Abstract
Background. Understanding the epidemiological dynamics of influenza virus is central to surveillance and vaccine strain selection. It has been suggested that tropical and subtropical regions represent the global source of influenza epidemics. However, our understanding of the epidemiological dynamics of influenza virus in these regions is limited by a relative lack of long-term data.
Methods. We analyzed epidemiological and virological data on influenza recorded over a period of 15 years from the metropolitan city of Shenzhen in subtropical southern China. We used wavelet analysis to determine the periodicity of influenza epidemics and molecular phylogeographic analysis to investigate the role of Shenzhen and southern China in the global evolution of influenza virus.
Results. We show that southern China is unlikely to represent an epicenter of global influenza activity, because activity in Shenzhen is characterized by significant annual cycles, multiple viral introductions every year, limited persistence across epidemic seasons, and viruses that generally are not positioned on the trunk of the global influenza virus phylogeny.
Conclusions. We propose that novel influenza viruses emerge and evolve in multiple geographic localities and that the global evolution of influenza virus is complex and does not simply originate from a southern Chinese epicenter.
Keywords: influenza, epidemiology, southern China, seasonality, phylogeography
Seasonal and pandemic influenza is associated with significant morbidity and mortality worldwide [1, 2]. Seasonal influenza epidemics have been well documented in temperate regions, where they exhibit strong seasonal winter peaks. However, the seasonality of influenza is less defined in many tropical/subtropical regions [3–8]. In temperate regions, most viral lineages go extinct following an epidemic, with few strains persisting between seasonal epidemics [9–11]. In contrast, viral strains that persist in tropical/subtropical regions are thought to be important in maintaining global genetic diversity by providing a reservoir population of virus that can be reimported into temperate regions each winter [9]. In particular, influenza viruses circulating in Southeast Asia have been proposed as the source of seasonal epidemics in temperate regions, the latter of which can be thought of as “sink” populations [9, 12]. Although this “source-sink” model is attractive, it was based on relatively few and temporally limited influenza virus data sets from subtropical regions, such as southern China.
To describe the epidemiological dynamics of influenza virus in a subtropical city in detail and to test the hypothesis that southern China represents a global source population of influenza virus, we characterized the epidemiology of influenza virus during 1995–2009 in the southern Chinese city of Shenzhen, at the border of China's subtropical and tropical regions. Because of Shenzhen's proximity to Hong Kong and status as the first of the special economic zones in China, the city plays an important role in connecting Mainland China to Hong Kong and to other countries in Southeast Asia. We used long-term epidemiological and virological data to determine the seasonality of influenza epidemics in the city. In addition, through a phylogenetic analysis of newly acquired influenza virus hemagglutinin (HA) gene sequences, we characterized the temporal and spatial dynamics of influenza viruses in this city and evaluated the persistence of influenza virus strains across epidemics in Shenzhen and in southern China.
METHODS
Influenza Surveillance Data From Shenzhen
Shenzhen has an estimated population of 14 million individuals and is located in subtropical China at latitude 22.6°N, bordering Hong Kong. In 1995, a prospective surveillance system was initiated to detect influenza among inpatients and outpatients admitted for upper respiratory infections (URIs) to 8 sentinel sites. Each sentinel site recorded daily numbers of consultations for URIs, defined as nasal congestion, cough, runny nose, sore throat, fever, facial pressure, and sneezing. In 2003, the case definition was changed to influenza-like illness (ILI) (defined by the World Health Organization [WHO] as sudden onset of fever [temperature, >38°C] and cough or sore throat in the absence of other diagnoses), and the system was expanded to 22 sentinel sites, including schools, general hospitals, public health units, and community healthcare centers [13]. Since 2005, a wider influenza surveillance network has been established, including 8 virology laboratories and 23 sentinel sites [14].
Collection of Clinical Specimens, Virus Culture, and Genotyping
Nasopharyngeal swab specimens from patients with URIs (before 2003) and patients with ILI (after 2003) who had not received antiviral treatment were collected within 3 days after symptom onset from the sentinel sites by district public health staff. Specimens were kept at 4°C and transported to one of the 7 virology laboratories maintained by the Shenzhen Center for Disease Control and Prevention, where they were stored at −80°C for subsequent virus isolation and identification. Clinical specimens from the patients were cultured in Madin-Darby canine kidney cells for 5–7 days or in embryonated chicken eggs for 2–3 days as described previously [15]. After incubation, a hemagglutination test was used to determine specimens that were positive for influenza virus [16]. The genotypes and subtypes of influenza virus isolates were analyzed by a hemagglutination inhibition test, using a WHO influenza diagnostic kit, and were confirmed by sequencing as described previously [17]. The influenza virus samples used in this study were collected as part of an ongoing and nationally approved influenza surveillance program. Because no patient data were used in this study, written consent was not required. All sequences generated here have been submitted to GenBank and were assigned accession numbers CY105951–CY106559.
Influenza Periodicity and Seasonality Analysis
We compiled weekly time series of ILI consultation rates (calculated as the weekly number of consultations for ILI divided by the weekly number of all consultations) in Shenzhen during 2003–2009 and monthly time series of the standardized number of influenza virus–positive specimens (calculated as the monthly number of influenza virus–positive specimens divided by the annual number of influenza virus–positive specimens) and the “percentage positivity” (calculated as the monthly number of influenza virus–positive specimens divided by the monthly number of all specimens tested) during 1995–2009. To characterize the seasonality and periodicity of epidemics in the city, we applied wavelet methods to ILI and influenza time series, as previously described for various infectious diseases data [18–20]. Briefly, wavelet analysis allows the identification of periodicities in time series data and can be used to distinguish between semiannual and annual outbreaks or to determine year-round activity. Such methods can also help track changes in periodicity over time and to quantify time lags between various indicators of epidemic activity. We used the wavelet package in R 2.12.0 [21] to conduct this analysis, as described in detail elsewhere [18]. All time series were square-root transformed prior to analysis to take into account sampling trends due to the expansion of the surveillance network.
To further quantify seasonality, we estimated the monthly proportion of viruses reported in each month of the year and used a Chi-square test to compare the proportion of different types of viruses identified in the summer (May–October) and winter (November–April).
Phylogenetic Analysis
We analyzed newly acquired HA1 sequences from human influenza viruses collected in Shenzhen from 1995 through 2009 (351 were from influenza A virus subtype H3N2 [A/H3N2], 104 were from influenza A virus subtype H1N1 [A/H1N1], and 154 were from influenza B virus [of which 2 were collected in 1994]), as well as all human influenza virus HA1 sequences that are available on GenBank for A/H1N1 (from 1918 through 2010), A/H3N2 (from 1968 through 2010), and influenza B virus (from 1940 through 2010). Sequences were downloaded from the National Center for Biotechnology Information Influenza Virus Resource, available on GenBank [22]. In total, we produced the following 3 global data sets of influenza virus: 9500 HA1 sequences of A/H3N2, 3632 sequences of A/H1N1, and 3133 sequences of influenza B virus. Maximum likelihood (ML) phylogenies were inferred by means of the rapid tree searching approach implemented in RAxML (version 7.04), using the general-time-reversal (GTR) model of nucleotide substitution with a gamma distribution (Γ) of among-site rate variation [23]. A total of 200 independent searches for ML phylogenies were performed with different random maximum parsimony starting trees. The phylogeny with the highest likelihood score was selected for further analysis. To provide a more geographically specific context for our study, 3 China-specific data sets were also generated for A/H3N2 (1320 sequences), A/H1N1 (374 sequences), and influenza B virus (700 sequences). These data sets included sequences collected from mainland China, Hong Kong, Macau, and Taiwan. In this case, phylogenetic trees were estimated using PhyML v3.412 [24] and the GTR + Γ substitution model. The robustness of the ML tree was assessed by bootstrap analyses of 500 pseudoreplicate data sets.
Phylogeographic Analysis
The geographic location of each sequence was first assigned a discrete geographic region, namely, Europe, North America, South America, Oceania, Africa, eastern Asia (excluding China), western Asia, Southeast Asia, southern Asia, southern China, and northern China. Southern China was defined here to include the provinces located south of the Yangtze River (including the region of Shenzhen), as well as 2 Special Administrative Regions—Hong Kong and Macau—but not Taiwan. With these data, we reconstructed the ancestral geographic region (ie, those at each node on the tree), using a parsimony procedure [25]. The trunk lineage of the phylogeny was defined as that originating from the root of the tree and ending at the most recently sampled cluster of isolates. The ancestral geographic regions on the trunk were estimated using 500 random resolutions of polytomies (ie, cases in which nodes split into >2 descendants). The frequency with which each geographic location fell on the trunk lineage was then calculated. This analysis was only conducted for the A/H3N2 phylogeny because there was insufficient temporal and geographic sampling of the A/H1N1 and influenza B virus data sets.
RESULTS
Seasonality and Periodicity of Influenza Virus Activity in Shenzhen
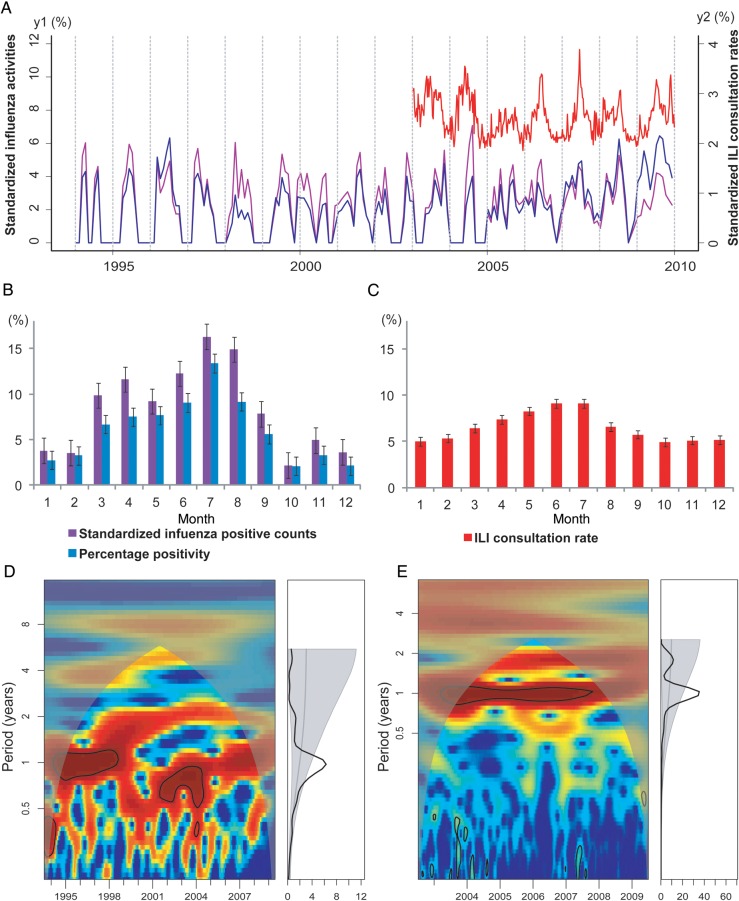
A total of 25 377 respiratory specimens were tested during 1995–2009 in Shenzhen, yielding 2678 influenza viruses (Table 1). Figure 1 shows the average seasonal patterns and periodicities of influenza virus activity and ILI in Shenzhen. Figure 1A shows the monthly time series of the number of influenza virus–positive specimens and the percentage positivity, overlaid with data on ILI activity. Influenza virus activity during 1995–2008 was characterized by a small peak, typically occurring during March–April, and a larger peak occurring in the summer, in July or August (Figure 1B). Data from the 2009 pandemic of influenza A virus subtype H1N1 (A/H1N1pdm09) infection were omitted from this analysis to focus on the average seasonal signature of interpandemic influenza. Importantly, there was a statistically significant annual cycle (P < .05) in both influenza virus–positive counts (Figure 1D) and percentage positivity (Supplementary Figure 1A) during 1995–2009. Both indicators of influenza virus activity were highly synchronized (average lag, <2 days; Supplementary Figure 1C).
Table 1.
Number of Cases of Influenza-Like Illness (ILI) and Influenza Virus–Positive Specimens, by Virus Subtype, Shenzhen, China, 1995–2009
| Year | URI or ILI Cases,No. (%a) | Specimens,No. | Positive Specimens, No. (%) | Influenza A Virus Subtype Positivity |
B Positivity,Proportion (%) | ||
|---|---|---|---|---|---|---|---|
| H3N2, No. | H1N1, No. | Overall, Proportion (%) | |||||
| 1995 | 37 509 (54) | 352 | 16 (5) | 7 | 1 | 8/16 (50) | 8/16 (50) |
| 1996 | 51 500 (79) | 241 | 33 (14) | 17 | 11 | 28/33 (85) | 5/33 (15) |
| 1997 | 50 294 (71) | 608 | 34 (6) | 2 | 5 | 7/34 (21) | 27/34 (79) |
| 1998 | 90 063 (71) | 2300 | 40 (0.2) | 39 | 0 | 39/40 (98) | 1/40 (2) |
| 1999 | 89 362 (72) | 1187 | 70 (9) | 36 | 0 | 36/70 (51) | 34/70 (49) |
| 2000 | 49 836 (75) | 1509 | 40 (3) | 10 | 11 | 21/40 (53) | 19/40 (47) |
| 2001 | 51 393 (75) | 1168 | 74 (6) | 1 | 50 | 51/74 (69) | 23/74 (31) |
| 2002 | 156 491 (68) | 815 | 34 (4) | 12 | 0 | 12/34 (35) | 22/34 (65) |
| 2003 | 27 268 (8) | 779 | 45 (6) | 41 | 0 | 41/45 (91) | 4/45 (9) |
| 2004 | 29 658 (7) | 773 | 20 (3) | 9 | 0 | 9/20 (35) | 17/20 (65) |
| 2005 | 25 097 (5) | 2325 | 133 (6)b | 33 | 51 | 84/133 (63) | 22/133 (17) |
| 2006 | 112 651 (6) | 2486 | 154 (6) | 3 | 103 | 106/154 (69) | 48/154 (31) |
| 2007 | 153 749 (7) | 2526 | 290 (11) | 172 | 11 | 183/290 (63) | 108/290 (37) |
| 2008 | 158 849 (6) | 3183 | 389 (12) | 48 | 177 | 225/389 (58) | 164/389 (42) |
| 2009 | 221 552 (7) | 5125 | 1306 (25) | 479 | 122; 450c | 1051/1306 (80) | 255/1306 (20) |
| Overall | 1 305 272 | 25377 | 2678 | 903 | 991 | 1894/2678 (71) | 757/2678 (28) |
Abbreviation: B, influenza B virus.
a Data indicate the proportion of visits for upper respiratory infection (URI) or ILI among all consultations. URI visits were monitored during 1995–2002 in Shenzhen, whereas ILI visits were monitored during 2003–2009.
b A total of 27 isolates in 2005 were not subtyped.
c In 2009, 122 H1N1 isolates were seasonal strains, while 450 of these isolates were the novel 2009 pandemic influenza virus A subtype H1N1 strain.
Figure 1.
Seasonality of influenza epidemics in Shenzhen, China, 1995–2009. A, Time series of the standardized number of influenza virus–positive specimens (calculated as the monthly number of influenza virus–positive specimens divided by the annual number of influenza virus–positive specimens; purple line, left y-axis), the “percentage positivity” (calculated as the monthly number of influenza virus–positive specimens divided by the monthly number of all specimens tested; blue line, left y-axis), and influenza-like illness (ILI) consultation rates (calculated as the weekly number of consultations for ILI divided by the weekly number of all consultations; red line, right y-axis). All time series have been square-root transformed. B, Monthly standardized number of influenza virus–positive specimens (purple) and percentage positivity (blue) during 1995–2008 (the 2009 pandemic influenza A virus subtype H1N1 season was omitted). Color bars represent the average proportion of influenza virus–positive patients identified in each month of the year. Error bars show standard errors based on variation between years. C, Same as B, but for monthly ILI consultation rate (in red) during 2003–2008. D, Wavelet power spectrum of standardized influenza positive counts during 1995–2009, identifying changes in periodicities over time (left) and average periodicity (right). Power increases from blue to red so that red indicates stronger periodicities. Black lines highlight periodicities reaching statistical significance (here, 1-year periodicities). Shaded areas indicate the presence of edge effects. E, Same as panel D, but for ILI consultation rate, 2003–2009.
We also explored patterns of seasonality in weekly ILI consultation rates during 2003–2009 (Figure 1C and Table 1). We observed a statistically significant annual cycle in ILI activity (P < .05), similar to patterns seen in laboratory-confirmed influenza data, with peaks occurring in the early summer months of May, June, and July (Figure 1C and 1E). Wavelet coherence analysis suggests that ILI activity consistently led influenza virus activity during 1995–2005, with a lag steadily declining over time (from 2.5 months to 0.2 months; Supplementary Figure 1B and 1C). Since 2006, the ILI consultation rate and influenza virus activity have been highly synchronized in Shenzhen, with an average lag of 4.5 days (Supplementary Figure 1C).
Subtype Distributions of Influenza Epidemics in Shenzhen
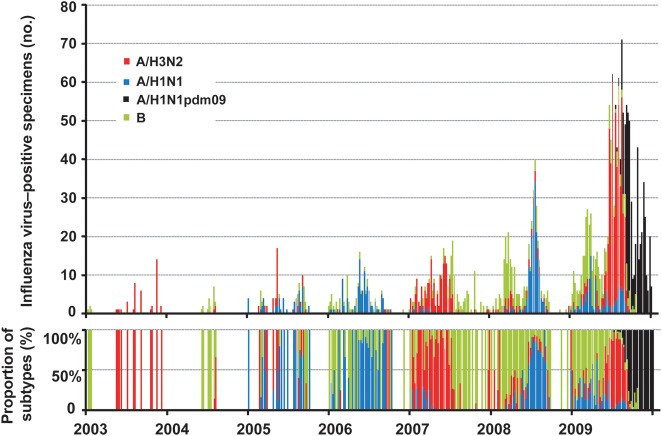
The predominant viral subtypes observed in Shenzhen during 1995–2009 are shown in Table 1. On the basis of HAI assay findings, 1894 of 2678 isolates were influenza A virus (541 were A/H1N1, 450 were A/H1N1pdm09 isolated in 2009, and 903 were A/H3N2), whereas 757 isolates were influenza B virus. Influenza A virus was predominant during most of the study period, except in 1995, 1997, 2002, and 2004. There were at least 2 subtypes cocirculating in the city each year. The seasonality of influenza virus infection varied by type, with influenza A virus more prevalent in the summer (64% of influenza A viruses were isolated during May–October), while influenza B virus was more prevalent in the winter (62% of influenza B viruses were isolated during November–April; χ2 = 78.195; P < .001; Figure 2).
Figure 2.
Weekly distribution of influenza virus subtypes in Shenzhen, China, 2003–2009. Blue: seasonal influenza A virus subtype H1N1 (A/H1N1); red: seasonal influenza A virus subtype H3N2 (A/H3N2); green: influenza B virus (B); black: 2009 pandemic influenza A virus subtype H1N1 (A/H1N1pdm09). A/H1N1 predominated in 2005, 2006, and 2008; A/H3N2 predominated in 2003, 2007, and in 2009 before the emergence of A/H1N1pdm09. B was predominant in 2004 but showed higher activity in late 2007 and the beginning of 2008.
A complicated circulation pattern was observed in 2009 because of the influenza pandemic. Influenza B virus predominated in the first 3 months of the year, after which A/H3N2 became more prevalent. A/H1N1pdm09 was introduced to Shenzhen in July 2009 and spread quickly in the subsequent months. Because of the cocirculation of A/H3N2 and A/H1N1pdm09, influenza A viruses accounted for >90% of influenza virus specimens in July and August 2009, and the percentage positivity reached its highest level at 40%.
Introductions and Persistence of Influenza Virus in Shenzhen
Phylogenetic trees of the HA sequences of global and Chinese A/H1N1, A/H3N2, and influenza B viruses showed that distinct lineages (ie, multiple phylogenetically distinct clusters) cocirculate in Shenzhen, indicative of multiple introductions (Supplementary Figure 2). In particular, Shenzhen viruses never formed a monophyletic group but were interspersed with those sampled from other locations, a signature of multiple introductions into this population. Although large monophyletic clusters of Shenzhen sequences were seen in the phylogenies of A/H1N1 and influenza B virus, this is likely due to the paucity of background sequences.
We estimated the number of separate introductions of A/H3N2, A/H1N1, and influenza B virus into Shenzhen as the number of phylogenetically distinct clusters present in each year (Table 2). Multiple viral introductions are defined as those Shenzhen sequences separated by viruses sampled in other geographic locations and by viruses from other seasons [26, 27]. There were >80 A/H3N2, 33 A/H1N1, and 39 influenza B virus lineages present in Shenzhen during 1995–2009 (Table 2). For years in which >2 samples were available for Shenzhen, we found 1 to >10 introductions of each subtype every year. Because clusters or individual sequences may represent multiple introduction events, this is likely an underestimate of the true number of introductions of influenza viruses into Shenzhen. We also investigated whether specific viral lineages persisted across years in Shenzhen (Table 2). Virus persistence occurred sporadically during the period of our study and for relatively short intervals of 2–3 years (A/H3N2: 1996–1997 and 2006–2007; A/H1N1: 2000–2001, 2005–2006, and 2006–2007; influenza B virus: 1999–2001, 2002–2003, 2004–2005, and 2005–2006). In most years, new viral lineages were introduced and seemingly replaced the previously circulating lineages.
Table 2.
Estimated Numbers of Introductions and Local Persistence of Influenza Viruses, Shenzhen, China, 1995–2009
| Influenza A Virus Subtype |
||||||
|---|---|---|---|---|---|---|
| H3N2 |
H1N1 |
Influenza B Virusa |
||||
| Year | Sequences, No. | Introductionsb | Sequences, No. | Introductionsb | Sequences, No. | Introductionsb |
| 1995 | 5 | 3 | 1 | 1 | 3 | 2 |
| 1996 | 7 | 6 | 5 | 2 | 2 | 2 |
| 1997 | 1 | 1 | 3 | 2 | 2 | 2 |
| 1998 | 7 | 4 | 0 | 0 | 1 | 1 |
| 1999 | 6 | 4 | 0 | 0 | 2 | 2 |
| 2000 | 9 | 5 | 10 | 4 | 3 | 2 |
| 2001 | 0 | 0 | 30 | 8 | 3 | 3 |
| 2002 | 10 | 9 | 0 | 0 | 3 | 2 |
| 2003 | 17 | 5 | 0 | 0 | 2 | 1 |
| 2004 | 9 | 2 | 0 | 0 | 2 | 2 |
| 2005 | 13 | 4 | 15 | 5 | 15 | 5 |
| 2006 | 3 | 2 | 11 | 3 | 14 | 8 |
| 2007 | 169 | >10 | 11 | 4 | 100 | 7 |
| 2008 | 38 | >10 | 18 | 4 | NA | NA |
| 2009 | 57 | >10 | NA | NA | NA | NA |
| Total | 351 | >80 | 104 | 33 | 152 | 39 |
| Years ofpersistence | 1996–1997, 2006–2007 | 2000–2001,2005–2006, 2006–2007 | 1999–2001, 2002–2003, 2004–2005, 2005–2006 | |||
Abbreviation: NA, sequence data is not available.
a Multiple viral introductions are defined as those Shenzhen sequences separated by viruses sampled in other geographic locations and by viruses from other seasons.
b No influenza B virus sequence data were available in 2008. In 2009, there was no seasonal H1N1 sequence available, as only 2009 pandemic influenza A virus subtype H1N1 was sequenced.
Spatial Dynamics of Influenza Virus in Southern China and Globally
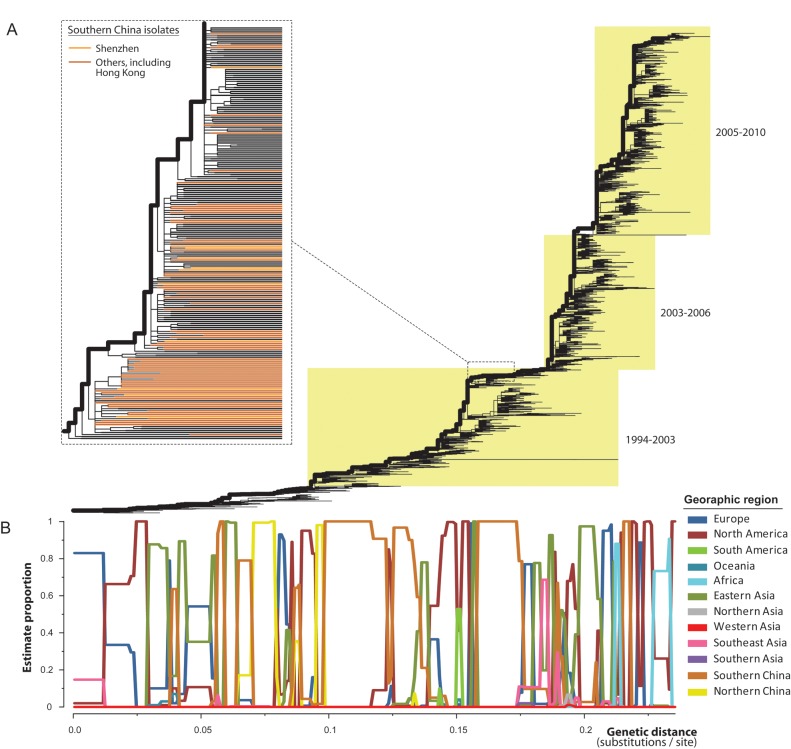
To better understand the contribution of influenza viruses from southern China to the global epidemiological dynamics of influenza, we estimated the frequency with which each geographic region was positioned on the main trunk lineage of global A/H3N2 phylogeny and, thus, which regions likely represent source populations. This analysis, which used data from 12 geographic regions, revealed that different geographic localities likely served as source populations at different time points (Figure 3). In particular, southern China, including Shenzhen, acted as a likely source population 3 times during 1994–2003 and once since 2003 (Figure 3B). Therefore, on the basis of these data, we found relatively little evidence that southern China represents a continuous “global” pool for influenza virus persistence. Indeed, other geographic regions, including those temperate zones, such as North America, eastern Asia (mainly Japan and South Korea), northern China, Europe, and Africa, also contributed to the gene pool of influenza viruses.
Figure 3.
Phylogeographic analysis of influenza A virus subtype H3N2. A, Maximum likelihood phylogeny of global isolates of the virus, with the trunk lineages shown by bold lines. Dashed lines indicate a magnification of that part of the tree where isolates from Shenzhen and other areas in southern China fall and indicated by light and dark orange colors, respectively (branches extended to the same level). B, Estimated proportions of geographic regions in the trunk lineages were plotted against their genetic distance from the tree root (units of nucleotide substitutions per site).
DISCUSSION
This is the first long-term study of the epidemiological and evolutionary dynamics of influenza virus in southern China. The epidemiological data demonstrated a consistent seasonal pattern of influenza virus infection across years, with high activity during July–August and a minor peak during March–April. Annual cycles were observed in both the laboratory-confirmed influenza and ILI activity data, and both disease indicators have become highly synchronous in recent years. The virological data revealed that multiple influenza virus subtypes cocirculated in the city and that the predominant subtype varied each year. Through phylogenetic analysis, we identified multiple introductions of each influenza virus subtype in Shenzhen every year, with relatively infrequent and only short-term persistence across epidemics. Finally, our phylogeographic analysis of A/H3N2 revealed that southern China was unlikely the sole source of influenza epidemics globally during the sampling period. Although southern China clearly contributes to the trunk of the influenza virus evolutionary tree, other geographic areas, including temperate regions, also act as sources in the global A/H3N2 migration network.
One argument in favor of tropical or subtropical regions (such as eastern Asia and Southeast Asia) as the global source of influenza virus is that there is a diversity of seasonal patterns of influenza within this highly connected region, which may serve as an endemic foci of influenza [9]. We show here that influenza epidemics in Shenzhen exhibit significant seasonality and annual cycles, with major peaks during July or August and an additional minor peak in March and April. This spring-summer seasonality pattern, especially obvious for influenza A virus, is out of phase with that of northern temperate regions, which have high influenza virus activity during December–March. While the seasonality of influenza virus infection in Shenzhen coincides with the rainy season, a period of reduced sunlight, warmer temperatures, and high humidity, these seasonal patterns contrast with those observed in other tropical or subtropical regions of Asia. For example, influenza virus activity in Cambodia and Myanmar occurs during the rainy season, between June and October or December, while the virus may circulate year-round in Singapore and Thailand [7, 8, 28, 29]. Most interestingly, influenza virus activity primarily occurs in the winter in Hong Kong, sometimes with a second smaller peak in the summer months (ie, June and July). Various environmental, viral, biological, social, and behavioral factors could be associated with seasonal variation in transmissibility and severity of influenza [5]. While Hong Kong and Shenzhen share the same humid subtropical climate and are geographically close, seasonal patterns of influenza virus infection differ markedly in the 2 cities, exemplifying the complexity of influenza seasonality [19].
Nonclimatic factors appear to be important drivers of the epidemiological dynamics of influenza virus in tropical or subtropical regions, including population movements and local social contacts [30, 31]. Shenzhen is the first special economic zone and a major manufacturing center in China. More than 70% of the Shenzhen population consists of migrant workers, who typically originate from rural areas in northern China and work in Shenzhen for part of the year, returning to the city in large numbers in March or April, after the Chinese New Year [32]. A migration of this scale may contribute to the initiation of influenza epidemics in Shenzhen in April and to the multiple introductions of influenza viruses in the city.
Our phylogenetic analysis suggests that southern China is unlikely to be the global epicenter of influenza virus activity, with viral lineages persisting only for short durations, continual reintroduction of outside viruses, and viral diversity that is infrequently located in the trunk region of the global phylogeny. Although multiple subtypes sometimes cocirculate in the city, the dominant subtype varies across years, and A/H3N2 and A/H1N1 continually disappear and reemerge, indicating reintroduction. Overall, this suggests that the dominant strains of epidemics are not persistent in the city but are introduced from other regions. This inference is further supported by the observation of multiple introductions of each subtype during an epidemic. Rather than a single viral lineage circulating in the city, multiple lineages of A/H3N2, A/H1N1, and influenza B virus were separately introduced and cocirculated each year, generating considerable genetic diversity among influenza viruses in Shenzhen. Similar observations have been made in supposed “sink” populations in North America, suggesting that multiple introductions and cocirculation may be universal characteristics of influenza virus ecology [26, 27].
We did not identify A/H3N2 or A/H1N1 that persisted across >2 years in Shenzhen, although certain influenza B viruses persisted for up to 3 years. Importantly, no major clade of viruses in a given season appears to have evolved in situ from those that circulated locally in the prior season, indicating that the genetic diversity of influenza virus in Shenzhen is mostly replenished at least every 1–2 years from an external gene pool. Finally, we observed widespread global gene flow into and out of southern China, rather than the ongoing in situ evolution expected of a source population.
A better understanding of the global dynamics of influenza virus has important implications for surveillance and vaccine strain selection, particularly to inform whether surveillance efforts should be concentrated in specific geographic regions. Our phylogeographic results are broadly consistent with a previous “equal contacts model” [33], which suggests that both temperate and tropical/subtropical regions contribute to the global diversity and evolution of A/H3N2 [34]. However, limited information is available on the evolutionary dynamics and seasonality of A/H1N1 and influenza B virus, and it remains unclear whether the persistence and global migrations patterns of these subtypes differ from those of A/H3N2. This is particularly important given the emergence of A/H1N1pdm09 in 2009, which may have shifted the dominance patterns and population impact of the different subtypes for the coming years.
Our study was unique in presenting long-term epidemiological, virological, and genetic data on influenza virus activity in Shenzhen, a prosperous and highly connected city of southern China. However, a complete understanding of the seasonality and global dynamics of influenza virus will require additional long-term studies from many other localities in tropical and subtropical areas of Asia, Africa, and the Americas.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. Author contributions were as follows: study design, E. C. H. and J. C.; data access: X. C., Y. T., M. H., and J. C.; data collection and experiments: X. C., M. H., X. L., J. H., S. Z., J. L., C. W, S. F., X. W., X. X., H. M., H. K., and J. C.; data analysis: Y. T. and T. T.-Y. L.; data interpretation: Y. T., T. T.-Y. L., M. I. N., C. V., and E. C. H.; and manuscript preparation: Y. T., T. T.-Y. L., M. I. N., C. V., and E. C. H.
Financial support. This work was supported by the Research Fund for the Control of Infectious Diseases Commissioned Study, Food and Health Bureau, Hong Kong SAR (RFCID, CU-09-01-01) and by the Influenza Program, Fogarty International Center, National Institutes of Health, which is funded by the Office of Global Affairs’ International Influenza Unit, US Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lipsitch M, Riley S, Cauchemez S, Ghani AC, Ferguson NM. Managing and reducing uncertainty in an emerging influenza pandemic. N Engl J Med. 2009;361:112–5. doi: 10.1056/NEJMp0904380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chowell G, Bertozzi SM, Colchero MA, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–9. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 3.Viboud C, Boëlle PY, Pakdaman K, Carrat F, Valleron AJ, Flahault A. Influenza epidemics in the United States, France, and Australia, 1972–1997. Emerg Infect Dis. 2004;10:32–9. doi: 10.3201/eid1001.020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipsitch M, Viboud C. Influenza seasonality: Lifting the fog. Proc Natl Acad Sci U S A. 2009;106:3645–6. doi: 10.1073/pnas.0900933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamerius J, Nelson MI, Zhou SZ, Viboud C, Miller MA, Alonso WJ. Global influenza seasonality: Reconciling patterns across temperate and tropical. Environ Health Perspect. 2011;119:439–45. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee VJ, Yap J, Ong JB, et al. Influenza excess mortality from 1950–2000 in tropical Singapore. PLoS One. 2009;4:e8096. doi: 10.1371/journal.pone.0008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waicharoen S, Thawatsupha P, Chittaganpitch M, Maneewong P, Thanadachakul T, Sawanpanyalert P. Influenza viruses circulating in Thailand in 2004 and 2005. Jpn J Infect Dis. 2008;61:321–3. [PubMed] [Google Scholar]
- 9.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008;453:615–9. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson MI, Holmes EC. The evolution of epidemic influenza. Nat Rev Genet. 2007;8:196–205. doi: 10.1038/nrg2053. [DOI] [PubMed] [Google Scholar]
- 11.Nelson MI, Simonsen L, Viboud C, Miller MA, Holmes EC. Phylogenetic analysis reveals the global migration of seasonal influenza A viruses. PLoS Pathog. 2007;3:1220–8. doi: 10.1371/journal.ppat.0030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell CA, Jones TC, Barr IG, et al. The global circulation of seasonal influenza A (H3N2) viruses. Science. 2008;320:340–6. doi: 10.1126/science.1154137. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. A practical guide to harmonizing virological and epidemiological influenza surveillance. http://www.wpro.who.int/internet/resources.ashx/CSR/Publications/GuideToHarmonizingInfluenzaSurveillance-revised2302.pdf. Accessed 20 September 2011. [Google Scholar]
- 14.Wang X, Cheng XW, Ma HW, et al. Influenza surveillance in Shenzhen, the largest migratory metropolitan city of China, 2006–2009. Epidemiol Infect. 2011;139:1551–9. doi: 10.1017/S0950268810002694. [DOI] [PubMed] [Google Scholar]
- 15.Schweiger B, Zadow I, Heckler R, Timm H, Pauli G. Application of a fluorogenic PCR assay for typing and subtyping of influenza viruses in respiratory samples. J Clin Microbiol. 2000;38:1552–8. doi: 10.1128/jcm.38.4.1552-1558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO manual on animal influenza diagnosis and surveillance. http://www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf. Accessed 20 September 2011. [Google Scholar]
- 17.Cheng X, Wu C, He J, et al. Serologic and genetic characterization analyses of a highly pathogenic influenza virus (H5N1) isolated from an infected man in Shenzhen. J Med Virol. 2008;80:1058–64. doi: 10.1002/jmv.21130. [DOI] [PubMed] [Google Scholar]
- 18.Johansson MA, Cummings DA, Glass GE. Multiyear climate variability and dengue–El Niño southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: a longitudinal data analysis. PLoS Med. 2009;6:e1000168. doi: 10.1371/journal.pmed.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Wong CM, Lau EH, Chan KP, Ou CQ, Peiris JS. Synchrony of clinical and laboratory surveillance for influenza in Hong Kong. PLoS One. 2008;3:e1399. doi: 10.1371/journal.pone.0001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrence C, Compo GP. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
- 21.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. http://www.R-project.org/ . Accessed 31 January 2012. [Google Scholar]
- 22.Bao Y, Bolotov P, Dernovoy D, et al. The influenza virus resource at the national center for biotechnology information. J. Virol. 2008;82:596–601. doi: 10.1128/JVI.02005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–90. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 24.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–21. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 25.Fitch WM. Toward defining the course of evolution: Minimum change for a specific tree topology. Systemat Zool. 1971;20:406–16. [Google Scholar]
- 26.Nelson MI, Simonsen L, Viboud C, et al. Stochastic processes are key determinants of short-term evolution in influenza A virus. PLoS Pathog. 2006;2:e125. doi: 10.1371/journal.ppat.0020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson MI, Edelman L, Spiro DJ, et al. Molecular epidemiology of A/H3N2 and A/H1N1 influenza virus during a single epidemic season in the United States. PLoS Pathog. 2008;4:e1000133. doi: 10.1371/journal.ppat.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mardy S, Ly S, Heng S, et al. Influenza activity in Cambodia during 2006–2008. BMC Infect Dis. 2009;9:168. doi: 10.1186/1471-2334-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa G, Kyaw Y, Danjuan L, et al. Influenza virus infections in Yangon, Myanmar. J Clin Virol. 2006;37:233–4. doi: 10.1016/j.jcv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Lowen A, Palese P. Transmission of influenza virus in temperate zones is predominantly by aerosol, in the tropics by contact: a hypothesis. PLoS Curr Influenza. 2009;1:RRN1002. doi: 10.1371/currents.RRN1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso WJ, Viboud C, Simonsen L, Hirano EW, Daufenbach LZ, Miller MA. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol. 2007;165:1434–42. doi: 10.1093/aje/kwm012. [DOI] [PubMed] [Google Scholar]
- 32.Garske T, Yu H, Peng Z, et al. Travel patterns in China. PLoS One. 2011;6:e16364. doi: 10.1371/journal.pone.0016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedford T, Cobey S, Beerli P, Pascual M. Global migration dynamics underlie evolution and persistence of human influenza A (H3N2) PLoS Pathog. 2010;6:e1000918. doi: 10.1371/journal.ppat.1000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahl J, Nelson MI, Chan KH, et al. Temporally structured metapopulation dynamics and persistence of influenza A H3N2 virus in humans. Proc Natl Acad Sci U S A. 2011;108:19359–64. doi: 10.1073/pnas.1109314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.