Abstract
Calcific aortic stenosis (CAS) is a pathological condition of the aortic valve characterized by dystrophic calcification of the valve leaflets. Despite the high prevalence and mortality associated with CAS, little is known about its pathogenetic mechanisms. Characterized by progressive dystrophic calcification of the valve leaflets, the early stages of aortic valve degeneration are similar to the active inflammatory process of atherosclerosis including endothelial disruption, inflammatory cell infiltration, lipid deposition, neo-vascularization and calcification. In the vascular system, the endothelium is an important regulator of physiological and pathological conditions; however, the contribution of endothelial dysfunction to valvular degeneration at the cellular and molecular level has received little attention. Endothelial cell (EC) activation and neo-vascularization of the cusps characterizes all stages of aortic valvular degeneration from aortic sclerosis to aortic stenosis. Here we reported the role of osteopontin (OPN) in the regulation of EC activation in vitro and in excised tissue from CAS patients and controls. OPN is an important pro-angiogenic factor in several pathologies. High levels of OPN have been demonstrated in both tissue and plasma of patients with aortic valve sclerosis and stenosis. The characterization of valvular ECs as a cellular target for OPN will help us uncover the pathogenesis of aortic valve degeneration and stenosis, opening new perspectives for the prevention and therapy of this prevalent disease.
Calcific aortic stenosis (CAS) is a slow but progressive pathological condition of the aortic valve characterized, in its final stage, by dystrophic calcification of the valve leaflets (Freeman and Otto, 2005; Goldbarg et al., 2007; Beckmann et al., 2010). It is the most frequent valvular disease, with a prevalence of 3–9%, and the main cause for valve replacement in the adult population (Bach et al., 2007). The aortic valve degeneration starts with a normal trileaflet aortic valve; initial phases of the disease include mild thickening of the leaflets whereas more advanced stages are associated with impaired leaflet motion and increased resistance to forward blood flow (Freeman and Otto, 2005). These conditions are known as aortic valve sclerosis (AVSc) and aortic valve stenosis (AVS), respectively.
Despite the high prevalence and mortality associated with aortic valve calcification, little is known about its pathogenetic mechanisms. As a result, the current treatment of choice for symptomatic AVS is surgical valve replacement (Cowell et al., 2004). Other treatment options, such as percutaneous valve replacement or aortic valvuloplasty, offer some benefits in terms of lower invasiveness and hospitalization time, but are not applicable to all patients (Balmer et al., 2004; Perin et al., 2009). Notably, surgical valve replacement in any of its forms leaves the underlying mechanism that caused the original valvular degeneration, untreated.
Degenerative lesions in human valves are characterized by endothelial cell (EC) disruption, increased cellularity and extracellular matrix deposition, accumulation of oxidized lipoproteins, non-foam cell and foam cell macrophages, and occasional T cells within the valve interstitium (O’Brien et al., 1996; Olsson et al., 1999). These histological findings resemble early sclerotic lesions of the vasculature, and together with the shared risk factors, suggest that, as in atherosclerosis, the initiation of aortic valvular sclerosis involves chronic inflammatory and neo-angiogenesis processes potentiated by systemic factors. In advanced calcific valvular lesions, prominent features include mineralized deposits composed of hydroxyapatite, several bone matrix proteins, and mature osteoblasts and osteoclasts (O’Brien et al., 1995; Mohler et al., 2001; Rajamannan et al., 2003) (Fig. S1). Therefore, valvular calcification, rather than being attributable to passive, unregulated precipitation of calcium phosphate, appears to be an active, highly regulated biomineralization process (Simmons et al., 2005).
Multiple biologic pathways are responsible for aortic valve degeneration. EC activation and neo-vascularization of the cusps characterize the valvular degeneration process since the aortic sclerosis phase. Pro-angiogenic factors are still detectable in the valve tissue when leaflet motion is impaired by calcium deposition (Collett and Canfield, 2005). The angiogenic process starts concomitantly with EC disruption, inflammation and lipid deposition (Pastor-Perez and Marin, 2009). Therefore, in the pathogenesis of CAS, injury to the endothelium is an important initial step causing thickening of the valve as a consequence of abnormal deposition of extracellular matrix, and thus preventing oxygen supply by diffusion. Decreased oxygen tension is a strong initiator of the angiogenic response through the induction of vascular endothelial growth factor (VEGF) gene expression (Ferrara and Davis-Smyth, 1997; Ferrara, 1999). The over-expression of VEGF receptors, Flk-1 and Flt-1, also increase with tissue hypoxia further supporting the importance of angiogenesis in the initiation and progression of CAS. The endothelium lining the surface of valve leaflets is presumed to be involved in valve homeostasis and pathology. Calcified aortic valve (CAV) cusps show a stronger angiogenic response than non-calcified valve (NCAV) cusps (Soini et al., 2003; Chalajour et al., 2004, 2007; Collett and Canfield, 2005). Our results, and other related studies, show the formation of tubular-like sprouts from human tissue explants of CAV leaflets using a three-dimensional (3D) culture system. Sprout formation points toward the angiogenic potential of these tissues during the process of valve degeneration. 3D collagen and immunohistochemical studies reveal that tissues from CAV leaflets have a high angiogenic response when compared to NCAV. The formation of angiogenic sprouts from stenotic valves occurs significantly faster than from non-stenotic valves (Soini et al., 2003; Chalajour et al., 2007).
Here, we first characterize the role of osteopontin (OPN) in the regulation of EC activation in vitro, and then analyzed its role on tissue excised from CAS patients and controls. OPN is a multifunctional glycol-phosphoprotein that is known for its regulatory function in bone remodeling. OPN is also involved in the inhibition of biomineralization of dystrophic and ectopic sites, including aortic valve tissue (Yu et al., 2009). Characterization of the biological process leading to valvular degeneration has been mostly focused on the formation of calcium nodules and the role of valvular interstitial cell transdifferentiation (Taylor et al., 2003). With this study, we intend to characterize the effect of OPN on cultured ECs and in excised tissue from CAS patients and controls. Studying the process of EC activation could bring to light important insights in the molecular mechanisms that drive the process of valve degeneration and help in the development of a therapeutic target before the calcification process occurs and surgical intervention becomes inevitable.
Methods
Cells, antibodies, and reagents
Human coronary artery smooth muscle cells, MEM Alpha medium, MEM medium, 10× DMEM, M200 medium, l-glutamine, fetal bovine serum, low serum growth supplement (LSGS) and penicillin/streptomycin (P/S) solutions were purchased from Invitrogen (Carlsbad, CA). Human recombinant OPN, anti-OPN neutralizing Ab and anti-VEGF neutralizing Ab were purchased from R&D systems (Minneapolis, MN), Ab anti-phospho-ERK1/2 and Ab anti-CD31 (PECAM-1) from Cell Signal (Beverly, MA), Ab anti-ERK 2, and secondary Ab goat anti-rabbit horseradish peroxidase-conjugated from Santa Cruz Biotechnology (Santa Cruz, CA), anti-αVβ3 neutralizing Ab from Millipore (Billerica, MA) and anti-CD44 neutralizing Ab from Ancell (Bayport, MN). Collagen I was purchased from BD Biosciences (San Jose, CA), VEGF from Peprotech (Rocky Hill, NJ) and UO126 from Calbiochem (San Diego, CA). For the phosphorylation study, casein kinase II was purchased from New England Biolabs (Ipswich, MA).
Wound healing assay
Mechanical endothelial damage was created in confluent human umbilical vein endothelial cells (HUVECs) growing in 12-well plates by using a sterile 200 μl tip. Cultures were reefed with low serum medium in the presence or absence of OPN (50 ng/ml), (p)OPN (50 ng/ml), NAb αvβ3 (10 μg/ml), NAb CD44 (10 μg/ml) or UO126 (10 nM). Images were taken at 0, 4, and 24 h time points. After wounding, cell migration in the recovery space was estimated by image analysis using an inverted Olympus CK2 microscope (Hitech Instruments, Inc., Broomall, CA). Wound closure was determined as the difference between wound width at 0 and 24 h.
In vitro calcification assay
In vitro calcification assay was performed using human coronary artery smooth muscle cells. Cells were cultured in Alpha MEM containing P/S, l-glutamine and10% Fetal Bovine Serum (FBS), until 70–80% confluent. Growth medium was replaced by calcification media (α-MEM containing 3 mM phosphate buffer) in all wells except the control. Fifty nanograms of recombinant OPN was used directly and casein kinase II phosphorylated recombinant OPN in separate wells in presence or absence of UO126, NAb αvβ3, or NAb CD44. Medium was replaced every 2 days. At day 8, the smooth muscle cells (SMCs) were incubated overnight in 0.6 NHCL at 4°C. Calcium was estimated using the calcium assay reagent (Biovision, Mountain View, CA). After washing the cells twice with 1× PBS, cells were harvested using I N NaOH + 3% SDS, and total protein was estimated. Amount of total calcium was expressed as mg of calcium/ml of medium. Pictures were taken at days 0 and 8 using an inverted Olympus CK2 microscope.
MAP kinase profile
Analysis of phospho-MAP kinases was carried out using the Human Phospho-MAPK Array Kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Briefly, the HUVECs and human coronary artery SMCs were grown to confluence and starved overnight with the growth medium supplemented with 0.5% FBS. Cells were then treated for 15 min with 50 ng/ml of non-phosphorylated and phosphorylated recombinant OPN. Cells were washed twice with PBS and lysates were prepared using the lysis buffer. Array included, 4 nitrocellulose membranes containing 21 different anti-kinase antibodies and 7 different controls printed in duplicate. These membranes were blocked for 2 h. One hundred micrograms of cell lysate was incubated with the membrane overnight at 4°C. Blots were washed twice and incubated with detection antibody for 2 h at room temperature. Finally, the blots were incubated with the Streptavidin-HRP for 30 min at room temperature on a rocking platform shaker. Blots were developed using the ECL substrate (Thermo Scientific). Phospho-MAPK Array data were quantitated by scanning the image and analyzing the array image file using Image J software.
Western blotting
After SDS–PAGE, proteins were transferred to PVDF membranes (Invitrogen). After transfer, membranes were blocked using 5% nonfat dried milk in TBS, pH 7.4 and incubated overnight at 4°C with the indicated antibodies. The membranes were washed three times with TBS–0.1% Tween 20 and then incubated with secondary antibodies (1:5,000) for 45 min at room temperature. After washing three-times with TBS–0.1% Tween 20, the immunoreactive bands were visualized using enhanced chemilumiescence (ECL) detection reagents. The membranes were scanned using the LAS-3000 Fujifilm Systems (Stamford, CT), and the labeled bands were quantified using the software Image J.
Tissue collection
Tissue samples were collected, through an IRB approved protocol from The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center. Patients were consented prior to intervention. The valve leaflets were collected at the time of surgical excision. The presence (or lack) of calcific AVS was determined through the analysis of cardiac catheterization, 2D-Echocardiography and clinical impression in situ. The samples were collected in media and kept at 4°C until embedded in collagen. Some control patient valves were collected through our collaboration with the Heart Failure Research Department at the University of Pennsylvania.
Baseline patients characteristics
On the total of 7 subjects, 43% were male. Their age was 61.3 ± 21.7 years. For the patients with CAS, the initial AVA was 0.8 ± 0.2, two had systemic hypertension, three had significant coronary artery disease and one was a current smoker. All patients had a comprehensive echocardiographic assessment including, M-mode, two-dimensional and color Doppler echocardiography. Supplemental Table I further characterizes the study groups. Aortic valve calcification score was 3.5 ± 0.59 in CAS patients and 1.3 ± 0.49 in controls (P < 0.001).
In vitro angiogenesis assay
Collagen I was prepared according to the manufacturer’s instruction: 80% Collagen I, 10% 10 × DMEM, and 10% 0.01 M NaOH. The solution was then titrated with 0.1 M NaOH to pH 7.4. In a 24-well plate, the tissue sample (1 mm2) was embedded in 1 ml of collagen solution and allowed to polymerize for 30 min at 37°C (5% CO2) prior to media addition. M200 medium was supplemented with 10% FBS, 1% L-Glu, 10% LSGS, and 1% P/S. The media were changed every other day. The following treatments were added: M200 Medium; VEGF 30 ng/ml; OPN 50 ng/ml; (p)OPN 50 ng/ml; UO126 10 μM; NAb CD44 10 μg/ml; NAb αVβ3 10 μg/ml; NAb OPN; and NAb VEGF. Pictures were taken every 3 days until day 14.
Immunohistochemistry
Paraffin embedded sections for both CAV and NCAV tissues were stained using the detection kit from DAKO (Glostrup, Denmark). A standard protocol was followed. Primary CD31 antibody concentration was optimized by titration and conjugated with a HRP linked secondary (DAKO). After color development using DAB+ (DAKO), a hematoxylin stain (Vector Laboratories Burlingame, CA) was applied. Slides were mounted with toluene and imaged. Alizarin Red and H&E staining were provided by the Hospital of the University of Pennsylvania’s Pathology Laboratory.
Results
Osteopontin promotes endothelial cell migration through ERK1/2 activation
Since the endothelium is one of the primary targets during valvular degeneration we first characterized the endothelial cell (EC) response to OPN in vitro (Fig. 1) and its intracellular signaling pathway (Figs. 2 and 4).
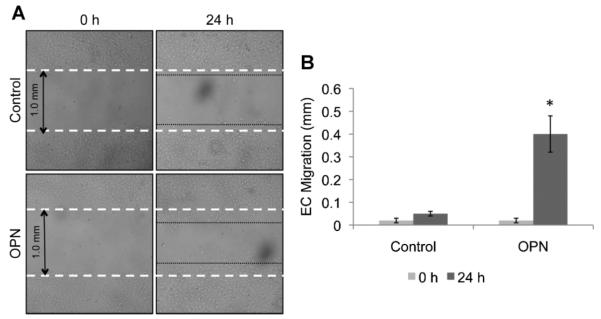
Fig. 1.
OPN induces in vitro endothelial cell migration. A: Wound healing assay of confluent HUVECs in the presence or absence of recombinant OPN (50 ng/ml) analyzed after 24 h. Magnification 4×. B: Bar graph representation of endothelial cell migration. The results of counted migrated cells are representative of three independent experiments each done in triplicate; P < 0.01
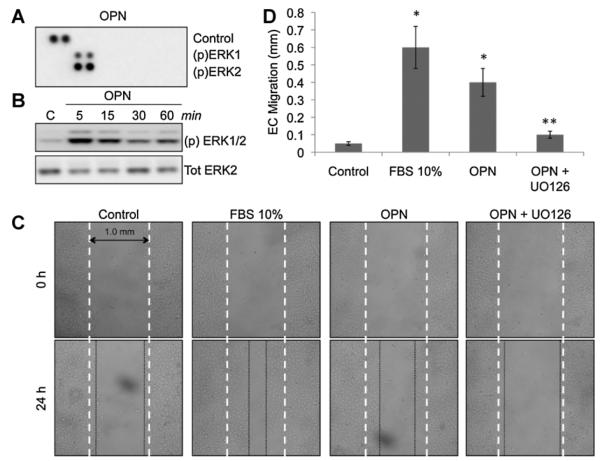
Fig. 2.
OPN promotes in vitro endothelial cell migration through ERK1/2 phosphorylation. A: Phospho-MAP kinase profile array of HUVECs treated with recombinant OPN (50 ng/ml) showing (p)ERK 1/2 activation. B: Western blotting for (p)ERK 1/2 of OPN-treated HUVECs. Tot ERK2 was used to normalize the results and as a loading control. C: Wound healing assay of confluent HUVECs treated with FBS10% as a positive control, and OPN (50 ng/ml) in the presence or absence of ERK1/2′s chemical inhibitor UO126 (10 μM). Endothelial cell migration was photographed after 24 h at 4× magnification. D: Bar graph representation of endothelial cell migration. The results of counted migrated cells are representative of three experiments each done in triplicate; P < 0.01.
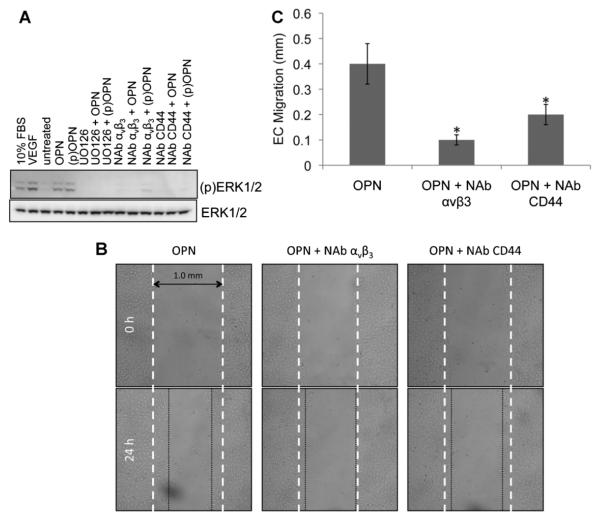
Fig. 4.
aVb3 and CD44 are necessary for OPN-induced endothelial cell migration. A: Western blotting of total protein extract of HUVECs to test ERK1/2 phosphorylation under different treatments. From left to right: FBS 10%, VEGF (30 ng/ml), untreated, OPN(50 ng/ml), (p)OPN(50 ng/ml), UO126 (10 mM) in the presence of OPN or (p)OPN, neutralizing antibody for αVβ3 (10 μg/ml) in the presence or absence of OPN or (p)OPN and neutralizing antibody for CD44 (10 μg/ml) in the presence or absence of OPN or (p)OPN. B: Wound healing assay of confluent HUVECs treated with OPN (50 ng/ml) in the presence of neutralizing antibody for CD44 or αVβ3. Endothelial cell migration was photographed after 24 h at 4× magnification. C: Bar graph representation of endothelial cell migration. The results of counted migrated cells are representative of three experiments each done in triplicate; P < 0.01.
To test the effect that OPN has on ECs, we performed a wound healing assay on cultured HUVECs and measure the EC response. As shown in Figure 1, OPN promotes EC migration in vitro. Differences in EC migration can be observed after 4 h of treatment (not shown) and are evident after 24 h of treatment (Fig. 1A,B). These experiments confirm that OPN has an active role in promoting EC migration in vitro. Similar experiments were performed with human aortic ECs, human microvascular ECs, and bovine ECs with similar results.
It has been reported that the positive effect of OPN on neo-vascularization is mediated by PI3K–ERK1/2 intracellular signaling pathway in vivo (Dai et al., 2009). To test the intracellular signaling pathway that mediates EC migration, we first analyzed the MAPK activation of HUVECs in the presence or absence of recombinant OPN. As reported in Figure 2A (and Supplement Fig. S2B) the MAPK profile array shows significant activation of ERK1/2 intracellular signaling pathway. To confirm these results, HUVECs were treated for the indicated time with recombinant OPN and then analyzed in Western blotting with phospho-specific ERK1/2 antibody (Fig. 2B). This experiment confirms ERK1/2 intracellular signaling activation under OPN treatment. We therefore utilized a widely used ERK1/2 chemical inhibitor (UO126) to test if EC migration in vitro was mediated by ERK1/2. In Figure 2, we demonstrated using the wound healing assay, that blocking ERK1/2 activation results in the abolishment of EC migration in vitro (Fig. 2C,D).
OPN biological activity is controlled by its phosphorylation status in both endothelial cells and smooth muscle cells
To further characterize the role of OPN on the regulation of EC migration, we considered the effects of OPN’s post-translational modifications on EC activation. Recombinant OPN was phosphorylated in vitro using casein kinase II (Fig. S2A). The extent of the phosphorylation of OPN, as well as, the phosphorylation occurring on specific sites, may play an important role in its physiological functions (Jono et al., 2000). It has been described that the ability of OPN to inhibit calcification depends on its phosphorylation status (Saad et al., 2008) (Fig. 3C,D). Since cultured smooth muscle cells (SMCs) and valvular interstitial cells (VICs) share many molecular and physiological characteristics (Supplemental Table II), an in vitro calcification assay has been extensively used as a model to test the biological activity of proteins involved in the calcium deposition related to aortic valve degeneration (Gericke et al., 2005; Speer et al., 2005).
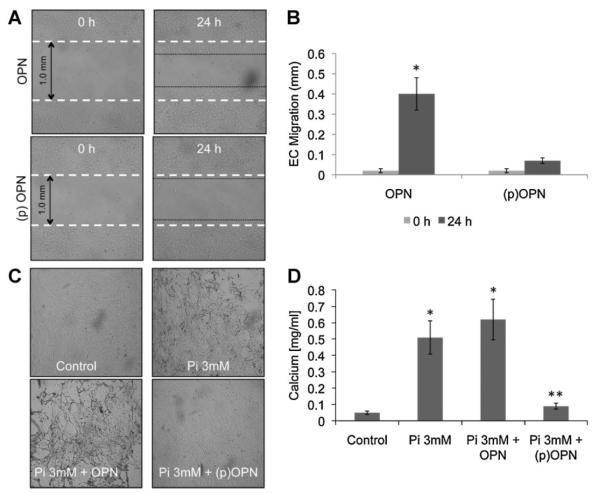
Fig. 3.
OPN phosphorylation status controls in vitro endothelial cell migration and in vitro smooth muscle cell calcification. A: Wound healing assay of confluent HUVECs in the presence or absence of recombinant OPN (50 ng/ml) or phosphorylated OPN (50 ng/ml) analyzed after 24 h.Magnification 4×. B: Bargraph representation of endothelial cell migration. The results of counted migrated cells were representative of three experiments each done in triplicate; P < 0.01. C: In vitro calcification of confluent smooth muscle cells treated with calcification medium (MEM containing 3 mM phosphate buffer) in the presence or absence of OPN (50 ng/ml) or (p)OPN (50 ng/ml). D: At day 8 of treatment, cells were washed twice with PBS and incubated overnight in 0.6 N HCl at 4°C. Calcium was estimated using the calcium assay reagent (Biovision, Mountain View, CA). Amount of total calcium is expressed as calcium mg/ml of medium. Pictures were taken at days 0 and 8 at 4× magnification. The results are representative of three independent experiments each done in triplicate; P < 0.01.
We phosphorylated the recombinant OPN in vitro and tested its ability to control calcium deposition on a SMC based calcification assay. As shown in Figure 3C,D, dephosphorylated recombinant OPN did not inhibit human SMC biomineralization, while phosphorylated recombinant OPN inhibits calcium deposition in vitro. These experiments confirm that the phosphorylation status controls the ability of OPN to inhibit biomineralization.
We then performed wound healing experiments in the presence or absence of in vitro phosphorylated OPN to investigate the role of this post-translational modification on EC activation. As shown in Figure 3A,B, phospho-OPN does not activate EC migration in vitro, whereas, as shown in Figure 1, dephosphorylated OPN promotes EC migration. These results suggest a different mechanism of action of OPN on different cellular populations. The phosphorylation status controls OPN’s biological activity on both ECs and SMCs: in SMC in vitro calcification assay OPN requires phosphorylation to inhibit calcium deposition; on the contrary, dephosphorylated OPN has no effects in preventing calcium deposition on the cellular surface. On ECs, dephosphorylated OPN promotes EC migration whereas phospho-OPN prevents EC migration.
Role of Osteopontin on CD44 and aVb3 signaling in endothelial cell migration compared to smooth muscle cell calcification
The primary receptors for OPN are those integrins that bind the central integrins attachment motif RGD. Since no specific OPN receptors have been identified so far, integrin αVβ3 was established as a primary receptor for OPN. In addition to the integrin binding, OPN also binds with a hyaluronic acid receptor, CD44 in an RGD-independent manner, inducing macrophage chemotaxis and engagement of β3-integrin receptors (Okamoto, 2007).
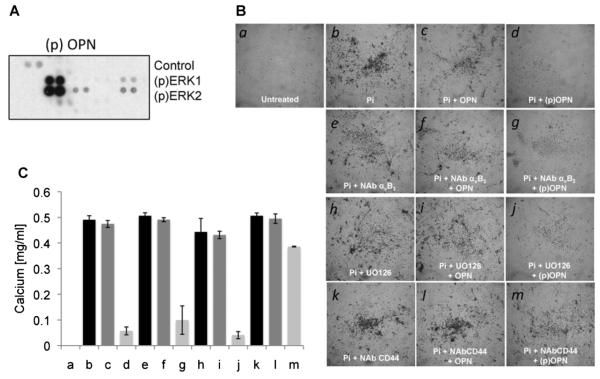
Since both CD44 and αVβ3 can mediate the biologic activities of OPN, we first analyzed the role of OPN on ERK1/2 activation in the presence or absence of neutralizing antibodies for CD44 and αVβ3 in endothelial and smooth muscle cells. Figure 4A shows that both neutralizing antibodies for CD44 and αVβ3 blocks ERK1/2 phosphorylation. As shown in Figure 5A, phospho-OPN activates ERK1/2 in smooth muscle cells.
Fig. 5.
CD44 but not αVβ3 regulated (p)OPN-induced smooth muscle cell bio-mineralization. A: Phospho-MAP kinase profile array of human smooth muscle cells treated with recombinant OPN (50 ng/ml) showing (p)ERK 1/2 activation. B: In vitro calcification of confluent smooth muscle cells treated with calcification medium (MEM containing 3 mM phosphate buffer) in the presence of the indicated treatments. C: At day 8 of treatment, calcium was estimated using the calcium assay reagent. Amount of total calcium is expressed as calcium mg/ml of medium. Pictures were taken at 4× magnification. The results are representative of three independent experiments each done in triplicate; P < 0.01.
Then, we characterized the biological effects of these two neutralizing antibodies on EC migration and SMC calcification. As reported in Figure 4B,C, we performed wound healing assays on HUVECs in presence or absence of neutralizing antibodies for CD44 and αVβ3. As shown, the neutralization of either CD44 or αVβ3 inhibits OPN effects on EC migration.
Based on these results and to complete the in vitro characterization of OPN’s biological activity, we tested the protective biological activity of OPN using an in vitro calcification assay in the presence or absence of UO126, and the neutralizing antibodies for CD44 and αVβ3. The inhibition of ERK1/2 with the chemical inhibitor UO126 does not disrupt the protective effect of phospho-OPN on the in vitro calcification assay. Similar results were obtained by blocking the activity of αVβ3 with a neutralizing antibody (Fig. 5B,C). On the contrary, a neutralizing antibody for CD44 reverts the inhibitory effects of OPN and allows for calcium deposition on the surface of cultured SMCs. These experiments suggest that the protective effect of phosphorylated OPN is independent of ERK1/2 and mainly mediated by the activity of CD44.
OPN controls endothelial cell migration of aortic valve tissue through ERK1/2
Angiogenesis is a key step in the remodeling of the aortic valve and the development of progressive valve calcification and degeneration. We decided to investigate the response of aortic valve tissue treated with OPN and the previously described intracellular signaling pathways. Although exposed to different hemodynamic conditions HUVECs and AV-derived ECs share many molecular and physiologic characteristics (Supplemental Table II). To evaluate the ability of valvular ECs to migrate and form tubular-like structures, leaflets of calcified aortic valves (CAV) and non-calcified aortic valves (NCAV) were embedded in a 3D collagen type I matrix as described in Methods. At day 14, formation of tubular-like structures was evaluated with a phase contrast microscope. From cultures of non-calcified leaflets, the endothelial migrating cells formed a monolayer around the valve tissue with few tubular-like structures. Calcified leaflets showed extensive tubular-like structures (Fig. 6A,B).
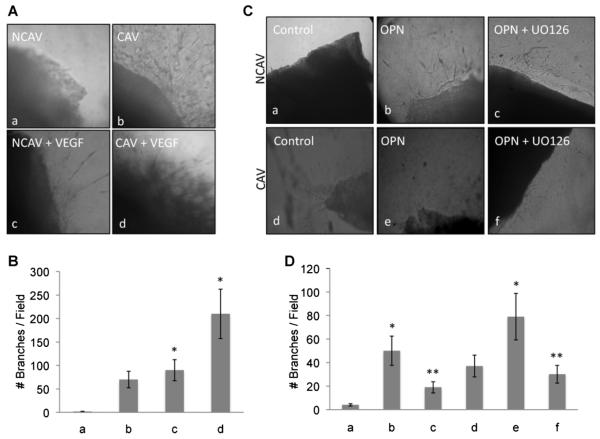
Fig. 6.
OPN induces in vitro angiogenesis of excised human aortic valve tissues through ERK1/2. A: In vitro angiogenesis assay of excised tissue from calcified and non-calcified aortic valve. Tissue was embedded in the collagen as described in the Methods Section and treated with VEGF (30 ng/ml). B: Bar graph representation of the in vitro angiogenesis assay. The number of tubular-like structures was counted as a number of branches per field. Pictures were taken at 10× magnification. C: Non-calcified and calcified tissue embedded in collagen and treated with OPN in the presence or absence of UO126 (10 μM). B: Bar graph representation of the in vitro angiogenesis assay. The number of tubular-like structures was counted as a number of branches per field. Pictures were taken at 10× magnification. The results are representative of three independent experiments each done in triplicate; P < 0.01.
Among the angiogenic factors, VEGF has been shown to play a major role in both physiologic and pathologic conditions, due to its unique biologic capacity to induce migration and proliferation of ECs, to enhance vascular permeability and to modulate thrombogenicity (Ferrara and Davis-Smyth, 1997). Accordingly, in Figure 6A,B we show that VEGF treatment of both CAV and NCAV enhances the angiogenic response of cultured valve tissues. These results confirm the angiogenic potential of ECs originating from aortic valve leaflets. CAV tissue shows more angiogenic activity than NCAV tissue.
To test if OPN has a similar effect on valvular ECs, we repeated the cell sprouting assay in the presence or absence of OPN and we measured the cell number, migration distance and the formation of tubular-like structures of ECs derived from both NCAV and CAV tissue. As reported in Figure 6C, OPN promotes migration and tubular-like formation in our 3D angiogenesis assay, suggesting that both HUVEC and valvular EC migration are controlled by OPN. Figure 6D is a bar graph representation of the number of branches per field analyzed.
We then tested the effect of OPN on valvular tissue to confirm the ERK1/2 mediation of the effects of OPN on valvular EC migration. As reported in Figure 6C,D, OPN enhances EC migration into the 3D collagen matrix and shows that UO126 (a chemical inhibitor of ERK1/2) blocks such migration. Together, these results suggest that both HUVECs and aortic valve-derived ECs are activated by OPN and that the activation is mediated by an ERK1/2 intracellular signaling pathway.
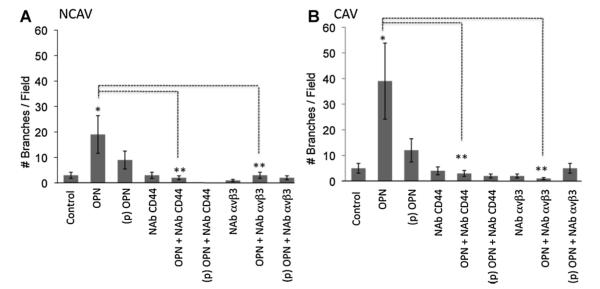
Finally, we tested the role of both CD44 and αVβ3 on the formation of tubular-like structures on aortic valve derived tissue (Fig. 7). In accord with our in vitro experiments, both CD44 and αVβ3 are necessary for EC migration and collagen sprouting. The neutralization of either CD44 or αVβ3 blocks the cellular reorganization into the collagen matrix. These results confirm that cultured ECs and aortic valve-derived tissue share the same cellular response when treated with OPN or phospho-OPN. The intracellular signaling pathway activated by OPN is mediated by CD44 and αVβ3; this results in ERK1/2 activation (Fig. 7A,B). VEGF, the major EC derived proangiogenic factor, increases OPN and αVβ3-integrin expression in HUVECs and stimulates integrin dependent EC motility (Senger et al., 1996). Recently, it has been reported that OPN increased VEGF expression in tumor cells stimulating neo-vascularization. In turn, VEGF also induced the overexpression of OPN in tumor cells (Chakraborty et al., 2008). Thus, experimental evidence suggests that OPN may affect angiogenesis by acting directly on ECs.
Fig. 7.
αVβ3 and CD44 are necessary for OPN-induced in vitro angiogenesis of excised human aortic valve tissues. A: Bar graph representation of the in vitro angiogenesis assay. Tissues were embedded in the collagen as described in the Methods Section and treated as indicated. The number of tubular-like structures was counted as the number of branches per field. Pictures were taken at 10× magnification. The results are representative of three independent experiments each done in triplicate; P < 0.01.
Since VEGF increases OPN and αVβ3-integrin expression in HUVECs and stimulates integrin dependent EC motility (Senger et al., 1996), we tested the effect of neutralizing anti-VEGF and neutralizing anti-OPN on cellular migration of AV-derived tissue. As expected, both treatments resulted in the inhibition of cellular motility (Fig. S3). Analyzing the cellular and molecular biology responses of EC activation could bring to light very important insights on the mechanisms that drive the process of valve degeneration and help in development of a therapeutic target before the calcification process occurs and surgical intervention becomes inevitable.
Discussion
With an estimated prevalence of 5.2 million affected people in the United States, CAS represents the most common type of valvular disease (Bach et al., 2007). The prevalence increases with age, so that approximately 30% of all individuals aged 65+ years have AVSc and 4% have AVS (Cowell et al., 2004). As a result of the steady population growth and rising life expectancy, the demand for aortic valve operations will significantly increase in the future (Northrup et al., 2002; Freeman and Otto, 2005; Thom et al., 2006; Beckmann et al., 2010). The current treatment of choice for AVS is surgical valve replacement (Cowell et al., 2004), either with mechanical or biological prostheses. Currently, there is no FDA-approved treatment to halt the progression of calcific aortic valve degeneration.
Although calcification is the most common problem associated with aortic valve disease, the cellular physiology and molecular events that underlie this process are not fully elucidated.
The assumption that CAS is only a passive, age-related disease has been strongly questioned by numerous studies showing that the pathogenesis of this disease actively requires the activation and transdifferentiation of several cellular components. The process of calcification is believed to be a highly programmed sequence of different intercellular signaling events, involving different cellular populations, which influence many fundamental cellular functions, such as proliferation, migration, gene transcription, cell–cell adhesion and cell–matrix adhesion (Gu and Masters, 2010).
Cardiac VICs, the primary cellular component involved in the biomineralization process, are a heterogeneous and dynamic population. They express molecular markers similar to those of SMCs performing a complex and highly sophisticated series of functions over a wide range of hemodynamic conditions (Taylor et al., 2003). Within the aortic valve leaflets, a number of cellular phenotypes can be distinguished; VICs represent the most abundant and more studied cellular population. However, a growing number of studies have been recently focused on the endothelium (El-Hamamsy et al., 2009; Simmons, 2009). These cells, actively interacting with the non-cellular component of the leaflets, are responsible for many valvular functions. Injury to the EC covering the valve leaflets is an important initial step in the pathogenesis of calcific aortic valve degeneration. As a result, EC activation and neo-vascularization within the leaflet tissue is a hallmark of aortic valve sclerosis and stenosis. The overexpression of pro-angiogenic factors such as VEGF, Flk-1 and Flt-1 further supports the assumption that angiogenesis is closely related to the progression of aortic valve degeneration.
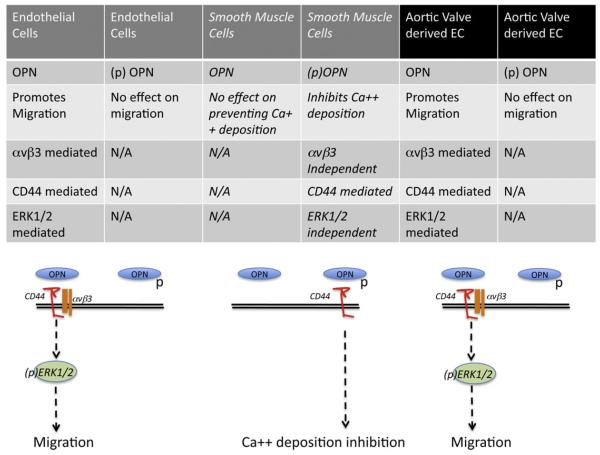
OPN is a phosphorylated, acidic, RGD-containing glycoprotein that binds certain CD44 variants and integrin receptors, including αVβ3 (Denhardt et al., 2001). The OPN–αVβ3 interaction is important for osteoclast migration and resorption, as well as, SMC migration and adhesion. OPN acts as a cytokine, playing important roles in the migration and invasion of several tumor cells (Rittling and Chambers, 2004; Tuck et al., 2007). In addition, OPN is regarded to be an important angiogenic factor in several pathologies. High levels of OPN have been demonstrated in both tissue and plasma of patients with aortic valve sclerosis and stenosis. Here we described the biological effects of OPN in vitro and in excised aortic valve tissue. Our results, combined with previously reported studies, confirm that the biological function of OPN on aortic valve degeneration is not exclusively related to the biomineralization process of VICs. OPN also plays an important role on different cellular targets, such as ECs. Calcified rheumatic valves have also been shown to express neo-angiogenesis factors (Rajamannan, 2005). Although angiogenesis is considered to be part of the early stages of calcification, pro-angiogenic factors are expressed even when the valve is completely calcified, suggesting that this mechanism is still present and active during the entire degeneration process. Immunohistochemistry for the EC markers CD31, CD34, von-Willebrand factor and Tie-2 and for the cell adhesion molecules, reveals an intense neovascularization of stenotic cusps in contrast to non-stenotic cusps. In Figure S1, we show immunohistochemistry staining of CD31 in NCAV and CAV tissue. Correspondingly, it has been shown that ECs of stenotic but not those of non-stenotic valves exhibit CEACAM1, a cell adhesion molecule expressed in angiogenic, but not in quiescent ECs (Ergun et al., 2000; Chalajour et al., 2004; Yetkin and Waltenberger, 2009). Chalajour et al. (2004) have demonstrated that the formation of angiogenic sprouts from calcified valves occurs significantly faster than from non-calcified valves. The capillary-like tubes sprouting from explanted tissue shows a positive staining for CD31. Immunoreactivity of CD44 and vWF has also been demonstrated in the tubular-like structures (Chalajour et al., 2004). We report our analysis of the role of OPN on cultured ECs and SMCs, two cellular phenotypes that, due to their physiological and molecular characteristics, resemble the two major cellular populations of the aortic valve leaflets. We then compared these results with the effects of OPN on aortic valve derived tissue form CAS patients and controls. Our analysis shows that OPN activated the same intracellular signaling pathways in HUVECs and excised aortic valve tissue. In addition, we reported a differential OPN activated intracellular signaling pathway in EC and SMC (Fig. 8).
Fig. 8.
Schematic representation of OPN and (p)OPN effect on endothelial cells, smooth muscle cells and excised aortic valve tissues from CAS patients and controls.
Based on the presented results, EC migration of both HUVEC and aortic valve derived cells is dependent on CD44 and αVβ3 and results in ERK1/2 phosphorylation. Since no specific OPN receptors have been identified, the receptors for OPN are those integrins that bind the central integrins attachment motif RGD, with the integrin αVβ3 considered one of the primary mediators of OPN’s activity on different cell types. In addition to integrin binding, OPN also binds CD44 in an RGD-independent manner, inducing macrophage chemotaxis and engagement of β3-integrin receptors (Okamoto, 2007). Interestingly, OPN’s biological effect on SMCs is dependent on the phosphorylation status of OPN and it is mediated exclusively by CD44. It is possible that different OPN post-translational modifications enable the protein to bind different receptors with variable affinity and therefore regulate a differential biological response.
In the aortic valve, lipid deposits and calcific lesions occur primarily in the fibrosa, the layer of the valve immediately beneath the endothelium on the aortic side of the valve (Otto et al., 1994; O’Brien et al., 1996; Simmons et al., 2005). The preferential susceptibility to lesion formation on the aortic rather than ventricular surface of the aortic valve may result from coordinated regulation of gene expression by the respective endothelia, resulting in side-specific endothelial phenotypes that favor or inhibit calcification. Furthermore, during the cardiac cycle, the aortic valve endothelium is subjected to complex fluid dynamics that are distinctly different on either side of the valve (Nicosia et al., 2003; Simmons et al., 2005). Thus, there is a spatial correlation between the nature of calcific lesions and the local hemodynamic environment, similar to that observed in the regions of large arteries more susceptible to atherogenesis. Local environmental factors and biomechanical forces may also contribute to the development of differential endothelial phenotypes with different susceptibility to focal calcification. This observation could generate the question of whether AV-derived cells exhibit differential properties when compared to AV tissue due to different mechanical stresses in response to directional flow. However, our preliminary histological and cytological analysis shows equivalence in the EC surface and intracellular signaling pathways activation on both sides of the aortic valve leaflets, suggesting that, although the hemodynamic conditions are different, the cellular response is similar. Knowledge of the complex mechanisms of cell–cell and cell–matrix interaction is essential in order to understand how different cell populations contribute to the progressive degeneration of the valve. These data provide new insights into the cellular and molecular mechanisms involving stenotic aortic valvular tissue and ECs. These results help us better understand the interaction between different cell populations within the valve tissue and bring new potential perspectives to the development of future preventive treatments for this condition.
Supplementary Material
Acknowledgments
This project is currently supported by award number RC1HL100035 from the National Heart, Lung, And Blood Institute, NIH (GF, JG, PP, and BF) and by the “Harrison Memorial Fund” of the University of Pennsylvania School of Medicine (RS and WS). We thank Kenneth B. Margulies, from the Heart Failure Research Department at the University of Pennsylvania for his precious collaboration in the collection of heart valve specimens.
Contract grant sponsor: National Heart, Lung and Blood Institute;
Contract grant number: RC1HL100035.
Contract grant sponsor: NIH.
Contract grant sponsor: University of Pennsylvania School of Medicine (Harrison Memorial Fund).
Footnotes
Additional Supporting Information may be found in the online version of this article.
Literature Cited
- Bach DS, Radeva JI, Birnbaum HG, Fournier AA, Tuttle EG. Prevalence, referral patterns, testing, and surgery in aortic valve disease: Leaving women and elderly patients behind? J Heart Valve Dis. 2007;16:362–369. [PubMed] [Google Scholar]
- Balmer C, Beghetti M, Fasnacht M, Friedli B, Arbenz U. Balloon aortic valvoplasty in paediatric patients: Progressive aortic regurgitation is common. Heart. 2004;90:77–81. doi: 10.1136/heart.90.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the use of biomarkers in calcific aortic valve disease. J Heart Valve Dis. 2010;19:441–452. [PMC free article] [PubMed] [Google Scholar]
- Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- Chalajour F, Treede H, Ebrahimnejad A, Lauke H, Reichenspurner H, Ergun S. Angiogenic activation of valvular endothelial cells in aortic valve stenosis. Exp Cell Res. 2004;298:455–464. doi: 10.1016/j.yexcr.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Chalajour F, Treede H, Gehling UM, Ebrahimnejad A, Boehm DH, Riemer RK, Ergun S, Reichenspurner H. Identification and characterization of cells with high angiogenic potential and transitional phenotype in calcific aortic valve. Exp Cell Res. 2007;313:2326–2335. doi: 10.1016/j.yexcr.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- Cowell SJ, Newby DE, Boon NA, Elder AT. Calcific aortic stenosis: Same old story? Age Ageing. 2004;33:538–544. doi: 10.1093/ageing/afh175. [DOI] [PubMed] [Google Scholar]
- Dai J, Peng L, Fan K, Wang H, Wei R, Ji G, Cai J, Lu B, Li B, Zhang D, Kang Y, Tan M, Qian W, Guo Y. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412–3422. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hamamsy I, Balachandran K, Yacoub MH, Stevens LM, Sarathchandra P, Taylor PM, Yoganathan AP, Chester AH. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol. 2009;53:1448–1455. doi: 10.1016/j.jacc.2008.11.056. [DOI] [PubMed] [Google Scholar]
- Ergun S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach JH, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell adhesion molecule 1: A potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5:311–320. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sorensen ES, Boskey AL. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbarg SH, Elmariah S, Miller MA, Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205–1213. doi: 10.1016/j.jacc.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Gu X, Masters KS. Regulation of valvular interstitial cell calcification by adhesive peptide sequences. J Biomed Mater Res A. 2010;93:1620–1630. doi: 10.1002/jbm.a.32660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- Mohler ER, III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- Nicosia MA, Cochran RP, Einstein DR, Rutland CJ, Kunzelman KS. A coupled fluid-structure finite element model of the aortic valve and root. J Heart Valve Dis. 2003;12:781–789. [PubMed] [Google Scholar]
- Northrup WF, III, Dubois KA, Kshettry VR, Teskey JM, Nicoloff DM. Trends in aortic valve surgery in a large multi-surgeon, multi-hospital practice, 1979–1999. J Heart Valve Dis. 2002;11:768–778. discussion 778–779. [PubMed] [Google Scholar]
- O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- O’Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Osteopontin and cardiovascular system. Mol Cell Biochem. 2007;300:1–7. doi: 10.1007/s11010-006-9368-3. [DOI] [PubMed] [Google Scholar]
- Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- Pastor-Perez F, Marin F. Hypertension, aortic sclerosis and the prothrombotic state: Understanding the complex interaction. J Hum Hypertens. 2009;23:287–288. doi: 10.1038/jhh.2008.103. [DOI] [PubMed] [Google Scholar]
- Perin MA, Brito FS, Jr., Almeida BO, Pereira MA, Abizaid A, Tarasoutchi F, Grube E. Percutaneous aortic valve replacement for the treatment of aortic stenosis: Early experience in Brazil. Arq Bras Cardiol. 2009;93:299–306. doi: 10.1590/s0066-782x2009000900015. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM. Calcific aortic stenosis: Medical and surgical management in the elderly. Curr Treat Options Cardiovasc Med. 2005;7:437–442. doi: 10.1007/s11936-005-0028-9. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad FA, Salih E, Glimcher MJ. Identification of osteopontin phosphorylation sites involved in bone remodeling and inhibition of pathological calcification. J Cell Biochem. 2008;103:852–856. doi: 10.1002/jcb.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- Simmons CA. Aortic valve mechanics: An emerging role for the endothelium. J Am Coll Cardiol. 2009;53:1456–1458. doi: 10.1016/j.jacc.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soini Y, Salo T, Satta J. Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum Pathol. 2003;34:756–763. doi: 10.1016/s0046-8177(03)00245-4. [DOI] [PubMed] [Google Scholar]
- Speer MY, Chien YC, Quan M, Yang HY, Vali H, McKee MD, Giachelli CM. Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc Res. 2005;66:324–333. doi: 10.1016/j.cardiores.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int J Biochem Cell Biol. 2003;35:113–118. doi: 10.1016/s1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr., Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Tuck AB, Chambers AF, Allan AL. Osteopontin overexpression in breast cancer: Knowledge gained and possible implications for clinical management. J Cell Biochem. 2007;102:859–868. doi: 10.1002/jcb.21520. [DOI] [PubMed] [Google Scholar]
- Yetkin E, Waltenberger J. Molecular and cellular mechanisms of aortic stenosis. Int J Cardiol. 2009;135:4–13. doi: 10.1016/j.ijcard.2009.03.108. [DOI] [PubMed] [Google Scholar]
- Yu PJ, Skolnick A, Ferrari G, Heretis K, Mignatti P, Pintucci G, Rosenzweig B, Diaz-Cartelle J, Kronzon I, Perk G, Pass HI, Galloway AC, Grossi EA, Grau JB. Correlation between plasma osteopontin levels and aortic valve calcification: Potential insights into the pathogenesis of aortic valve calcification and stenosis. J Thorac Cardiovasc Surg. 2009;138:196–199. doi: 10.1016/j.jtcvs.2008.10.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.