Abstract
T-cell prolymphocytic leukemia is a rare and unusual malignancy characterized by the proliferation of small- to medium-sized prolymphocytes of postthymic origin with distinctive clinical, morphologic, immunophenotypic, and cytogenetic features. Involvement of the peripheral blood, bone marrow, lymph nodes, liver, spleen, and skin can occur. The clinical course is typically very aggressive with poor response to conventional chemotherapy and short survival rates, and the only potential long-term curative treatment is hematopoietic stem cell transplantation. We report the case of a man with de novo T-cell prolymphocytic leukemia and discuss the distinctive clinical, morphologic, immunophenotypic, and cytogenetic features of this entity.
Prolymphocytic leukemia (PLL) is a rare lymphocytic disorder characterized by marked lymphocytosis and splenomegaly and represents only 2% of all mature lymphocytic leukemias in adults over the age of 30. PLL is clearly defined into subtypes as B-cell prolymphocytic leukemia (B-PLL) and T-cell prolymphocytic leukemia (T-PLL), with T-PLL representing approximately 20% of the cases (1). While these subtypes have their similarities, T-cell and B-cell PLL are two distinct diseases with different clinical and laboratory features. T-PLL is more rare and more rapidly progressive and aggressive than B-PLL (2). T-PLL is generally resistant to conventional chemotherapy, and historically the median survival has been about 7 months, while that for B-CLL is 30 to 50 months (3–5). We report a case of de novo T-PLL and discuss the typical clinical, morphologic, immunophenotypic, and cytogenetic features of this entity.
CASE PRESENTATION
A 75-year-old African American man was hospitalized at Baylor University Medical Center at Dallas in May 2012 after a recent fall and a 4-month history of progressive weakness. An evaluation by an oncologist 18 months earlier had disclosed a white cell count of 47,000 K/uL, hematocrit of 44%, and platelet count of 165,000 K/uL. A differential count at that time revealed many convoluted, atypical lymphocytes comprising 91% of the circulating mononuclear cells. Peripheral blood flow cytometry confirmed a T-PLL with lymphocytes expressing CD2, CD3, CD4, CD5, CD7, CD26, and CD52. This population was negative for CD1a, CD8, and TdT. The clonal T-cell receptor beta gene rearrangement assays were positive. Cytogenetics confirmed a complex karyotype including an inversion of chromosome 14 and isochromosomes 8q. Fluorescence in situ hybridization also revealed 11q22 deletion and 17p13 loss. The patient was lost to follow up, and he did not return for marrow assessment or to discuss treatment options.
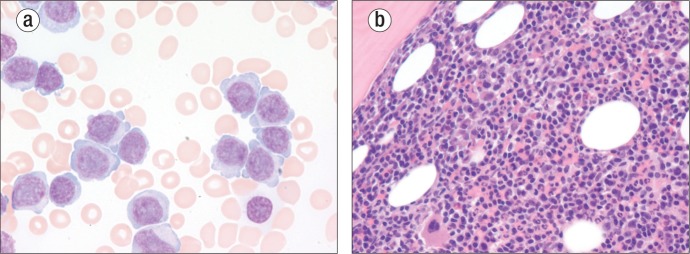
On this admission, he was lethargic and appeared chronically ill. Medications included amlodipine, rosuvastatin, and ranitidine. There was no peripheral lymphadenopathy. Auscultation of the chest revealed diffuse rhonchi, normal heart sounds, and a 2/6 systolic murmur. His spleen was palpable 7.0 cm below the costal margin. His hematocrit was 29% and his white blood cell count was 476,000 K/uL with 96% lymphocytes and 3% neutrophils. Many atypical large lymphocytes with slightly convoluted nuclei containing prominent nucleoli and copious nongranular cytoplasm were present (Figure 1a). The platelet count was 47,000 K/uL. Blood cultures on admission were positive for coagulase-negative Staphylococcus. Uric acid was 7.9 mg/dL, and lactate dehydrogenase was markedly elevated at 2369 u/L (reference range 185–249). Creatinine was 1.6 mg/dL, and liver function studies were normal. A computed tomography scan of the chest revealed mediastinal lymphadenopathy, and an abdominal sonogram confirmed the splenomegaly.
Figure 1.
Morphology of T-cell prolymphocytic leukemia. (a) Peripheral blood stained with Wright's stain (original magnification under oil 1000×). (b) Bone marrow stained with hematoxylin and eosin (original magnification 400×).
On the second hospital day, a bone marrow biopsy (Figure 1b) along with flow cytometry and cytogenetics confirmed T-PLL. During his hospitalization he was treated with a 7-day course of 2-chlorodeoxyadenosine at 0.1 mg/kg intravenously daily by continuous infusion. He was maintained on broad-spectrum antibiotics but developed renal insufficiency and recurrent gastrointestinal bleeding secondary to a benign esophageal ulcer documented on endoscopy. His white blood cell count only marginally improved with his chemotherapy, with a white count of 199,000 K/uL after 6 days of chemotherapy. At that time intensive care was withheld, comfort care measures were instituted, and the patient died on the 10th hospital day.
DISCUSSION
T-PLL, a rare hematological malignancy, was first described in 1973. It represents <2% of mature lymphocytic leukemias (1, 6, 7). T-PLL primarily affects older adults with an average age at presentation of 65 years with a slight male predominance (8). Most patients present with hepatosplenomegaly (splenomegaly in 82% to 92%) and generalized lymphadenopathy (1). Other common findings include skin lesions (27%) and pleural serous effusions (14%) (7).
The peripheral blood commonly exhibits anemia and thrombocytopenia with a marked lymphocytosis and lymphocyte counts frequently >100,000 K/uL (7). A distinctive hematologic aspect is a rapidly rising white blood cell count with a doubling time of weeks to months (9). The key morphologic feature in the diagnosis of T-PLL is a population of prolymphocytes in the peripheral blood. The typical morphology consists of prolymphocytes of medium size with condensed nuclear chromatin, a single prominent nucleolus, and intensely basophilic nongranular cytoplasm with cytoplasmic protrusions or “blebs.” The nuclei can be round, oval, or irregular (10, 11). In 25% of cases, the cell size is smaller and the nucleolus may not be visible by light microscopy (small cell variant) (12). In 5% the nuclear outline is markedly irregular and can even be cerebriform, mimicking Sézary cells (13). Both of these variants are otherwise similar to typical T-PLL, including immunophenotype and cytogenetics, and thus it is justified that all three are grouped together in a single category (11).
The bone marrow is diffusely infiltrated by prolymphocytes in most cases with variable residual hematopoiesis. Reticulin fibrosis is almost always present (10, 11). When the spleen is involved, histology finds a dense red pulp infiltrate with invasion into the splenic capsule, blood vessels, and extension into the atrophied white pulp. In lymph nodes, the involvement is diffuse with paracortical expansion by T prolymphocytes, sometimes with sparing of follicles (14). Multiple prominent high-endothelial venules are often infiltrated by neoplastic cells (10). Skin involvement differs from that seen in mycosis fungoides and Sézary syndrome, with dermal infiltrates primarily around the appendages and without epidermotropism (7, 15).
Immunophenotypically, T prolymphocytes are mature postthymic peripheral T cells that do not express TdT and the cortical thymic marker CD1a. The cells are positive for CD2, CD3, and CD5 and have strong CD7 staining. This strong CD7 intensity is in contrast to other mature T-cell malignancies, where this marker may be weak or negative. The membrane expression of CD3 may be weak or even negative in occasional cases, but T-cell receptor-beta/gamma chain genes are always rearranged. CD52 is usually expressed at high density in T prolymphocytes and can be used as a target of therapy by the monoclonal antibody alemtuzumab (8, 16). In 65% of patients, the cells are CD4+, CD8−, and in 13% they are CD4−, CD8+. In 21% the T prolymphocytes coexpress CD4 and CD8, which is a feature almost unique to T-PLL. The distinctive coexpression of CD4 and CD8 together with the weak CD3 membrane expression and the strong CD7 expression suggest that the T-PLL cell may be at an intermediate stage of differentiation between a cortical thymocyte and a circulating mature T cell (7). The most specific markers for T-PLL by immunophenotyping are CD26 and TCL-1 protein expression, which are not detected in the other mature T-cell leukemia/lymphomas (11). The overexpression of the oncogene TCL1 is useful for detecting residual T-PLL in bone marrow sections after therapy (9).
T-PLL is characterized by complex chromosomal abnormalities, which suggests that chromosomal aberrations might occur progressively during the course of the disease, thus explaining the aggressive nature of this condition. Recurrent changes mainly affect chromosomes 14, 8, 11, and X (17). The most common characteristic chromosome abnormality, seen in 80% of cases, is inversion of chromosome 14 with breakpoints in the long arm at q11 and q32 (inv (14)(q11;q32)). Reciprocal tandem translocations between the two chromosomes 14 occur in 10% (t14;14)(q11;q32) (17, 18). These two rearrangements involve the 14q11 and 14q32.1 loci, where the genes coding for TCRα and the protooncogene TCL-1 are localized, respectively. The rearrangements result in juxtaposition of these two genes and lead to expression and activation of TCL-1 (19). About 20% of patients have the translocation t(X;14)(q28;q11), which results in rearrangement of the MTCP1 gene (20). Both TCL-1 and MTCP-1 have oncogenic properties, as both can induce a T-cell leukemia (CD4−/CD8+) in transgenic mice (19, 21, 22). Abnormalities involving both arms of chromosome 8 are frequent, t(8;8)(p11-12;q12) as well as trisomy 8q, with both being seen in 70% to 80% of cases. Other alterations seen in T-PLL include deletions of 12p13 and 11q22, with the latter being the locus for the ataxia telangiectasia mutated gene. Abnormalities of chromosome 6 and 17 and deletion of the TP53 gene are also not uncommonly encountered (10).
T-PLL is aggressive and often resistant to therapy. The overall prognosis is poor, with a median survival of approximately 7 months in patients treated with conventional regimens. Recently the average survival has been extended to >2 years following the introduction of newer therapies (11). The initial treatment of choice for most patients is the monoclonal antibody alemtuzumab (anti-CD52); the best responses have been seen with this agent, but responses are still transient and further disease progression is inevitable (3, 23). Purine analogues also have activity in this disease (24, 25). Both autologous and allogeneic stem cell transplants in patients who achieve remission have been used and are associated with more durable outcomes (26). Because of the severe immunosuppression associated with alemtuzumab, the purine analogue 2-chlorodeoxyadenosine was selected for the reported patient's treatment.
References
- 1.Robak T, Robak P. Current treatment options in prolymphocytic leukemia. Med Sci Monit. 2007;13(4):RA69–RA80. [PubMed] [Google Scholar]
- 2.Madaris L. T-cell prolymphocytic leukemia: a rare disease in an elderly female. J Am Acad Nurse Pract. 2010;22(12):648–653. doi: 10.1111/j.1745-7599.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 3.Dearden CE, Matutes E, Cazin B, Tjønnfjord GE, Parreira A, Nomdedeu B, Leoni P, Clark FJ, Radia D, Rassam SM, Roques T, Ketterer N, Brito-Babapulle V, Dyer MJ, Catovsky D. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98(6):1721–1726. doi: 10.1182/blood.v98.6.1721. [DOI] [PubMed] [Google Scholar]
- 4.Del Giudice I, Davis Z, Matutes E, Osuji N, Parry-Jones N, Morilla A, Brito-Babapulle V, Oscier D, Catovsky D. IgVH genes mutation and usage, ZAP-70 and CD38 expression provide new insights on B-cell prolymphocytic leukemia (B-PLL) Leukemia. 2006;20(7):1231–1237. doi: 10.1038/sj.leu.2404238. [DOI] [PubMed] [Google Scholar]
- 5.Ruchlemer R, Parry-Jones N, Brito-Babapulle V, Attolico I, Wotherspoon AC, Matutes E, Catovsky D. B-prolymphocytic leukaemia with t(11;14) revisited: a splenomegalic form of mantle cell lymphoma evolving with leukaemia. Br J Haematol. 2004;125(3):330–336. doi: 10.1111/j.1365-2141.2004.04913.x. [DOI] [PubMed] [Google Scholar]
- 6.Catovsky D, Galetto J, Okos A, Galton DA, Wiltshaw E, Stathopoulos G. Prolymphocytic leukaemia of B and T cell type. Lancet. 1973;2(7823):232–234. doi: 10.1016/s0140-6736(73)93135-8. [DOI] [PubMed] [Google Scholar]
- 7.Matutes E, Brito-Babapulle V, Swansbury J, Ellis J, Morilla R, Dearden C, Sempere A, Catovsky D. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood. 1991;78(12):3269–3274. [PubMed] [Google Scholar]
- 8.Dearden CE. T-cell prolymphocytic leukemia. Med Oncol. 2006;23(1):17–22. doi: 10.1385/MO:23:1:17. [DOI] [PubMed] [Google Scholar]
- 9.Herling M, Khoury JD, Washington LT, Duvic M, Keating MJ, Jones D. A systematic approach to diagnosis of mature T-cell leukemias reveals heterogeneity among WHO categories. Blood. 2004;104(2):328–335. doi: 10.1182/blood-2004-01-0002. [DOI] [PubMed] [Google Scholar]
- 10.Catovsky D, Ralfkiaer E, Muller-Hermelink HK. T-cell prolymphocytic leukemia. Pathology and genetics of tumours of haemopoietic and lymphoid tissues. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organisation Classification of Tumours. Lyon, France: IARC Press; 2008. pp. 270–271. [Google Scholar]
- 11.Dearden CE. T-cell prolymphocytic leukemia. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S239–S243. doi: 10.3816/CLM.2009.s.018. [DOI] [PubMed] [Google Scholar]
- 12.Matutes E, Garcia Talavera J, O'Brien M, Catovsky D. The morphological spectrum of T-prolymphocytic leukaemia. Br J Haematol. 1986;64(1):111–124. doi: 10.1111/j.1365-2141.1986.tb07579.x. [DOI] [PubMed] [Google Scholar]
- 13.Pawson R, Matutes E, Brito-Babapulle V, Maljaie H, Hedges M, Mercieca J, Dyer M, Catovsky D. Sezary cell leukaemia: a distinct T cell disorder or a variant form of T prolymphocytic leukaemia? Leukemia. 1997;11(7):1009–1013. doi: 10.1038/sj.leu.2400710. [DOI] [PubMed] [Google Scholar]
- 14.Osuji N, Matutes E, Catovsky D, Lampert I, Wotherspoon A. Histopathology of the spleen in T-cell large granular lymphocyte leukemia and T-cell prolymphocytic leukemia: a comparative review. Am J Surg Pathol. 2005;29(7):935–941. doi: 10.1097/01.pas.0000160732.43909.3f. [DOI] [PubMed] [Google Scholar]
- 15.Mallett RB, Matutes E, Catovsky D, Maclennan K, Mortimer PS, Holden CA. Cutaneous infiltration in T-cell prolymphocytic leukaemia. Br J Dermatol. 1995;132(2):263–266. doi: 10.1111/j.1365-2133.1995.tb05023.x. [DOI] [PubMed] [Google Scholar]
- 16.Matutes E. T-cell prolymphocytic leukemia. Cancer Control. 1998;5(1):19–24. doi: 10.1177/107327489800500102. [DOI] [PubMed] [Google Scholar]
- 17.Maljaei SH, Brito-Babapulle V, Hiorns LR, Catovsky D. Abnormalities of chromosomes 8, 11, 14, and X in T-prolymphocytic leukemia studied by fluorescence in situ hybridization. Cancer Genet Cytogenet. 1998;103(2):110–116. doi: 10.1016/s0165-4608(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 18.Brito-Babapulle V, Catovsky D. Inversions and tandem translocations involving chromosome 14q11 and 14q32 in T-prolymphocytic leukemia and T-cell leukemias in patients with ataxia telangiectasia. Cancer Genet Cytogenet. 1991;55(1):1–9. doi: 10.1016/0165-4608(91)90228-m. [DOI] [PubMed] [Google Scholar]
- 19.Pekarsky Y, Hallas C, Isobe M, Russo G, Croce CM. Abnormalities at 14q32.1 in T cell malignancies involve two oncogenes. Proc Natl Acad Sci U S A. 1999;96(6):2949–2951. doi: 10.1073/pnas.96.6.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stern MH, Soulier J, Rosenzwajg M, Nakahara K, Canki-Klain N, Aurias A, Sigaux F, Kirsch IR. MTCP-1: a novel gene on the human chromosome Xq28 translocated to the T cell receptor alpha/delta locus in mature T cell proliferations. Oncogene. 1993;8(9):2475–2783. [PubMed] [Google Scholar]
- 21.Gritti C, Dastot H, Soulier J, Janin A, Daniel MT, Madani A, Grimber G, Briand P, Sigaux F, Stern MH. Transgenic mice for MTCP1 develop T-cell prolymphocytic leukemia. Blood. 1998;92(2):368–373. [PubMed] [Google Scholar]
- 22.Virgilio L, Lazzeri C, Bichi R, Nibu K, Narducci MG, Russo G, Rothstein JL, Croce CM. Deregulated expression of TCL1 causes T cell leukemia in mice. Proc Natl Acad Sci U S A. 1998;95(7):3885–3889. doi: 10.1073/pnas.95.7.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keating MJ, Cazin B, Coutré S, Birhiray R, Kovacsovics T, Langer W, Leber B, Maughan T, Rai K, Tjønnfjord G, Bekradda M, Itzhaki M, Hérait P. Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol. 2002;20(1):205–213. doi: 10.1200/JCO.2002.20.1.205. [DOI] [PubMed] [Google Scholar]
- 24.Palomera L, Domingo JM, Agulló JA, Soledad Romero M. Complete remission in T-cell prolymphocytic leukemia with 2-chlorodeoxyadenosine. J Clin Oncol. 1995;13(5):1284–1285. doi: 10.1200/JCO.1995.13.5.1284. [DOI] [PubMed] [Google Scholar]
- 25.Mercieca J, Matutes E, Dearden C, MacLennan K, Catovsky D. The role of pentostatin in the treatment of T-cell malignancies: analysis of response rate in 145 patients according to disease subtype. J Clin Oncol. 1994;12(12):2588–2593. doi: 10.1200/JCO.1994.12.12.2588. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan B, Else M, Tjonnfjord GE, Cazin B, Carney D, Carter J, Ketterer N, Catovsky D, Ethell M, Matutes E, Dearden CE. Stem cell transplantation after alemtuzumab in T-cell prolymphocytic leukaemia results in longer survival than after alemtuzumab alone: a multicentre retrospective study. Br J Haematol. 2010;149(6):907–910. doi: 10.1111/j.1365-2141.2010.08134.x. [DOI] [PubMed] [Google Scholar]