Abstract
Retinochoroiditis manifests in patients infected with Toxoplasma gondii. Here, we assessed 30 sibships and 89 parent/case trios of presumed ocular toxoplasmosis (POT) to evaluate associations with polymorphisms in the NOD2 gene. Three haplotype-tagging single-nucleotide polymorphisms (tag-SNPs) within the NOD2 gene were genotyped. The family-based association test showed that the tag-SNP rs3135499 is associated with retinochoroiditis (P = .039). We then characterized the cellular immune response of 59 cases of POT and 4 cases of active ocular toxoplasmosis (AOT). We found no differences in levels of interferon γ (IFN-γ) and interleukin 2 produced by T-helper 1 cells when comparing patients with AOT or POT to asymptomatic individuals. Unexpectedly, we found an increased interleukin 17A (IL-17A) production in patients with POT or OAT. In patients with POT or AOT, the main cellular source of IL-17A was CD4+CD45RO+T-bet−IFN-γ− T-helper 17 cells. Altogether, our results suggest that NOD2 influences the production of IL-17A by CD4+ T lymphocytes and might contribute to the development of ocular toxoplasmosis.
Keywords: NOD2, IL-17, Th17, T lymphocytes, ocular toxoplasmosis, Toxoplasma gondii
Lifelong infection with the obligate intracellular protozoan Toxoplasma gondii affects one-third of the human population globally [1]. Although toxoplasmosis is asymptomatic in the majority of cases, T. gondii infection is the most common cause of posterior uveitis worldwide [2, 3]. Its importance is even greater in Brazil, where the prevalence and severity of ocular disease is higher than that in the rest of the world [4–6]. The immune responses induced by T. gondii infection are initiated by the activation of innate immune cells and the induction of proinflammatory cytokines through activation of Toll-like receptors and development of T-helper 1 cells, exclude lymphocytes [7, 8]. A profile that includes Th1 cytokines, such as interferon-γ (IFN-γ) and interleukin 5, interleukin 6 (IL-6), and interleukin 17A (IL-17A) has also been associated with acute ocular toxoplasmosis in humans [9, 10]. However, the innate immune receptors that interact directly with T. gondii molecules triggering local inflammation in the eye remain to be identified.
The nucleotide-binding oligomerization domain containing 2 (NOD2) is an intracellular pattern-recognition receptor grouped in the NOD-like receptor family of proteins [11]. NOD2 is known to recognize bacterial peptidoglycan and to be expressed in different cell types, including neutrophils, monocytes, macrophages, and dendritic cells [12, 13]. Activation of NOD2 triggers the release of NF-κB, which induces the production of proinflammatory mediators, upregulation of major histocompatibility complex class II, and activation of antigen-specific CD4+ T-cell responses, including memory T cells [12, 14]. In mice, T. gondii infection was shown to activate NOD2, providing a T cell–intrinsic signal necessary for generating Th1-mediated immunity [15].
In the present study, we evaluated the involvement of the NOD2 receptor in susceptibility to ocular toxoplasmosis. For this purpose, we genotyped 3 haplotype-tagging single-nucleotide polymorphisms (tag-SNPs) within the NOD2 gene and tested for their association with the ocular disease [16]. Since NOD2 appears to be involved in eliciting both Th1 and T-helper 17 cell immune responses, we also characterized the response of T. gondii–specific CD4+ T cells in terms of cytokine production. We report that the tag-SNP rs3135499 is associated with presumed ocular toxoplasmosis (POT). Interestingly, the levels of IL-17A produced by parasite antigen–specific CD4+CD45RO+T-bet−IFN-γ Th17 lymphocytes were higher in patients with POT or active ocular toxoplasmosis (AOT). These individuals also present a high frequency of CD4+CD45RO+T-bet−IFN-γ− cells that produce IL-17A. Thus, altogether, our results suggest the involvement of NOD2 and Th17 lymphocytes in the development of ocular toxoplasmosis.
MATERIALS AND METHODS
Cohorts and Patients
Genetic analysis was performed in a combined cohort (Table 1) of parent/offspring and sibship trios from Vale do Jequitinhonha, in Minas Gerais state (the MG cohort), and from Campos dos Goytacazes, in Rio de Janeiro state (the RJ cohort). The seroprevalence of toxoplasmosis in these cohorts is 43% and 53% [17], respectively. The MG cohort comprises 66 cases of POT distributed in 61 families. The RJ cohort comprise 68 cases of POT distributed in 30 nuclear families and 30 sibships with at least one affected child and is described elsewhere [18]. The 2 cohorts were combined to increase the power to detect allelic associations with the genotyped SNPs. DNA was extracted from 301 individuals in total. Immunophenotyping assays were performed for individuals from the MG cohort only. Individuals were separated into four groups, including: seropositive asymptomatic or displaying POT or AOT and seronegative controls. All patients provided written informed consent prior to inclusion, and all procedures were performed in person, in accordance with institutional and National Institutes of Health guidelines.
Table 1.
Baseline Characteristics of the Minas Gerais Cohort and Rio de Janeiro Cohort Used in Family Based Allelic Association Test
| Cohort | Value |
|---|---|
| Minas Gerais | |
| No. of families | 13 |
| No. of nuclear families | 61 |
| No. of families with 1 affected generation | 51 |
| No. of families with 2 affected generations | 10 |
| No. of families with 1 affected offspring | 54 |
| No. of families with 2 affected offspring | 6 |
| Total no. of affected individuals | 66 |
| Total no. of individuals | 141 |
| Rio de Janeiro | |
| No. of nuclear families | 30 |
| No. of families with one affected offspring | 28 |
| No. of families with two affected offspring | 2 |
| No. of sibships | 30 |
| No. of sibships with 1 affected offspring | 25 |
| No. of sibships with 2 affected offspring | 4 |
| No. of sibships with 3 affected offspring | 1 |
| Total no. of affected individuals | 68 |
| Total no. of individuals | 160 |
Clinical Phenotypes for POT and AOT
Fundus examination was performed in seropositive individuals from the MG and RJ cohorts, under the same criteria by a group of ophthalmologists, coordinated by Dr Wesley Campos. The posterior retinal/retinochoroidal-scarred, healed lesions were classified as A or B (Figure 1) according to their morphological aspects, as described elsewhere [19]. Briefly, class A lesions present well-marked boundaries, usually surrounded by a pigmented halo and extensive destruction of the retina and choroid. Class B lesions are characterized by a surrounding hypopigmented halo and a smaller degree of tissue destruction, both of which fulfill the morphological criteria of presumed T. gondii infection. Individuals presenting with POT were examined at the time of survey and 6–10 months after the first examination. No difference was observed in any aspect of the lesions during the period. The additional AOT cases displayed healed, scarred lesions and active retinochoroiditis located adjacently to the previous lesion, as well as high avidity anti–T. gondii immunoglobulin G and no immunoglobulin M (Supplementary Table 1), all of which are consistent with reactivation of ocular toxoplasmosis. These patients also underwent visual acuity evaluation, tonometry, biomicroscopy, and ophthalmoscopy [20].
Figure 1.
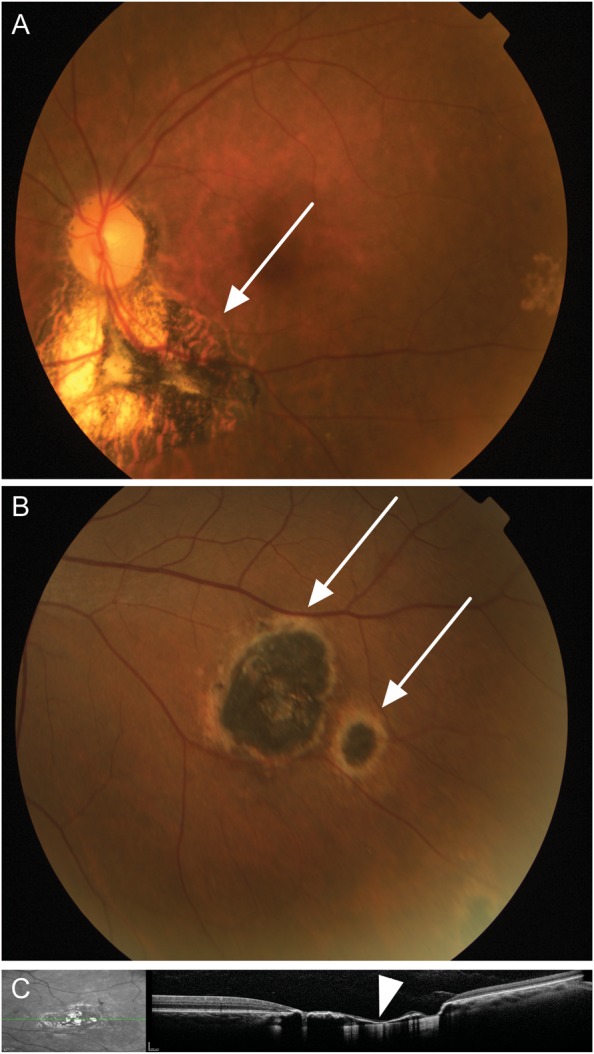
Ocular examination in the Minas Gerais cohort. A, A representative retinography of an individual with a type A scarred lesion (arrow), highly suggestive of toxoplasmosis, characterized by sharply demarcated pigmented borders and a hypopigmented central portion with extensive destruction of the retina and choroid. B, A representative retinography of type B scarred ocular lesions (arrows), suggestive of toxoplasmosis, characterized by a hyperpigmented central area surrounded by a hypopigmented halo with smaller degree of tissue destruction. C, Spectral domain optical coherence tomography of an ocular lesion (left), showing disorganization of the retinal layers and thinning of the retina (arrow head, right).
Serodiagnosis for Toxoplasmosis by Enzyme-Linked Immunosorbent Assay
High binding microtiter plates Immulon-2 (Dynatech Laboratories, CT) were coated with 1 μg/mL of F3, an extract enriched for the surface components of T. gondii tachyzoites and developed as previously described [21].
Genotyping and Family-Based Allelic Association Tests
The tag-SNPs in the NOD2 gene were selected from the Hapmap project, release 27 (http://www.hapmap.org), using 5 kb of flanking sequence on each side of the gene. To select the tag-SNPs, the tagger tool [22] within Haploview software v4.2 [23] was used, setting a minor allele frequency cutoff of 0.2 in CEU and YRI populations and an r2 threshold of 0.8. Genotyping was performed using the Taqman (Applied Biosystems, CA) technology for NOD2 SNPs at rs2076753 (intronic), rs2111235 (intronic), and rs3135499 (3′ untranslated region). The tag-SNPs were in Hardy-Weinberg equilibrium in genetically unrelated founders in the Brazilian families (P > .05). The power to detect association in the trios/sibships is 70.8%, at an odds ratio of 1.5 and a nominal P value of .05. Family-based allelic association tests were performed within the family based association test [16], which is based on the transmission disequilibrium test but allows for analyses under different genetic models and with incomplete parental data [16, 24]. Analyses were performed using a genotype model and under the null hypothesis of “no linkage and no association.” Nominal P values are presented.
T-Cell Immunophenotyping and Intracellular Cytokine Measurement
In the immunological study, 105 individuals from the MG cohort were used and included 21 anti-T. gondii–seronegative individuals, 59 cases of POT, and 25 cases of asymptomatic toxoplasmosis (Supplementary Table 2). Additional 4 cases of AOT were also analyzed.
PBMCs were prepared from heparinized venous blood from adult volunteers by Ficoll-Hypaque density gradient centrifugation (Sigma Aldritch). All cultures were performed using Roswell Park Memorial Institute 1640 medium supplemented with 5% heat-inactivated fetal calf serum (Gibco, NY) and penicillin (200 U/mL) in the presence or absence of stimulus at 37°C in 5% CO2. Cells were stimulated for 20 hours with anti-CD3 (1.0 μg/mL) and anti-CD28 (0.5 μg/mL) (BD Biosciences, CA) or with soluble tachyzoite antigens (STAg) prepared from the RH strain of T. gondii (10 μg/mL). For that propose, tachyzoites were obtained by peritoneal washing of infected SWISS mice, and STAg was produced as previously described [21, 25]. In the last 8 hours of culture, brefeldin A (GolgiPlug Protein Transport Inhibition, BD Biosciences) was added to each well. After incubation, cells were washed and stained for surface molecules for 15 minutes at room temperature. The cells were washed, permeabilized (Cytofix/Cytoperm, BD Biosciences), and fixed in 200 µL of phosphate-buffered saline–2% paraformaldehyde. At least 90 000 gated events were acquired for analysis using FACSCalibur with Cellquest or LSR II with Diva (BD Biosciences). The antibodies used for staining were immunoglobulin controls, anti-CD3(UCTH1)-FITC, anti-CD4(OKT4)-FITC or –APC, anti-CD4(RPA-T4)-APC-eFluor780 or PerCPCy5.5, anti-CD45RO(UCHL1)-PerCP-eFluor710, anti-TNF-α(MAb11)-PE, anti-IL-17A(eBio64DEC17)-PE, anti-IL-17A(eBio64CAP17)-AlexaFlour647, anti-IFN-γ(4SB3)-PECy7, anti-TNF-α(MAb11)-AlexaFluor700, and anti-T-bet(eBio4B10)-PE, all from eBioscience; and anti-IL-2(MQ117H12)-APC (BD Biosciences). FlowJo (v8.8.6) and GraphPad Prism (v5.0b) were used for data analysis and graphic presentation.
Proliferation Assay
For the proliferation assay, cells were stained with 1.25 μM CFSE at 1 × 107 cells/mL for 8 minutes. Cells were then equally distributed into 3 wells containing STAg, anti-CD3/anti-CD28, or medium in the absence of stimulus. The cells were cultured at 37°C in 5% CO2. After 5 days of culture, the cells were stained with the following antibodies: anti-HLADR(LN3)-PE, anti-CD8(OKT8)-PerCP, and anti-CD4(OKT4)-APC, as described above.
Determination of Cytokine Levels
To determine the IL-17A, interleukin 2 (IL-2), interleukin 4 (IL-4), IFN-γ, interleukin 10 (IL-10), and tumor necrosis factor α (TNF-α) levels, the BD Cytometric Bead Array Human Th1/Th2/Th17 Cytokine Kit was used according to manufacturer's instructions. Briefly, 1 × 106 PBMCs/well were cultured with STAg, with anti-CD3/anti-CD28, or with no stimulus for 12 hours at 37°C in 5% CO2. The supernatants were collected and kept at −20°C. The concentration of cytokines was calculated using the BD FCAP Array software (v1.0.1).
Statistical Analyses
Statistical analyses for the in vitro assays were performed using the unpaired Student t test with Welch's correction, for parametric data, or the Mann–Whitney U test, for nonparametric data.
RESULTS
SNP rs3135499 in the NOD2 Gene Is Associated With Ocular Toxoplasmosis
To test the hypothesis that polymorphisms at NOD2 might influence susceptibility to ocular toxoplasmosis in the Brazilian cohorts (Table 1), we genotyped 3 tag-SNPs distributed throughout the human NOD2 gene (Figure 2) in 91 parent/offspring trios and 30 sibships with cases of POT. The selected SNPs had an r2 threshold of 0.8, a minor allele frequency of >0.2, and were in Hardy-Weinberg equilibrium in the unrelated parents. By use of the family based association test [16], we found evidence for association between ocular toxoplasmosis and the SNP rs3135499 (2-sided P = .039; Table 2). Under a genotype model of inheritance, the homozygous genotype CC is associated with protection against the development of ocular toxoplasmosis (Z statistic = −2.06). An unknown causal SNP in strong linkage disequilibrium with this marker could account for the observed association with the ocular disease.
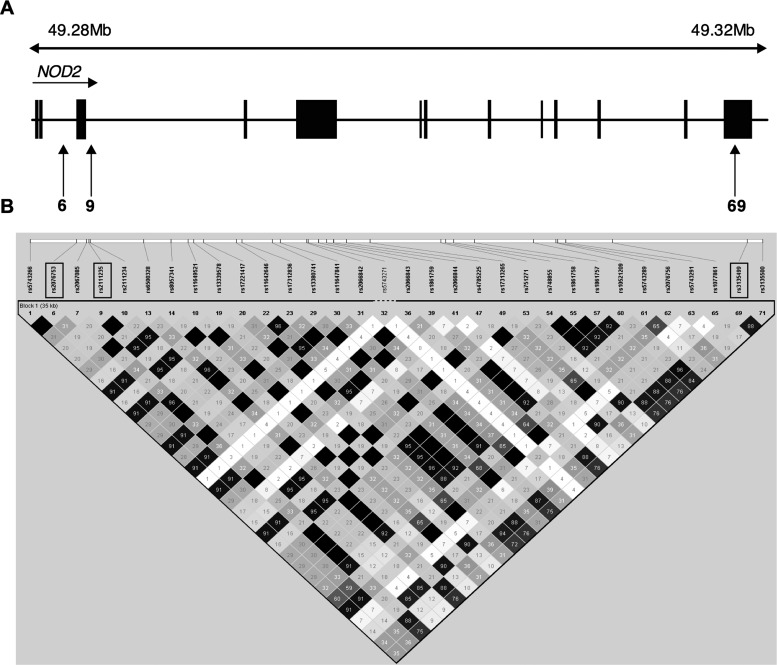
Figure 2.
Haploview analysis for r2 pairwise measures of linkage disequilibrium between NOD2 single-nucleotide polymorphisms (SNPs) in unrelated family founders in Brazil. A, The NOD2 genomic structure, indicating the position of the 3 genotyped tag-SNPs (vertical arrows). In the diagram, 6 indicates the SNP rs2076753, 9 indicates the SNP rs2111235, and 69 indicates the SNP rs3135499. Exons are represented as black rectangles. B, Linkage disequilibrium plots showing pairwise r2 linkage disequilibrium measures across the 3 NOD2 SNPs genotyped in the study. Linkage disequilibrium estimates were determined in Haploview software v4.2, using unrelated individuals from the Brazilian families. r2 values are represented in white for r2 = 0, with intermediate values for 0 < r2 < 1 indicated by shades of grey. The numbers within the squares represent the r2 scores for pairwise linkage disequilibrium.
Table 2.
Results of Family Based Allelic Association Tests (FBAT) Between NOD2 Single-Nucleotide Polymorphisms (SNP) and Presumed Ocular Toxoplasmosis in Samples from Brazilian Patients
| SNP Characteristic |
FBAT Parameterb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Location | Physical Positiona | MAF | Genotype | Genotype Frequency | No. of Familiesc | S | E(S) | Var(S) | Zd | Pe |
| NOD2rs3135499 | 3′ UTR | 50766127 | 0.45 | CC | 0.211 | 19 | 3.00 | 7.17 | 4.09 | −2.06 | .039 |
| CA | 0.487 | 53 | 34.00 | 28.38 | 13.09 | 1.55 | .121 | ||||
| AA | 0.302 | 44 | 18.00 | 19.45 | 10.25 | −0.45 | .651 | ||||
| NOD2rs2111235 | Intron 1 | 50733969 | 0.32 | GG | 0.488 | 40 | 17.00 | 17.73 | 9.53 | −0.24 | .814 |
| GA | 0.381 | 46 | 27.00 | 23.55 | 11.67 | 1.01 | .312 | ||||
| AA | 0.131 | 19 | 5.00 | 7.73 | 4.42 | −1.30 | .194 | ||||
| NOD2rs2076753 | Intron 2 | 50733374 | 0.24 | TT | 0.044 | 4 | 2.00 | 1.50 | 0.875 | 0.535 | .593 |
| TG | 0.383 | 26 | 11.00 | 12.20 | 6.174 | −0.483 | .629 | ||||
| GG | 0.573 | 26 | 14.00 | 13.30 | 6.049 | 0.285 | .776 | ||||
Abbreviations: E(S), expected transmission of the allele; MAF, minor allele frequency; S, observed transmission of the allele; UTR, untranslated region; Var(S), variance.
a Physical positions of markers, in base pairs, are given according to Build 37.3 of the human genome; alleles in the positive strand are shown for all markers. All 3 SNPs were in Hardy-Weinberg equilibrium in unaffected founders of the families.
b Single point FBAT analysis under a genotype model of inheritance for associations between NOD2 polymorphisms and ocular toxoplasmosis in the Brazilian cohort (Rio de Janeiro trios and Minas Gerais cohort taken together).
c No. of families informative for FBAT.
d A positive Z score indicates association with disease (overtransmission of the genotype under consideration in cases), and a negative Z score indicates the nonassociated or protective genotype.
e Bold indicates a significant association at a nominal P value of ≤ .05.
Activation and Cytokine Production by CD4+ T Lymphocytes From Patients With Scarred Toxoplasmic Retinochoroiditis
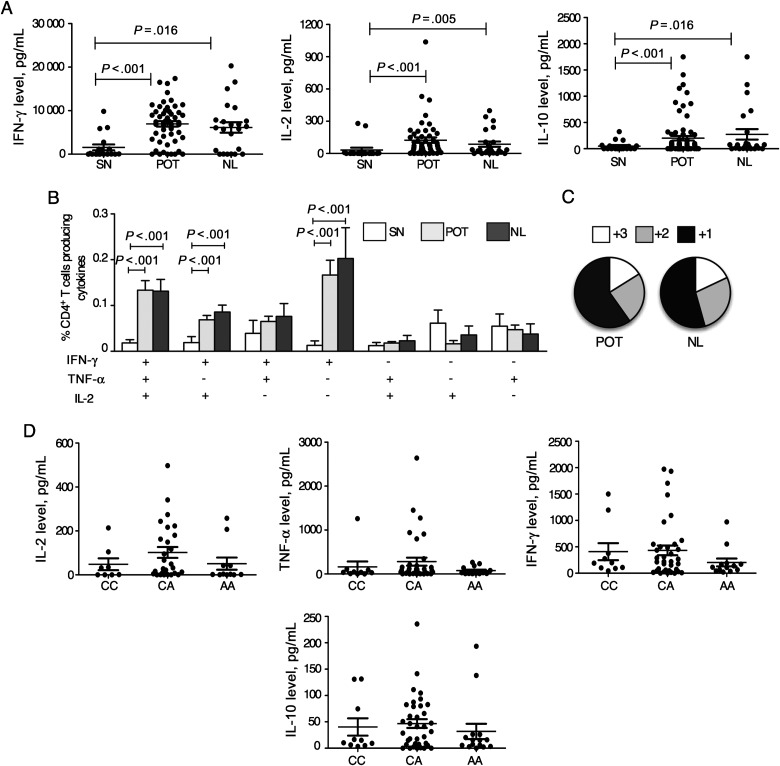
Earlier studies suggest that T cells from patients with ocular toxoplasmosis display an altered responsiveness to T. gondii antigens [26]. Thus, we investigated this question by using PBMCs from seropositive individuals from the MG cohort who did or did not have POT. On STAg stimulation, the HLA-DR+CD4+ T lymphocytes from seropositive individuals with POT (30.6% ± 3.0%) or no eye lesions (NL; 35.9 ± 4.9) presented similar proliferative response, as assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution. To access the production of cytokines in the 3 different groups of subjects, total PBMCs were cultured in the presence of STAg for 12 hours. PBMCs from seropositive individuals produced significantly higher amounts of IFN-γ, IL-2, and IL-10, compared with seronegative individuals (Figure 3A). The levels of these cytokines were similar among the seropositive individuals (the POT and NL groups), regardless of retinochoroiditis.
Figure 3.
Unimpaired function of T-helper cell 1 lymphocytes from Toxoplasma gondii–infected individuals bearing the susceptible NOD2 rs3135499 genotype. A, Peripheral blood mononuclear cells (PBMCs) were harvested from seronegative (SN) individuals and patients with chronic toxoplasmosis with ocular scars (POT) or no eye lesions (NL). PBMCs were then cultured for 8 hours in the presence or absence of parasite antigen (STAg), and cytokine levels were measured in the culture supernatants by a cytometric bead array assay. B, PBMCs were cultured for 20 hours in the presence or absence of STAg and stained for CD4 and the cytokines interleukin 2 (IL-2), interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α). Bars represent mean frequencies (±standard error of the mean) of CD4+ T cells producing any of the 7 possible combinations of the cytokines IL-2, IFN-γ, and TNF-α in the SN group (white bars), POT group (grey bars), and NL group (black bars). C, Fraction of the total response comprising cells expressing all 3 cytokines (3), any 2 cytokines (2), or any 1 cytokine (1). D, Production of IFN-γ, IL-2, TNF-α, and interleukin 10 (IL-10) is plotted against the 3 possible genotypes for each of the rs3135499 SNPs for which we found evidence for association with ocular toxoplasmosis.
We next evaluated the frequency of STAg-specific CD4+ T cells simultaneously producing IFN-γ, IL-2, or TNF-α in individuals with POT. Seropositive individuals had significantly higher frequencies of IFN-γ/TNF-α/IL-2–producing cells (named multifunctional), IL-2/IFN-γ–producing cells, and IFN-γ–producing CD4+ T cells, compared with seronegative individuals. However, these frequencies did not differ between NL and POT seropositive groups (Figure 3B). The proportions of single, double, and triple producers were similar between the NL and POT individuals (Figure 3C). These results indicate that, considering the cytokine production and frequency of multifunctional T cells, the Th1 response specific for T. gondii antigens are similar between NL and POT patients.
Unimpaired Function of Th1 Lymphocytes From T. gondii–Infected Individuals Bearing the Susceptible NOD2 rs3135499 Genotype
It has been shown that during T. gondii infection, NOD2 plays a role in the differentiation of Th1 lymphocytes by triggering the production of IL-2 by naive T cells and leading to their commitment toward the Th1 phenotype. Despite the normal production of interleukin 12p40 in NOD2−/− mice, the frequency of IFN-γ–producing Th1 cells is lower than in wild-type mice [15]. Since we found evidence for association between the NOD2 rs3135499 SNP and POT, we then analyzed the cytokine profile produced by CD4+ T cells from individuals with and individuals without POT. PBMCs derived from individuals bearing the possible genotypes for the rs3135499 locus produced similar levels of the Th1 cytokines (IL-2, IFN-γ, TNF-α, and IL-10) (Figure 3D). Altogether, our results indicate that the rs3135499 SNP in the NOD2 gene is not associated with an altered function of Th1 lymphocytes.
Polymorphism rs3135499 in the NOD2 Gene Is Associated With Enhanced Production of IL-17A by T. gondii–specific CD4+ T Lymphocytes
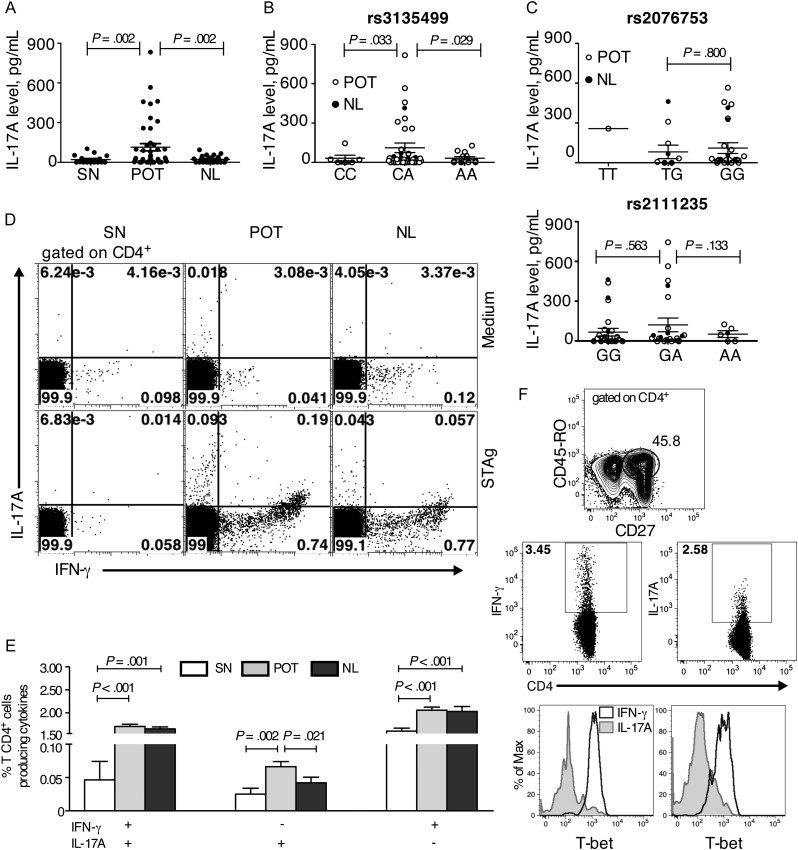
Apart from the contribution of NOD2 to the development of Th1 lymphocytes, it has also been shown that NOD2 plays a role in inducing the production of IL-17A by human memory T cells [14]. Thus, the levels of IL-17A were analyzed in supernatant of PBMCs cultured for 5 days in the presence of STAg. PBMCs from individuals with POT produced significantly higher amounts of IL-17A, compared with individuals in the NL group (Figure 4A). We then analyzed the production of IL-17A within the different genotypes for the 3 tag-SNPs in the NOD2 gene. The seropositive individuals were classified into 3 groups on the basis of 3 possible genotypes for each SNP (Figure 4B). There was no significant difference in the production of IL-17A among the genotypes for the 2 tag-SNPs (rs2076753 and rs2111235) not associated with the ocular disease (Table 2 and Figure 4C). On the contrary, we found higher production of IL-17A in individuals bearing the heterozygous genotype for the rs3135499 tag-SNP, comparison to both homozygotes (P = .029 compared to AA; P = .033 compared to CC). Among the individuals bearing the CA genotype, individuals with POT produced higher levels of IL-17A (POT group, 87.1 pg/ml ± 28.7 pg/ml; NL group, 24.8 p/ml ± 7.8 pg/ml; P = .045). These results suggest a possible role for polymorphisms at the NOD2 gene and IL-17A production with the development of ocular disease in human toxoplasmosis.
Figure 4.
Individuals with the heterozygous genotype for the single-nucleotide polymorphism (SNP) rs3135499 in the NOD2 gene produce higher levels of interleukin 17A (IL-17A) and have a high frequency of Toxoplasma gondii–specific T-helper cell 17 (Th17) lymphocytes. A, Production of IL-17A by antigen-stimulated peripheral blood mononuclear cells (PBMCs) from groups of individuals who were seronegative (SN), had presumed ocular toxoplasmosis (POT), or were asymptomatic (NL). B, Production of IL-17A plotted against the 3 possible genotypes for SNP rs3135499 that we found evidence of an association with ocular toxoplasmosis (P = .039). C, Production of IL-17A plotted against the 3 possible genotypes for the SNPs rs2076753 (top panel) and rs2111235 (bottom panel), both of which were not associated with the ocular disease. Seropositive individuals in the POT and NL groups are presented as white and black dots, respectively. PBMCs from the individuals were stimulated with or without parasite antigen (STAg) for 20 hours and stained either for CD4, IL-17A, and IFN-γ (D and E); or for CD3, CD4, CD45RO, CD27, T-bet, IL-17A, and interferon γ (IFN-γ; F). D, Distribution of CD4+ T cells producing IL-17A, IFN-γ, or both after culture in the absence (top row) or presence (bottom row) of STAg. Each density plot shows concatenated files of 2 SN (left panels), 4 POT (middle panels), and 3 NL (right panels) patients. E, Mean frequencies (±standard error of the mean) of CD4+T cells that produce the possible combinations of IFN-γ and IL-17A. F, Panel on the top shows the CD27+CD45RO+ gate on the CD4+ T lymphocytes and middle panels show IFN-γ–producing CD4+CD27+CD45RO+ and IL-17A–producing CD4+CD27+CD45RO+IL-17A+ T cells in PBMCs from a single subject displaying POT. Bottom panels show representative histograms of T-bet expression in IFN-γ– and IL-17A–producing CD4+CD27+CD45RO+ T cells from 2 of 6 POT patients.
Enhanced Frequency of Th17 Lymphocytes in Patients Susceptible to Ocular Toxoplasmosis
We then defined the cellular source of IL-17A in PBMCs from individuals with POT. It has been shown that Th17 can produce cytokines other than IL-17A, including TNF-α and limited amounts of IFN-γ [27]. They can be distinguished from Th1 cells by the expression of T-bet, a Th1 cell–specific transcription factor [28]. After 20 hours of incubation with or without STAg, PBMCs were stained for different cell surface markers (CD4, CD27, and CD45RO), the transcription factor (T-bet), and cytokines (IFN-γ and IL-17A). Individuals from seropositive groups presented higher frequency of CD4+ T cells producing only IFN-γ, only IL-17A, or both IFN-γ/IL-17A, compared with seronegative individuals (Figure 4D and 4E). The main source of IL-17A in individuals with POT was CD4+/T-bet−/IFN-γ− (Th17) cells (Figure 4F). Interestingly, the proportion of the Th17 lymphocytes was significantly higher in individuals with POT, compared with asymptomatic seropositive individuals (Figure 4E). These results indicate that Th17 lymphocytes and IL-17A may be involved in the development of the ocular lesions during acute, inflammatory phases of T. gondii infection.
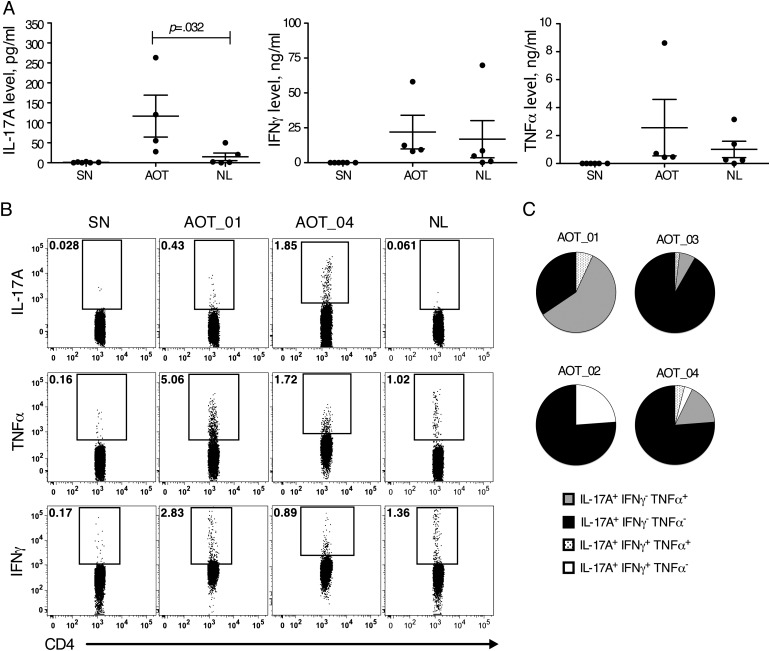
IL-17A Production and Th17 Lymphocytes Are Also Augmented in Individuals With Active Toxoplasmic Retinochoroiditis
To address the question that IL-17A could be involved in the inflammatory process leading to active retinochoroiditis, we also evaluated the production of IL-17A in 4 individuals displaying AOT (Supplementary Table 1). STAg-stimulated PBMCs from subjects with AOT produced significantly higher levels of IL-17A, compared with those from asymptomatic individuals (Figure 5A). In addition, subjects with AOT presented a higher frequency of CD4+CD45RO+ T cells producing IL-17A but not TNF-α and IFN-γ, compared with the asymptomatic seropositive group (P=.035, .064, and .555 for CD4+CD45RO+ T cells producing IL-17A, TNF-α, and IFN-γ, respectively) (Figure 5B). Looking specifically at the IL-17A–producing CD4+CD45RO+T-bet− T cells, defined as Th17 lymphocytes (Figure 5C), the majority were only IL-17A producers. Although these cells were the most frequent, CD4+CD45RO+T-bet+ T cells that also produced TNF-α or IFN-γ were found in patients with active lesions. These results indicate that together with Th1 cells, Th17 cells may contribute to the inflammatory process leading to toxoplasmic retinochoroiditis.
Figure 5.
Interleukin 17A (IL-17A)–producing CD4+ T cells are also present in a higher frequency in individuals with active ocular toxoplasmosis. A, IL-17A, interferon γ IFN-γ), and tumor necrosis factor α (TNF-α) production by peripheral blood mononuclear cells (PBMCs) from active ocular toxoplasmosis (AOT) lesions cultured in the presence or absence of parasite antigen (STAg). B, Frequencies of antigen-stimulated CD3+CD4+CD45RO+T lymphocytes expressing IL-17A, TNF-α, or IFN-γ from 1 patient in the seronegative group (SN; top panels), 1 in the asymptomatic group (NL; bottom panels), or 2 in the AOT group (AOT_01 and AOT_04; middle panels). C, Distribution of subgroups of IL-17A–producing CD4+ T lymphocytes expressing the 4 different sets of cytokines, combining TFN-α, IFN-γ, and IL-17A in 4 patients with AOT. The proportions of CD4+CD45RO+ T lymphocytes that are IL-17A+IFN-γ+TNF-α+, IL-17A+IFN-γ−TNF-α−, IL-17A+IFN-γ+TNF-α−, and IL-17A+IFN-γ−TNF-α+ are represented in dotted pattern, black, white and grey, respectively.
DISCUSSION
T. gondii is the main infectious cause of human posterior retinochoroiditis, the most frequent clinical manifestation of congenital and acquired toxoplasmosis [2, 29]. The disease is typically presented as unilateral focus of retinochoroiditis, in the presence of adjacent chorioretinal scars, usually associated with intraocular inflammation followed by levels of necrotizing retinopathy [3] The severity and prevalence of the disease vary greatly and is believed to be under the influence of the status of host immune system [9], the genotype of infective parasite strains [30], and the host genetic background [31]. In this sense, polymorphisms in genes of the innate immune system, such as TLR9 (which encodes a protein that recognizes unmethylated CpG motives [18]), NALP-1 (which encodes a member of the NOD-like receptor family involved in inflammasome formation [32]), and P2X7 (which encodes a purinergic receptor for adenosine triphosphate that is also important for inflammasome formation [33]), have been associated with susceptibility to manifestations of congenital infection and ocular toxoplasmosis. Here, we found evidence for association of 1 SNP in the NOD2 gene with toxoplasmic retinochoroiditis. The observed association could be due to strong linkage disequilibrium between rs3135499 and the true causal variant in the NOD2 gene, which encodes an innate immune receptor, that influences cytokine production by T lymphocytes [13, 15, 34].
A recent report demonstrates that NOD2 is an important mediator of host resistance to infection with T. gondii [15]. NOD2-deficient mice resist during the acute stage of infection but succumb at the beginning of the chronic phase. Shaw and collaborators demonstrated that NOD2−/− mice show normal IL-12 production, but impaired Th1 responses during T. gondii infection, with a lower frequency of CD4+T cells producing IFN-γ and IL-2 [15]. However, experiments performed by our group show that decreased cytokine production and enhanced susceptibility is not observed in NOD2−/− mice infected with the ME49 strain of T. gondii [35]. Consistently with our findings in the rodent model of toxoplasmosis, the different alleles of the 3 SNPs of NOD2 gene were not associated with changes in the levels of IL-2, IFN-γ, TNF-α, and IL-10 produced by antigen-stimulated PBMCs or CD4+ T lymphocytes from patients chronically infected with T. gondii, regardless of the presence of ocular disease. Nevertheless, we found higher levels of IL-17A produced by PBMCs from patients with POT or AOT. Furthermore, we observed that the frequency of parasite antigen–specific Th17 (CD4+T-bet−IL-17A+IFN-γ−) lymphocytes was increased in PBMCs from patients with retinochoroiditis. We hypothesize that Th17 and IL-17A contribute to the inflammatory process and development of ocular toxoplasmosis.
The role of Th17 and IL-17A in rodent and human toxoplasmosis was addressed by a few studies. After oral infection with T. gondii, mice lacking the IL-17A receptor develop a diminished inflammatory process in the ileum, compared with wild-type mice, after oral infection with T. gondii [36]. Additionally, in mice infected with T. gondii, the lack of interleukin 27 signaling, a cytokine that inhibits Th17 differentiation, results in severe neuropathology associated with production of high levels of IL-17A, IL-6, and TNF-α [37]. This indicates a role for IL-17A in the immunopathology and inflammatory processes triggered by T. gondii infection. In humans, IL-17A has been detected in aqueous humour specimens from individuals with AOT, although the source of IL-17A has not yet been identified [10].
Importantly, NOD2 has been implicated in IL-17A production by memory T cells. In human dendritic cells primed with bacteria, stimulation with muramyl dipeptide enhanced the production of IL-23 and IL-1β triggered by activation of Toll-like receptor 2 (TLR2). In turn, IL-23 promoted IL-17A release by memory T cells [14]. It is noteworthy that glycosylphosphatidylinositol anchors derived from T. gondii tachyzoites were also shown to activate TLR2 [38]. T. gondii molecules that are recognized by NOD2 receptor are yet to be identified. Additionally, the ocular surface has an environment enriched with cytokines (ie, TGF-β, IL-6, IL-23, and IL-1β) that are important for the Th17 cell differentiation [39, 40]. In fact, Th17 lymphocytes or IL-17A are also known to be involved in ocular diseases, including uveitis and scleritis of various etiologies [10, 41]. Furthermore, mutations in the NOD2 gene are also causative of uveitis in individuals with Blau syndrome, an autosomal dominant condition that presents as granulomatous inflammation affecting the eyes, skin, and joints [42]. In experimental autoimmune uveitis, the intensity of IL-17A production by Th17 cells correlates with susceptibility to disease [43].
It has been shown both in vitro and in the mouse model that the infective parasite strain is an important factor influencing the T-cell development, cytokine responses, and mouse resistance to experimental infection with T. gondii [44]. Thus, considering the high frequency of virulent parasites strains and acquired ocular toxoplasmosis found in Brazil [6, 45], it is tempting to speculate that the observed variability of the immune responses and the association of inflammatory Th17 lymphocytes with ocular disease are influenced by the infective parasite strains. However, the translation of findings in the rodent model to human toxoplasmosis is elusive, especially considering that functional TLR11 [46] and the whole family of genes encoding IFN-γ–inducible GTPases [47], which are critical elements in host resistance to infection in mice, are mostly absent in humans.
Nevertheless, cytokines produced by T cells are of critical importance for host resistance to infection with T. gondii, both in the murine model and in humans [25, 48, 49]. By controlling parasite replication, TNF-α and IFN-γ prevent systemic dissemination of tachyzoites during acute infection and prevent the reactivation of chronic toxoplasmosis in the central nervous system [48]. In this study, we found similar level of Th1 cytokines and similar frequencies of CD4+ T cells producing IL-2, IFN-γ, and TNF-α in response to STAg in seropositive individuals, regardless of the presence of ocular disease. In contrast, the frequency of IL-17A-producing Th17 cells was augmented in patients with POT or AOT. Hence, it is possible that Th17 lymphocytes contribute to the development of ocular disease by promoting a deleterious inflammatory process, rather than by interfering with parasite control. Consistently, inflammation has been recognized as a critical process that mediates the ocular lesions in patients infected with T. gondii [50]. Together, our results demonstrate an association between NOD2 and the production of IL-17A by inflammatory Th17 lymphocytes and the development of ocular toxoplasmosis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are most grateful to Dr Fernando Oréfice, Dr Mário Carlos Lemos, Dr Sydney Rocha Lemos, Dr Gustavo Hering, and Dr Juliana Oréfice, who also contributed to this study by obtaining histories from and performing physical and eye examinations on individuals who participated in this study; Ms Maria Bernadete Santério Inocêncio, for collecting the blood from the patients with AOT; Dr Ricardo W. Vítor, from the Department of Parasitology of the Universidade Federal de Minas Gerais, who kindly provided the RH parasite strain from which the STAg was obtained; and the patients and their families, for participating and for permitting us to follow their progress.
Financial support. This work was supported in part by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (R01 AI071319-01), the National Institute of Science and Technology for Vaccines (INCTV/CNPq/FAPEMIG), the Carlos Chagas Filho Research Support Foundation FAPERJ (E-26/112.045/2008 and E-26/110.869/2009), and the National Council for Scientific and Technological Development–CNPq (scholarships to M. S. D. and S. R. B) R. T. G. and L. R. A. are research fellows from CNPq.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J. 2011;30:1056–61. doi: 10.1097/INF.0b013e3182343096. [DOI] [PubMed] [Google Scholar]
- 3.Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004;137:1–17. [PubMed] [Google Scholar]
- 4.Portela RW, Bethony J, Costa MI, et al. A multihousehold study reveals a positive correlation between age, severity of ocular toxoplasmosis, and levels of glycoinositolphospholipid-specific immunoglobulin A. J Infect Dis. 2004;190:175–83. doi: 10.1086/421505. [DOI] [PubMed] [Google Scholar]
- 5.Glasner PD, Silveira C, Kruszon-Moran D, et al. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol. 1992;114:136–44. doi: 10.1016/s0002-9394(14)73976-5. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert RE, Freeman K, Lago EG, et al. Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis. 2008;2:e277. doi: 10.1371/journal.pntd.0000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–88. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6:895–906. doi: 10.1038/nri1978. [DOI] [PubMed] [Google Scholar]
- 9.Garweg JG, Candolfi E. Immunopathology in ocular toxoplasmosis: facts and clues. Mem Inst Oswaldo Cruz. 2009;104:211–20. doi: 10.1590/s0074-02762009000200014. [DOI] [PubMed] [Google Scholar]
- 10.Lahmar I, Abou-Bacar A, Abdelrahman T, et al. Cytokine profiles in toxoplasmic and viral uveitis. J Infect Dis. 2009;199:1239–49. doi: 10.1086/597478. [DOI] [PubMed] [Google Scholar]
- 11.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–8. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 12.Cooney R, Baker J, Brain O, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–7. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 14.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–9. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Shaw MH, Reimer T, Sanchez-Valdepenas C, et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat Immunol. 2009;10:1267–74. doi: 10.1038/ni.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvath S, Xu X, Laird NM. The family based association test method: strategies for studying general genotype–phenotype associations. Eur J Hum Genet. 2001;9:301–6. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- 17.Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Orefice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peixoto-Rangel AL, Miller EN, Castellucci L, et al. Candidate gene analysis of ocular toxoplasmosis in Brazil: evidence for a role for toll-like receptor 9 (TLR9) Mem Inst Oswaldo Cruz. 2009;104:1187–90. doi: 10.1590/s0074-02762009000800019. [DOI] [PubMed] [Google Scholar]
- 19.Bahia-Oliveira LM, Silva JA, Peixoto-Rangel AL, et al. Host immune response to Toxoplasma gondii and Ascaris lumbricoides in a highly endemic area: evidence of parasite co-immunomodulation properties influencing the outcome of both infections. Mem Inst Oswaldo Cruz. 2009;104:273–80. doi: 10.1590/s0074-02762009000200021. [DOI] [PubMed] [Google Scholar]
- 20.Jabs DA, Nussenblatt RB, Rosenbaum JT Standardization of Uveitis Nomenclature Working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caetano BC, Bruna-Romero O, Fux B, Mendes EA, Penido ML, Gazzinelli RT. Vaccination with replication-deficient recombinant adenoviruses encoding the main surface antigens of Toxoplasma gondii induces immune response and protection against infection in mice. Hum Gene Ther. 2006;17:415–26. doi: 10.1089/hum.2006.17.415. [DOI] [PubMed] [Google Scholar]
- 22.de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–23. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 23.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 24.Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(Suppl 1):S36–42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Gazzinelli RT, Bala S, Stevens R, et al. HIV infection suppresses type 1 lymphokine and IL-12 responses to Toxoplasma gondii but fails to inhibit the synthesis of other parasite-induced monokines. J Immunol. 1995;155:1565–74. [PubMed] [Google Scholar]
- 26.Yamamoto JH, Vallochi AL, Silveira C, et al. Discrimination between patients with acquired toxoplasmosis and congenital toxoplasmosis on the basis of the immune response to parasite antigens. J Infect Dis. 2000;181:2018–22. doi: 10.1086/315494. [DOI] [PubMed] [Google Scholar]
- 27.Church LD, Filer AD, Hidalgo E, et al. Rheumatoid synovial fluid interleukin-17-producing CD4 T cells have abundant tumor necrosis factor-alpha co-expression, but little interleukin-22 and interleukin-23R expression. Arthritis Res Ther. 2010;12:R184. doi: 10.1186/ar3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller SA, Weinmann AS. Molecular mechanisms by which T-bet regulates T-helper cell commitment. Immunol Rev. 2010;238:233–46. doi: 10.1111/j.1600-065X.2010.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delair E, Latkany P, Noble AG, Rabiah P, McLeod R, Brezin A. Clinical manifestations of ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19:91–102. doi: 10.3109/09273948.2011.564068. [DOI] [PubMed] [Google Scholar]
- 30.Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, Sibley LD. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc Natl Acad Sci U S A. 2011;108:9631–6. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mack DG, Johnson JJ, Roberts F, et al. HLA-class II genes modify outcome of Toxoplasma gondii infection. Int J Parasitol. 1999;29:1351–8. doi: 10.1016/s0020-7519(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 32.Witola WH, Mui E, Hargrave A, et al. NALP1 influences susceptibility to human congenital toxoplasmosis, proinflammatory cytokine response, and fate of Toxoplasma gondii-infected monocytic cells. Infect Immun. 2011;79:756–66. doi: 10.1128/IAI.00898-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamieson SE, Peixoto-Rangel AL, Hargrave AC, et al. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010;11:374–83. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werts C, le Bourhis L, Liu J, et al. Nod1 and Nod2 induce CCL5/RANTES through the NF-kappaB pathway. Eur J Immunol. 2007;37:2499–508. doi: 10.1002/eji.200737069. [DOI] [PubMed] [Google Scholar]
- 35.Caetano BC, Biswas A, Lima DS, Jr, et al. Intrinsic expression of Nod2 in CD4+ T lymphocytes is not necessary for the development of cell-mediated immunity and host resistance to Toxoplasma gondii. Eur J Immunol. 2011;41:3627–31. doi: 10.1002/eji.201141876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guiton R, Vasseur V, Charron S, et al. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J Infect Dis. 2010;202:427–35. doi: 10.1086/653738. [DOI] [PubMed] [Google Scholar]
- 37.Stumhofer JS, Laurence A, Wilson EH, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 38.Debierre-Grockiego F, Campos MA, Azzouz N, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–37. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 39.Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci. 2010;51:3083–91. doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng X, Bian F, Ma P, et al. Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol. 2010;222:95–102. doi: 10.1002/jcp.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amadi-Obi A, Yu CR, Liu X, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 42.Okafuji I, Nishikomori R, Kanazawa N, et al. Role of the NOD2 genotype in the clinical phenotype of Blau syndrome and early-onset sarcoidosis. Arthritis Rheum. 2009;60:242–50. doi: 10.1002/art.24134. [DOI] [PubMed] [Google Scholar]
- 43.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melo MB, Jensen KD, Saeij JP. Toxoplasma gondii effectors are master regulators of the inflammatory response. Trends Parasitol. 2011;27:487–95. doi: 10.1016/j.pt.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreira Ade M, Vitor RW, Gazzinelli RT, Melo MN. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol. 2006;6:22–31. doi: 10.1016/j.meegid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Yarovinsky F, Zhang D, Andersen JF, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–9. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 47.Hunn JP, Feng CG, Sher A, Howard JC. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mamm Genome. 2011;22:43–54. doi: 10.1007/s00335-010-9293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gazzinelli RT, Brezin A, Li Q, Nussenblatt RB, Chan CC. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-alpha and IFN-gamma. Exp Parasitol. 1994;78:217–29. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 49.Gazzinelli RT, Wysocka M, Hayashi S, et al. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–43. [PubMed] [Google Scholar]
- 50.London NJ, Hovakimyan A, Cubillan LD, Siverio CD, Jr, Cunningham ET., Jr Prevalence, clinical characteristics, and causes of vision loss in patients with ocular toxoplasmosis. Eur J Ophthalmol. 2011;21:811–9. doi: 10.5301/EJO.2011.6403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.