Abstract
Although hypertension remains the most potent and widespread cardiovascular risk factor, its pharmacological treatment has achieved only limited success. The chromogranin A derived fragment catestatin inhibits catecholamine release by acting as an endogenous nicotinic cholinergic antagonist, and can “rescue” hypertension in the setting of CHGA targeted ablation. Here we undertook novel peptide chemistry to synthesize isomers of catestatin: normal/wild-type (W-T) as well as a retro-inverso (R-I) version, with not only inversion of chirality (L→D amino acids) but also reversal of sequence (carboxyl→amino). The R-I peptide was entirely resistant to proteolytic digestion, and displayed enhanced potency as well as preserved specificity of action towards nicotinic cholinergic events: catecholamine secretion, agonist desensitization, secretory protein transcription, and cationic signal transduction. Structural modeling suggested similar side chain orientations of the W-T and R-I isomers, while CD spectroscopy documented inversion of chirality. In vivo, the R-I peptide “rescued” hypertension in two mouse models of the human trait: monogenic Chga targeted ablation, with prolonged efficacy of the R-I version; and a polygenic model, with magnified efficacy of the R-I version. These results may have general implications for generation of metabolically stable mimics of biologically active peptides for cardiovascular pathways. The findings also point the way toward a potential new class of drug therapeutics for an important risk trait, and more generally open the door to broader applications of the retro-inverso strategy in other pathways involved in cardiovascular biology, with the potential for synthesis of diagnostic and therapeutic probes for both physiology and disease.
Keywords: Hypertension, peptides, cardiovascular diseases
INTRODUCTION
Hypertension is the most common and lethal of cardiovascular risk factors 1, and despite pharmacological advances it remains inadequately controlled by antihypertensive medications 2. Here we targeted a novel catecholamine storage and release pathway for therapeutic potential, by synthesizing and deploying a novel stabilized peptidomimetic agent.
Chromogranin A (CHGA), a 48-kDa acidic glycoprotein, is the major soluble protein in catecholamine storage vesicles 3, 4. Upon stimulation, CHGA is co-secreted, by exocytosis, along with catecholamines and their co-transmitters. CHGA serves as a precursor of biologically active peptides including catestatin (human CHGA352-372), which acts at nicotinic cholinergic receptors as a potent autocrine inhibitor of catecholamine secretion 5, 6. In human essential (hereditary) hypertension, diminished level of plasma catestatin concentration implies an early deficiency of this peptide might play a role for the subsequent development of the disease 7–9. Recent studies documented that a naturally occurring human variant of catestatin alters autonomic function and blood pressure 10.
Monogenic models of hypertension illustrate the role of particular physiological pathways on BP. Targeted ablation of the chromogranin A (CHGA) locus in the mouse results in unbridled severe hypertension 11 that can be “rescued” by administration of the catecholamine release inhibitory “catestatin” fragment of CHGA 11.
The retro-inverso (R-I) peptide synthetic modification 12 involves both inversion of amino acid alpha-carbon chirality and reversal of peptide bonds (i.e., reversal of primary amino acid sequence) 12–14, with the goal of increasing peptide stability while preserving or reconstituting side chain orientations.
Since the catestatin fragment of CHGA exerts both antihypertensive 11, 15, 16 and vasodilatory 17, 18 actions in vivo, we synthesized a novel R-I isomer of human catestatin (hCHGA352-372) and tested its stability, conformation, mechanistic specificity for inhibition of events triggered by nicotinic cholinergic stimulation, as well as antihypertensive activity in vivo. Our results suggest that R-I catestatin is entirely impervious to proteolytic digestion, while retaining potency and specificity for nicotinic cholinergic-stimulated catecholamine release, and finally enhanced duration as an antihypertensive agent in vivo.
METHODS
See the online-only data supplement
RESULTS
Nicotinic cholinergic-evoked catecholamine secretion
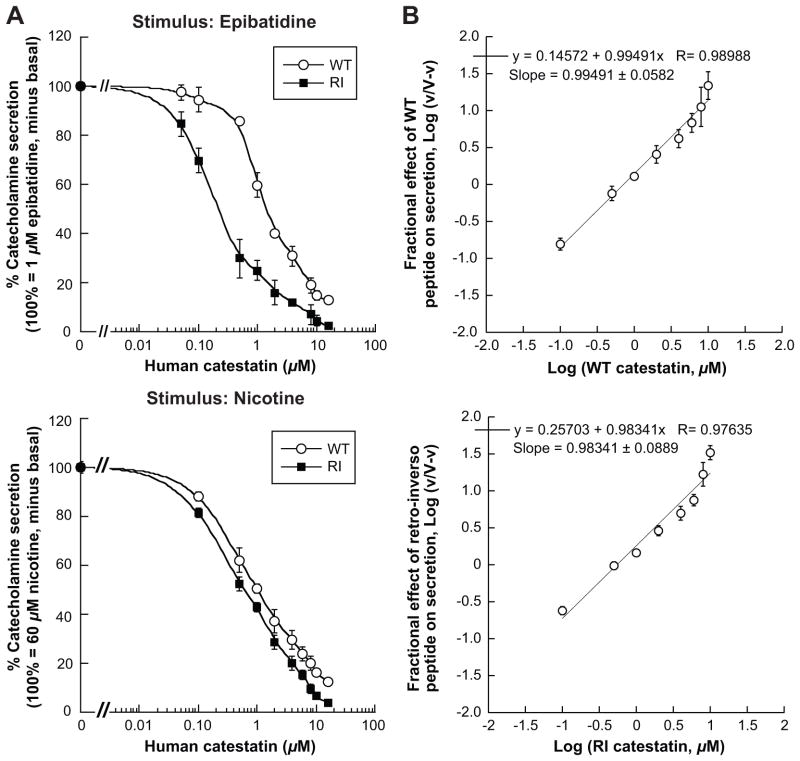
In rat pheochromocytoma PC12 cells we tested W-T and R-I peptides for inhibition of the nicotinic cholinergic agonists epibatidine (1 μmol/L, Figure 1A, left upper panel) or nicotine (Figure 1A, left lower panel). On epibatidine, compared to W-T (IC50 ~1.36 μmol/L), the R-I displayed ~6-fold enhanced potency with an IC50 of ~0.22 μmol/L. On nicotine, the IC50 was ~1.0 μmol/L for W-T and ~0.6 μmol/L for R-I.
Figure 1. Catestatin peptidomimetics: Potency for secretory inhibition during nicotinic cholinergic stimulation.
(A) During agonist [epibatidine (left upper panel) or nicotine (left lower panel)] induced catecholamine secretion, the responses were inhibited by human catestatins: wild type or retro-inverso peptides. PC12 cells were labeled with [3H]-norepinephrine and were incubated with epibatidine (1 μmol/L) in absence or presence of ascending doses (0.05, 0.1, 0.5, 1, 2, 4, 8, 10, 16 μmol/L) of W-T or R-I peptides. Control (100%) net epinephrine release represents the release in the presence of epibatidine alone. Results were analyzed by two-way ANOVA evaluating the effect of peptide (F=154.5; p<0.0001), dose (F=184.9; p<0.0001) and peptide/dose interaction (F=9.22; p<0.0001). In the left lower panel, PC12 cells were labeled with [3H]-norepinephrine and then incubated with 60 μmol/L nicotine, either alone or in combination with ascending doses of each peptide (0.1 to 16 μmol/L), just as done in the upper panel. Results were analyzed by two-way repeated-measure ANOVA evaluating the effect of peptide (F=45.84, p<0.0001) and dose (F=292.4, p<0.0001).
(B) Testing for cooperativity of the catestatin peptides, W-T (upper panel) and R-I (lower panel) in the inhibition of secretion: Hill plots. The plot assess the fractional effect of ascending doses (as above) of catestatin peptides to inhibit catecholamine release triggered by 60 μmol/L nicotine from PC12 cells. The slope is the Hill coefficient represents the cooperativity of binding of catestatin ligand, where a slope = 1 indicates non-cooperativity.
To test a role for cooperativity in catestatin peptides’ inhibition of secretion, we examined the fractional effect of peptide to inhibit nicotine stimulated catecholamine secretion as a function of log10 of peptide dose (Figure 1B). For each peptide, the plots were linear over a wide range of catestatin concentrations, and the Hill slopes were near unity for the each peptide, indicating non-cooperative actions in each case.
Nicotinic agonist desensitization
Nicotinic cholinergic responses are subject to desensitization after repeated exposure to agonist, and catestatin can block this phenomenon 19–21. Here we found that both W-T and R-I peptides blocked prior agonist-induced desensitization of catecholamine secretion, in dose-dependent fashion (Supplemental Figure S1A) with IC50 values of ~0.61 and ~0.25 μmol/L respectively.
Nicotinic agonist-stimulated secretory protein transcription
Nicotinic cholinergic stimulation triggers not only the release of catecholamines, CHGA and catestatin, but also stimulate the re-synthesis of the just-released transmitters, by the process of “stimulus-secretion-synthesis coupling”. We used nicotine to trigger transcription of a CHGA promoter/luciferase reporter transfected into chromaffin cells, achieving a ~2-fold activation over basal transcription. Both W-T and R-I peptides inhibited the transcriptional activation significantly, by ~28% and ~33% respectively (Supplemental Figure S1B).
Specificity for nicotinic cholinergic stimulation
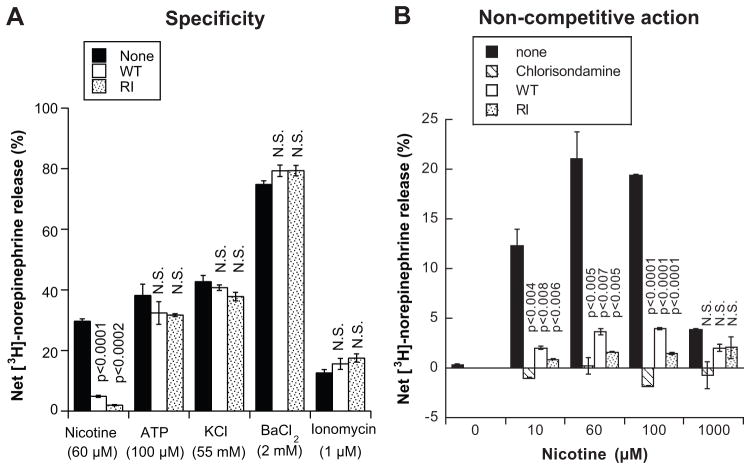
In addition to the nicotinic-cholinergic stimulation, we tested several secretagogues that act at later stages in the secretory pathway than the nicotinic receptor, including membrane depolarization (by KCl), or blockade of repolarization (by Ba2+). In addition, we used secretagogues that act on different targets from the nicotinic-cholinergic receptor, such as P2x (ATP) or artificial Ca2+ pores (Ca2+ ionophore ionomycin). Both W-T and R-I peptides significantly inhibited catecholamine release, but only when triggered by nicotine (Figure 2A), and not when secretion was caused by agents acting distal to the receptor, or on other receptor classes.
Figure 2. Specificity of catestatin effects on chromaffin cell catecholamine secretion.
(A) Inhibitory effect of W-T and R-I peptide on catecholamine secretion is specific for nicotinic cholinergic stimulation. PC12 cells were labeled with [3H]-norepinephrine and were then treated with different secretagogue [nicotinic cholinergic stimulation by 60 μmol/L nicotine, membrane depolarization by 55 mmol/L extracellular K+; P2x receptor activation by 100 μmol/L ATP; K+ channel blockade by 2 mmol/L BaCl2; calcium ionophore 1 μmol/L ionomycin, either alone or combination with W-T or R-I peptide (10 μmol/L each) and harvested after 30 min for measurement of norepinephrine secretion. Results were analyzed by one-way ANOVA evaluating the effect of peptide on nicotine-stimulated norepinephrine secretion (F=1038.3, p<0.0001). The effect of peptide on other secretagogue was not significant. P values compare each peptide point to the secretion in absence of peptide for specific secretagogue.
(B) W-T and R-I peptide acts as a non-competitive nicotinic cholinergic antagonist. PC12 cells were labeled with [3H]-norepinephrine, and secretion over a 30 min time course was studied in response to different doses of nicotine (10, 60, 100, 1000 μmol/L), either alone or in combination with W-T or R-I peptide (10 μmol/L each), or positive control the non-competitive nicotinic antagonist chlorisondamine (10 μmol/L). Results were analyzed by one-way ANOVA evaluating the effect of antagonist (F=24.2, p<0.0001).
Non-competitive nicotinic antagonist behavior
To demonstrate the noncompetitive nature of inhibition, we treated PC12 cells with ascending doses of nicotine (10, 60, 100 and 1000 μmol/L) alone or with the antagonists; W-T or R-I peptide (10 μmol/L) or chlorisondamine (an established non-competitive neuronal nicotinic cholinergic antagonist; 10 μmol/L) for 30 min, after which cells were harvested for measurement of norepinephrine release (Figure 2B). Even very high doses of nicotine (100–1000 μmol/L) could not overcome W-T or R-I peptide inhibition of norepinephrine release, functionally establishing the non-competitive nature of the inhibition. Although W-T and R-I peptides were not as potent as chlorisondamine to inhibit catecholamine release, both of them reduced norepinephrine secretion significantly over nicotine concentrations of 10 to 100 μmol/L (Figure 2B).
Nicotinic cholinergic cationic signal transduction: Ca2+ influx
Nicotinic cholinergic secretory responses in the chromaffin cells are associated with an early influx of calcium. To test the ability of the R-I peptide to interfere at this early stage of signal transduction, PC12 cells were stimulated with nicotine (60 μmol/L) in the presence or absence of W-T or R-I peptides, and agonist-mediated uptake of 45Ca2+ was measured. The R-I peptide inhibited the uptake of 45Ca2+ in a dose dependent manner (Supplemental Figure S2), suggesting that the initial interaction with nicotinic receptors remained intact for the R-I peptide. The IC50 was ~0.62 μmol/L for W-T and ~0.81 μmol/L for R-I peptide.
Peptide stability: Proteolytic digestion and MALDI-TOF mass spectrometry
Stability is a major hurdle for peptide therapeutics, to enable both absorption and persistence (half-life). Thus, the stability of the R-I catestatin was compared with W-T (natural sequence), inverso (all-D), and retro (carboxyl→amino) peptides (Table-1), by subjecting them to pronase digestion and subsequent MALDI-TOF mass spectrometry. All 4 peptides displayed a major peak at mass 2326.7 (MH+ corresponding to human CHGA352-372), when incubated in the absence of pronase (Supplemental Figure S3, panel C). In the presence of high pronase concentration (substrate : enzyme ratio, 50:1), W-T and retro-catestatins (all L-amino acid peptides) underwent complete digestion (Supplemental Figure S3, panel A), whereas inverso- and R-I (all-D) peptides showed complete resistance toward pronase (Supplemental Figure S3, panel A). With reduced enzyme concentration (substrate : enzyme, 500:1), low molecular mass peptides (~1000 Da) could be detected from W-T and retro (all-L) peptides, but the complete removal of the parent peptide(s) (at MH+ 2326.7) reinforces the susceptibility of all-L peptides toward pronase digestion (Supplemental Figure S3, panel B). Thus, inversion of chirality (with D-amino acids) in the inverso and R-I peptides yielded remarkable increments in stability.
Table 1.
Human catestatin (CHGA352-372) Retro-Inverso synthetic sequences:
| Wild-type | Amino-SSMKLSFRARAYGFRGPGPQL-carboxyl |
| Inverso | Amino-ssmklsfrarayGfrGpGpql-carboxyl |
| Retro | Amino-LQPGPGRFGYARARFSLKMSS-carboxyl |
| Retro-inverso | Amino-lqpGpGrfGyararfslkmss-carboxyl |
Human catestatin (CHGA352-372) was synthesized in 4 isomeric versions: wild-type, inverso (all D-amino acids), retro (reversing sequence from amino→carboxyl, to carboxyl→amino), and retro-inverso (R-I, reversing sequence, as well as inverting chirality to all D-amino acids). Upper-case = L-amino acid (one-letter code), Lower-case = D-amino acid (except Gly/G, which has no chirality).
Structural biology: Molecular modeling and CD (circular dichroism) spectroscopy of the R-I peptide
Since the R-I peptide conserved inhibitory activity comparable to the W-T, we suspected that the R-I isomer maintains similar overall side-chain topology to the W-T peptide. To probe structural similarities between W-T and R-I, we began with molecular modeling and energy minimization (Figure 3A & Supplemental Figure S4A), in which the superimposition of the R-I with W-T in anti-parallel fashion results in an overall similarity in spatial distribution of side chains. CD spectroscopy of the W-T revealed substantial (~67.5%) right-handed alpha-helix, as indicated by double minima at 208 and 222 nm and a maximum at 195 nm (Figure 3B); the R-I isomer displayed a mirror-image CD spectrum, consistent with global inversion of lpha -carbon chirality.
Figure 3. Structural biology.
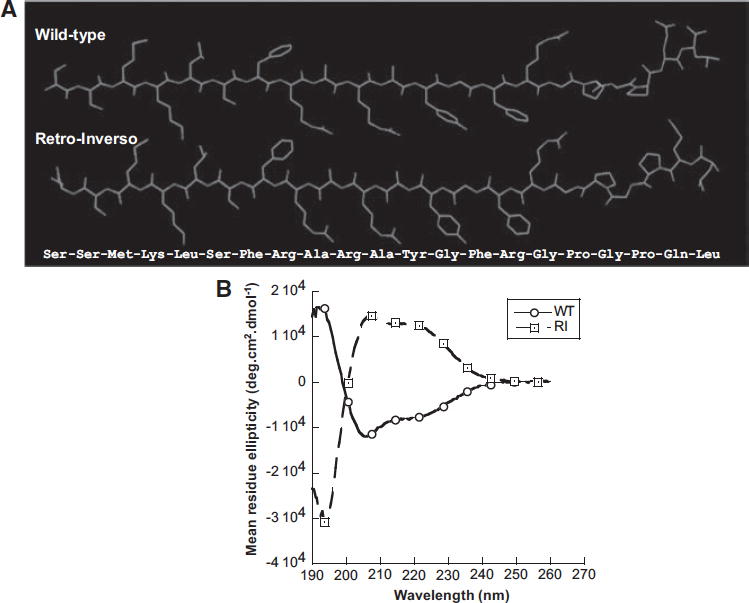
(A) Molecular modeling of W-T and R-I peptides. The structures were created in ChemDraw and the inversion of chirality was achieved in Chem3D, followed by energy minimization (MM2 force field).
(B) Circular Dichroism (CD) spectroscopy of W-T vs. R-I synthetic catestatin regions from human CHGA. CD spectra of 21-amino acid catestatin peptides (hCgA352-372) at 0.125 mg/ml in 98% 2,2,2-trifluoroethanol were taken over 190–260 nm.
Autonomic/cardiovascular physiology/pharmacology
Prolonged “rescue” of a monogenic model of hypertension: Chga−/− mice
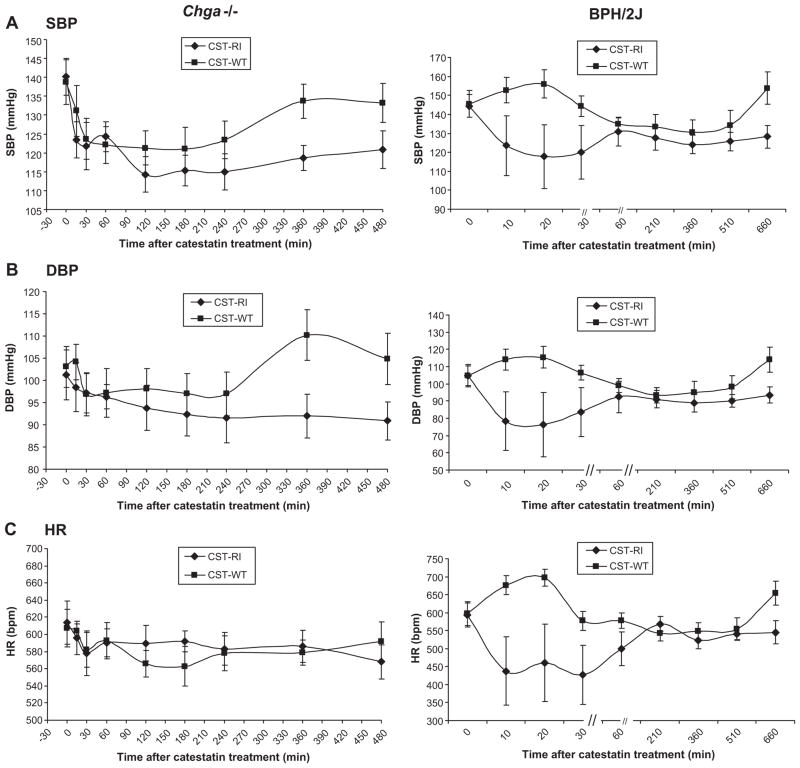
The remarkable stability as well as selective inhibitory profile of R-I peptide led us to test the potency of this peptide in vivo, in an animal model of hypertension as a consequence of catestatin deficiency: the Chga−/− mouse 11, 15 (with initial SBP/DBP 139.5±0.8/102.2±0.9 mmHg). Treatment with either W-T or R-I peptides resulted in substantial reductions of the elevated SBP/DBP in Chga−/− mice (Figure 4A and 4B, left panels). There was no difference in SBP/DBP reduction between the two peptides for the first ~240 min of treatment, but thereafter (up to ~480 min, or completion of monitoring) SBP/DBP declines were maintained only after R-I treatment; here the fall in BP was unaccompanied by reflex tachycardia (Figure 4C, left panel) 15.
Figure 4. Effect of the catestatin R-I peptide to “rescue” hypertension in monogenic Chga−/−(left) and polygenic BPH/2J (right) hypertensive mice.
Results are analyzed by two-way, repeated-measures ANOVA, evaluating the effects of drug and time.
Rescue from elevated SBP (A.) and DBP (B.) by catestatin peptides; SBP and DBP were monitored by telemetry before and after administration of catestatin peptides in Chga−/− mice (left panel, n=7) or in BPH/2J mice (right panel, n=7).
(C.) Change in heart rate after administration of W-T or R-I catestatin peptides in hypertensive Chga−/− (left panel) and BPH/2J (right panel) mice.
| Strain | Trait | Drug | Time |
|---|---|---|---|
| Chga−/− | SBP | F=6.4, p=0.01 | F=3.5, p=0.001 |
| DBP | F=6.8, p=0.01 | F=0.71, p=0.68 | |
| HR | F=0.08, p=0.77 | F=0.68, p=0.71 | |
| BPH/2J | SBP | F=15.3, p=0.0001 | F=1.9, p=0.05 |
| DBP | F=15.5, p=0.0001 | F=1.7, p=0.09 | |
| HR | F=18.1, p<0.0001 | F=2.5, p=0.008 |
Polygenic model of hypertension: BPH/2J mice
Hypertensive BPH/2J mice (with initial SBP/DBP 145.0±0.6/104.9±0.1 mmHg) demonstrated substantial reductions of both SBP and DBP after treatment with the R-I peptide; the declines were apparent by 10–30 min and were maintained for up to ~660 min (Figure 4A and 4B, right panel). By contrast, after the W-T peptide, SBP/DBP did not fall demonstrably until ~60 min after the dose, and by ~660 min both had risen to pre-treatment levels. Once again, the fall in BP after the R-I peptide was not accompanied by reflex tachycardia; indeed, HR fell by ~150 beats/min (Figure 4C, right).
DISCUSSION
Overview
In this report, we characterize a novel retro-inverso isomer of the catecholamine release inhibitory peptide catestatin, which exhibits enhanced stability with retained potency and selectivity to block multiple nicotinic cholinergic processes, culminating in prolonged antihypertensive activity in two mouse models of human hypertension.
Catestatin: Formation and physiological actions
Catestatin is an endogenous 21-amino acid peptide 22 derived from CHGA by proteolytic cleavage in hormone storage vesicles 23–27. Since its discovery, catestatin’s actions as an endogenous nicotinic cholinergic antagonist have been documented in cultured sympathochromaffin cells 5, animal models 17 (i.e., targeted ablation of the mouse Chga gene, or Chga−/−) 11, 28, and finally in humans, including observations of deficiency in hypertension 7, 9, direct evidence of vasodilation 18, and effects of catestatin endogenous genetic variation (especially Gly364Ser) on autonomic physiology and BP 10. Direct effects of catestatin on the heart have also been described 16, 29.
Such observations prompted our investigation of catestatin as a potential therapeutic agent for autonomic and cardiovascular disease, and especially systemic hypertension, and we have thus searched for more stable and/or potent versions of this peptide. Since inversion of chirality at alpha-carbons in amino acids (yielding all-D peptides) generates extreme resistance of the peptide/amide bond (C-N) to proteolytic attack, we set out to test the potency of an all-D catestatin peptide, with reversal of sequence in a retro-version, to inhibit nicotinic cholinergic processes (Table-1).
Catestatin R-I isomer
The secretory inhibition demonstrated by the R-I isomer, especially its specificity and non-competitive nature, suggests similar actions to the W-T peptide in binding to nicotinic receptors with subsequent downstream effects. In the R-I peptide, only D-amino acids were employed, and the change in chirality was counteracted by reversing the primary amino acid sequence, thus preserving the major side-chain orientations of the peptide, likely underpinning its ability to mimic the parent/W-T isoform. Retro-inverso peptides may thus exhibit improved bioavailability because of enhanced stability to proteolysis; indeed, increased stability has been demonstrated for R-I peptides mimicking enkephalin, substance P, gastrin and atrial natriuretic peptide 30.
We also documented remarkable stability of the R-I catestatin version during in vitro pronase digestion (Supplemental Figure S3), an extreme test of peptide stability. Some R-I peptides have also displayed improved blood-brain-barrier permeability 31. Finally, the catestatin R-I isomer was effective in “rescuing” (reducing) the high blood pressure phenotype in Chga−/− mice, a monogenic model of hypertension, and the therapeutic BP effect was sustained for a substantially longer interval (>8 hrs during our in vivo study) by the R-I versus W-T isoforms, likely reflecting enhanced stability of the R-I peptide in the circulation. In short, we discovered a more effective catestatin variant that showed potency over longer period, thus illustrating its therapeutic potential.
This study in context: R-I peptidomimetics
Attempts to develop synthetic R-I peptides date back ~3 decades 12, 30, with “isosteric” modification of the peptide bond, including its reversal. Here, rather than individually reversing each peptide bond, we undertook a novel and more rapid strategy, using all D-amino acid monomers and retroversion of the amino acid sequence: amino→carboxyl becoming carboxyl→amino 14. The overall side chain spatial distribution of the resulting R-I isomer seemed to recapitulate side chain orientation in the W-T version, both on molecular modeling (Figure 3A & Supplemental Figure S4A) and the CD spectrum (Figure 3B). Nonetheless, we have not created a perfect image of the original W-T peptide, for two reasons 14: first of all, the end-groups (carboxyl and amino) have not been adjusted properly; and second, the core alpha-carbons possess inverted chirality; indeed, such inversion likely accounts for resistance to proteolytic cleavage 12.
Metabolically stable and functionally active D-peptides have been described in other settings: an inhibitor of amyloid Aβ oligomerization 32, transcriptional repressor BCL6 33, inhibitors of HIV-1 integrase 34, and an antiviral octapeptide against feline immunodeficiency virus 35. A chemokine receptor CXCR4 D-peptide inhibited cellular entry of HIV-1 36. An R-I p53 effectively inhibits the tumor suppressor p53/oncoprotein MDM2 interaction 37. In certain cases, D peptides were not as effective as their parent L-peptide templates; for example, the D-isomer of JNK inhibitory peptide has reduced cytoprotective effects in pancreatic islet β cells 38, indicating structural limits to the efficacy or fidelity of peptide R-I isomers 39.
Advantages and limitations of this study
We have documented the actions of this novel catestatin R-I isomer in several settings: cellular catecholamine and transcriptional responses mediated specifically by the nicotinic cholinergic receptor, structure and physical stability, and enhanced/prolonged actions on hypertension on two mouse models in vivo. The use of neuronal nicotinic cholinergic antagonists (or “ganglionic blockers”) to treat hypertension has been problematic because of autonomic side effects. Such ganglionic blockers act mainly at neuronal nicotinic type cholinergic receptors in sympathetic and parasympathetic autonomic ganglia. Standard ganglionic blockers (such as trimethaphan) cause reflex tachycardia because they inhibit parasympathetic neurotransmission 40, the predominant autonomic influence on heart rate 41–44. By contrast, the antihypertensive response to catestatin and its peptidomimetic in experimental animals (Figure 4C) is associated with a fall in heart rate, likely representing the influence of sympathetic nervous system on heart in these two mouse models; however, we have not yet studied the effect of catestatin on heart rate in humans. We have not yet evaluated enteral delivery (in liquid or solid food) or more chronic administration of the compound: safety or toxicity after repeated administration of such isomers in vivo remains to be investigated. The number of catestatin residues requiring D-isomerization has not yet been optimized, and the R-I end-groups remain to be normalized
Conclusions and perspectives
A novel retro-inverso isoform of catestatin displays enhanced stability, preserved mechanism and potency, and prolonged in vivo actions on BP in two rodent models of human hypertension. These findings may point the way towards a potential new class of drug therapeutics for an important risk trait, and more generally open the door to broader applications of the retro-inverso strategy in other peptide mechanisms in cardiovascular biology, such as the angiotensin-II, bradykinin or endothelin pathways, wherein synthesis of a broader array of probes would be desirable for diagnostic and therapeutic intervention into physiology and disease.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new? Here we demonstrate use of a novel class of peptidomimetics to control blood pressure.
What is relevant? Hypertension remains the most potent and widespread cardiovascular risk factor, and its pharmacological treatment has achieved only limited success. Here we have explored novel peptide chemistry to develop catestatin isomers with increased stability and potency to inhibit catecholamine release as well as reduce BP in vivo.
Summary. The retro-inverso isomer of catestatin peptide demonstrated enhanced potency and stability, with preserved mechanistic specificity, and prolonged antihypertensive efficacy in vivo. The results validate a strategy to generate metabolically stable mimetics of biologically active peptides for cardiovascular therapeutics.
Acknowledgments
Support: National Institutes of Health, Department of Veterans Affairs.
GLOSSARY OF ABBREVIATIONS
- BP
Blood pressure
- CD
Circular Dichroism
- CHGA
Chromogranin A
- Chga−/−
Targeted ablation (knockout) of the mouse Chga locus
- MALDI-TOF
Matrix Assisted Laser Desorption Ionization/Time Of Flight
- R-I
Retro-Inverso
- TFA
Trifluoroacetic acid
- TFE
Trifluoroethanol
- W-T
Wild-Type
Footnotes
Disclosures: None to report.
References
- 1.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 2.Panos Kanavos JO, Weber Michael A, editors. A global assessment of current efforts to control high blood pressure and an analysis of future options to prevent a silent epidemic affecting hundreds of millions worldwide. Ruder Finn, InC; New York: 2007. High blood pressure and health policy. Where we are and where we need to go next. [Google Scholar]
- 3.O’Connor DT, Frigon RP. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem. 1984;259:3237–3247. [PubMed] [Google Scholar]
- 4.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 5.Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin A fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahata SK, Mahata M, Wakade AR, O’Connor DT. Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A(344-364)): Identification of amino acid residues crucial for activity. Mol Endocrinol. 2000;14:1525–1535. doi: 10.1210/mend.14.10.0531. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PA, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB. Heritability and genome-wide linkage in us and australian twins identify novel genomic regions controlling chromogranin A: Implications for secretion and blood pressure. Circulation. 2008;118:247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. The catecholamine release-inhibitory “catestatin” region of chromogranin A: Early decline in humans at genetic risk of hypertension. Ann N Y Acad Sci. 2002;971:533–535. doi: 10.1111/j.1749-6632.2002.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O’Connor DT. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352–372)): Naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 11.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallai PV, Richman S, Struthers RS, Goodman M. Approaches to the synthesis of retro-inverso peptides. Int J Pept Protein Res. 1983;21:84–92. doi: 10.1111/j.1399-3011.1983.tb03081.x. [DOI] [PubMed] [Google Scholar]
- 13.Chorev M, Goodman M. Partially modified retro-inverso peptides. Comparative curtius rearrangements to prepare 1,1-diaminoalkane derivatives. Int J Pept Protein Res. 1983;21:258–268. [PubMed] [Google Scholar]
- 14.Chorev M. The partial retro-inverso modification: A road traveled together. Biopolymers. 2005;80:67–84. doi: 10.1002/bip.20219. [DOI] [PubMed] [Google Scholar]
- 15.Gayen JR, Gu Y, O’Connor DT, Mahata SK. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin A null mouse. Endocrinology. 2009;150:5027–5035. doi: 10.1210/en.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. The antihypertensive chromogranin A peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 18.Fung MM, Salem RM, Mehtani P, Thomas B, Lu CF, Perez B, Rao F, Stridsberg M, Ziegler MG, Mahata SK, O’Connor DT. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin Exp Hypertens. 2010;32:278–287. doi: 10.3109/10641960903265246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochoa EL, Chattopadhyay A, McNamee MG. Desensitization of the nicotinic acetylcholine receptor: Molecular mechanisms and effect of modulators. Cell Mol Neurobiol. 1989;9:141–178. doi: 10.1007/BF00713026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 21.Mahata SK, Mahata M, Parmer RJ, O’Connor DT. Desensitization of catecholamine release. The novel catecholamine release-inhibitory peptide catestatin (chromogranin A344-364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem. 1999;274:2920–2928. doi: 10.1074/jbc.274.5.2920. [DOI] [PubMed] [Google Scholar]
- 22.Taylor CV, Taupenot L, Mahata SK, Mahata M, Wu H, Yasothornsrikul S, Toneff T, Caporale C, Jiang Q, Parmer RJ, Hook VY, O’Connor DT. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A. Determination of proteolytic cleavage sites in hormone storage granules. J Biol Chem. 2000;275:22905–22915. doi: 10.1074/jbc.M001232200. [DOI] [PubMed] [Google Scholar]
- 23.Eskeland NL, Zhou A, Dinh TQ, Wu H, Parmer RJ, Mains RE, O’Connor DT. Chromogranin A processing and secretion: Specific role of endogenous and exogenous prohormone convertases in the regulated secretory pathway. J Clin Invest. 1996;98:148–156. doi: 10.1172/JCI118760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parmer RJ, Mahata M, Gong Y, Mahata SK, Jiang Q, O’Connor DT, Xi XP, Miles LA. Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J Clin Invest. 2000;106:907–915. doi: 10.1172/JCI7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Q, Taupenot L, Mahata SK, Mahata M, O’Connor DT, Miles LA, Parmer RJ. Proteolytic cleavage of chromogranin A (CgA) by plasmin. Selective liberation of a specific bioactive CgA fragment that regulates catecholamine release. J Biol Chem. 2001;276:25022–25029. doi: 10.1074/jbc.M101545200. [DOI] [PubMed] [Google Scholar]
- 26.Biswas N, Vaingankar SM, Mahata M, Das M, Gayen JR, Taupenot L, Torpey JW, O’Connor DT, Mahata SK. Proteolytic cleavage of human chromogranin A containing naturally occurring catestatin variants: Differential processing at catestatin region by plasmin. Endocrinology. 2008;149:749–757. doi: 10.1210/en.2007-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas N, Rodriguez-Flores JL, Courel M, Gayen JR, Vaingankar SM, Mahata M, Torpey JW, Taupenot L, O’Connor DT, Mahata SK. Cathepsin L colocalizes with chromogranin A in chromaffin vesicles to generate active peptides. Endocrinology. 2009;150:3547–3557. doi: 10.1210/en.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gayen JR, Zhang K, RamachandraRao SP, Mahata M, Chen Y, Kim HS, Naviaux RK, Sharma K, Mahata SK, O’Connor DT. Role of reactive oxygen species in hyperadrenergic hypertension: Biochemical, physiological, and pharmacological evidence from targeted ablation of the chromogranin A (Chga) gene. Circ Cardiovasc Genet. 2010;3:414–425. doi: 10.1161/CIRCGENETICS.109.924050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahapatra NR. Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure. Cardiovasc Res. 2008;80:330–338. doi: 10.1093/cvr/cvn155. [DOI] [PubMed] [Google Scholar]
- 30.Chorev M, Goodman M. Recent developments in retro peptides and proteins--an ongoing topochemical exploration. Trends Biotechnol. 1995;13:438–445. doi: 10.1016/S0167-7799(00)88999-4. [DOI] [PubMed] [Google Scholar]
- 31.Taylor EM, Otero DA, Banks WA, O’Brien JS. Retro-inverso prosaptide peptides retain bioactivity, are stable in vivo, and are blood-brain barrier permeable. J Pharmacol Exp Ther. 2000;295:190–194. [PubMed] [Google Scholar]
- 32.Taylor M, Moore S, Mayes J, Parkin E, Beeg M, Canovi M, Gobbi M, Mann DM, Allsop D. Development of a proteolytically stable retro-inverso peptide inhibitor of beta-amyloid oligomerization as a potential novel treatment for alzheimer’s disease. Biochemistry. 2010;49:3261–3272. doi: 10.1021/bi100144m. [DOI] [PubMed] [Google Scholar]
- 33.Cerchietti LC, Yang SN, Shaknovich R, Hatzi K, Polo JM, Chadburn A, Dowdy SF, Melnick A. A peptomimetic inhibitor of BCL6 with potent antilymphoma effects in vitro and in vivo. Blood. 2009;113:3397–3405. doi: 10.1182/blood-2008-07-168773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zawahir Z, Neamati N. Inhibition of HIV-1 integrase activity by synthetic peptides derived from the HIV-1 HXB2 pol region of the viral genome. Bioorg Med Chem Lett. 2006;16:5199–5202. doi: 10.1016/j.bmcl.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 35.D’Ursi AM, Giannecchini S, Di Fenza A, Esposito C, Armenante MR, Carotenuto A, Bendinelli M, Rovero P. Retroinverso analogue of the antiviral octapeptide C8 inhibits feline immunodeficiency virus in serum. J Med Chem. 2003;46:1807–1810. doi: 10.1021/jm034012h. [DOI] [PubMed] [Google Scholar]
- 36.Zhou N, Luo Z, Luo J, Fan X, Cayabyab M, Hiraoka M, Liu D, Han X, Pesavento J, Dong CZ, Wang Y, An J, Kaji H, Sodroski JG, Huang Z. Exploring the stereochemistry of CXCR4-peptide recognition and inhibiting HIV-1 entry with D-peptides derived from chemokines. J Biol Chem. 2002;277:17476–17485. doi: 10.1074/jbc.M202063200. [DOI] [PubMed] [Google Scholar]
- 37.Sakurai K, Chung HS, Kahne D. Use of a retroinverso p53 peptide as an inhibitor of MDM2. J Am Chem Soc. 2004;126:16288–16289. doi: 10.1021/ja044883w. [DOI] [PubMed] [Google Scholar]
- 38.Fornoni A, Cobianchi L, Sanabria NY, Pileggi A, Molano RD, Ichii H, Rosero S, Inverardi L, Ricordi C, Pastori RL. The l-isoform but not d-isoforms of a jnk inhibitory peptide protects pancreatic beta-cells. Biochem Biophys Res Commun. 2007;354:227–233. doi: 10.1016/j.bbrc.2006.12.186. [DOI] [PubMed] [Google Scholar]
- 39.Rai J. Interaction energy analysis of peptide can predict the possibilities of mimetics by its retroinverso isomer. Chem Biol Drug Des. 2009;74:483–487. doi: 10.1111/j.1747-0285.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 40.Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, Lin MC, Li J, Grim CE, Wright FA, O’Connor DT. Chromogranin A in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 41.Chadman KK, Woods JH. Cardiovascular effects of nicotine, chlorisondamine, and mecamylamine in the pigeon. J Pharmacol Exp Ther. 2004;308:73–78. doi: 10.1124/jpet.103.057307. [DOI] [PubMed] [Google Scholar]
- 42.Janssen BJ, Leenders PJ, Smits JF. Short-term and long-term blood pressure and heart rate variability in the mouse. Am J Physiol Regul Integr Comp Physiol. 2000;278:R215–225. doi: 10.1152/ajpregu.2000.278.1.R215. [DOI] [PubMed] [Google Scholar]
- 43.Mestivier D, Dabire H, Chau NP. Effects of autonomic blockers on linear and nonlinear indexes of blood pressure and heart rate in SHR. Am J Physiol Heart Circ Physiol. 2001;281:H1113–1121. doi: 10.1152/ajpheart.2001.281.3.H1113. [DOI] [PubMed] [Google Scholar]
- 44.Turkkan JS, Kadden RM. Classically conditioned heart rate responses in macaca mulatta after beta-adrenergic, vagal and ganglionic blockade. J Auton Nerv Syst. 1979;1:211–227. doi: 10.1016/0165-1838(79)90018-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.