Abstract
Elevated levels of pterins and nitric oxide (NO) are observed in patients with septic shock and bacterial meningitis. We demonstrate that Escherichia coli K1 infection of human brain microvascular endothelial cells (HBMECs) induces the expression of guanosine triphosphate cyclohydrolase (GCH1), the rate-limiting enzyme in pterin synthesis, thereby elevating levels of biopterin. DAHP (2,4-diamino hydroxyl pyrimidine), a specific inhibitor of GCH1, prevented biopterin and NO production and invasion of E. coli K1 in HBMECs. GCH1 interaction with Ecgp96, the receptor for outer membrane protein A of E. coli K1, also increases on infection, and suppression of Ecgp96 expression prevents GCH1 activation and biopterin synthesis. Pretreatment of newborn mice with DAHP prevented the production of biopterin and the development of meningitis. These results suggest a novel role for biopterin synthesis in the pathogenesis of E. coli K1 meningitis.
Keywords: Biopterin; outer membrane protein A; endothelial cells; brain; invasion, inducible nitric oxide; Escherichia coli K1
Escherichia coli (E. coli) K1 is the bacterium that most commonly causes neonatal meningitis in industrialized countries [1]. The morbidity and mortality rates associated with neonatal meningitis have not changed for last 2 decades despite the use of effective antibiotics and supportive care [2]. This poor outcome is attributed to an incomplete understanding of the pathogenesis and pathophysiology of the disease, which inhibits the development of novel therapeutic strategies for prevention. Besides antimicrobial therapy, prevention of E. coli K1 traversal to the brain has become another area of focus for preventing meningitis. E. coli K1 translocation of the blood-brain barrier and invasion of human brain microvascular endothelial cells (HBMECs), a single-cell lining of the blood-brain barrier, requires unique interactions between the bacterial determinants and their cognate host receptors. Previous studies have demonstrated that Ecgp96, a 96-kDa glycoprotein, serves as a receptor in HBMEC to outer membrane protein A (OmpA) of E. coli K1 for binding to and invasion of the bacterium [3].
Additionally, E. coli K1 induces tight junction disruption and blood-brain barrier leakage by triggering iNOS activation and consequently nitric oxide (NO) production [4]. Tetrahydrobiopterin (BH4), a pterin analogue and an obligate cofactor for all the isoforms of NOS, primarily controls NO production [5]. Pterin (biopterin and neopterin) production occurs mainly by de novo synthesis, using guanosine triphosphate (GTP) as a source and GTP cyclohydrolase (GCH1; EC 3.5.4.16) is the first and rate-limiting enzyme in this reaction [6]. Human GCH1 exists in its native form (approximately 28 kDa) and assembles to a homo-decamer to catalyze GTP to pterins [7]. The vital role of GCH1 and pterins in cell differentiation, pain modulation and mRNA stability is well documented [8–10]. Furthermore, elevated levels of pterin are used as a diagnostic marker in infections caused by intracellular pathogens and malignant tumors [11]. Lipopolysaccharide (LPS), interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) stimulate de novo synthesis of pterins in various immune and endothelial cells [12].
During sepsis, iNOS-dependent NO production and subsequent vasodilation is a major factor responsible for persistent hypotension [13]. Of note, elevated pterin levels in cerebrospinal fluid (CSF) of pediatric patients with bacterial meningitis were also observed [14]. Therefore, we hypothesize that E. coli K1 triggers NO production not only by activating iNOS expression but also by modulating pterin synthesis. Since endothelial cells have been reported to produce low levels of neopterin [15], we have focused only on biopterin production in this study. Here, our studies demonstrate that E. coli K1 invasion of HBMECs depends on the expression of GCH1 and biopterin synthesis. Importantly, we found that pretreatment of newborn mice with the GCH1-specific inhibitor 2,4-diamino hydroxyl pyrimidine (DAHP) protects the animals from E. coli K1 meningitis.
METHODS
Bacterial Strains, Antibodies, and Other Reagents
E. coli (OmpA+ E. coli), a spontaneous rifampin-resistant mutant of strain RS 218 (serotype O18:K1:H7), was isolated from the CSF of a neonate with meningitis [3]. OmpA− E. coli is a mutant of RS218 that does not express OmpA or invade HBMECs. Antibodies to GCH1, iNOS, and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Ecgp96 antibody was generated as previously described [16]. Fluorescent-tagged secondary antibodies were purchased from Invitrogen (Carlsbad, CA). DAHP and Lipofectamine were obtained from Sigma (St. Louis, MO). Griess reagent was purchased from Promega (Madison, WI). We purchased 6-methylpterin (internal standard), d-neopterin, and l-biopterin from Shricks Laboratories (Jona, Switzerland). Ascorbic acid was obtained from Calbiochem (La Jolla, CA). Lugol's iodine was purchased from Electron Microscopy Sciences (Hatfield, PA). Antibodies to GFAP and MPO were obtained from Leica Microsystems (Buffalo Grove, IL).
HBMEC Maintenance and E. coli Invasion Assays
HBMECs were isolated, cultured, and maintained as described elsewhere [17]. The frozen stocks of HBMECs were revived, characterized for brain endothelial cell markers and used for invasion assays. Total cell association (represented as binding) and invasion assays were described previously [3]. Cytotoxicity of DAHP was assessed using a Cytotox 96 assay kit (Promega) according to the manufacturer's protocol.
Determination of Biopterin Levels
The total biopterin level was measured using the protocol of Fukushima and Nixon, with minor modifications [18]. The whole sample preparation was performed in a dark environment, using brown centrifuge tubes. Total lysates of HBMECs and brain tissues were treated with 50 µL of 10% trichloroacetic acid on ice for 30 minutes. Samples were centrifuged at 10 000 × g at 4°C for 10 minutes, and the supernatants were subjected to iodine oxidation. A total of 125 µL of sample and the standard containing 200 nM neopterin, 100 nM biopterin, and 10 µL of internal standard (6-methylpterin) were mixed. Thirty microliters of 1 N HCl was added, and the oxidation was started by the addition of 10 µL of acidic iodine solution. Tubes were kept at room temperature for 60 minutes, and then 15 µL of ascorbic acid was added to reduce excess iodine. A total of 100 µL of the solution was subjected to high-performance liquid chromatography (HPLC; Thermo Fisher Scientific, Hercules, CA). Separation was performed on a C18 Spherisorb 5-µm precolumn (10 × 4.6 mm) and a ODS-1 Spherisorb analytical column (250 × 4.6 mM; Waters, Milford, MA), using 1.25 mmol/L potassium hydrogen phosphate buffer, with 6% (v/v) methanol at a flow rate 1.3 mL/minutes.
Plasma Membrane Isolation, Immunoprecipitation, and Western Blotting
Plasma membrane and cytosolic fractions from HBMECs were isolated as described earlier, using the BioVision kit [19]. For immunoprecipitation, cytosolic fractions (300 µg) or plasma membrane fractions (80–100 µg) were incubated with appropriate antibodies overnight at 4°C, washed, and incubated for 2 hours with protein A agarose beads. The beads with bound immune complexes were washed, boiled for 10 minutes in sodium dodecyl sulfate (SDS) sample buffer, and separated by 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE). For identification of iNOS dimerization, samples were dissolved in sample buffer (devoid of β-mercaptoethanol) without boiling, and SDS-PAGE was performed at 4°C [20]. Western blotting was done as described previously [19]. Protein expression levels were quantitated using ImageJ software (http://www.rsbweb.nih.gov/ij/).
Flow Cytometry
To detect the expression of GCH1 and Ecgp96, HBMECs were infected with OmpA+ E. coli and OmpA− E. coli, with or without DAHP pretreatment for various periods, and the cells were subjected to flow cytometry as described previously [19].
Suppression of Ecgp96 Expression by Small Interfering RNA (siRNA)
HBMECs at 60% confluence were transfected with 40 pmol of Ecgp96 siRNA (catalog no. HSS110955; Invitrogen) by use of Lipofectamine, according to the manufacturer's instructions. The efficiency of silencing was verified by Western blotting of total HBMEC lysates.
Estimation of NO as Nitrite by Griess Assay
Total nitrite (NO2–) content was used as an index of NO production. Nitrite levels were determined in supernatants of cell cultures by spectrophotometry, using Griess reagent (Promega, Madison, WI) and using sodium nitrite as a standard according to the manufacturer's protocol.
Newborn Mouse Model of Meningitis
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Children's Hospital of Los Angeles and followed guidelines for the performance of animal experiments, as mandated by the office Laboratory Animal Welfare at the National Institutes of Health. C57BL/6J timed-pregnant mice were purchased from Jackson Laboratories (Bar Harbor, ME). One litter of 3-day-old mouse pups was infected intranasally with 103 colony-forming unites (CFU) of bacteria as previously described [21]. Control mice received pyrogen-free saline through the same route. To evaluate the effect of DAHP on OmpA+ E. coli meningitis, DAHP (100 mg/kg body weight) was injected intraperitoneally 6 hours before, at the time of, and 6 hours after infection. On the basis of a previous report [22] and dose optimization studies, a dose of 100 mg/kg was used for this study. CSF samples were collected aseptically under anesthesia by cisternal puncture and were directly inoculated into rifampicin-containing LB. Growth of E. coli in broth from CSF samples was considered positive for meningitis. Whole brain was aseptically removed. One half of the brain was homogenized in sterile phosphate-buffered saline to determine the bacterial count, and the other half was stored in formalin for histopathologic analysis. Bacterial counts in brain were determined by plating 10-fold serial dilutions on rifampicin LB agar plates.
Statistical Analysis
All data were derived from at least 3 independent experiments. Statistical analyses were conducted using SigmaPlot software (version 11.0). Significant differences (P < .05) between the groups were determined using the unpaired Student t test. For animal studies, statistical significance was determined using analysis of variance.
RESULTS
Infection of HBMECs With OmpA+ E. coli K1 Induces GCH1 Expression and Biopterin Synthesis
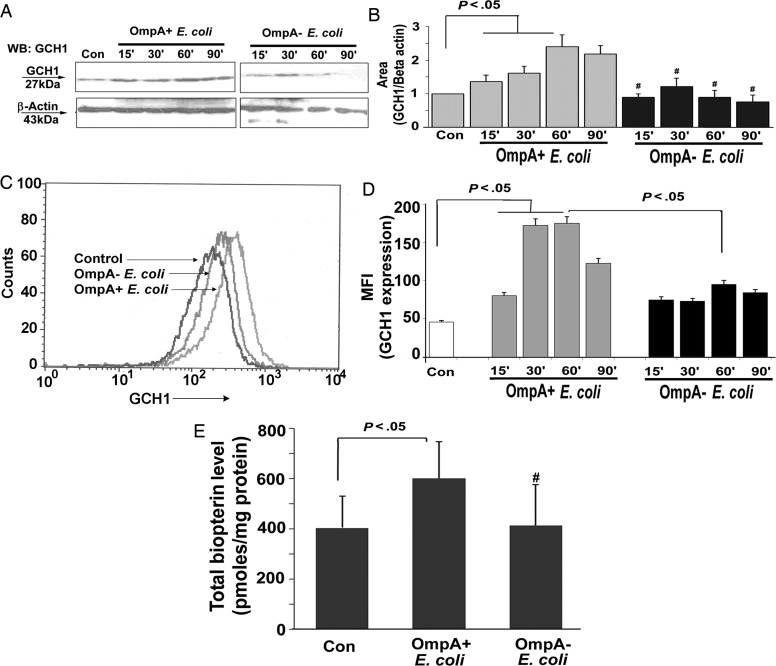
Our previous findings showed that the expression of OmpA in E. coli K1 is important for the invasion of HBMECs [3]. Therefore, we examined the effect of OmpA+ E. coli and OmpA− E. coli K1 infection on GCH1 expression and biopterin production. OmpA+ E. coli K1 infection significantly induced GCH1 expression in a time-dependent manner, which peaked 60 minutes after infection and started declining by 90 minutes (Figure 1A). OmpA− E. coli K1 infection showed only a moderate effect on GCH1 expression. The increase in GCH1 expression was 2.5-fold greater during OmpA+ E. coli K1 infection than in control cells, after normalizing to β-actin levels, as measured by densitometry of the protein bands (Figure 1B). Flow cytometry of HBMECs infected with the bacteria after staining with anti-GCH1 antibodies revealed that OmpA+ E. coli induced greater levels of GCH1 expression 30 and 60 minutes after infection as compared to control or OmpA− E. coli–infected cells (Figure 1C and 1D). Next, the total biopterin level was measured by HPLC 60 minutes after infecting HBMECs with OmpA+ E. coli or OmpA− E. coli K1. Cells infected with OmpA+ E. coli K1 showed a significant increase in the quantities of total biopterin as compared to uninfected control HBMECs (P < .05). However, no significant increase in biopterin levels was observed in HBMECs infected with OmpA− E. coli K1 (Figure 1E). These results suggest that E. coli K1 infection stimulates biopterin synthesis by inducing GCH1 expression and that the presence of OmpA in E. coli is critical for this event to occur.
Figure 1.
OmpA+ Escherichia coli infection of human brain microvascular endothelial cells (HBMECs) induces guanosine triphosphate cyclohydrolase (GCH1) expression and biopterin production. A, Total cell lysates of HBMECs infected with OmpA+ E. coli or OmpA− E. coli were subjected to Western blotting with antibodies to GCH1 and β-actin. B, Integrated density values of the bands were measured using ImageJ software and graphed. Bars represent means ± SD of fold-differences relative to GCH1 expression in uninfected HBMECs taken as one. #P > .05, with respect to control. C, Confluent monolayers of HBMECs were either left alone, infected with OmpA+ E. coli or Omp− E. coli for varying periods, and cells were subjected to flow cytometry using anti-GCH1 antibodies, as described in Materials and Methods. An overlay image generated from the data at 90 minutes after infection is shown as a representative panel. D, Mean fluorescence intensities (MFIs) of GCH1 expression obtained from the experiments described in panel C were graphed after subtracting the isotype-matched control values. Omp-, OmpA negative; OmpA+, OmpA positive. E, In separate experiments, confluent monolayers of HBMECs were treated with Omp+ E. coli or Omp- E. coli for 60 minutes, and total cell lysates were prepared. Total biopterin levels were measured in the lysates, using high-performance liquid chromatography, as described in Materials and Methods. Values are means ± SD of 3 different experiments. #P > .05, compared with control (Con). Abbreviations: Con, control; Omp−, OmpA negative; OmpA+, OmpA positive.
Inhibition of Biopterin Synthesis by DAHP Prevents NO Production and Binding to and Invasion of HBMECs by E. coli K1
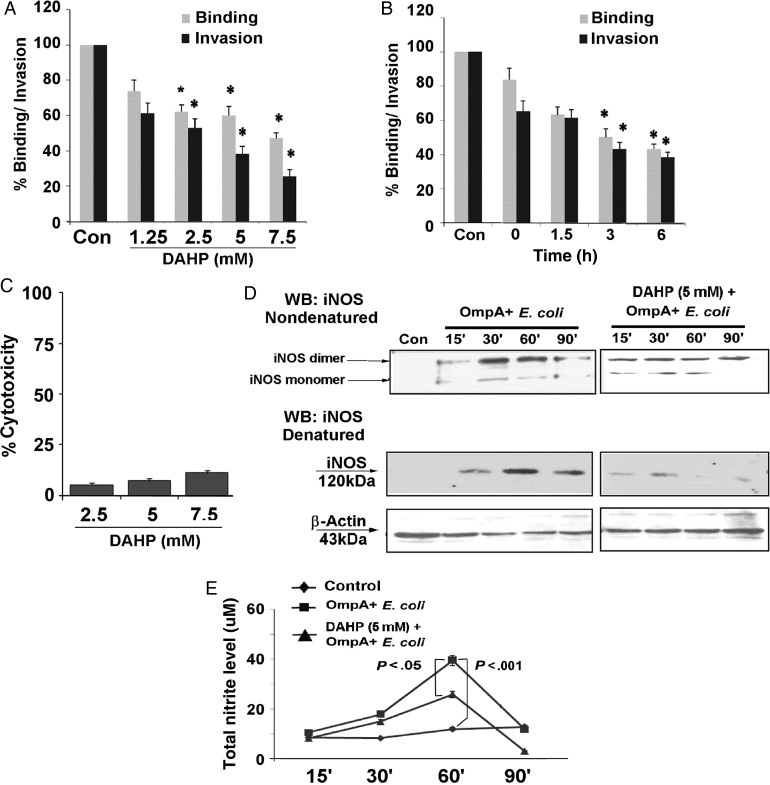
To analyze the role of GCH1 and biopterin in binding to and invasion of HBMECs by E. coli K1, the cells were pretreated with DAHP for various times and at different concentrations. A time- and dose-dependent inhibition in both binding and invasion of E. coli K1 was observed (Figure 2A and B). HBMECs treated with 5 mM DAHP for 3 hours reduced the binding and invasion of E. coli K1 by 45.6% and 56.2%, respectively, compared with control cells (mean ± SD CFU/well, 1.93 × 103 ± 0.4 × 103; P < .05). Interestingly, DAHP cotreatment (0-hour treatment) along with E. coli K1 infection also showed significant inhibition of binding and invasion (16.5% and 46.8%, respectively). DAHP treatment of HBMECs for 6 hours showed minimal cytotoxicity, even at a concentration of 7.5 mM (Figure 2C) and no antimicrobial activity (data not shown). Pretreatment of HBMECs with 5 mM of DAHP for 3 hours was found to be the minimal condition for 50% inhibition of invasion by E. coli K1. Hence, the same time and dose of DAHP treatment were used for further experiments.
Figure 2.
Pretreatment of human brain microvascular endothelial cells (HBMECs) with 2,4-diamino hydroxyl pyrimidine (DAHP) prevents Omp+ Escherichia coli from binding to and invading the cells by inhibiting iNOS expression and NO production. A, HBMEC monolayers were pretreated with different doses of DAHP (1.25–7.5 mM) for 3 hours and infected with OmpA+ E. coli for 90 minutes. Percentage binding and invasion as compared to untreated HBMECs were calculated. *P < .05, compared with control (Con). B, Cells were pretreated with 5 mM of DAHP for various times prior to OmpA+ E. coli infection, and percentage binding and invasion were calculated. Values are mean ± SD of 2 different experiments performed in triplicate. *P < .05, compared with control (Con). C, HBMEC monolayers were treated with various concentrations of DAHP for 6 hours, and the cytotoxicity percentage was measured as described in Materials and Methods. D, To analyze iNOS dimerization, total lysates of Omp+ E. coli–infected HBMECs, with or without DAHP pretreatment (5 mM for 3 hours), were subjected to low-temperature Western blotting under nondenatured conditions. Uninfected HBMECs were used as a control. The same lysates were denatured and subjected to conventional Western blotting to examine total expression (monomer) of iNOS. β-actin was used to normalize the iNOS expression. E, HBMECs were cultured in 96-well plates and infected with Omp+ E. coli, with or without DAHP pretreatment (5 mM for 3 hours). The total nitrite level was measured in the cell supernatants by the Griess method. Values are mean ± SD of 2 different experiments performed in triplicate. *P < .05. Abbreviation: WB, Western blotting.
We have previously reported that E. coli K1 triggers NO production via iNOS for invading HBMECs [4]. Of note, dimer formation is critical for sustained enzymatic activity of iNOS and mainly depends on a cofactor like BH4 [23]. Since E. coli K1 infection stimulates total biopterin production, we analyzed the monomer/dimer status of iNOS in HBMECs. A time-dependent increase in iNOS dimer formation was observed in HBMECs infected with OmpA+ E. coli K1 (Figure 2D). Similarly, the total iNOS expression also increased, beginning 30 minutes after infection. Pretreatment of HBMECs with DAHP (5 mM) for 3 hours attenuates both iNOS monomer and dimer expression. In parallel, production of NO (measured as the total nitrate level) induced by E. coli K1 was significantly diminished by DAHP pretreatment (Figure 2E). These results suggest that biopterin synthesis is necessary for increased production of NO during OmpA+ E. coli infection of HBMECs.
Expression and Association of Ecgp96 With GCH1 Is Necessary for Biopterin Production
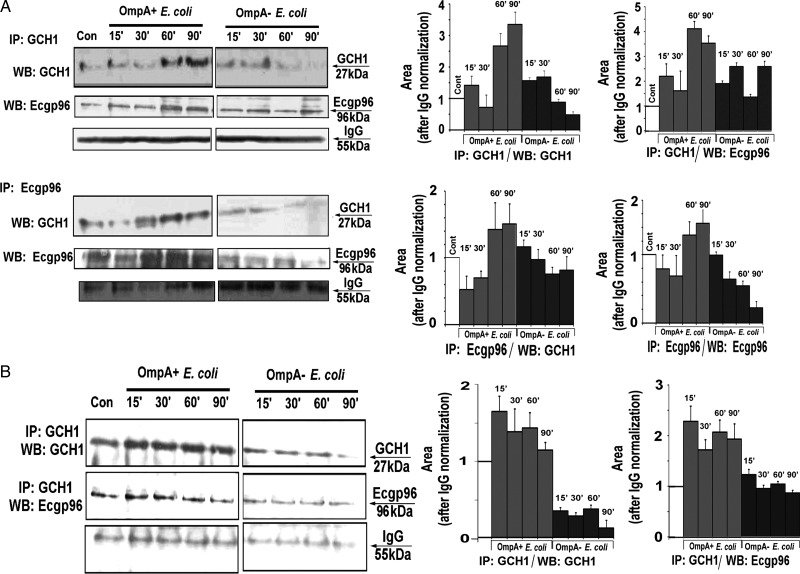
It was demonstrated that only the GCH1 decamer exhibits enzyme activity for catalysis of GTP to pterin [24]. Chaperones play a vital role in protein maturation, folding, and multimerization [25], and Ecgp96 (also known as “HSP90β,” “gp96,” and “GRP94”) is a very abundant molecular chaperone in endoplasmic reticulum [26]. Hence, we hypothesized that the association of Ecgp96 and GCH1 is essential for the enzymatic activity of GCH1. To explore this, cytosolic and membrane fractions were prepared from HBMECs infected with OmpA+ E. coli or OmpA− E. coli and were immunoprecipitated with anti-GCH1 antibodies, followed by immunoblotting with anti-Ecgp96 antibodies. In cytosolic fractions, a significant association of GCH1 and Ecgp96 was observed in OmpA+ E. coli–infected cells, with maximal interaction at 60 and 90 minutes, as determined by densitometric analysis (Figure 3A). Since OmpA− E. coli showed minimal stimulation of GCH1 expression and Ecgp96, it was not surprising that a slight increase in association of GCH1 and Ecgp96 was also observed at 30 minutes. To reconfirm this observation, reverse immunoprecipitation was performed using anti-Ecgp96 antibodies, followed by immunoblotting with anti-GCH1 antibodies. Maximum association of Ecgp96 with GCH1 was observed in HBMECs 60 and 90 minutes after infection with OmpA+ E. coli.
Figure 3.
Association of guanosine triphosphate cyclohydrolase (GCH1) and Ecgp96 in human brain microvascular endothelial cells (HBMECs). A, Cytosolic fractions (300 µg) of uninfected HBMECs or HBMECs infected with OmpA+ Escherichia coli or Omp− E. coli were immunoprecipitated (IP) with anti-GCH1 antibodies. The immune complexes were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted with anti-Ecgp96 antibodies. Similarly, total lysates were immunoprecipitated with anti-Ecgp96 antibody and immunoblotted with anti-GCH1 antibodies. Con, control. B, Plasma membrane fractions from total lysates of uninfected HBMECs or HBMECs infected with Omp+ E. coli or OmpA− E. coli were prepared. Membrane proteins (100 µg) were immunoprecipitated (IP) with anti-GCH1 antibodies and immunoblotted with antibodies to GCH1 or Ecgp96. The experiments were performed at least 3 times, and representative blots are shown here. The densities of protein bands from each blot were determined, normalized to the densities of immunoglobulin G (IgG), and graphed. The control (Cont) values were taken as one. Abbreviation: WB, Western blotting.
Our previous studies showed surface translocation of Ecgp96 from cytosol during E. coli K1 infection in HBMECs [16]. Thus, it was of interest to analyze whether these 2 proteins also associate at the membrane level. Immunoprecipitation of membrane fractions of infected cells revealed a significant association of GCH1 and Ecgp96. However, unlike the interaction observed in the cytosol, the maximum association began at 15 minutes and was maintained until 90 minutes after OmpA+ E. coli infection (Figure 3B). In contrast, OmpA− E. coli–infected HBMECs only showed a basal level of association. Collectively, these data demonstrate that increased expression and association of Ecgp96 and GCH1 occurs in the cytoplasm and that a portion of the complexes translocate to the plasma membrane of HBMECs during infection with OmpA+ E. coli.
Inhibition of GCH1 Reduces Total and Surface Expression of Ecgp96
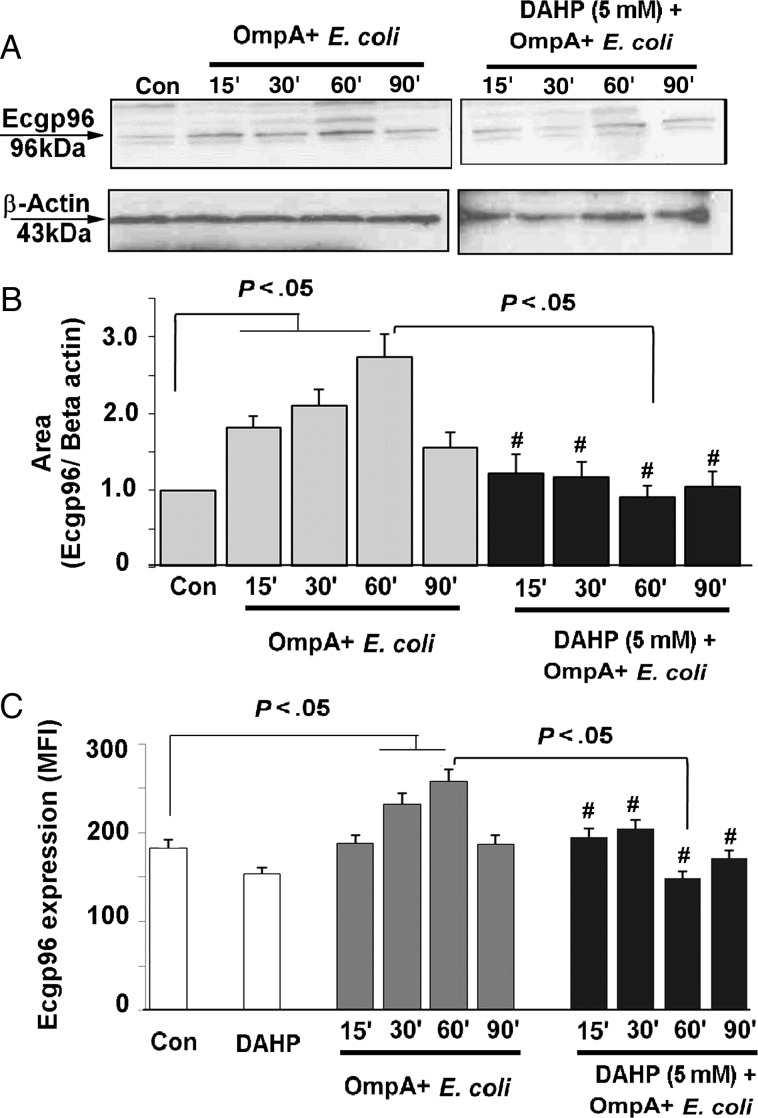
OmpA+ E. coli infection induces total and cell surface expression of Ecgp96 in HBMECs, mediated by increased production of NO [4]. Since pretreatment of DAHP attenuates NO production, we sought to analyze the effect of DAHP on the total and surface expression of Ecgp96. As shown in Figure 4A, OmpA+ E. coli activates total expression of Ecgp96 15–60 minutes after infection. Pretreatment of HBMECs with 5 mM of DAHP for 3 hours significantly reduced the expression of Ecgp96 despite infection with OmpA+ E. coli. Densitometric analysis of the protein bands revealed a 2.5-fold increase in the expression of Ecgp96 in HBMECs infected with OmpA+ E. coli, compared with control, which was inhibited by treating the cells with DAHP (Figure 4B). Flow cytometry of HBMECs revealed increased expression of Ecgp96 on the membranes 30–60 minutes after infection, while DAHP-treated and infected HBMECs exhibited significantly reduced expression of Ecgp96 by 60 minutes after infection (P < .05; Figure 4C). These results suggest that GCH1 regulates Ecgp96 expression and plasma membrane translocation via NO production.
Figure 4.
Effect of 2,4-diamino hydroxyl pyrimidine (DAHP) on total and membrane expression of Ecgp96. A, Total lysates of uninfected and OmpA+ Escherichia coli–infected human brain microvascular endothelial cells (HBMECs), with or without DAHP pretreatment (5 mM for 3 hours), were prepared and subjected to Western blotting with anti-Ecgp96 or anti-β-actin antibodies. Con, control. B, Integrated density values of Ecgp96 and β-actin bands were measured using ImageJ software, normalized to β-actin, and graphed. Bars represent the means ± SD of fold-differences relative to Ecgp96 expression in uninfected HBMECs. #P > .05, compared with control (Con). OmpA+, OmpA positive. C, Membrane expression of Ecgp96 in uninfected and OmpA+ E. coli–infected HBMECs, with or without DAHP pretreatment (5 mM for 3 hours), were analyzed using flow cytometry with anti-Ecgp96 antibodies. The mean fluorescence intensity (MFI) of Ecgp96 expression was calculated after subtracting the MFI value of isotype-matched antibodies as a control. Values are mean ± SD of 3 different experiments. #P > .05, compared with control (Con). Abbreviation: OmpA+, OmpA positive.
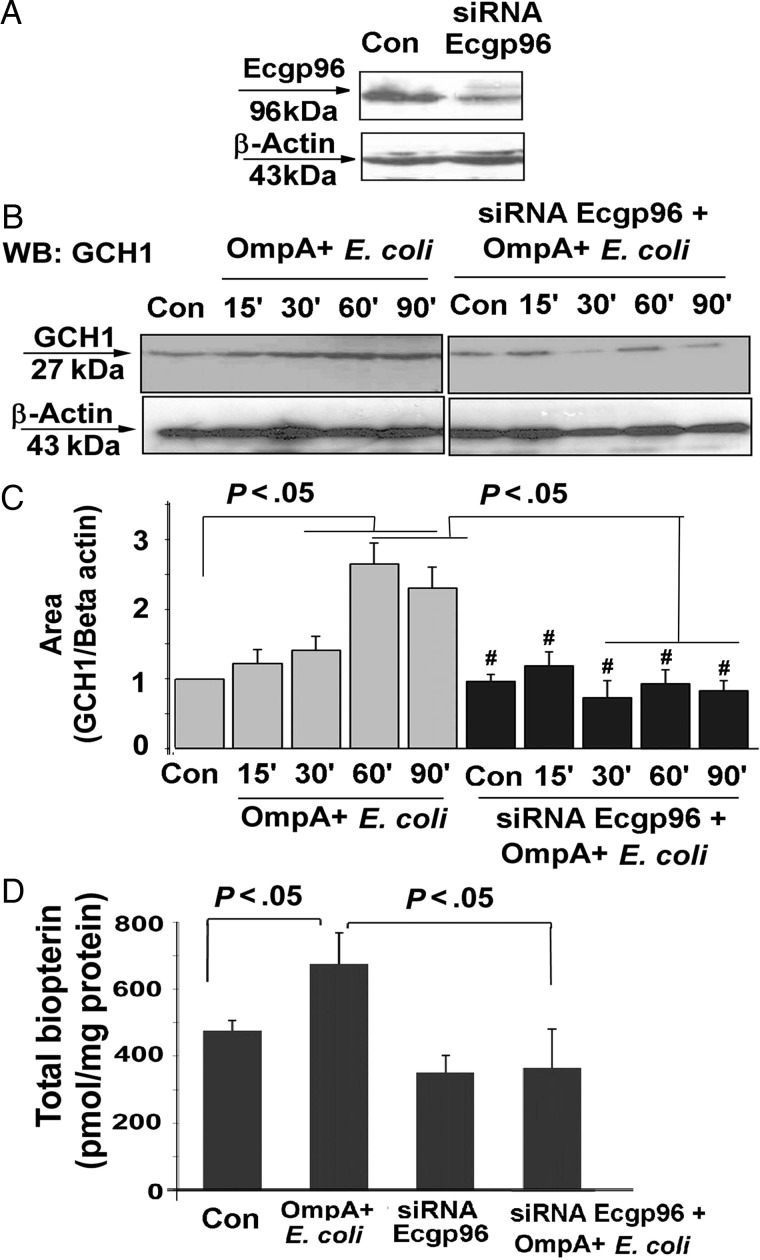
Silencing Ecgp96 by siRNA Affects GCH1 Expression and Biopterin Synthesis During E. coli K1 Infection
We next sought to evaluate GCH1 expression and biopterin synthesis after silencing Ecgp96 expression, using siRNA. HBMECs transfected with 40 pmol of Ecgp96 siRNA suppressed the expression of Ecgp96 by approximately 75%, on the basis of densitometric analysis (Figure 5A). Untransfected HBMECs revealed increased expression of GCH1 with E. coli K1 infection, beginning 15 minutes after infection. However, such an increase of GCH1 expression was inhibited in Ecgp96-siRNA/HBMECs, despite infection with OmpA+ E. coli (Figure 5B). Densitometry analysis of GCH1 expression revealed a 2.5-fold decrease in expression, which was similar to that for control cells (Figure 5C). Furthermore, Ecgp96-siRNA–transfected cells with and cells without OmpA+ E. coli infection were analyzed for total biopterin production (Figure 5D). In control HBMECs, the total biopterin level was found to be 476.2 pmol/mg of protein, and the level decreased to 351.6 pmol/mg protein on silencing of Ecgp96 expression. Similarly, in HBMECs infected with OmpA+ E. coli, the total biopterin level declined from 676.8 pmol/mg protein to 365.6 pmol/mg protein on introduction of Ecgp96-siRNA into HBMECs (P < .05). Together, these results support our hypothesis that Ecgp96 plays vital role in cellular biopterin production by regulating the activity of GCH1.
Figure 5.
Role of Ecgp96 on guanosine triphosphate cyclohydrolase (GCH1) expression and biopterin production. A, Human brain microvascular endothelial cells (HBMECs) were transfected with 40 pmol of Ecgp96 small interfering RNA (siRNA), and the expression of Ecgp96 was analyzed by Western blot using anti-Ecgp96 antibodies. Con, control. B and D, GCH1 expression (B) and total biopterin levels (D) were examined in uninfected and Omp+ Escherichia coli–infected HBMECs, with or without transfection with Ecgp96 siRNA, by Western blotting (WB) and high-performance liquid chromatography, respectively. Con, control. C, Quantitative analysis of GCH1 expression after normalization to β-actin levels was performed by ImageJ software. Bars represent means ± SD of fold-differences relative to GCH1 expression in uninfected and untransfected HBMECs. Total biopterin values are means ± SD of 3 different experiments. #P > .05, compared with control (Con). Abbreviation: OmpA+, OmpA positive.
GCH1 Inhibition by DAHP Prevents Meningitis in Newborn Mice by E. coli K1
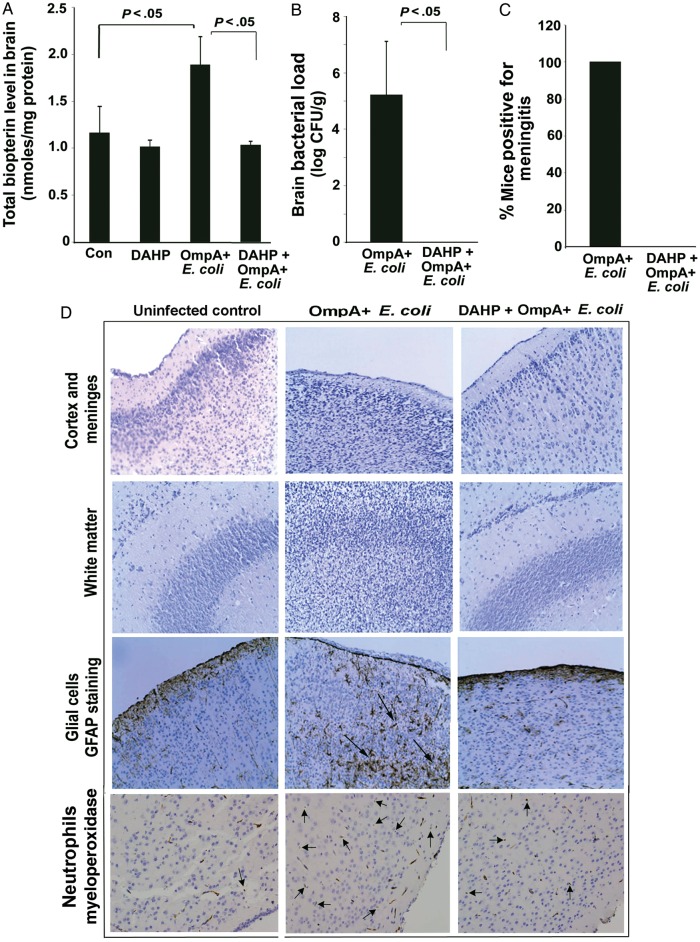
We further examined the role of biopterin synthesis in our well-established newborn mouse model of meningitis. A significant elevation in total biopterin production was noticed in the brains of OmpA+ E. coli–infected pups, compared with controls (Figure 6A). Additionally, the mean bacterial load ( ± SD) in the brains of OmpA+ E. coli–infected newborn mice was found to be 5.22 ± 1.9 log CFU/g brain tissue at 72 hours (Figure 6B). In agreement with this finding, 100% of infected pups had CSF cultures that were positive for E. coli K1 (which indicates positivity for meningitis; Figure 6C). Of note, brains of newborn mice treated with DAHP had no positive CSF cultures and had decreased levels of biopterin induced by OmpA+ E. coli infection. Analysis of brain tissue sections of OmpA+ E. coli–infected pups showed significant brain damage by extensive infiltration of glial cells and neutrophils, compared with brain sections of uninfected pups (Figure 6D). DAHP pretreatment prevented the abnormalities induced by OmpA+ E. coli infection in the brains of newborn mice. These results suggest that GCH1 activity is important for the development of meningitis due to E. coli K1 in newborn mice.
Figure 6.
2,4-diamino hydroxyl pyrimidine (DAHP) prevents the occurrence of meningitis in newborn mice. Newborn mice at day 3 were infected intranasally with 103 colony-forming units (CFU) of OmpA+ Escherichia coli, with or without receiving DAHP (100 mg/kg body weight, intraperitoneally) pretreatment. At the end of the study, whole brain was aseptically removed. Half of the brain was analyzed for total biopterin production by high-performance liquid chromatography (A) and bacterial load (B) by plating the tissue homogenates on rifampicin LB agar plates. Samples of cerebrospinal fluid (CSF) were collected by cisternal puncture and directly inoculated into LB containing rifampicin to examine the presence of bacteria (C). A positive CSF culture is considered as positive for meningitis. Another half of the brain from each animal was paraffin embedded, sectioned, and stained with hematoxylin-eosin or subjected to immunostaining with anti-GFAP or anti-MPO antibodies (D). The saline-treated and uninfected group was used as a control (Con) to compare the pathological alterations. Arrows indicate the infiltration of either glial cells or neutrophils. Representative images of each group (n = 6) are shown. Original magnification ×20.
DISCUSSION
Our previous studies have demonstrated that E. coli K1 induces NO production via OmpA interaction with the receptor Ecgp96 in HBMECs [4]. Here, we show that OmpA+ E. coli infection of HBMECs enhances the expression of GCH1, biopterin synthesis, and, concurrently, NO production. Although LPS has been shown to increase GCH1 expression, we observed only a moderate effect during OmpA− E. coli infection. Our unpublished data revealed that OmpA+ E. coli induces the expression of Toll-like receptor 2/Ecgp96 complexes on HBMEC plasma membranes, whereas OmpA− E. coli enhances mainly Toll-like receptor 4 (TLR4) expression. Therefore, it is possible that the moderate increase in GCH1 expression and biopterin production observed in OmpA− E. coli–infected HBMECs could be due to the interaction of LPS with TLR4. Since GTP is the substrate for cellular pterin synthesis, it should be noted that the GTP level is inversely proportional to the cellular pterin level, which in turn affects G-protein signaling and small-GTPase activation. This sequence of events could be the basis of our earlier observation that OmpA+ E. coli decreases the activity of the small GTPase Rac1 for cytoskeleton rearrangements [27].
Homo-decamerization of GCH1 is a critical factor in its activation, indicating that assembly of the GCH1 monomer into larger units is necessary for function. Hwu et al have reported the role of Hsp90, a heat shock protein, in regulating GCH1 expression and activity [28]. Although Ecgp96 is a paralogue of the Hsp90β form, this study revealed that Ecgp96 also associates with GCH1 and that their levels increased significantly on OmpA+ E. coli infection of HBMECs. In addition, Ecgp96/GCH1 complexes also translocated to the cell surface during the invasion process. Coordinated expression of GCH1 and iNOS under inflammatory conditions has been reported in various models [29, 30]. Furthermore, the reduced forms of biopterin are critical for iNOS dimerization and NO production [31]. Consistent with these reports, OmpA+ E. coli induced total expression, dimer formation of iNOS, and subsequent NO production 30–60 minutes after infection of HBMECs. Of note, NO-mediated Ecgp96 expression via iNOS was observed in HBMECs infected with OmpA+ E. coli. In DAHP-treated HBMECs, a significant decrease in total dimeric iNOS and in NO production was observed and, concurrently, reduced the total and surface expression of Ecgp96 during OmpA+ E. coli infection. A significant decrease in the total biopterin levels in HBMECs transfected with Ecgp96 siRNA and decreased expression of GCH1 induced by OmpA+ E. coli infection were also observed.
We translated our in vitro observations to a neonatal mouse model to further investigate the role of biopterin synthesis in OmpA+ E. coli infection. A beneficial role of DAHP in preventing septic shock induced by gram-positive and gram-negative pathogens was suggested by several studies [32–34]. However, this is the first report to show the effect of DAHP in preventing bacterial entry into brain. NO production by iNOS during infection has been reported to be 100–1000-fold higher than that of constitutive forms of NOS [35] and to cause severe brain injury and increased intracranial pressure [36]. Additionally, elevated GCH1 expression and pterin metabolites were found to produce persistent neuropathic pain [9]. OmpA+ E. coli–induced abnormalities in brain pathology completely resolved, and the total biopterin production was reduced in DAHP-treated newborn mice. In addition, the observation of a significant decrease in blood bacteremia among infected newborn mice (data not shown) indicates that the inhibition of biopterin synthesis reduced the bacterial load in the blood.
In sum, this study demonstrates that OmpA+ E. coli infection stimulates biopterin synthesis by manipulating the expression and activity of GCH1 for efficient brain translocation. Furthermore, the stimulation of biopterin synthesis also favors iNOS expression, dimerization, and subsequent NO production (Figure 7). Since iNOS-mediated NO production was triggered by many clinically important pathogens, it is of interest to examine the role of GCH1 and biopterin production in other infectious diseases.
Figure 7.
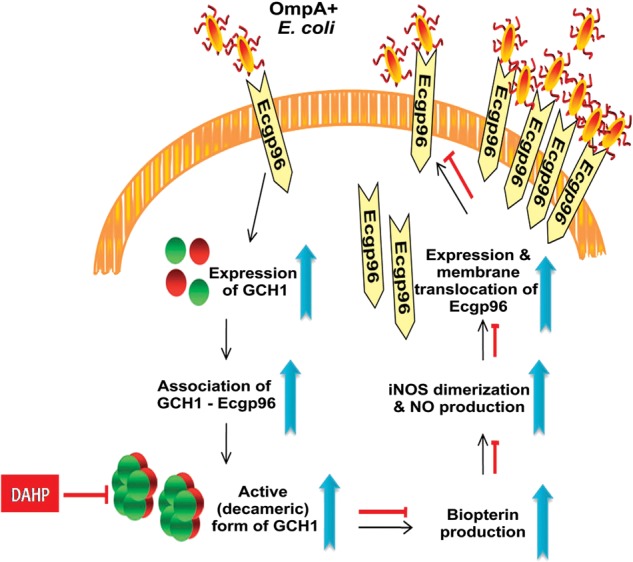
A proposed model for the pivotal role of biopterin synthesis in Escherichia coli K1 invasion of human brain microvascular endothelial cells (HBMECs). OmpA+ E. coli infection of HBMECs enhances both Ecgp96 and guanosine triphosphate cyclohydrolase (GCH1) expression and GCH1-Ecgp96 association for formation of an enzymatically active homo-decamer to stimulate biopterin synthesis. Elevated GCH1 expression and biopterin production induces NO production by stimulating iNOS expression and dimerization. The formation of NO further stimulates Ecgp96 expression and membrane translocation that enables more OmpA+ E. coli interaction with Ecgp96 for subsequent invasion. 2,4-diamino hydroxyl pyrimidine blocks the entire signaling cascade induced by OmpA+ E. coli by functionally inhibiting GCH1 and biopterin production.
Notes
Acknowledgments. We thank Barbara Driscoll, for a critical reading of the manuscript, and members of Dr Bellusci's laboratory, for assistance in immunohistochemistry. We are indebted to Larry Wang, Department of Pathology at CHLA, for evaluating the brain sections.
Financial support. This work was supported by the National Institutes of Health (grants AI40567 and NS73115 to N. V. P.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scheld WM, Koedel U, Nathan B, Pfister HW. Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis. 2002;186(Suppl 2):S225–33. doi: 10.1086/344939. [DOI] [PubMed] [Google Scholar]
- 2.Unhanand M, Mustafa MM, McCracken GH, Jr., Nelson JD. Gram-negative enteric bacillary meningitis: a twenty-one-year experience. J Pediatr. 1993;122:15–21. doi: 10.1016/s0022-3476(05)83480-8. [DOI] [PubMed] [Google Scholar]
- 3.Prasadarao NV, Wass CA, Weiser JN, Stins MF, Huang SH, Kim KS. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–53. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal R, Prasadarao NV. Nitric oxide/cGMP signalling induces Escherichia coli K1 receptor expression and modulates the permeability in human brain endothelial cell monolayers during invasion. Cell Microbiol. 2010;12:67–83. doi: 10.1111/j.1462-5822.2009.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thony B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347(Pt 1):1–16. [PMC free article] [PubMed] [Google Scholar]
- 7.Swick L, Kapatos G. A yeast 2-hybrid analysis of human GTP cyclohydrolase I protein interactions. J Neurochem. 2006;97:1447–55. doi: 10.1111/j.1471-4159.2006.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka K, Kaufman S, Milstien S. Tetrahydrobiopterin, the cofactor for aromatic amino acid hydroxylases, is synthesized by and regulates proliferation of erythroid cells. Proc Natl Acad Sci U S A. 1989;86:5864–7. doi: 10.1073/pnas.86.15.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–77. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 10.Linscheid P, Schaffner A, Schoedon G. Modulation of inducible nitric oxide synthase mRNA stability by tetrahydrobiopterin in vascular smooth muscle cells. Biochem Biophys Res Commun. 1998;243:137–41. doi: 10.1006/bbrc.1998.8072. [DOI] [PubMed] [Google Scholar]
- 11.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 12.Hattori Y, Nakanishi N, Kasai K, Shimoda SI. GTP cyclohydrolase I mRNA induction and tetrahydrobiopterin synthesis in human endothelial cells. Biochim Biophys Acta. 1997;1358:61–6. doi: 10.1016/s0167-4889(97)00052-9. [DOI] [PubMed] [Google Scholar]
- 13.Hauser B, Bracht H, Matejovic M, Radermacher P, Venkatesh B. Nitric oxide synthase inhibition in sepsis? Lessons learned from large-animal studies. Anesth Analg. 2005;101:488–98. doi: 10.1213/01.ANE.0000177117.80058.4D. [DOI] [PubMed] [Google Scholar]
- 14.Azumagawa K, Suzuki S, Tanabe T, Wakamiya E, Kawamura N, Tamai H. Neopterin, biopterin, and nitric oxide concentrations in the cerebrospinal fluid of children with central nervous system infections. Brain Dev. 2003;25:200–2. doi: 10.1016/s0387-7604(02)00217-6. [DOI] [PubMed] [Google Scholar]
- 15.Franscini N, Blau N, Walter RB, Schaffner A, Schoedon G. Critical role of interleukin-1beta for transcriptional regulation of endothelial 6-pyruvoyltetrahydropterin synthase. Arterioscler Thromb Vasc Biol. 2003;23:e50–3. doi: 10.1161/01.ATV.0000099785.65848.F1. [DOI] [PubMed] [Google Scholar]
- 16.Prasadarao NV, Srivastava PK, Rudrabhatla RS, Kim KS, Huang SH, Sukumaran SK. Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect Immun. 2003;71:1680–8. doi: 10.1128/IAI.71.4.1680-1688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima T, Nixon JC. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal Biochem. 1980;102:176–88. doi: 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan S, Fernandez GS, Sacks DB, Prasadarao NV. IQGAP1 mediates the disruption of adherens junctions to promote Escherichia coli K1 invasion of brain endothelial cells. Cell Microbiol. 2012;14:1415–33. doi: 10.1111/j.1462-5822.2012.01805.x. doi:10.1111/j.1462-5822.2012.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai K, Suzuki H, Oda H, et al. Phosphoinositide 3-kinase in nitric oxide synthesis in macrophage: critical dimerization of inducible nitric-oxide synthase. J Biol Chem. 2006;281:17736–42. doi: 10.1074/jbc.M601896200. [DOI] [PubMed] [Google Scholar]
- 21.Mittal R, Krishnan S, Gonzalez-Gomez I, Prasadarao NV. Deciphering the roles of outer membrane protein A extracellular loops in the pathogenesis of Escherichia coli K1 meningitis. J Biol Chem. 2011;286:2183–93. doi: 10.1074/jbc.M110.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Hasegawa H, Kaneko E, Ichiyama A. Gastrointestinal serotonin: depletion due to tetrahydrobiopterin deficiency induced by 2,4-diamino-6-hydroxypyrimidine administration. J Pharmacol Exp Ther. 1991;256:773–9. [PubMed] [Google Scholar]
- 23.Eissa NT, Yuan JW, Haggerty CM, Choo EK, Palmer CD, Moss J. Cloning and characterization of human inducible nitric oxide synthase splice variants: a domain, encoded by exons 8 and 9, is critical for dimerization. Proc Natl Acad Sci U S A. 1998;95:7625–30. doi: 10.1073/pnas.95.13.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yim JJ, Brown GM. Characteristics of guanosine triphosphate cyclohydrolase I purified from Escherichia coli. J Biol Chem. 1976;251:5087–94. [PubMed] [Google Scholar]
- 25.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–49. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 26.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–6. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 27.Rudrabhatla RS, Selvaraj SK, Prasadarao NV. Role of Rac1 in Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microbes Infect. 2006;8:460–9. doi: 10.1016/j.micinf.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwu WL, Lu MY, Hwa KY, Fan SW, Lee YM. Molecular chaperones affect GTP cyclohydrolase I mutations in dopa-responsive dystonia. Ann Neurol. 2004;55:875–8. doi: 10.1002/ana.20122. [DOI] [PubMed] [Google Scholar]
- 29.Togari A, Arai M, Mogi M, Kondo A, Nagatsu T. Coexpression of GTP cyclohydrolase I and inducible nitric oxide synthase mRNAs in mouse osteoblastic cells activated by proinflammatory cytokines. FEBS Lett. 1998;428:212–6. doi: 10.1016/s0014-5793(98)00531-6. [DOI] [PubMed] [Google Scholar]
- 30.Frank S, Madlener M, Pfeilschifter J, Werner S. Induction of inducible nitric oxide synthase and its corresponding tetrahydrobiopterin-cofactor-synthesizing enzyme GTP-cyclohydrolase I during cutaneous wound repair. J Invest Dermatol. 1998;111:1058–64. doi: 10.1046/j.1523-1747.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- 31.Geller DA, Di Silvio M, Billiar TR, Hatakeyama K. GTP cyclohydrolase I is coinduced in hepatocytes stimulated to produce nitric oxide. Biochem Biophys Res Commun. 2000;276:633–41. doi: 10.1006/bbrc.2000.3537. [DOI] [PubMed] [Google Scholar]
- 32.Li HY, Yao YM, Shi ZG, et al. Effect of 2,4-diamino-6-hydroxy-pyrimidine on postburn Staphylococcus aureus sepsis in rats. Crit Care Med. 2002;30:2520–7. doi: 10.1097/00003246-200211000-00020. [DOI] [PubMed] [Google Scholar]
- 33.van Amsterdam JG, van den Berg C, Zuidema J, te Biesebeek JD, Rokos H. Effect of septicaemia on the plasma levels of biopterin and nitric oxide metabolites in rats and rabbits. Biochem Pharmacol. 1996;52:1447–51. doi: 10.1016/s0006-2952(96)00511-4. [DOI] [PubMed] [Google Scholar]
- 34.Melichar B, Malirova E, Bures J, et al. Gastric juice neopterin in Helicobacter pylori infection. FEMS Immunol Med Microbiol. 1995;10:335–8. doi: 10.1111/j.1574-695X.1995.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 35.Villavicencio RT, Liu S, Kibbe MR, et al. Induced nitric oxide inhibits IL-6-induced stat3 activation and type II acute phase mRNA expression. Shock. 2000;13:441–5. doi: 10.1097/00024382-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Terpolilli NA, Zweckberger K, Trabold R, et al. The novel nitric oxide synthase inhibitor 4-amino-tetrahydro-L-biopterine prevents brain edema formation and intracranial hypertension following traumatic brain injury in mice. J Neurotrauma. 2009;26:1963–75. doi: 10.1089/neu.2008.0853. [DOI] [PubMed] [Google Scholar]