
Figure 5.
a) Cellular localization of AT1A in HEK cells cotransfected with expression plasmids encoding AT1A receptor fused with fluorescent mCherry (AT1A, magenta, I, III, IV and VI) and, with different plasmids encoding fluorescent markers for endoplasmic reticulum (Calreticulin, green, II and III) or Golgi apparatus (GalNAc, green, V and VI). The fluorescent constructs were visualized after 24 hours by live-cell confocal microscopy. AT1A receptors localize in the plasma membrane and partially associate with the Golgi apparatus. The results are representative of 2 or more independent experiments. b) Detection of AT1 receptors in HEK cells transfected with varying amounts (0, 2, 4 and 8 μg) of a plasmid encoding the His-tagged AT1A receptor (AT1A-His DNA) using different antibodies. Anti: His: penta HIS antibody, Qiagen 34660; Anti: AT1 receptor #1: Alomone AAR-011; Anti: AT1 receptor #2: Santa Cruz sc-1173 (n-10); Anti: AT1 receptor #3: AbCam 18801. Red boxes indicate bands corresponding to the AT1AR protein. Asterisk indicates a band recognized by the HIS antibody in non-transfected cells, suggesting endogenous HIS-containing protein.
We next tested the ability of the commercial antibodies to detect AT1AR increments at different levels of over-expression. For this, we subcloned the AT1AR sequence from plasmid AT1A-mCherry into the pcDNA3.1 His vector in frame with the His epitope (AT1A-His), and different amount of plasmidic DNA were transfected into HEK cells. For detection of the exogenous His-tagged proteins by Western blot an anti-His antibody was utilized. The anti-His antibody detected multiple bands close to 39 kDa in cells transfected with 2 μg plasmid. The intensity of these bands (relative to GAPDH) increased by 83 ± 40 % and 193 ± 39 % (n=3) in cells transfected with 4 and 8 μg DNA (lower red box on Fig 5b). In contrast, this pattern was not reproduced by utilizing any of the anti-AT1R antibodies (Fig 5b).
Discussion
In this study we tested three different rabbit polyclonal commercial antibodies that have been used in published reports to detect AT1R protein. Antibodies #1and #2 were raised against a short sequence (15 amino-acids) of the extracellular amino terminus of the AT1R protein that is identical among rat and mouse. This region is ~95% identical between AT1A and AT1B receptors (accession numbers NP_796296.1 and EDL34899.1). Antibody #3 was raised against intracellular carboxy terminus sequences also identical between rat and mouse. In this case, two synthetic peptides specific for each receptor subtype, AT1A and AT1B were used. The AT1A and AT1B receptors are each 359 amino acids, highly homologous (sharing <94% amino acid identity) with predicted molecular masses of 41 kDa. Therefore, the antibodies used in these studies should not distinguish AT1A and AT1B receptor sub-types. Since both AT1R isoforms undergo post-translational glycosylation, a single ~41 kDa band should represent the non-glycosylated AT1A or AT1B receptor whereas higher molecular bands would be expected for the glycosylated forms.12
By performing direct side-by-side comparisons of the bands recognized by each antibody, we concluded that each antibody binds to distinct unknown proteins of diverse molecular sizes and raised the concern that these antibodies cross-react with proteins other than the AT1R. Experiments using kidney tissue from mice with genetic deficiencies of the major murine AT1R isoform (AT1A) or lacking both receptor sub-types (AT1A and AT1B) revealed that, all three antibodies showed the same pattern of bands on Western blots whether the proteins were derived from WT, AT1AKO and AT1ABKO mice. Inclusion of samples from the double knockout (AT1ABKO) eliminated the possibility that the positive signal in the AT1AKO was due to up-regulation of the homologous AT1BR. This suggests that none of these antibodies recognize the AT1R protein with sufficient specificity in kidney tissue by Western blot.
Our immunohistochemical staining studies revealed apparent AT1R localization in the renal vasculature and proximal and distal tubules. However, identical staining patterns were observed when kidneys from mice lacking both AT1R sub-types (AT1ABKO) were utilized. The positive AT1R immunostaining in the renal microvasculature of the AT1ABKO kidneys was unexpected since these tissues lack renal vasoconstrictor responses to angiotensin II in in vitro and in vivo.13, 14 Additionally, the apparent positive AT1R immunostaining was also observed in the liver vasculature of AT1ABKO mice indicating that the non-specific positive antibody staining observed in the vascular smooth muscle of the AT1ABKO is not restricted to kidney. The identity of the proteins recognized by these antibodies remains elusive. We conducted immunoprecipitation of kidney homogenates (using antibodies #1 and #2) followed by mass spectrometry and were unable to identify either the AT1R or any other protein in the 41 kDa range. Two possibilities may explain these inconclusive results: 1) the antibodies are not suitable for immunoprecipitation experiments; and 2) the non-specific protein has yet to be reported therefore does not exist in the current peptide database. Nonetheless, despite our inability to identify the specific protein or proteins identified by these antibodies, the cumulative from our studies provide compelling evidence that this protein is not the AT1R.
We were confident that these findings were intrinsic to the antibodies and not to the presence of AT1R in our knockout lines as we verified the absence of AT1R mRNA expression and functional responses to angiotensin II in randomly selected mice from our colony. Based on the strategy of gene disruption utilized to generate our KO mice14, 15 where many stop codons were introduced into the early portions of the coding sequence of each gene, it is theoretically possible that very small, truncated forms of the receptors might be generated. However, it is highly unlikely that these protein fragments would reach the cell surface. Moreover, if present, they should appear as unique bands in the knockout mice with sizes substantially smaller than that predicted for the native AT1R protein.14, 15
We used HEK cells over-expressing AT1A receptors to test the antibodies again. Our confocal fluorescence images in living HEK cells show proper plasma membrane expression of the AT1A receptor. In addition, AT1A –mCherry fluorescence was not significant in the ER, indicating that processed AT1AR proteins were properly sorted to the Golgi apparatus for further secretion, as shown by colocalization with the marker for this organelle. These findings indicate that HEK cells are able to transcribe, translate, and normally process the AT1AR, which localizes normally to the plasma membrane. Accordingly, we used HEK cells transfected with the AT1AR-His plasmid. Using an anti-His antibody, we detected distinct amounts of His-tagged AT1AR proportional to the amount of DNA transfected under each experimental condition. Bands of molecular weights in the ~39 kDa and ~65 kDa range were detected only in cells transfected with the AT1A-His plasmid (red boxes on Fig 5b). A single band at ~51 kDa was present in all samples including the mock-transfected cells and likely represents endogenous His proteins expressed by HEK cells (marked with an asterisk on Fig 5b). Nevertheless, the ~39 kDa band is consistent with the non-glycosylated form of the AT1R12 even though one may question the apparent molecular weight of AT1R “monomer” because this band appears to be slightly smaller than the predicted 41 kDa mass of this protein. This small apparent difference in size could be accounted for by an altered migration velocity due to the positively charged histidines added to the carboxyl-terminal tail. Furthermore, the multiple bands apparent at ~39 could also be proteolytic degradation products of the AT1R protein. Additionally, while the multiple banding at ~65 kDa is consistent with the different degrees of AT1 receptor glycosylation reported for the rat kidney16, it is also possible that these bands represent ubiquitinated receptor which the mechanism whereby the AT1R is degraded. Nevertheless, in contrast to the anti-His antibody, these bands were not identified by any of the anti-AT1R antibodies. Since we do not observe specific reactivity of the antibodies to AT1R protein expressed at high levels in HEK cells, the failure of the antibodies to detect the AT1R in Western blots does not appear to be an issue of insufficient sensitivity. It is important to note that the non-specific bands recognized by the anti-AT1R antibodies in HEK cells are different in number and size compared to those observed in the kidney tissues (Fig 2) which provides additional evidence for the lack of specificity of these antibodies. Although the inability of the commercial antibodies to recognize the AT1R protein could be explained by potential masking of the epitope by the His tag, we believe this is unlikely to occur due to the denaturing conditions used to unfold the proteins in our Western blotting technique. Additionally the His tag is attached at the carboxy terminus region of the protein and at least 2 of the antibodies tested are raised against the amino terminus sequence.
Other investigators attempted to generate custom-made antibodies without success. The Daugherty laboratory8 generated several antisera against potential new antigenic sites within the AT1R receptor and they were not able to demonstrate any specific interaction to the AT1A or AT1B receptors by either Western blotting or immunostaining of tissue sections. In a different report by Hoffmann and colleagues17, investigators generated antibodies using the last 20 amino acids of the carboxy terminus tail of each AT1A and AT1B receptors. These antibodies yielded bands of different molecular mass in neural cells compared to mammalian tissues. Although these bands were absent when pre-incubating the antibodies with the immunizing synthetic peptides, this maneuver alone is insufficient to demonstrate specificity for the intact receptor protein and thus, the specificity of such antibody remains elusive. Finally, using a monoclonal antibody (6313/G2) raised against and N-terminal peptide (residues 8-17), we previously localized the AT1R protein in renal vascular smooth muscle cells, proximal tubule and more distal nephron segments in kidneys of adult rats.16, 18 The specificity of this antibody was confirmed by Western blotting of COS-7 cells transfected with an AT1R expressing plasmid.19 Thus, it is possible that identification of the AT1R by antibody-based techniques is suitable for rat but not mouse tissues. In contrast, the Bernstein laboratory raised a polyclonal antibody against N-terminal peptide residues 15-24, that stained mainly vascular smooth muscle cells, brush border and thick ascending limbs.20 Unlike our report, this antibody failed to demonstrate immunoreactivity in distal collecting ducts. The origin of this discrepancy is still unknown but it may be explained by some degree of antibody cross-reactivity. Unfortunately, the identity of the peptide being recognized in such studies has not been verified likely because AT1KO mice were not available at the time.
Perspectives
We report here that commercially available antibodies are erroneous tools to detect the AT1R protein. Generation of highly specific antibodies for G-protein coupled receptors has reportedly been difficult 21-26 and the reasons behind this are yet to be understood. One explanation is potential differences in structure and charge between the glycosylation (naturally carried in the AT1R) and the synthetic peptides utilized when raising these antibodies. Successful strategies to generate reliable anti-AT1R antibodies are urgently needed. Investigators should utilize alternative methods such as ligand-binding1, epitope-tagging27, Northern blot 28, or quantitative RT-PCR when studying the biology of AT1R. Interpretations of previously published work relying solely on quantitative and qualitative assessments of the AT1R protein using these antibodies should be viewed with extreme caution.
Supplementary Material
Novelty and Significance.
What Is New
Antibody-based detection of AT1R is unreliable due to the lack of specificity of commercial antibodies which recognize proteins different than the AT1R.
What Is Relevant
The AT1R mediates the hypertensive actions of angiotensin II. Understanding AT1R protein tissue distribution, abundance and interaction with other molecules is crucial to elucidate the pathophysiology of cardiovascular and renal diseases and develop new pharmacologic tools for their treatment.
Many investigators have published studies using these unreliable antibodies.
Caution must be used when interpreting such reports and designing experimental approaches to understand the biology of these receptors.
Summary
Our studies indicate that commercially available antibodies may not always be suitable for detecting AT1R protein.
Inclusion of appropriate positive and negative controls is essential when using antibodies-based techniques.
Investigators should be wary of using these tools when designing new experimental approaches to understand the biology and pathophysiology of AT1 receptors.
Acknowledgments
Sources of Funding This work was supported by National Institutes of Health Grants HL056122 (T.M.C) and F32DK081333 (M.H). Part of this work was done in the laboratory of Suzanne Jackowski, PhD, Department of Infectious Diseases, St. Jude Children’s Research Hospital and funded by National Institutes of Health Grant GM062896 (S.J).
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1a angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowley SD, Zhang J, Herrera M, Griffiths RC, Ruiz P, Coffman TM. The role of AT1 receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2011;30:F1124–F1130. doi: 10.1152/ajprenal.00305.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, Wilcox CS. P47(phox) is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension. 2012;59:415–420. doi: 10.1161/HYPERTENSIONAHA.111.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda T, Muto S, Fujisawa G, Iwazu Y, Kimura M, Kobayashi T, Nonaka-Sarukawa M, Sasaki N, Watanabe Y, Shinohara M, Murakami T, Shimada K, Kobayashi E, Kusano E. Heart angiotensin II-induced cardiomyocyte hypertrophy suppresses coronary angiogenesis and progresses diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2012;302:H1871–H1883. doi: 10.1152/ajpheart.00663.2011. [DOI] [PubMed] [Google Scholar]
- 6.Polichnowski AJ, Lu L, Cowley AW., Jr Renal injury in angiotensin II+l-name-induced hypertensive rats is independent of elevated blood pressure. Am J Physiol Renal Physiol. 2011;300:F1008–1016. doi: 10.1152/ajprenal.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varagic J, Frohlich ED, Susic D, Ahn J, Matavelli L, Lopez B, Diez J. AT1 receptor antagonism attenuates target organ effects of salt excess in SHRs without affecting pressure. Am J Physiol Heart Circ Physiol. 2008;294:H853–858. doi: 10.1152/ajpheart.00737.2007. [DOI] [PubMed] [Google Scholar]
- 8.Rateri DL, Moorleghen JJ, Balakrishnan A, Owens AP, 3rd, Howatt DA, Subramanian V, Poduri A, Charnigo R, Cassis LA, Daugherty A. Endothelial cell-specific deficiency of ang II type 1a receptors attenuates ang II-induced ascending aortic aneurysms in ldl receptor-/- mice. Circ Res. 2011;108:574–581. doi: 10.1161/CIRCRESAHA.110.222844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwai N, Yamano Y, Chaki S, Konishi F, Bardhan S, Tibbetts C, Sasaki K, Hasegawa M, Matsuda Y, Inagami T. Rat angiotensin II receptor: Cdna sequence and regulation of the gene expression. Biochem Biophys Res Commun. 1991;177:299–304. doi: 10.1016/0006-291x(91)91982-i. [DOI] [PubMed] [Google Scholar]
- 10.Kakar SS, Sellers JC, Devor DC, Musgrove LC, Neill JD. Angiotensin II type-1 receptor subtype cdnas: Differential tissue expression and hormonal regulation. Biochem Biophys Res Commun. 1992;183:1090–1096. doi: 10.1016/s0006-291x(05)80302-x. [DOI] [PubMed] [Google Scholar]
- 11.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cdna encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 12.Deslauriers B, Ponce C, Lombard C, Larguier R, Bonnafous JC, Marie J. N-glycosylation requirements for the AT1a angiotensin II receptor delivery to the plasma membrane. Biochem J. 1999;339(Pt 2):397–405. [PMC free article] [PubMed] [Google Scholar]
- 13.Park S, Bivona BJ, Harrison-Bernard LM. Compromised renal microvascular reactivity of angiotensin type 1 double null mice. Am J Physiol Renal Physiol. 2007;293:F60–67. doi: 10.1152/ajprenal.00049.2007. [DOI] [PubMed] [Google Scholar]
- 14.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1a angiotensin ii receptor gene. Proc Natl Acad Sci U S A. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliverio MI, Kim HS, Ito M, Le T, Audoly L, Best CF, Hiller S, Kluckman K, Maeda N, Smithies O, Coffman TM. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both at1a and AT1b receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison-Bernard LM, Imig JD, Carmines PK. Renal AT1 receptor protein expression during the early stage of diabetes mellitus. Int J Exp Diabetes Res. 2002;3:97–108. doi: 10.1080/15604280214483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann A, Cool DR. Characterization of two polyclonal peptide antibodies that recognize the carboxy terminus of angiotensin II AT1a and AT1b receptors. Clin Exp Pharmacol Physiol. 2005;32:936–943. doi: 10.1111/j.1440-1681.2005.04288.x. [DOI] [PubMed] [Google Scholar]
- 18.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of ang II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273:F170–177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 19.Barker S, Marchant W, Ho MM, Puddefoot JR, Hinson JP, Clark AJ, Vinson GP. A monoclonal antibody to a conserved sequence in the extracellular domain recognizes the angiotensin II AT1 receptor in mammalian target tissues. J Mol Endocrinol. 1993;11:241–245. doi: 10.1677/jme.0.0110241. [DOI] [PubMed] [Google Scholar]
- 20.Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE. Immunohistochemical localization of rat angiotensin ii at1 receptor. Am J Physiol. 1993;264:F989–995. doi: 10.1152/ajprenal.1993.264.6.F989. [DOI] [PubMed] [Google Scholar]
- 21.Pradidarcheep W, Labruyere WT, Dabhoiwala NF, Lamers WH. Lack of specificity of commercially available antisera: Better specifications needed. J Histochem Cytochem. 2008;56:1099–1111. doi: 10.1369/jhc.2008.952101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26:8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu X, Bartfai T. Analyzing the validity of galr1 and galr2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:417–420. doi: 10.1007/s00210-009-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamdani N, van der Velden J. Lack of specificity of antibodies directed against human beta-adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:403–407. doi: 10.1007/s00210-009-0392-1. [DOI] [PubMed] [Google Scholar]
- 26.Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:397–402. doi: 10.1007/s00210-009-0393-0. [DOI] [PubMed] [Google Scholar]
- 27.Roy SF, Laporte SA, Escher E, Leduc R, Guillemette G. Epitope tagging and immunoreactivity of the human angiotensin II type 1 receptor. Can J Physiol Pharmacol. 1997;75:690–695. [PubMed] [Google Scholar]
- 28.Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1a and 1b in mouse. Am J Physiol. 1994;267:E260–267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.