Abstract
A 57-year-old woman, who had undergone Roux-en-Y gastric bypass surgery 9 years earlier, was admitted to the intensive care unit because of pneumonia. Despite antibiotic therapy, she died 40 days later, apparently because of sepsis and organ failure related to the pneumonia. However, the patient's family requested an autopsy, which revealed that her death was due to perforation of the Roux limb of her gastric bypass, which had resulted in severe peritonitis. The perforation was caused by a nasogastric tube inserted for enteral nutrition. We discuss ways nasogastric tubes might be inserted more safely after gastric bypass, the response of Baylor University Medical Center at Dallas to this complication, and the role of autopsy in improving the quality of hospital care.
In 2005, it was estimated that approximately 1 million people in the United States had undergone Roux-en-Y gastric bypass (RYGB) for treatment of severe obesity (1). When RYGB functions well for a number of years, it may be overlooked or forgotten when a patient is admitted to a hospital for a nongastrointestinal problem. Some of these patients, especially those admitted in intensive care units (ICUs), will receive nasogastric or orogastric tubes for enteral nutrition (2), often according to hospital protocol under the direction of nutrition services and nursing staff.
This article describes a case where insertion of a nasogastric tube caused intestinal perforation in a patient who had previously undergone RYGB. Like most of the nasogastric tubes used for enteral nutrition or for removal of gastric contents by suction, the tube that caused intestinal perforation in our patient was made of polyvinylchloride (PVC). Such tubes are flexible, but they are stiff enough to permit advancement through the nose or mouth into the stomach or duodenum.
CASE REPORT
A 57-year-old woman was brought to the emergency department with a 3-day history of productive cough and confusion. She had been on chronic immunosuppression with adalimumab and methotrexate for rheumatoid arthritis and was started on prednisone about 1 month earlier for Hashimoto's encephalopathy. She had had RYGB 9 years earlier for severe obesity. A chest radiograph showed an infiltrate in the right middle and upper lobe. While in the emergency department, she developed severe respiratory distress, and an endotracheal tube was inserted. A 16 Fr (5.3 mm) PVC orogastric tube was also inserted, presumably for routine gastric decompression to prevent aspiration. A subsequent radiograph was interpreted by a radiologist as showing that the tube was “apparently in the stomach.” (A retrospective review of the film showed that the proximal aperture of the tube was below the gastroesophageal junction and that the tip was in the upper part of the Roux limb; Figure 1a.) She was then admitted to the ICU with a preliminary diagnosis of health care–associated pneumonia and started on broad-spectrum intravenous antibiotics.
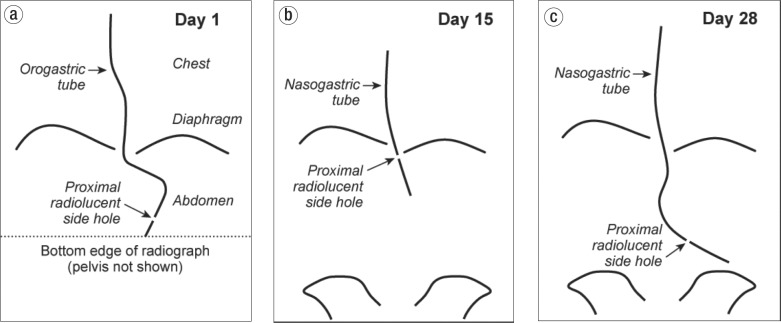
Figure 1.
Schematic diagram of the radiographs obtained on (a) Day 1, (b) Day 15, and (c) Day 28 depicting the location and course of the orogastric or nasogastric tubes within the lower esophagus and abdominal cavity.
Sputum staining showed gram-negative rods, and the cultures grew Escherichia coli and methicillin-sensitive Staphylococcus aureus. Blood cultures were negative. The patient was hemodynamically stable on hospital day 2, and the nutrition service recommended enteral tube feedings. On hospital day 3, tube feedings were started using the orogastric tube that had been inserted in the emergency department.
On hospital day 15, the patient removed her endotracheal and orogastric tubes. She developed hypoxemia shortly thereafter and the endotracheal tube was replaced. A new 16 Fr PVC nasogastric tube was also inserted, and a portable abdominal radiograph was interpreted as showing that the tip of the tube was in the stomach. (A retrospective review of this film showed that the proximal radiolucent side hole of the tube was located near the gastroesophageal junction [Figure 1b]; the distal 8 cm of tube containing smaller holes was presumably located in the gastric pouch and Roux limb.) Tube feedings were resumed. Over the next 2 weeks, the patient remained in the ICU and slowly improved.
On hospital day 28, the nasogastric tube could not be aspirated or flushed; it was therefore removed and a new 16 Fr PVC tube was inserted. Three portable supine abdominal radiographs were utilized during this tube insertion. The first and second radiographs revealed that the tip of the tube had not passed the apparent esophagogastric junction, and the radiologist recommended advancement of the tube after each of the two films. After the second advancement, a third film was interpreted as follows: “Nasogastric tube courses into the stomach with the proximal side hole visualized well below the gastroesophageal junction.” Retrospective review of the final abdominal radiograph taken on day 28 showed that the inserted nasogastric tube took a different course within the abdominal cavity than the orogastric tube that was inserted on day 1 and that it deviated leftward and extended about 20 cm beyond the assumed location of the gastrojejunostomy (Figure 1c). There was no evidence of free air in the peritoneal cavity on the three supine abdominal radiographs taken on day 28.
After interpretation of the third radiograph on day 28, Oxepa® tube feeding was instituted (35 to 50 mL/hour). Approximately 12 liters of tube feedings were infused through the tube during the next 11 days. “Gastric residual volumes” were zero on multiple occasions. Bowel sounds were present, and initially the abdomen was recorded to be nondistended. However, the patient's clinical condition gradually worsened with hypotension, hypoxemia, fever, leukocytosis, renal failure requiring dialysis, and diarrhea. Fecal fluid tested negative for Clostridium difficile toxin. On day 39, the abdomen was noted to be distended and firm. After a family meeting on day 39, the decision was made to withdraw life support measures, including continuous venovenous hemodialysis, vasopressors, mechanical ventilation, and nasogastric feeding. The nasogastric tube was therefore withdrawn. The patient expired about an hour after the vasopressors were stopped. The clinical diagnosis at the time of death was pneumonia, sepsis, and multiorgan failure. Subsequent to her death, the patient's family requested an autopsy.
Autopsy
Examination of the lungs revealed intraalveolar fibrosis and organizing diffuse alveolar damage consistent with resolving pneumonia. There was no evidence of aspiration of foreign material.
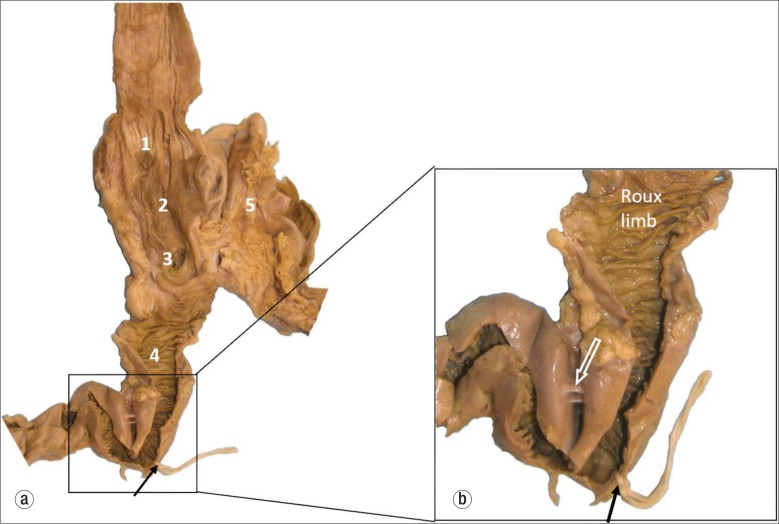
Examination of the abdominal cavity revealed adhesed loops of small intestine encased in approximately 1600 mL of purulent fluid within a loculated area in the left upper quadrant. A well-demarcated 4- to 5-mm circular perforation was visible on the external surface of one of the loops of small intestine (Figure 2). Further dissection revealed that the small intestinal perforation was in the jejunal Roux limb 14 cm distal to the gastrojejunostomy, immediately proximal to a hairpin turn of the jejunum (Figure 3). This hairpin turn of jejunum was caused by adhesions between two loops of the jejunal Roux limb. Microscopic refractile material was entrapped in the serosal inflammatory infiltrate, consistent with food or pill particles.
Figure 2.
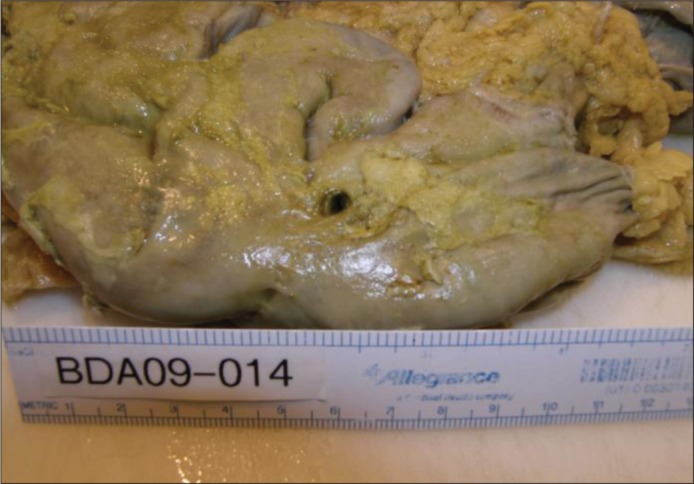
View at autopsy of formalin-fixed external surface of loops of small intestine bound together by adhesions and containing a 5 mm small intestinal perforation.
Figure 3.
(a) The dissected upper gastrointestinal tract: 1) gastroesophageal junction; 2) gastric pouch; 3) opening of the blind Roux loop; 4) Roux limb; 5) excluded (bypassed) stomach. The black arrow and string show the site of perforation. (b) A closer view of the hairpin turn of the Roux limb caused by adhesions (white arrow).
The round and sharply circumscribed transmural perforation was not associated with histological evidence of peptic ulcer, vasculitis, or transmural ischemic necrosis of the surrounding bowel wall. The pathological findings were most compatible with perforation from an inserted nasogastric tube (which had been withdrawn just prior to death). Subsequent review of radiographs (Figure 1) also led to the conclusion that the perforation was the result of insertion of the nasogastric tube on day 28.
The pathologist concluded that the immediate cause of death was purulent peritonitis and sepsis due to perforation of the Roux limb of a gastric bypass procedure that had been performed years earlier. As stated above, the perforation was caused by a nasogastric tube inserted for nutritional support. The pneumonia that precipitated her admission to the hospital was resolving at the time of death.
DISCUSSION
The fact that this patient had had an RYGB procedure 9 years prior to her admission was noted in the past surgical history, but was not mentioned in daily progress notes, nursing notes, or nutrition service notes during her 40-day hospital stay. A review of the hospital records revealed no evidence that the radiologists who interpreted nasogastric tube positions were made aware of the previous RYGB.
When this complication was discussed at a clinical case conference, it became clear that physicians, nurses, and dietitians at our hospital did not know of any possible increased risks of nasogastric tube insertion in patients who previously had an RYGB. Moreover, in reviewing medical and nutritional publications, we found no case reports or guidelines stating that insertion of any type of nasogastric tube was dangerous in patients who had a remote history of RYGB. However, a search of Internet discussion boards on bariatric surgery revealed that some patients who have undergone RYGB fear complications from nasogastric tubes or have been cautioned by their surgeon not to receive a nasogastric tube (3, 4). Wikipedia has an article on nasogastric intubation that states that “use of an NG tube is also contraindicated in patients who have had gastric bypass surgery” (5). This statement appears to have been added in July 2008, about a year before our patient was admitted with pneumonia.
Our single case does not prove that patients with RYGB have a higher risk of complications from nasogastric tube insertion than patients who have not had RYGB. However, we believe that the complication experienced by our patient, when combined with knowledge of RYGB anatomy and pathophysiology, logically suggests that insertion of nasogastric tubes after RYGB may be more dangerous.
Nasogastric or orogastric tubes are designed for intubation of an anatomically normal stomach, which is large, highly distensible, and can hold an air volume of >1600 mL with little or no increase in intragastric pressure (6). A standard PVC nasogastric tube in our hospital is 122 cm in length, and its distal 8 cm contains multiple side holes through which fluids can be aspirated or infused. The entirety of the distal 8 cm of the tube can easily fit within a normal stomach. If excess tube length is inserted, the tube can coil within the normal stomach with little risk of causing significant damage. In contrast, the volume of the gastric pouch following RYGB is only about 30 mL, and its height is only about 4 cm (7, 8). There is no room for tube coiling, and it is unlikely that all of the holes in the distal 8 cm could be within the gastric pouch at the same time. Either some proximal holes of the tube would be in the lower esophagus, or some of the distal holes would have traversed the gastrojejunal anastomosis and be located in the Roux limb.
In the normal stomach, there are no recognized anatomic sites that are highly vulnerable to injury by a nasogastric tube, whereas in patients with RYGB there are several vulnerable sites. One of these is the narrow anastomosis between the gastric pouch and jejunum. This gastrojejunal anastomosis is usually about 10 mm in diameter, only about twice the diameter of a 16 Fr tube (5.3 mm), and it is often poorly vascularized, making it susceptible to ulceration and injury (9, 10). Another location prone to injury is the blind loop of the Roux limb, which has no direct exit (Figure 4) (11). The wall of this blind loop is much thinner than the stomach wall. Still another vulnerable area is the proximal Roux limb, also known as the alimentary limb, which carries ingested food through the upper intestine. Like the blind loop, its wall is thin. Moreover, due to the operation that creates an RYGB, or due to anastomotic leaks in the early postoperative period, or due to previous unrelated abdominal surgical procedures, serosal adhesions may develop and produce kinks in the Roux limb. In our patient, fibrous serosal adhesions caused a hairpin turn 14 cm below the gastrojejunostomy, and this prevented the inserted tube from moving distally within the jejunum, facilitating perforation when the tube was advanced.
Figure 4.
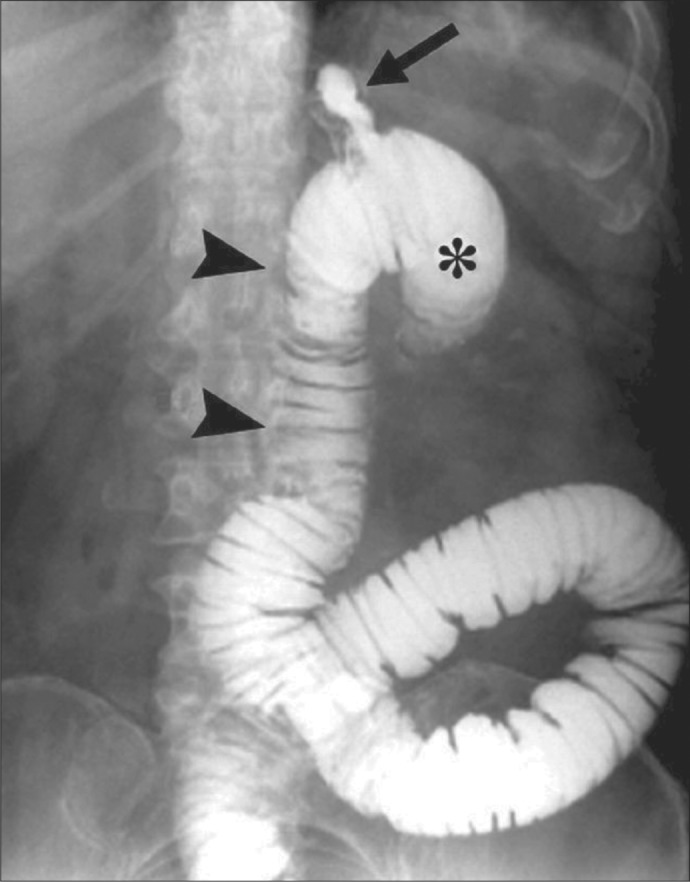
Upper gastrointestinal radiograph after ingestion of contrast in a previously published patient who had had an RYGB. The upper part of the Roux limb is identified by the two arrowheads; the gastric pouch, by the arrow; and the blind limb, by the asterisk. The blind limb and the Roux limb are distended in this case because an internal hernia had caused obstruction of the jejunum. This film illustrates the ease with which a nasogastric tube could be advanced into the blind loop. Reprinted with permission from Merkle et al, 2005 (11).
Despite the possible increased risk of perforation, in several clinical situations use of a nasogastric tube is therapeutically essential in RYGB patients—most notably in those who have intestinal obstruction or prolonged ileus. Moreover, our single case report does not justify a ban on the use of PVC nasogastric tubes for nutritional support in patients who have previously received RYGB. However, our case does suggest that extra caution be employed when intubating patients who have had an RYGB.
Based on our experience with this case, we have several suggestions. The nurse or physician inserting the tube and the radiologist should know if the patient has previously had an RYGB, and they should have knowledge of bypass anatomy and the vulnerable sites noted above. They should not advance a PVC tube against resistance. They should recognize the substantial variation in distances between the nares and the upper part of the small intestine in people with an intact stomach (ranging from 51 to 74 cm) (12) and how these distances are altered by RYGB.
In adult patients with normal gastric anatomy, the length of tube, measuring from the nose, that has to be inserted so that the tip of the tube would lie in the body of the stomach can be estimated by using the formula {(NEX − 50)/2} +50, where NEX is nose to earlobe to xiphoid length in centimeters (13). This calculated length of tube can be inserted and then proper position can be verified using a radiograph. For many patients this calculated distance for initial insertion is about 58 cm, and presumably for this reason the PVC nasogastric tubes in our hospital are ink marked at 58 cm from the tip of the tube. Although this is probably an entirely safe length of tube to blindly insert in patients with normal gastric anatomy, in our opinion it is 10 to 15 cm longer than should be initially inserted into a patient who has previously had RYGB.
The final position of the tube, following adjustments based on radiography, should probably have the proximal side hole of the tube just below the estimated location of the lower esophageal sphincter. If the tube is not inserted far enough, nutrient solutions will be infused into the esophagus through the proximal holes of the tube, with risk of aspiration. (This problem could be mitigated somewhat by using a nasogastric tube with a single opening in the most distal part of the tube, although this might reduce efficacy of suctioning.) If too much tube is inserted, the end of the tube will move far down in the Roux limb, which has an unpredictable course.
The risk of perforation with PVC tubes could probably be reduced by using fluoroscopic guidance, which allows visualization of the tip of the tube as the tube is advanced. Using polyurethane or silicone nasogastric tubes, which are softer and more flexible than PVC tubes, would further reduce the risk. These tubes are more difficult to insert, a problem that can be mitigated by use of a guide wire if needed (14).
Hospital response
Following autopsy, a conference was held between Baylor physicians and the patient's family. The autopsy results were fully explained, and the family was told that death of the patient was caused by perforation of the small intestine during insertion of a nasogastric tube.
This complication was discussed extensively at a special case conference. It was decided that prior bariatric surgery would be added to the list of conditions in which a physician, rather than a nurse, would insert nasogastric tubes. The revised Baylor policy for nasogastric/orogastric tube insertion is as follows: “In patients with altered physiology of the nares, oropharynx, esophagus, or stomach, such as occurs with bariatric surgery, other gastric surgery, nasal deformity or surgery, esophageal varices, or chronic epistaxis, nurses will not insert nasogastric or orogastric tubes, but consult with the physician to perform the procedure.” Two years after the new policy, one of the authors of this report interviewed 10 Baylor nurses from different floors and ICUs, and 9 of them were aware of the requirement for a physician to insert gastric tubes in patients who have had a gastric bypass. It was pointed out that this policy is also conveyed to newly hired nursing staff during their orientation.
The authors also requested the medical safety officer of Baylor University Medical Center at Dallas to consider adding a requirement that a radiologist who interprets the position of gastric tubes be informed when patients have previously received bariatric surgery.
The role of autopsy in this case
This case illustrates, once again, the value of a traditional hospital autopsy for discovery of clinically unanticipated findings and how such information may lead to useful modifications of hospital policies and procedures and provide the family with the true cause of death.
Autopsies on patients who die in the hospital are mainly done at the request of the patient's family or physicians in order to clarify the cause of death, to assess clinical care, and occasionally for other purposes (15). The average hospital autopsy rate declined in the United States from 16.9% in 1972 to 4.3% in 2007 (16, 17). At the present time, autopsy rates remain near 20% in only a few hospitals, including Brigham and Women's Hospital (personal communication, Gayle Winters, MD, August 23, 2012), Mayo Clinic (personal communication, Joseph J. Maleszewski, MD, August 23, 2012), and The Johns Hopkins Hospital (18). At Mayo Clinic, a concerted effort is under way to raise the autopsy rate from 25% to 50% (personal communication, Joseph J. Maleszewski, MD, August 23, 2012). At Baylor University Medical Center at Dallas, autopsy rates from 2006 to 2011 were relatively constant and averaged 4.4%. In our opinion, with rates this low, it is impossible to accurately calculate the frequency of therapeutic complications and misdiagnoses, and the quality of teaching programs and health care improvement programs is compromised.
References
- 1.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294(15):1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 2.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G A.S.P.E.N. Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. JPEN J Parenter Enteral Nutr. 2009;33(3):277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 3.Thinner Times Forum. Medical alert bracelets? Available at www.thinnertimesforum.com/topic/43543-medical-alert-bracelets/www.thinnertimesforum.com/topic/55652-anyone-told-to-wear-a-medical-id-bracelet/; accessed August 23, 2012.
- 4.ObesityHelp. Medical alert bracelet? NG tube? Available at www.obesityhelp.com/forums/rny/3403505/Medical-Alert-Bracelet-NG-Tube/; accessed August 23, 2012.
- 5.Wikipedia. Nasogastric intubation. (revised July 7, 2012). Available at http://en.wikipedia.org/wiki/Nasogastric_intubation; accessed August 23, 2012.
- 6.McNally EF, Kelly JE, Jr, Ingelfinger FJ. Mechanism of belching: effects of gastric distension with air. Gastroenterology. 1964;46:254–259. [PubMed] [Google Scholar]
- 7.Alva S, Eisenberg D, Duffy A, Roberts K, Israel G, Bell R. Virtual three-dimensional computed tomography assessment of the gastric pouch following laparoscopic Roux-Y gastric bypass. Obes Surg. 2008;18(4):364–366. doi: 10.1007/s11695-008-9438-6. [DOI] [PubMed] [Google Scholar]
- 8.Roberts K, Duffy A, Kaufman J, Burrell M, Dziura J, Bell R. Size matters: gastric pouch size correlates with weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2007;21(8):1397–1402. doi: 10.1007/s00464-007-9232-x. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen NT, Stevens CM, Wolfe BM. Incidence and outcome of anastomotic stricture after laparoscopic gastric bypass. J Gastrointest Surg. 2003;7(8):997–1003. doi: 10.1016/j.gassur.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Printen KJ, Scott D, Mason EE. Stomal ulcers after gastric bypass. Arch Surg. 1980;115(4):525–527. doi: 10.1001/archsurg.1980.01380040147026. [DOI] [PubMed] [Google Scholar]
- 11.Merkle EM, Hallowell PT, Crouse C, Nakamoto DA, Stellato TA. Roux-en-Y gastric bypass for clinically severe obesity: normal appearance and spectrum of complications at imaging. Radiology. 2005;234(3):674–683. doi: 10.1148/radiol.2343030333. [DOI] [PubMed] [Google Scholar]
- 12.Ahrens EH, Jr, Blankenhorn DH, Hirsch J. Measurement of the human intestinal length in vivo and some causes of variation. Gastroenterology. 1956;31(3):274–284. [PubMed] [Google Scholar]
- 13.Hanson RL. Predictive criteria for length of nasogastric tube insertion for tube feeding. JPEN J Parenter Enteral Nutr. 1979;3(3):160–163. doi: 10.1177/014860717900300310. [DOI] [PubMed] [Google Scholar]
- 14.Carrasquilla C, Weiss M, Gianos J. Safe intestinal decompression in fresh postoperative gastric bypass. Obes Surg. 2006;16(9):1256–1260. doi: 10.1381/096089206778392293. [DOI] [PubMed] [Google Scholar]
- 15.Autopsy Committee of the College of American Pathologists. The autopsy, medicine, and mortality statistics. Vital Health Stat. 2001;3(32):1–42. [PubMed] [Google Scholar]
- 16.Hoyert DL. The changing profile of autopsied deaths in the United States, 1972-2007. NCHS Data Brief. 2011. pp. 1–8. 67. [PubMed]
- 17.Nemetz PN, Tanglos E, Sands LP, Fisher WP, Jr, Newman WP, 3rd, Burton EC. Attitudes toward the autopsy—an 8-state survey. MedGenMed. 2006;8(3):80. [PMC free article] [PubMed] [Google Scholar]
- 18.March D. Traditional physical autopsies—not high-tech “virtopsies”—still “gold standard” for determining cause of death [press release] Baltimore, MD: Johns Hopkins Medicine; January 16, 2012. Available at www.hopkinsmedicine.org/news/media/releases/traditional_physical_autopsies___not_high_tech_virtopsies___still_gold_standard_for_determining_cause_of_deathwww.hopkinsmedicine.org/news/media/releases/traditional_physical_autopsies___not_high_tech_virtopsies___still_gold_standard_for_determining_cause_of_death; accessed August 23, 2012. [Google Scholar]