Abstract
Background. Infection with pandemic H1N1 influenza A viruses (IAVs) containing hemagglutinin (HA) proteins with globular heads that differ substantially from seasonal strains results in a boost in broadly cross-reactive antibodies that bind to the HA stalk. Boosting these antibodies has become an attractive strategy for creating a universal IAV vaccine. Therefore, it was essential to determine whether vaccines containing H1N1 viruses whose head domains differ substantially compared to seasonal strains could also achieve this boost.
Methods. Prospective samples of subjects who had received the A/New Jersey/1976 (NJ/76) vaccine and healthy, age-matched controls were assessed for the presence of anti-HA stalk antibodies before and after receiving the A/California/04/2009 (Cal/09) vaccine between October 2009 and January 2010.
Results. Individuals who received either the NJ/76 vaccine or the Cal/09 vaccine experienced a robust boost in HA stalk-reactive, neutralizing antibodies similar to what has been observed in individuals infected with Cal/09.
Conclusions. These results demonstrate that vaccines containing viruses whose HA head domains that differ substantially from seasonal strains are capable of boosting titers of HA stalk antibodies. Furthermore, anti-HA stalk antibodies elicited by vaccination appear to be long-lived and therefore could be targeted for the generation of a universal IAV vaccine.
Keywords: influenza virus, universal vaccine, stalk antibodies, hemagglutinin, Vaccination
Rapid antigenic shift and drift of influenza A viruses (IAVs) result in annual epidemics and periodic pandemics that place a major strain on global healthcare systems and pose a significant threat to the global economy [1]. Recent work has focused on characterizing a new class of IAV antibodies that bind to the hemagglutinin (HA) stalk domain [2–11]. These antibodies typically exhibit much broader reactivity and neutralizing activity than antibodies that bind to conventional antigenic sites on the HA head. HA stalk antibodies are thought to be boosted most efficiently in the context of infection when individuals are exposed to HAs whose head domains differ substantially from previous exposures, but whose stalk domains remain conserved [12]. We have recently postulated that the elicitation of such antibodies may have contributed to the extinction of seasonal IAV strains [12]. Indeed, we have demonstrated that individuals infected with pandemic 2009 (p2009) IAV experienced a boost in virus-neutralizing antibodies specific to the HA stalk [13]. This phenomenon has also been recently confirmed in a mouse model of sequential infection [14]. Importantly, other groups have observed naturally occurring HA stem antibodies in individuals who received a seasonal vaccine containing H1 and H3 viruses [15] or an H5N1 vaccine. Likewise, recent studies have shown that monoclonal antibodies and antibody-producing cells specific to the HA stem could be isolated from individuals who received the p2009 IAV vaccine [16, 17].
The HA segment of A/California/04/09 (Cal/09) virus descends from a separate lineage than that of previously circulating seasonal H1N1 strains. However, it is closely related to the HA segments of the A/New Jersey/1976 (NJ/76) virus and to the 1918 “Spanish Flu” [18, 19]. We therefore reasoned that individuals who received the NJ/76 vaccine or the Cal/09 vaccine may have also experienced a boost in cross-neutralizing antibodies specific to the HA stalk.
To investigate this possibility, we examined 2 cohorts of subjects for the presence of HA stalk antibodies. One cohort consisted of individuals who received the NJ/76 vaccine as well as the Cal/09 vaccine. The second cohort of age-matched controls received the Cal/09 vaccine only. We demonstrate that individuals who received the NJ/76 vaccine had elevated levels of anti-HA stalk antibodies prior to receiving the Cal/09 vaccine. Vaccination with Cal/09 boosted titers of anti-HA stalk antibodies only in subjects who had not been vaccinated with NJ/76. Importantly, receipt of the NJ/76 vaccine or the Cal/09 vaccine led to an enhanced neutralization response against a virus containing a homologous HA stalk and a heterosubtypic HA head. These findings confirm that anti-HA stalk antibodies were elicited by the NJ/76 and Cal/09 vaccines and enhance our understanding of the mechanisms through which these antibodies are generated naturally. This raises the exciting possibility that generation of vaccine constructs designed to specifically boost titers of HA stalk antibodies may lead to a universal influenza virus vaccine, capable of broad and long-lasting protection against diverse IAV strains.
METHODS
Human Serum Samples
Serum samples were collected in October 2009 from subjects who had received the NJ/76 vaccine (n = 20, average age, 62 years) and from age-matched controls (n = 15, average age, 57 years) with the assistance of the Mount Sinai General Clinical Research Center (GCRC). Subjects who had experienced influenza-like illness within the 4 months prior to the beginning of the study were excluded. Both groups of subjects had similar recent vaccination histories: 18 of 20 NJ/76 vaccinees and 14 of 15 control subjects had received at least one influenza vaccination in the previous 5 years. All subjects were administered the monovalent Cal/09-like vaccine between October 2009 and January 2010. Six to eight months after receiving the Cal/09 vaccine, subjects returned to have blood drawn (5 of 20 NJ/76 vaccinees; 7 of 15 control subjects). Due to the limited amounts of serum available, equal volumes of pre- and post-Cal/09 vaccination serum samples were pooled from individuals for whom both samples were available (NJ/76 vaccinees = 5; control subjects = 7). These pools were then tested in multiple assays after validating that the pools accurately reflected the data gathered from the individual subjects within each pool. A pool of pre-Cal/09 vaccination serum from all available subjects from each cohort was also used to test seroconversion to NJ/76. Samples were collected in accordance with institutional review board of Mount Sinai School of Medicine and the Mount Sinai School of Medicine Grants and Contracts Office (study number 09-0554 0001 01 MI). All subjects provided informed consent.
Cells and Viruses
Madin-Darby Canine Kidney (MDCK) cells were obtained from the ATCC and were maintained in Dulbecco Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal calf serum (FCS, Hyclone) and 100 U/mL penicillin and streptomycin (Gibco). Cal/09 was propagated on MDCK cells in DMEM containing 1 µg/mL l-1-tosylamide-2-phenylethyl chloromethyl ketone-treated (TPCK)-trypsin (Sigma-Aldrich). A/duck/France/MB42/1976 (France/76) virus was propagated in 10-day-old embryonated chicken eggs. The cH5/1 N3 virus was generated using a reverse genetics system described elsewhere [20–22]. The reverse genetics plasmids that encode viral RNA and messenger RNA include the 6 wild-type viral segments from A/Puerto Rico/8/34 (PR8) as well as plasmids encoding cH5/1 HA [22] and N3 NA from A/Swine/Missouri/4296424/06 virus (Miss/06), kindly supplied by Randy A. Albrecht). The sequence of the cH5/1 and N3 RNA was confirmed by sequencing of reverse-transcription polymerase chain reaction products. All infections were performed using DMEM supplemented with 1 µg/mL TPCK-trypsin (infection media).
Expression and Purification of Recombinant Influenza Virus Proteins
Coding sequences for the N-terminal ectodomain of HAs from PR8, A/New Caledonia/20/99 (NC/99) virus and cH6/1 [13, 22]. HAs were cloned into a modified pFastBac vector (Invitrogen) with a C-terminal hexahistidine-tag and T4 trimerization domain. Recombinant baculovirus (rBV) was generated according to the manufacturer's recommendations. BTI-TN5B1-4 (High Five) [23] cells grown in HyClone SFX insect cell media (Thermo Fisher Scientific) were infected with rBV expressing HAs at a multiplicity of infection (MOI) of 10 and a cell density of 1 × 106 cells/mL in 500 mL shaker flasks. Cells were harvested 72–96 hours postinfection. Supernatant (250 mL) was collected and incubated with Ni-NTA resin (Qiagen) for 2 hours at 4°C. The slurry was loaded on to columns and washed 3 times with washing buffer (50 mM Na2HCO3, 300 mM NaCl, 20 mM imidazole, pH 8). Protein was eluted with elution buffer (50 mM Na2HCO3, 300 mM NaCl, 300 mM imidazole, pH 8). Fractions containing protein were pooled and concentrated using Amicon Ultracell (Millipore) centrifugation units with a cutoff of 30 kDa, and buffer was exchanged to phosphate-buffered saline (PBS) of pH 7.4. Protein purity and identity was tested by SDS-PAGE, Coomassie staining, and western blot. Protein concentration was determined with Bradford reagent.
Immunoglobulin G (IgG) Endpoint Titer Determination
IgG endpoint titers were determined by enzyme-linked immunosorbent assay (ELISA). The 96-well plates (Immulon 2; Nunc) were coated with 2 µg/mL of purified recombinant HA or with bovine serum albumin (BSA) in carbonate/bicarbonate buffer, pH 9, overnight at 4°C. Plates were blocked with 5% nonfat milk and were washed with PBS/0.025% Tween-20 (PBS-T). Serum was diluted serially in 5% nonfat milk. Plates were incubated for 1 hour at RT and then were washed with PBS-T. Goat antihuman IgG-horseradish peroxidase (HRP; Meridian Life Science Inc) was diluted 1:5000 in 5% nonfat milk before adding to wells and incubating for 1 hour at RT. Plates were again washed with PBS-T prior to the addition of peroxidase substrate (SigmaFAST OPD, Sigma-Aldrich). Reactions were stopped by the addition of 3 M HCl. Optical density measurements were taken at 490 nm. Optical densities gathered against HAs were subtracted from those measured against BSA to normalize against nonspecific signal. Background signal was calculated for each specific antigen based on the reactivity of secondary antibody alone. Endpoint titers were defined as having an optical density at least 3 standard deviations above background after subtraction of nonspecific (BSA) signal.
Hemagglutination Inhibition (HAI) Assays
Serum samples were treated with 0.5 volumes 8 mg/mL TPCK-trypsin (Sigma-Aldrich) at 56°C for 30 minutes. Samples were cooled to RT prior to the addition of three volumes of 11 mM potassium periodate solution (Sigma-Aldrich). After incubation for 15 minutes at RT, 3 volumes of 1% glycerol saline solution was added to samples that were again incubated for 15 minutes at RT. Finally, 1.5 volumes of 0.85% saline were added to samples prior to use. All volumes are in relation to the starting volume of serum. Virus and antibodies were mixed and incubated for 30 minutes at RT. Chicken red blood cells were then added to wells, and plates were incubated on ice for approximately 30 minutes prior to reading.
Microneutralization Assays
In total, 200 TCID50/100 µL of virus was added to wells of serially diluted serum, which had been pretreated with TPCK-trypsin as described earlier. Serum and viruses were incubated for 1 hour at 37°C. Serum/virus mixtures were transferred onto 96-well plates of confluent MDCK cells, which were incubated for 1 hour at 37°C, 5% CO2. Plates were washed with PBS and were reincubated for 20 hours with infection media containing equivalent concentrations of diluted serum. Cells were fixed with 80% acetone and were blocked with 3% hydrogen peroxide and 5% nonfat milk. Cells were probed with a 1:2000 dilution of biotin-conjugated mouse anti-NP (Millipore) followed by a 1:5000 dilution of secondary HRP-conjugated streptavidin (Millipore). Peroxidase substrate (SigmaFAST, Sigma-Aldrich) was added to wells, and reactions were stopped with 3M HCl. Neutralization titers were defined as the dilution of serum that resulted in at least 50% inhibition of infectivity.
RESULTS
NJ/76 Vaccinees Had Elevated Titers of HA Stalk Antibodies Prior to Cal/09 Vaccination
An amino acid sequence comparison of HA0 from A/Fort Warren/1/50 (FW/50, seasonal), NJ/76 (swine-origin), NC/99 (seasonal) and Cal/09 (swine-origin) was performed to examine the degree of amino acid sequence identity in the head and stalk domains of each HA (Table 1). Cal/09 and NJ/76 HAs share overall 79.9% and 82.5% amino acid sequence identity, respectively, when compared to the HA of seasonal strain NC/99. Similarly, the degree of identity between NJ/76 and the precirculating seasonal strain FW/50 was 83.0%. The greatest degree of identity among these proteins is found in the HA stalk domains, while the head domains of the swine-origin H1s differ substantially from that of the seasonal H1s. Therefore, it was possible that in a manner analogous to Cal/09 infection [13], recipients of the NJ/76 vaccine may have experienced a boost in HA stalk antibodies prior to Cal/09 exposure due to the substantial antigenic differences between the NJ/76 HA and that of the previously circulating seasonal strains.
Table 1.
Hemagglutinin (HA) Amino Acid Sequence Comparison of Strains Cal/09, NJ/76, NC/99, and FW/50
| Comparison | Whole Protein (HA0) (% Identity) | Globular Head Residues 52–277 H3 Numbering (% Identity) | Stalk (% Identity) |
|---|---|---|---|
| Cal/09 vs NJ/76 | 91.0 | 85.9 | 94.6 |
| Cal/09 vs NC/99 | 79.9 | 67.1 | 88.9 |
| NJ/76 vs NC/99 | 82.5 | 69.7 | 91.6 |
| NJ/76 vs FW/50 | 83.0 | 69.8 | 90.9 |
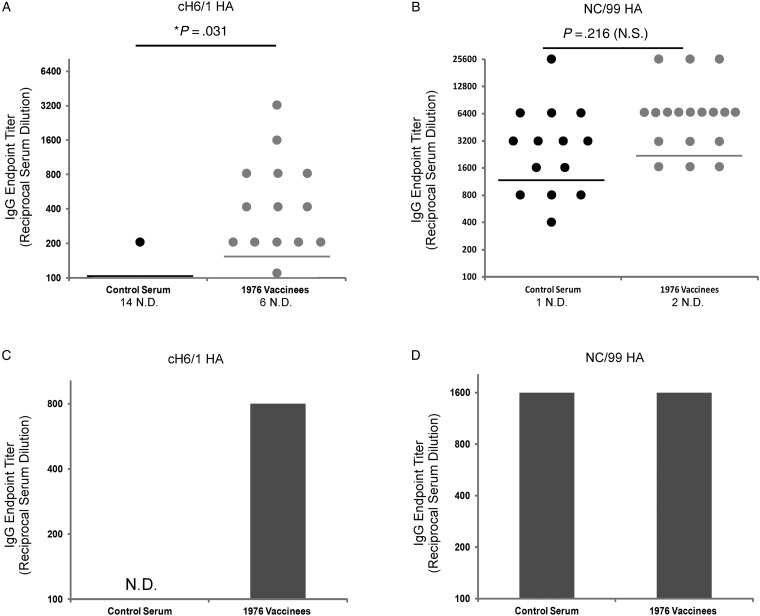
Therefore, serum samples from individuals who received the 1976 vaccine (n = 20) were compared to serum samples from age-matched individuals who did not receive the vaccine (n = 15). Endpoint IgG titers against cH6/1 and NC/99 were determined by ELISA. The cH6/1 HA protein contains an H1 (PR8) stalk, but an H6 head. The PR8 stalk exhibits approximately 90% amino acid sequence identity to the H1 viruses used in this study, similar to the natural variability between H1 stalk sequences shown in Table 1 (Table 2). This protein serves as a useful tool for the detection of group 1 HA stalk-binding antibodies, as we have recently shown [13]. Strikingly, NJ/76 vaccinees had significantly elevated IgG endpoint titers compared to control subjects against cH6/1 HA (Figure 1A). No significant difference existed between the 2 groups in IgG endpoint titers against the seasonal NC/99 HA, demonstrating that this phenomenon was specific for HA stalk antibodies (Figure 1B). Due to the scarcity of samples available, pre- and post-Cal/09 vaccination serum pools were then generated from all patients in both groups for whom both of the samples were available (NJ/76 vaccinees = 5/20; control subjects = 7/15). IgG endpoint titers from the pre-Cal/09 vaccination pools from each group were then determined against cH6/1 and NC/99 HAs, to ensure that they accurately reflected the data collected from individual patients (Figure 1A and 1B). Indeed, the NJ/76 vaccinee pool displayed a substantially elevated endpoint titer against cH6/1 HA compared to the control pool (Figure 1C). However, no differences existed in IgG endpoint titers against NC/99 HA between the 2 groups (Figure 1D). These data demonstrate that NJ/76 vaccinees had elevated titers of anti-HA stalk antibodies prior to receiving the Cal/09 vaccine.
Table 2.
PR8 Hemagglutinin (HA) Stalk Amino Acid Comparison With Cal/09, NJ/76, and NC/99
| Comparison | Stalk (% Identity) |
|---|---|
| PR8 vs Cal/09 | 87.8 |
| PR8 vs NJ/76 | 90.7 |
| PR8 vs NC/99 | 93.6 |
Figure 1.
NJ/76 vaccine recipients had elevated anti-hemagglutinin (HA) stalk antibodies prior to Cal/09 vaccination. Serial dilutions of serum from NJ/76 vaccinees (n = 20) or age-matched control subjects (n = 15) were tested for their reactivity to (A) cH6/1 HA or (B) NC/99 HA by ELISA and immunoglobulin G (IgG) endpoint titers where calculated. Due to limited quantities of available serum, pre-Cal/09 vaccination IgG endpoint titers were also determined for pooled NJ/76 vaccinees (N = 5) and control subjects (n = 7) against (C) cH6/1 and (D) NC/99. Each pool consisted of all individuals from each group for whom both pre- and post-Cal/09 vaccination samples were available. Horizontal lines indicate the geometic mean titer. Endpoint titer limit of detection = 100. Unpaired Student t-tests were performed and 2-tailed P values < .05 were considered statistically significant. Abbreviations: ND, not detected; NS, not significant. *statistically significant.
NJ/76 Vaccinees Had Protective HAI Titers Against Cal/09 Prior to Cal/09 Vaccination
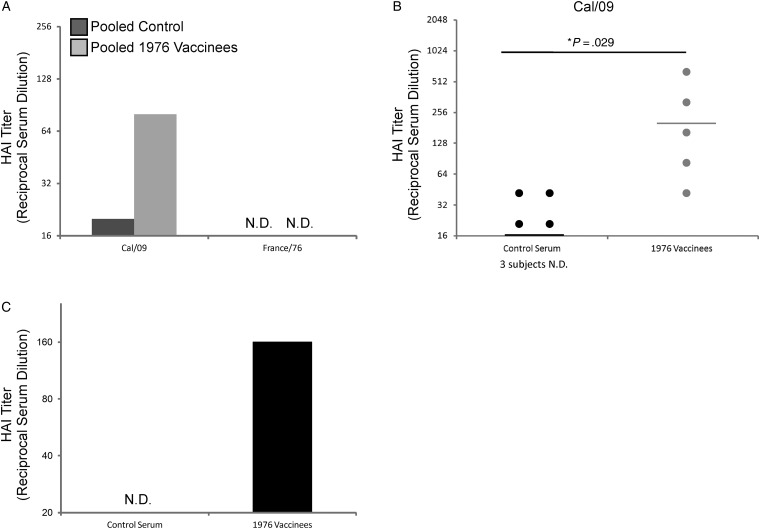
Prospective studies [24], retrospective studies [25–27], and studies in animal models [28–31] have suggested that individuals vaccinated or infected with NJ/76 virus had protective levels of cross-neutralizing antibodies to the 1918 pandemic influenza virus and to the pandemic Cal/09 strain due to conserved antigenic sites present in the HA head. Likewise, vaccination with trivalent vaccine containing Cal/09 could protect ferrets from challenge with 1918 IAV [32]. Only NJ/76 vaccine recipients had protective HAI titers against Cal/09, whereas unvaccinated subjects did not (Figure 2A). To again ensure the reliability of the pooled HAI results, HAI assays were performed against Cal/09 with serum from each individual included in the pools. Results were consistent and significant, demonstrating that the pooled serum gave an accurate representation of the population (Figure 2B). As expected, NJ/76 vaccinees were seropositive for NJ/76 virus prior to receiving the Cal/09 vaccine, whereas control subjects were not (Figure 2C). No HAI activity was observed against France/76 virus (H6N4) in either sample, confirming that none of the subjects had previous exposure to H6 viruses and that there was no substantial cross-reactivity between the H6 head domain and that of the H1s tested in this study (Figure 2A). Taken together, these results confirm that NJ/76 vaccinees experienced a boost in HAI antibodies, which bind to the globular head of Cal/09 HA prior to Cal/09 vaccination.
Figure 2.
NJ/76 vaccine recipients had elevated hemagglutination inhibition (HAI) titers against Cal/09 prior to Cal/09 vaccination. (A) HAI titers were determined for pre-Cal/09 vaccination pooled serum samples from NJ/76 vaccinees (n = 5) and control subjects (n = 7) against Cal/09 and France/76 using chicken red blood cells. (B) HAI assays against Cal/09 were also performed using serum samples corresponding to the individual subjects from within each pool in order to ensure that the pooled results were representative of the group as a whole. (C) Pre-Cal/09 vaccination serum was pooled from all control subjects and NJ/76 vaccines and was used to perform an HAI assay against NJ/76. Horizontal lines indicate the geometic mean titer. HAI limit of detection = 20. Unpaired Student t-tests were performed, and 2-tailed P values < .05 were considered statistically significant. Abbreviation: ND, not detected. *statistically significant.
Anti-HA Stalk Antibody Titers Were Boosted in Control Subjects Subsequent to Cal/09 Vaccination
The observation that individuals infected with p2009 IAV [13] or immunized with the NJ/76 vaccine had elevated titers of anti-HA stalk antibodies led us to investigate whether vaccination with Cal/09 would also boost anti-HA stalk antibody titers. Therefore, both control subjects and NJ/76 vaccinees were administered the monovalent Cal/09 vaccine between October 2009 and January 2010. Subjects returned to have blood drawn 6–8 months later. Endpoint IgG titers against NC/99 HA and cH6/1 HA were determined for pooled postvaccination serum from both groups by ELISA. These were compared to the prevaccination values determined in Figure 1B and 1D in order to calculate the fold-change in IgG titers against each HA protein post-Cal/09 vaccination (Table 3). Endpoint IgG titers of control subjects rose greater than 2-fold against cH6/1, whereas IgG endpoint titers of NJ/76 vaccinees did not increase. No boost was observed against NC/99 HA in either group, as would be expected. In fact, a decrease in NC/99 reactivity was observed in the serum samples of NJ/76 vaccinees post-Cal/09 vaccination. Although we do not fully understand the reason for this decrease, it may be due to displacement of NC/99 antibody-producing cells with those reactive against Cal/09. These data indicate that Cal/09 vaccination is also capable of boosting titers of anti-HA stalk antibodies but only in individuals who had not been previously exposed to NJ/76.
Table 3.
Post-Cal/09 Vaccination IgG Endpoint Titer Changes Against NC/99 HA and cH6/1 HA
| HAProtein | Control |
1976 Vaccinees |
||||
|---|---|---|---|---|---|---|
| Pre-Cal/09 Vaccination | Post-Cal/09 Vaccination | Fold Change | Pre-Cal/09 Vaccination | Post-Cal/09 Vaccination | Fold Change | |
| cH6/1 | ND | 200 | >2 | 800 | 800 | 1 |
| NC/99 | 1600 | 1600 | 1 | 1600 | 800 | 0.5 |
Abbreviations: HA, hemagglutinin; IgG, immunoglobulin G; ND, not detected.
Vaccination With NJ/76 or Cal/09 Boosted Neutralizing Antibodies Against Virus Containing Homologous HA Stalk and a Heterologous HA Head
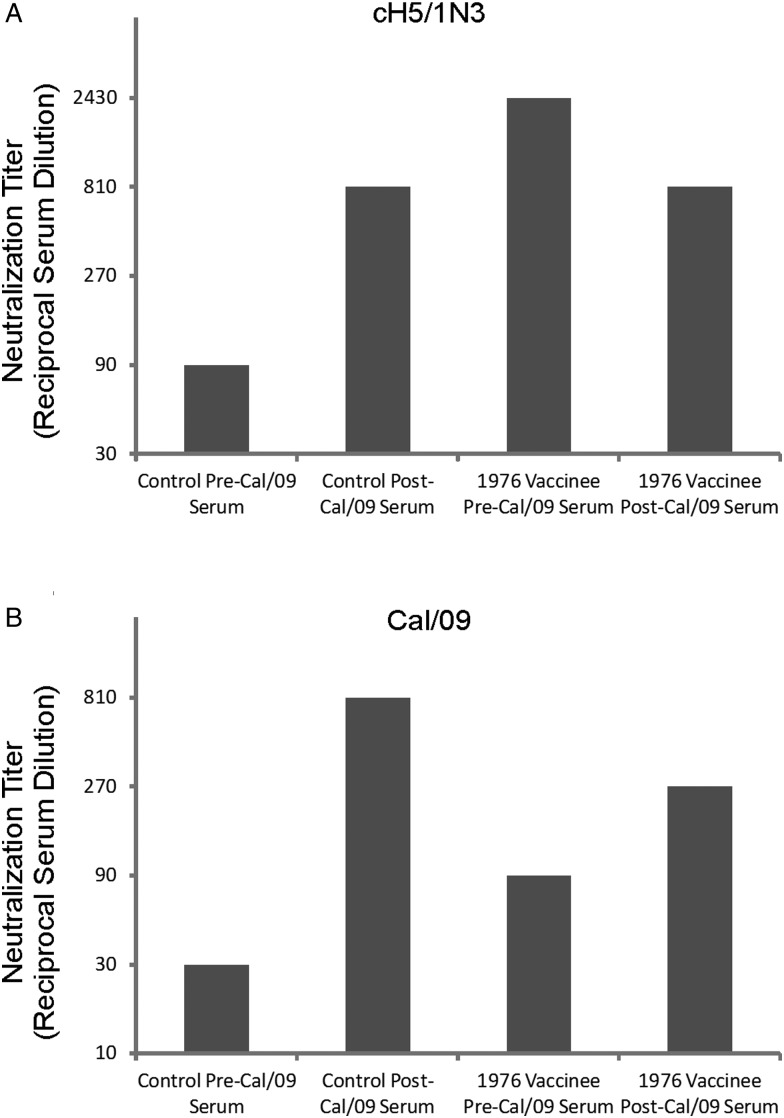
It was important to determine whether the boost in anti-HA stalk antibodies experienced after NJ/76 or Cal/09 vaccination corresponded to enhanced neutralization titers against virus containing a heterosubtypic HA head domain. To test this, we performed a microneutralization assay against a cH5/1 N3 virus using our pooled serum samples. The cH5/1 N3 virus was used to detect the presence of HA stalk neutralizing antibodies, as it contains an H5 HA head domain, a PR8 HA stalk, and an N3 from Miss/06. A cH6/1N3 virus would also have been interchangeable for the purposes of this assay but was not available. In agreement with the IgG endpoint titer data against cH6/1, serum samples from NJ/76 vaccinees exhibited markedly more potent neutralization titers against cH5/1 N3 than did control subjects prior to vaccination with Cal/09 (2430 vs 90). However, only control subjects experienced a boost in neutralizing antibodies subsequent to vaccination (810, up from 90; Figure 3A). As expected, NJ/76 vaccinees had neutralization titers against Cal/09 that were 3-fold more potent than control subjects prior to Cal/09 vaccination (90 vs 30). Both groups experienced a boost in neutralizing antibody titers against Cal/09 subsequent to Cal/09 vaccination (Figure 3B). Taken together, these data demonstrate that the anti-HA stalk antibodies boosted by vaccination with 1976 and 2009 H1N1 viruses correspond to an enhanced capacity to neutralize virus harboring a homologous HA stalk and a heterosubtypic HA head domain.
Figure 3.
NJ/76 and Cal/09 vaccines boosted broadly neutralizing antibodies. Microneutralization assays were performed on MDCK against (A) cH5/1 N3 and (B) Cal/09 virus using TPCK-trypsin-treated, pooled serum samples collected from NJ/76 vaccinees (n = 5) and control subjects (n = 7) before and after Cal/09 vaccination. Following infection, cells were stained with an anti-NP antibody and an HRP-conjugated secondary antibody. Neutralization titers were defined as the lowest serum dilution that resulted in at least 50% reduction in specific signal. Microneutralization assay limit of detection = 30.
DISCUSSION
The most recent IAV pandemic began in 2009 and was caused by a swine-origin H1N1 virus [18, 33]. The HA lineage of this virus was shared by the 1976 swine-origin H1N1 virus that caused an outbreak in Fort Dix, New Jersey [24]. Vaccination of mice with NJ/76-based vaccines was able to protect against lethal challenge with p2009 virus or 1918 IAV [28, 30]. In these studies, the authors demonstrate that the NJ/76 vaccine is able to elicit antibodies that have HAI activity against p2009 IAV. Therefore, virus neutralization and protection was attributed to cross-reactive antibodies directed against antigenic sites present in the HA head domain. Interestingly, recent work has shown that a mutation in HA2, proximal to the globular head, may also influence cross-reactivity of these antibodies [26].
An increasing number of studies have focused on a new class of antibodies that bind to the HA stalk domain. Murine antibodies, including C179 [3] and 6F12 [9], and prototypical human antibodies, including CR6261 [5], F10 [2], and FI6 [8], seem to bind particularly well-conserved epitopes composed of membrane proximal regions of HA1 and HA2. These antibodies are able to neutralize virus but do not exhibit HAI activity typical of neutralizing antibodies that bind to the HA head.
Recent work from our lab has shown that individuals infected with p2009 IAV experienced a boost in HA stalk-reactive antibodies [13]. We postulated that due to their broadly neutralizing characteristics, these antibodies may be responsible for the extinction of the previous seasonal H1N1 virus. This led us to question whether this phenomenon would also occur upon exposure to vaccines containing viruses whose HA head domain differed substantially from seasonal strains. The elevated HA stalk antibody titers observed in NJ/76 vaccinees not only suggested that the NJ/76 vaccine was indeed capable of inducing HA stalk-reactive antibodies but demonstrated for the first time to our knowledge that these antibodies are long lived, similar to neutralizing antibodies that bind to the HA head domain. Critically, this in turn indicates that development of novel broadly protective IAV vaccines may also provide long-lasting protection. Of course, it remains uncertain to which degree subsequent vaccinations or infections may have contributed to maintaining or enhancing antibodies boosted by the NJ/76 vaccine.
Like the NJ/76 vaccination and Cal/09 infection [13], the Cal/09 vaccine was capable of boosting titers of HA stalk-reactive antibodies but only in control subjects who had not received the NJ/76 vaccine. This supports the notion that efficient boosting of stalk antibodies requires exposure to an antigenically dissimilar HA head and would explain why multiple exposures to seasonal IAV variants does not appear to result in robust levels of stalk antibodies [12]. The lack of protective preexisting HAI titers against Cal/09 in control subjects, the absence of reported illness in both groups in the months prior to the study, strongly suggest that these individuals had not been infected with Cal/09 prior to vaccination.
Finally, the data demonstrate that individuals who have received swine-origin H1 vaccines developed elevated levels of antibodies capable of neutralizing virus containing a heterosubtypic HA head domain. Together, these findings advance our understanding of the natural scenarios in which anti-stalk antibodies are elicited and may enhance our understanding of why influenza virus strains die out. The fact that not only infection, but also vaccination, is capable of boosting titers of anti-HA stalk antibodies, and that these antibodies appear to be long-lived, raises the prospect that a successful universal IAV vaccine strategy that elicits high levels of neutralizing HA stalk antibodies will be successful. The boost in stalk antibodies elicited by NJ/76 or Cal/09 vaccination was amazingly robust relative to the impressive boost observed in patients who were infected with p2009 IAV [13]. Enhancing the magnitude of anti-HA stalk antibody boost elicited by immunization should therefore be a central consideration in vaccine design. Such a vaccine would substantially reduce the annual global burden associated with IAV infections and reduce the risk of a new IAV pandemic arising in the future.
Notes
Acknowledgments. The authors thank M. Klotman and S. Mubareka for assistance in preparing the IRB protocol, and J. S. Mymryk for critical reading of the manuscript.
Financial support. This work was supported by PATH and by the National Institutes of Health (grant HHSN26620070010C to P. P.; grants AI085306 and UL1RR029887 to C. F. B.). M. S. M. was supported by a Canadian Institutes of Health Research Post-Doctoral Fellowship. F. K. was supported by an Erwin Schrödinger fellowship (J 3232) from the Austrian Science Fund (FWF).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Field's Virology. 5th ed. Vol. 2. Philadelphia: Lippincott; 2007. [Google Scholar]
- 2.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67:2552–8. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Throsby M, van den Brink E, Jongeneelen M, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3:e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekiert DC, Bhabha G, Elsliger MA, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekiert DC, Friesen RH, Bhabha G, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–50. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TT, Tan GS, Hai R, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107:18979–84. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corti D, Voss J, Gamblin SJ, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–6. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 9.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol. 2012;86:6179–88. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–93. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap AK, Steel J, Oner AF, et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci U S A. 2008;105:5986–91. doi: 10.1073/pnas.0801367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palese P, Wang TT. Why do influenza virus subtypes die out? A hypothesis. MBio. 2011;2(5):e00150–11. doi: 10.1128/mBio.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pica N, Hai R, Krammer F, et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109:2573–8. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krammer F, Pica N, Hai R, Tan GS, Palese P. Hemagglutinin stalk reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J Virol. 2012;86:10302–7. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corti D, Suguitan AL, Jr, Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120:1663–73. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson CA, Wang Y, Jackson LM, et al. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front Microbiol. 2012;3:1–19. doi: 10.3389/fimmu.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–52. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: a review. Vaccine. 2010;28:4895–902. doi: 10.1016/j.vaccine.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–73. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–82. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann G, Watanabe T, Ito H, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96:9345–50. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hai R, Krammer F, Tan GS, et al. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol. 2012;86:5774–81. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, Grabherr R. Trichoplusia ni cells (High Five) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol Biotechnol. 2010;45:226–34. doi: 10.1007/s12033-010-9268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clin Infect Dis. 2010;50:1487–92. doi: 10.1086/652441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie H, Li X, Gao J, et al. Revisiting the 1976 “swine flu” vaccine clinical trials: cross-reactive hemagglutinin and neuraminidase antibodies and their role in protection against the 2009 H1N1 pandemic virus in mice. Clin Infect Dis. 2011;53:1179–87. doi: 10.1093/cid/cir693. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Anderson CM, De Feo CJ, et al. Cross-neutralizing antibodies to pandemic 2009 H1N1 and recent seasonal H1N1 influenza A strains influenced by a mutation in hemagglutinin subunit 2. PLoS Pathog. 2011;7:e1002081. doi: 10.1371/journal.ppat.1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 28.Easterbrook JD, Kash JC, Sheng ZM, et al. Immunization with 1976 swine H1N1- or 2009 pandemic H1N1-inactivated vaccines protects mice from a lethal 1918 influenza infection. Influenza Other Respi Viruses. 2011;5:198–205. doi: 10.1111/j.1750-2659.2010.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min JY, Chen GL, Santos C, Lamirande EW, Matsuoka Y, Subbarao K. Classical swine H1N1 influenza viruses confer cross protection from swine-origin 2009 pandemic H1N1 influenza virus infection in mice and ferrets. Virology. 2010;408:128–33. doi: 10.1016/j.virol.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manicassamy B, Medina RA, Hai R, et al. Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines. PLoS Pathog. 2010;6:e1000745. doi: 10.1371/journal.ppat.1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kash JC, Qi L, Dugan VG, et al. Prior infection with classical swine H1N1 influenza viruses is associated with protective immunity to the 2009 pandemic H1N1 virus. Influenza Other Respi Viruses. 2010;4:121–7. doi: 10.1111/j.1750-2659.2010.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce MB, Belser JA, Gustin KM, et al. Seasonal trivalent inactivated influenza vaccine protects against 1918 Spanish influenza virus in ferrets. J Virol. 2012 doi: 10.1128/JVI.00674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan M. World now at the start of 2009 influenza pandemic. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html. Accessed 28 March 2012. [Google Scholar]