Abstract
In recent years, access to information regarding acquisition and synthesis of newer designer drugs has been at an all-time high due largely to the Internet. As these drugs have become more prevalent, laboratory techniques have been developed and refined to identify and screen for this burgeoning population of drugs. This provides a unique opportunity for learning about many of these methods. Laboratory testing techniques and instrumentation are obscure to many health care professionals, yet their results are crucial. Here, we present a case of an overdose of an uncommon designer drug (2C-E) and discuss the basics of liquid chromatography and mass spectrometry, two important techniques used in isolating and identifying the drug. Although often overlooked and taken for granted, these techniques can play a pivotal role in the diagnosis and subsequent management of select patients.
The drug scene is constantly changing and evolving. Traditionally, drugs of abuse are associated with popular street drugs such as marijuana, heroin, cocaine, and methamphetamine. In the 1990s, several other drugs were added to this list, including the so-called “club drugs”: ecstasy, ketamine, and gamma hydroxybutyric acid. In more recent years, “designer drugs” have emerged, which are either chemically altered natural substances or completely synthetic molecular structures that have psychotropic effects. Due to the widespread use of the Internet, information regarding synthesis of and access to novel compounds is more accessible than ever. This poses new challenges to the medical community in terms of treatment as well as identification of the abused substance, especially in patients unable to communicate. Here we present a case of a fatal overdose of the designer drug known as 2C-E (4-ethyl-2, 5-dimethoxy-b-phenethylamine), a phenethylamine derivative. Although previously published, the prior case report focused on radiographic findings (1). In the present article, we focus on how 2C-E is detected in the urine using basic chromatography and mass spectrometry principles.
CASE REPORT
A 26-year-old white man with known polysubstance abuse and psychiatric problems was found unresponsive at a friend's house. Initial emergency responders intubated the patient and treated him with naloxone with no change in clinical status. According to the patient's friend, he had ingested a drug known as 2C-E, but was not aware of any other drug or alcohol use. The patient had prescriptions for various psychiatric medications including sertraline, clonazepam, gabapentin, and zolpidem.
In the emergency department, his clinical status remained unchanged. An initial urine toxicology screen was positive for marijuana metabolites and benzodiazepines. A head computed tomography scan was negative, and electroencephalography showed diffuse slowing. The patient's initial laboratory studies are shown in Table 1.
Table 1.
Initial laboratory values
| Analyte | Value | Reference range | Flag |
|---|---|---|---|
| Glucose (mg/dL) | 295 | 70–99 | High |
| Sodium (mEq/L) | 143 | 136–145 | |
| Potassium (mEq/L) | 4.5 | 3.5–5.1 | |
| Chloride (mEq/L) | 104 | 98–107 | |
| Carbon dioxide (mEq/L) | 29 | 21–32 | |
| Anion gap (mEq/L) | 10 | 6–16 | |
| Blood urea nitrogen (mg/dL) | 15 | 7–18 | |
| Creatinine (mg/dL) | 2.4 | 0.6–1.3 | High |
| Calcium (mg/dL) | 8.2 | 8.5–10.1 | Low |
| Total protein (g/dL) | 6.9 | 6.4–8.2 | |
| Albumin (g/dL) | 3.7 | 3.4–5.0 | |
| Globulin (g/dL) | 3.2 | 2.4–3.5 | |
| Total bilirubin (mg/dL) | 0.2 | 0.2–1.0 | |
| Alkaline phosphatase (U/L) | 66 | 50–136 | |
| Aspartate transaminase (U/L) | 124 | 15–37 | High |
| Alanine transaminase (U/L) | 122 | 12–78 | High |
| Amylase (U/L) | 76 | 25–115 | |
| Lipase (U/L) | 174 | 73–393 | |
| Lactic acid (mmol/L) | 3.7 | 0.9–1.7 | High |
On admission, the patient had acute kidney failure and leukocytosis, and he subsequently developed a right lower lobe pneumonia, which resolved with antibiotics. Although the patient remained obtunded, his brain stem reflexes remained intact throughout his hospitalization. After discussing the patient's prognosis in detail, the family decided to withdraw life-supporting therapy. The patient died about 2 weeks after his initial admission. Subsequent specialized urine drug screens were positive for the compound known as 2C-E.
DISCUSSION
The 2C family of designer drugs is a large group of chemicals classified as ring-substituted phenethylamines, most of which were first synthesized in the 1970s by the chemist Alexander Shulgin (2). They are characterized by methoxy groups at positions 2 and 5 of the benzene ring. Within the 2C family, there are differences in the substitution of the fourth position on the benzene ring. For instance, at position 4, 2C-I substitutes iodine while 2C-B substitutes bromine. For 2C-E in particular, an ethyl group is substituted at position 4 (Figure 1). A full list of 2C compounds published by Shulgin is provided in Table 2.
Figure 1.
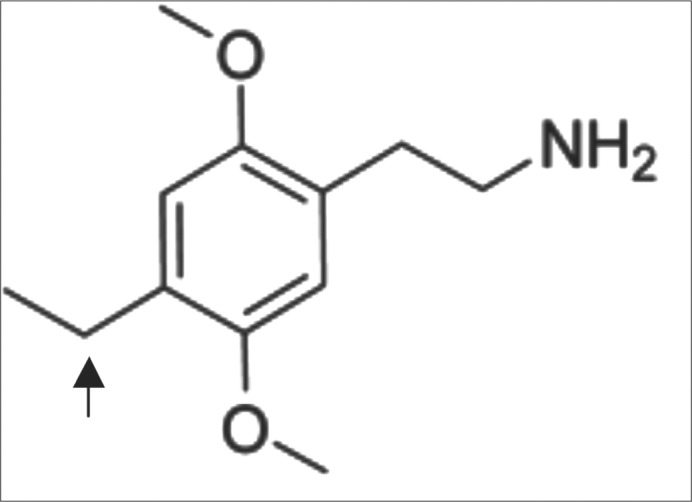
Structure of 2C-E. The arrow denotes the ethyl group attached to the fourth position of the benzene ring.
Table 2.
The 2C family of phenethylamine derivatives∗
| 2C family | |
|---|---|
| 2C-B | 4-bromo-2, 5-dimethoxy-phenethylamine |
| 2C-C | 4-chloro-2, 5-dimethoxy-phenethylamine |
| 2C-D | 4-methyl-2, 5-dimethoxy-phenethylamine |
| 2C-E | 4-ethyl-2, 5-dimethoxy-phenethylamine |
| 2C-F | 4-fluoro-2, 5-dimethoxy-phenethylamine |
| 2C-G | 3, 4-dimethyl-2, 5-dimethoxy-phenethylamine |
| 2C-G-3 | 3, 4-trimethylene-2, 5-dimethoxy-phenethylamine |
| 2C-G-4 | 3, 4-tetramethylene-2, 5-dimethoxy-phenethylamine |
| 2C-G-5 | 3, 4-norbornyl-2, 5-dimethoxy-phenethylamine |
| 2C-G-N | 1, 4-dimethoxynaphthyl-2-ethylamine |
| 2C-H | 2, 5-dimethoxy-phenethylamine |
| 2C-I | 4-iodo-2, 5-dimethoxy-phenethylamine |
| 2C-N | 4-nitro-2, 5-dimethoxy-phenethylamine |
| 2C-O-4 | 4-isopropoxy-2, 5-dimethoxy-phenethylamine |
| 2C-P | 4-propyl-2, 5-dimethoxy-phenethylamine |
| 2C-SE | 4-methylseleno-2, 5-dimethoxy-phenethylamine |
| 2C-T | 4-methylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-2 | 4-ethylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-4 | 4-isopropylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-7 | 4-propylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-8 | 4-cyclopropylmethylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-9 | 4-(t)-butylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-13 | 4-(2-methoxyethylthio)-2, 5-dimethoxy-phenethylamine |
| 2C-T-15 | 4-cyclopropylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-17 | 4-(s)-butylthio-2, 5-dimethoxy-phenethylamine |
| 2C-T-21 | 4-(2-fluoroethylthio)-2, 5-dimethoxy-phenethylamine |
Reprinted with permission from Shulgin (2).
Pharmacologically, 2C-E (and the 2C family) shows strong efficacy for the 5-HT2C receptor, accounting for its hallucinogenic effects (3). Accounts from users typically report a dosage range from 10 to 30 mg (2). Although little is known of the pharmacokinetics of the drug, it seemingly takes effect within seconds of insufflation, with a slightly delayed action if taken orally. The drug displays a marked dosage effect, and hallucinogenic activity typically lasts for 4 to 8 hours (2). Metabolism of 2C-E appears to have several pathways, although the main ones appear to be via hepatic oxidative deamination and/or O-demethylation (4–6).
The clinical presentation of users of the 2C group can be varied. At lower doses, the drugs act as a stimulant (2). However, at higher doses (>10 mg), there is a marked hallucinogenic and psychoactive effect. Deaths have also been reported following the usage of 2C compounds including, but not limited to, 2C-T-7 (7) and 2C-I (8).
For diagnosis, an analytic method for detection and quantification is required for clinical chemistry and forensic toxicology. Many different techniques have been developed to screen for these newer designer drugs, including capillary electrophoresis–mass spectrometry (9–12), capillary electrophoresis coupled with a diode array detector (13), gas chromatography–mass spectrometry (14, 15), and liquid chromatography–mass spectrometry (LC-MS) (16). In more recent years, LC-MS in tandem (LC-MS/MS) has become the detection test of choice due to its ability to separate and identify small molecules with similar structures in one run (17).
In order to understand LC-MS/MS, it is easiest to break it up into its separate analytic components. The liquid chromatography phase, specifically high-performance liquid chromatography, separates chemicals by running a solvent (mobile phase) containing the chemicals of interest through a column (stationary phase). The chemical (2C-E) binds to the column through hydrophobic interactions. As can be expected, different molecules elute through the column at different times due to differences in molecular structure and hydrophobic interactions. This technique is very good at separating chemicals (even molecules with very similar masses); however, it does not necessarily identify the compound, especially if there are many unknown chemicals in the sample. This is where coupling of mass spectrometry is useful.
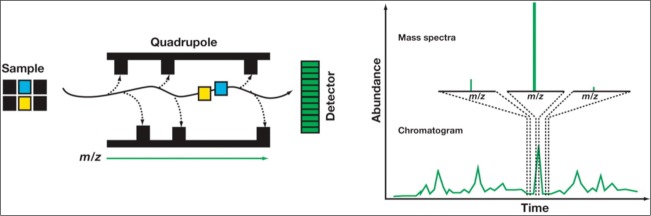
In typical non-tandem mass spectrometry, these unknown compounds enter the mass spectrometer, where they are initially ionized and given either a positive or a negative charge. Mass spectrometers identify the molecules present in a sample based on their mass-to-charge ratio (m/z). The molecules then move into the quadrapole chamber, which consists of four parallel magnetic rods arranged in a square formation (Figure 2) (18). The rods are calibrated in such a way that only the ions of interest are allowed to pass unimpeded through the chamber and hit the detector. For instance, 2C-E has a molecular weight of approximately 209 and a charge of 1+ following ionization; therefore, the m/z ratio will be 209. The mass spectrometer only allows compounds with this specific m/z ratio to pass through and be detected. However, other compounds can have very similar m/z ratios, detracting from the specificity of a mass spectrometer. For example, morphine and hydromorphine have an identical m/z ratio of 286, thus making separation of the two compounds difficult by non-tandem mass spectrometry. Running mass spectrometry in tandem can help alleviate this issue.
Figure 2.
Schematic of a quadrapole mass spectrometer. The quadrapole is set to transmit only ions with a specific mass-to-charge ratio (m/z) that matches the analytes of interest. Because only ions matching the selected m/z are transmitted, the resultant chromatogram and mass spectra are clean and easy to interpret. However, because the two analytes of interest have identical m/z, the mass spectrometer is unable to differentiate between them. Reprinted with permission from Hill et al, 2011 (18).
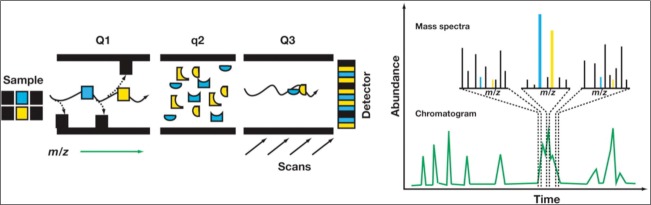
Mass spectrometry in tandem is characterized by multiple quadrapoles (typically three) that are arranged in a series (Figure 3) (18). The first and third quadrapoles are similar in function to those described previously. The middle quadrapole differs in that it functions as the “collision cell.” It uses a combination of inert gases and changing frequencies. As the ions from the first quadrapole mass spectrometer enter this second chamber, they collide with the inert gases and fragment into even smaller ions. These molecules then enter the third quadrapole, where they are detected. By fragmenting parent ions with similar m/z ratios, new ions are created with differing m/z ratios, which are sent and detected in the third quadrapole. This functions to identify the parent molecule with great specificity.
Figure 3.
Schematic of a mass spectrometer in tandem. Q1 is set to only transmit ions with a specific mass-to-charge ratio (m/z) that matches the analytes of interest. The collision cell, q2, produces fragments from these transmitted ions, while Q3 constantly scans across the entire m/z range, allowing all fragments produced in q2 to reach the detector. The recording of all fragments results in a complex chromatogram and mass spectra but has allowed for the unique identification of each analyte at the appropriate sampled time point, indicated in blue and yellow. Reprinted with permission from Hill et al, 2011 (18).
References
- 1.Sacks J, Ray MJ, Williams S, Opatowsky MJ. Fatal toxic leukoencephalopathy secondary to overdose of a new psychoactive designer drug 2C-E (“Europa”) Proc (Bayl Univ Med Cent) 2012;25(4):374–376. doi: 10.1080/08998280.2012.11928883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1991. [Google Scholar]
- 3.Acuña-Castillo C, Villalobos C, Moya PR, Sáez P, Cassels BK, Huidobro-Toro JP. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 2002;136(4):510–519. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmo H, Hengstler JG, de Boer D, Ringel M, Remião F, Carvalho F, Fernandes E, dos Reys LA, Oesch F, de Lourdes Bastos M. Metabolic pathways of 4-bromo-2,5-dimethoxyphenethylamine (2C-B): analysis of phase I metabolism with hepatocytes of six species including human. Toxicology. 2005;206(1):75–89. doi: 10.1016/j.tox.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Rohanová M, Pálenícek T, Balíková M. Disposition of 4-bromo-2,5-dimethoxyphenethylamine (2C-B) and its metabolite 4-bromo-2-hydroxy-5-methoxyphenethylamine in rats after subcutaneous administration. Toxicol Lett. 2008;178(1):29–36. doi: 10.1016/j.toxlet.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr Drug Metab. 2010;11(5):468–482. doi: 10.2174/138920010791526042. [DOI] [PubMed] [Google Scholar]
- 7.Curtis B, Kemp P, Harty L, Choi C, Christensen D. Postmortem identification and quantitation of 2,5-dimethoxy-4-n-propylthiophene-thylamine using GC-MSD and GC-NPD. J Anal Toxicol. 2003;27(7):493–498. doi: 10.1093/jat/27.7.493. [DOI] [PubMed] [Google Scholar]
- 8.Drees JC, Stone JA, Wu AH. Morbidity involving the hallucinogenic designer amines MDA and 2C-I. J Forensic Sci. 2009;54(6):1485–1487. doi: 10.1111/j.1556-4029.2009.01199.x. [DOI] [PubMed] [Google Scholar]
- 9.Boatto G, Nieddu M, Carta A, Pau A, Palomba M, Asproni B, Cerri R. Determination of amphetamine-derived designer drugs in human urine by SPE extraction and capillary electrophoresis with mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;814(1):93–98. doi: 10.1016/j.jchromb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Boatto G, Nieddu M, Dessì G, Manconi P, Cerri R. Determination of four thiophenethylamine designer drugs (2C-T-series) in human plasma by capillary electrophoresis with mass spectrometry detection. J Chromatogr A. 2007;1159(1-2):198–202. doi: 10.1016/j.chroma.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Boatto G, Nieddu M, Pirisi MA, Dessì G. Simultaneous determination of new thioamphetamine designer drugs in plasma by capillary electrophoresis coupled with mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(22):3716–3720. doi: 10.1002/rcm.3273. [DOI] [PubMed] [Google Scholar]
- 12.Nieddu M, Boatto G, Dessì G. Determination of 4-alkyl 2,5 dimethoxy-amphetamine derivatives by capillary electrophoresis with mass spectrometry detection from urine samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852(1-2):578–581. doi: 10.1016/j.jchromb.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Nieddu M, Boatto G, Carta A, Sanna A, Pisano M. Simultaneous determination of ten amphetamine designer drugs in human whole blood by capillary electrophoresis with diode array detection. Biomed Chromatogr. 2005;19(10):737–742. doi: 10.1002/bmc.508. [DOI] [PubMed] [Google Scholar]
- 14.Peters FT, Schaefer S, Staack RF, Kraemer T, Maurer HH. Screening for and validated quantification of amphetamines and of amphetamine- and piperazine-derived designer drugs in human blood plasma by gas chromatography/mass spectrometry. J Mass Spectrom. 2003;38(6):659–676. doi: 10.1002/jms.483. [DOI] [PubMed] [Google Scholar]
- 15.Habrdova V, Peters FT, Theobald DS, Maurer HH. Screening for and validated quantification of phenethylamine-type designer drugs and mescaline in human blood plasma by gas chromatography/mass spectrometry. J Mass Spectrom. 2005;40(6):785–795. doi: 10.1002/jms.853. [DOI] [PubMed] [Google Scholar]
- 16.Nieddu M, Boatto G, Pirisi MA, Azara E, Marchetti M. LC-MS analysis of trimethoxyamphetamine designer drugs (TMA series) from urine samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867(1):126–130. doi: 10.1016/j.jchromb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Wohlfarth A, Weinmann W, Dresen S. LC-MS/MS screening method for designer amphetamines, tryptamines, and piperazines in serum. Anal Bioanal Chem. 2010;396(7):2403–2414. doi: 10.1007/s00216-009-3394-4. [DOI] [PubMed] [Google Scholar]
- 18.Strathmann FG, Hoofnagle AN. Current and future applications of mass spectrometry to the clinical laboratory. Am J Clin Pathol. 2011;136(4):609–616. doi: 10.1309/AJCPW0TA8OBBNGCK. [DOI] [PubMed] [Google Scholar]