Abstract
The usefulness of vaccine-based strategies to prevent lethal bacterial infection in a host with neutropenia is not well-defined. Here, we show in a neutropenic mouse model that immunity induced by mucosal vaccination with a live-attenuated Pseudomonas aeruginosa vaccine is protective against lethal P. aeruginosa pneumonia caused by both vaccine-homologous and vaccine-heterologous strains, whereas passive immunization confers only vaccine-homologous protection. Cells in the macrophage lineage served as crucial innate cellular effectors in the neutropenic host after active immunization. Vaccine efficacy was CD4+ T-cell dependent and associated with accumulation of macrophage-lineage cells in the alveolar space after infection, as well as with enhanced P. aeruginosa clearance from the lung. Adaptive CD4+ T cells produced granulocyte-macrophage colony-stimulating factor (GM-CSF) on restimulation in vitro, and local GM-CSF was critical for vaccine efficacy. Thus, collaboration between the innate and adaptive effectors induced by mucosal vaccination can overcome neutropenia and confer protection against lethal bacterial infection in the profoundly neutropenic host.
Keywords: Pseudomonas aeruginosa, vaccines, infection, Pneumonia, immunocompromise, neutropenia, macrophage, monocyte, CD4, GM-CSF
Infectious diseases are among the most frequent and serious complications in cancer patients receiving chemotherapy and in stem cell transplant recipients [1]. Pseudomonas aeruginosa is a leading cause of bacteremic pneumonia in neutropenic cancer patients, among whom it is associated with significant morbidity and mortality [2]. Infections with P. aeruginosa are becoming increasingly difficult to treat because of antibiotic resistance, which is associated with poor outcomes [3, 4]. Accordingly, there is a pressing need for new strategies, including vaccines and passive immunotherapies, to combat these infections. Vaccine-based strategies for infectious diseases in these high-risk populations, however, are hampered by the decrease in number and function of multiple immune effectors, particularly neutrophils, which are one of the most critical arms of host defense against P. aeruginosa [5].
In a nonneutropenic setting, we have previously shown that mucosal immunization of mice with P. aeruginosa live-attenuated vaccines induces a broad range of protective immune effectors, such as lipopolysaccharide (LPS)– and outer membrane protein–targeted opsonophagocytic antibodies, and also cellular effectors, such as CD4+ T cells that secrete the cytokine interleukin 17 (IL-17), called T-helper 17 (Th17) cells [6, 7]. The latter immune mechanism allows for rapid recruitment of neutrophils and their efficient killing of bacteria. This is essential for protection against acute lethal pneumonia, particularly when levels of opsonophagocytic antibodies to the LPS O antigen are low or absent, which occurs with infections due to LPS O-antigen–heterologous strains (ie, strains having a different LPS serogroup from that of the vaccine strain) [6, 7]. Th17 cells have the potential to secrete proinflammatory cytokines other than IL-17, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), and it has recently been shown that Th17-derived GM-CSF is a key mediator of experimental autoimmune encephalitis [8, 9]. However, it is unclear whether GM-CSF has a role in vaccine-induced host defense against acute infectious processes.
Little is known about the optimal form of acquired immunity that might protect a host with profound neutropenia against lethal bacterial pneumonia. We hypothesized that a mucosal vaccination strategy could lead to maximal use of lung macrophages as critical phagocytes that could be orchestrated by vaccine-induced CD4+ T cells and thus could create protective immunity to lethal P. aeruginosa pneumonia that is independent of neutrophils.
MATERIALS AND METHODS
A detailed description of the methods for histologic analysis, immunofluorescent staining, in vitro cytokine secretion assays, and intracellular cytokine staining is available in the Supplementary Materials.
Bacterial Strains
The bacterial strains used in this study are listed in Table 1. Of note, the live-attenuated vaccine strain PAO1ΔaroA is cleared from the lung of nonneutropenic mice by 100 hours after immunization [10]. PAO1ΔaroA is also highly attenuated in its virulence in neutropenic mice [11].
Table 1.
Bacterial Strains Used in This Study
| Strain | Description | Reference or Source |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild-type strain, LPS smooth, non–cytotoxic, serogroup O2/O5, subtype epitopes O2a, O2d | M. Vasila |
| PAO1ΔaroA | aroA deletion mutant of PAO1, LPS smooth, serogroup O2/O5, subtype epitopes O2a, O2d | [10] |
| 170003 | Wild-type strain, LPS smooth, non–cytotoxic, serogroup O2/O5, subtype epitopes O2a, O2b | [40] |
| IT7 | Wild-type strain, LPS smooth, non–cytotoxic, serogroup O2/O5, subtype epitope O2a | [40] |
| IT4 | Clinical isolate (bacteremia), LPS smooth, serogroup O1 | [41] |
| 6294 | Clinical isolate (corneal infection), LPS smooth, noncytotoxic, serogroup O6 | BPEIb and [41, 42] |
| E. coli HB101 | supE44, hsdS20(r-Bm-B), recA13, ara-14, proA2 lacY1, galK2, rpsL20, xyl-5, mtl-1 | [43] |
Abbreviations: E. coli, Escherichia coli; LPS, lipopolysaccharide; P. aeruginosa, Pseudomonas aeruginosa.
a M. Vasil, University of Colorado Health Sciences Center, Aurora, CO.
b Bascom-Palmer Eye Institute, Miami, FL.
Immunization and Infection During Neutropenia
Mice used in these studies were C3H/HeN (sex, female; age, 6–8 weeks at the beginning of each experiment) obtained from Harlan Sprague-Dawley Farms (Chicago, IL). Animal experiments complied with institutional and federal guidelines regarding the use of animals in research.
For active immunization, mice were anesthetized intraperitoneally with ketamine/xylazine, and P. aeruginosa live-attenuated vaccine strain PAO1ΔaroA or Escherichia coli strain HB101 (control) was given intranasally once per week for 3 weeks at escalating doses of 108, 5 × 108, and 109 colony-forming units (CFU) [10]. For passive immunization, 0.2 mL of hyperimmunized rabbit sera was administered intraperitoneally to mice [10]. Pneumonia was induced by intranasal inoculation of anesthetized mice with P. aeruginosa strains during the fourth week after the final active immunization dose or 24 hours after passive immunization [5, 12]. Before challenge, mice were made neutropenic by intraperitoneal receipt of either a 150-mg/kg dose of cyclophosphamide (CY; Sigma-Aldrich) every other day for 3 doses (with the last dose received on the day before bacterial challenge) or a single 0.2-mg dose of anti-Gr-1 monoclonal antibodies (mAb; RB6–8C5) 1 day before challenge. We have previously shown in this model that the absolute neutrophil count in mouse peripheral blood is <50 cells/mm3 for at least 4 days from the day after the third dose of CY (ie, the day of bacterial challenge in the present study) [5]. The absolute neutrophil count is <100 cells/mm3 for 5 days from the day after anti-Gr-1 mAb administration [11]. Mice were maintained on drinking water containing 0.15 mg/mL gentamicin (Research Product International) during induction of neutropenia and until the day of bacterial challenge, when drinking water was changed back to sterile water.
In Vivo Depletion or Supplementation of Immune Effectors
For depletion of macrophages/monocytes in the alveolar space, clodronate-containing liposomes (100 µL/mouse in 2 divided doses) were given intranasally to anesthetized mice 1 day before bacterial challenge [5]. Liposomes containing phosphate-buffered saline (PBS) were given intranasally to control mice. Clodronate, which was kindly provided by Roche Diagnostics (Mannheim, Germany), was used to assemble clodronate-containing (250 mg/mL) liposomes, as previously described [13]. For in vivo CD4+ T-cell depletion, anti-CD4 mAb (GK1.5, BioXCell) was given to anesthetized mice both intranasally and intraperitoneally (100 µg/dose and 500 µg/dose, respectively) 72 and 24 hours before bacterial challenge; we have previously shown that this leads to >98% reduction in total lung CD4+ T cells 24 and 72 hours after live bacterial challenge [7]. Control mice received the same doses of rat immunoglobulin G (IgG; Sigma–Aldrich) by the same route at the same time points. For GM-CSF neutralization, 100 µg of anti-GM-CSF mAb (MP1-22E9, eBioscience) was administered intranasally [14] to anesthetized mice 8 hours before infection. For GM-CSF supplementation experiments, a 1-µg dose of murine recombinant GM-CSF (Invitrogen) was administered intranasally [15] to anesthetized mice 3 and 0 hours before infection.
Quantification of Immune Cells in Bronchoalveolar Lavage (BAL) Fluid
After euthanasia by carbon dioxide inhalation, BAL fluid was obtained by exposing the trachea and then twice injecting, and then removing, 1 mL of PBS containing 0.5 mM ethylenediaminetetraacetic acid. After treatment with red blood cell lysis buffer (R&D systems), part of each BAL fluid sample was stained with Trypan blue to enumerate viable cells. The rest of each sample was centrifuged onto microscope slides, air-dried, stained with a modified Wright-Giemsa stain (Diff-Quick, Baxter Scientific, Miami, FL), and evaluated for differential leukocyte counts (100 leukocytes per sample; original magnification ×400) on the basis of standard morphologic criteria. To assess the proportion of neutrophils in the total lung leukocytes, cells were isolated from collagenase-digested lungs [16]; stained with V500-labeled CD45.2 (clone 104, BD Biosciences), APC-labeled Ly6G (1A8, BioLegend), and APC-eFluor780–labeled CD11b (M1/70, eBioscience); and analyzed by FACS (BD LSR Fortessa).
Quantification of P. aeruginosa Levels in the Lung
For CFU measurements, lungs were weighed, homogenized in 1% proteose peptone (BD, Franklin Lakes, NJ), and serial diluted and plated on MacConkey agar. Non–lactose fermenting, oxidase-positive colonies were enumerated after overnight growth at 37°C.
Histologic and Immunofluorescence Analyses
Lung histologic analysis and immunofluorescent staining of immune cells from BAL fluid and collagenase-digested whole lung for MOMA-2 and Ki-67 antigen were performed by standard methods, as described in detail in the Supplementary Materials.
In Vitro GM-CSF Secretion From Adaptive Versus Naive T cells and Intracellular Cytokine Staining
Please see the Supplementary Materials for details. Briefly, splenic and lung T cells were isolated either from PAO1ΔaroA-immunized, E. coli–immunized, or unimmunized mice, using CD3+ T-cell selection columns (R&D Systems), as previously reported [6]. T cells were cocultured for 7 days with gentamicin-killed P. aeruginosa strain PAO1 as antigen and irradiated splenocytes as feeder cells in complete cell culture media, with or without anti-CD4 mAb, as described in the Supplementary Materials. GM-CSF levels in the culture supernatants were measured using an enzyme-linked immunosorbent assay (R&D Systems). Staining of intracellular GM-CSF and IL-17 from in vitro–stimulated splenocytes isolated from immunized or naive mice was performed using standard methods, as described in the Supplementary Materials. Gentamicin-killed P. aeruginosa strain PAO1 was used as antigen for in vitro T-cell assays because P. aeruginosa strain IT4 is gentamicin resistant. We previously showed that the use of gentamicin-killed P. aeruginosa strains having different LPS serogroups in these assays yielded similar T-cell responses in terms of IL-17 and interferon γ secretion [6].
Statistical Analyses
BAL fluid cell counts and P. aeruginosa CFU were evaluated by the Mann–Whitney U test or the Kruskal–Wallis test with the Dunn multiple comparison test for pairwise comparisons involving ≥3 groups. In vitro GM-CSF levels were analyzed by analysis of variance with the Dunnett multiple comparison test, using PAO1ΔaroA/I.C. group as the control comparator group. Survival data were analyzed by the Fisher exact test or by survival analysis with the Kaplan-Meier method, followed by Bonferroni corrections for multiple comparisons. Analyses were performed using Prism software (GraphPad Software, San Diego, CA).
RESULTS
Efficacy of Active Versus Passive Immunization During Neutropenia
We first evaluated the efficacy of a P. aeruginosa aroA–deleted, live-attenuated vaccine [10], denoted PAO1ΔaroA, against lethal P. aeruginosa pneumonia in the setting of neutropenia induced either by CY or by anti-Gr-1 mAb. Active immunization with PAO1ΔaroA protected neutropenic mice from acute lethal pneumonia caused not only by the LPS-homologous (serogroup O2/O5) P. aeruginosa strains PAO1, 170003, and IT7, but also by the LPS-heterologous strains IT4 (serogroup O1) and 6294 (serogroup O6) (Table 2). Passive immunization with rabbit hyperimmune antiserum raised to PAO1ΔaroA [17] conferred protection only against the LPS-homologous strain PAO1, not against the LPS-heterologous strains IT4 or 6294 (Table 2). We previously demonstrated the absence of opsonophagocytic antibodies against strains IT4 and 6294 in sera of mice actively immunized with PAO1ΔaroA [10]. These data collectively suggest a critical contribution of cellular components for full-fledged vaccine-induced protection. For the rest of the current studies, we used this model of active immunization followed by vaccine-heterologous challenge to investigate the cellular mechanisms of protection.
Table 2.
Survival of Neutropenic Mice During Experimental Pseudomonas aeruginosa Pneumonia After Active or Passive Immunization
| Challenge Strain (Serogroup) | Inoculum, CFU/Mouse | Agent of Neutropenia Induction | No. of Survivors/No. of Mice Challenged |
|||
|---|---|---|---|---|---|---|
| Active Immunization |
Passive Immunization |
|||||
| PAO1ΔaroA | Control (E. coli) | Rabbit Antiserum to PAO1ΔaroA | Control (Rabbit antiserum to E. coli) | |||
| PAO1 (O2/O5) | 1000 | Cyclophosphamide | 6/6a | 0/6 | … | … |
| PAO1 (O2/O5) | 1000 | Anti-Gr-1 | 6/8a | 0/8 | 5/9a | 0/10 |
| 170003 (O2/O5) | 100 | Cyclophosphamide | 6/6a | 1/6 | … | … |
| IT7 (O2/O5) | 80 | Cyclophosphamide | 4/5a | 0/5 | … | … |
| IT4 (O1) | 60 | Cyclophosphamide | 7/7a | 0/7 | … | … |
| IT4 (O1) | 70 | Anti-Gr-1 | 7/7a | 2/7 | 0/8 | 0/10 |
| 6294 (O6) | 60 | Cyclophosphamide | 6/8a | 0/9 | … | … |
| 6294 (O6) | 60 | Anti-Gr-1 | 3/8b | 0/9 | 0/7 | 0/8 |
Abbreviations: CFU, colony-forming units; E. coli, Escherichia coli.
a P < .05, by the Fisher exact test.
b P < .05 by the long-rank test only (P > .05, by the Fisher exact test).
Macrophage-Lineage Cells Are Indispensable Innate Cellular Effectors of Protection
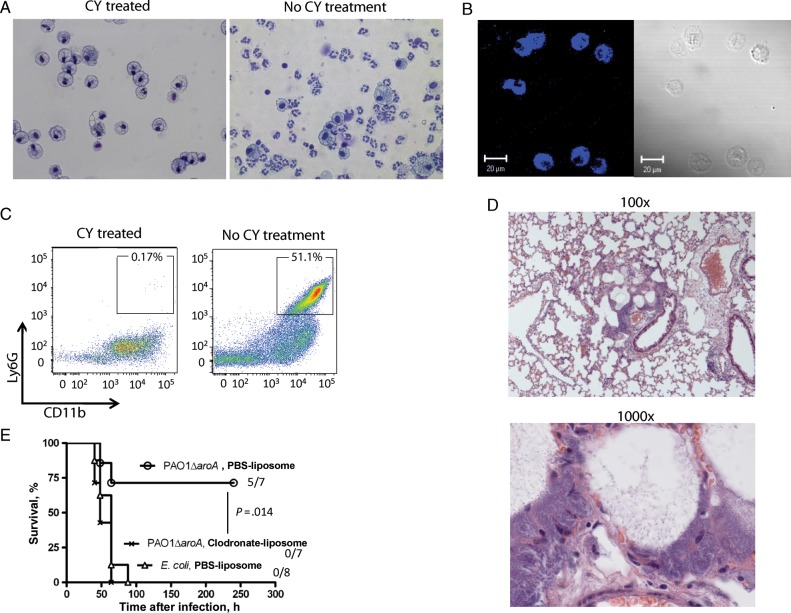
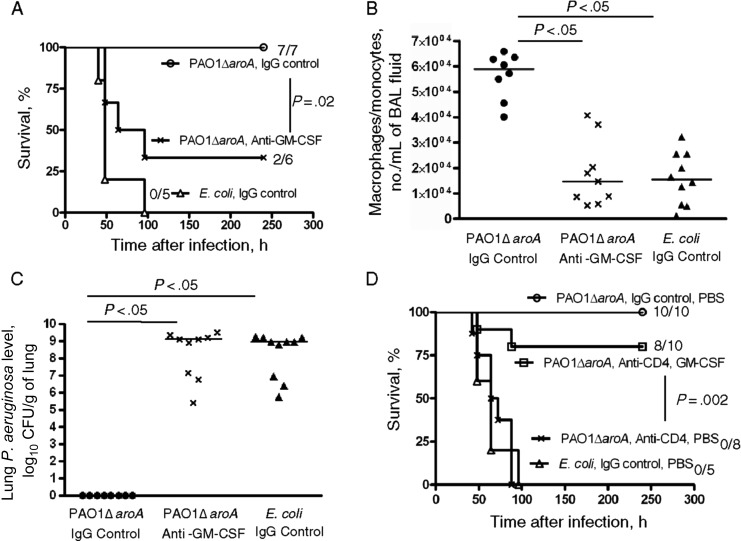
We next evaluated the leukocyte differentials in BAL fluid from PAO1ΔaroA-immunized or control E. coli–immunized mice that were made neutropenic and then challenged by the vaccine-heterologous strain IT4. BAL fluid was analyzed at 6, 18, 24, and 30 hours after infection during anti-Gr-1 mAb–induced neutropenia and at 30 hours after infection during CY-induced neutropenia (Figure 1A). Polymorphonuclear cells composed <1% of leukocytes in the BAL fluid at all time points tested. Instead, the cells in the monocyte/macrophage lineage composed >90% of the leukocytes in BAL fluid at all points except 6 hours after infection in anti-Gr-1 mAb–induced neutropenia, when monocytes/macrophages composed a median of 84.5% (interquartile range [IQR], 78.5%–86.6%) and 80.9% (IQR, 77.2%–86.9%) of the leukocytes in BAL fluid of PAO1ΔaroA-immunized mice and control E. coli–immunized mice, respectively (P = .72, by the Mann–Whitney U test). Cells with standard monocyte/macrophage morphology from representative samples were verified by positive staining for MOMA-2, a pan-monocyte/macrophage lineage intracellular marker [18, 19] (Figure 1B). We also assessed the degree of neutrophil depletion within the infected lung tissue of the neutropenic mice. Neutrophils (defined as Ly6G high, CD11b high cells) composed <0.3% of total lung leukocytes (CD45+ cells) 24 hours after infection during CY-induced neutropenia (Figure 1C). The median proportion of neutrophils in lung leukocytes was not different between PAO1ΔaroA-immunized mice (0.13% [IQR, 0.074%–0.19%]) and E. coli–immunized mice (0.20% [IQR, 0.044%–0.25%]; P = .83, by the Mann–Whitney U test). Lung histopathologic analysis done 30 hours after infection in anti-Gr-1–induced neutropenia revealed an accumulation of rod-shaped bacterial cells in alveolar spaces but no evidence of PMN infiltration (Figure 1D). Finally, in vivo depletion of monocytes/macrophages in the alveolar space by intranasal administration of clodronate-containing liposomes that started 24 hours before infection abolished vaccine efficacy (Figure 1E). These data suggest that cells in the macrophage lineage serve as indispensable innate cellular effectors of vaccine-induced protection in the context of profound neutrophil depletion in the infected tissue.
Figure 1.
Monocytes/macrophages are indispensable innate cellular effectors in the lung for vaccine-induced protection against Pseudomonas aeruginosa pneumonia during neutropenia. A, At left, Wright-Giemsa stain of bronchoalveolar lavage (BAL) fluid from PAO1ΔaroA-immunized mice with cyclophosphamide (CY)–induced neutropenia at 30 hours after challenge (strain IT4, 70 colony-forming units [CFU]). Representative images are from 10 mice. At right, BAL fluid from PAO1ΔaroA-immunized nonneutropenic mice (without CY treatment) 30 hours after sublethal P. aeruginosa infection. Original magnification ×400. B, Immunofluorescent staining for the monocyte/macrophage marker MOMA-2 (blue) in BAL fluid cells from mice described in the left side of panel A. C, At left, dot plot showing proportion of neutrophils (Ly6G high, CD11b high) in total lung leukocytes (CD45 gate) from PAO1ΔaroA-immunized mice with CY-induced neutropenia at 24 hours after infection (strain IT4, 80 CFU). Representative data are from 5 mice. At right, dot plot showing the proportion of neutrophils from PAO1ΔaroA-immunized nonneutropenic mice (without CY treatment) 24 hours after sublethal P. aeruginosa challenge. A total of 2 × 105 events were acquired per each mouse, followed by selection of CD45+ cells. D, Lung histopathologic findings for Escherichia coli–immunized mice with anti-Gr-1–induced neutropenia at 30 hours after infection (strain IT4, 70 CFU). Representative images are from 3 mice. Original magnification ×100 and ×1000. E, Survival of immunized mice with neutropenia (anti-Gr-1 induced) after P. aeruginosa infection (strain IT4, 70 CFU), with or without local monocyte/macrophage depletion by intranasal clodronate-containing liposomes or phosphate-buffered saline (PBS)–containing control liposomes. Numbers on right are number of surviving mice/number of mice challenged. The P value was calculated by the log-rank test with Bonferroni correction and compares the survival of clodronate-treated mice with that of control liposome-treated PAO1ΔaroA-immunized mice.
Vaccine-Induced Protection Is Associated With Monocyte/Macrophage Accumulation After Infection and With Enhanced P. aeruginosa Clearance From Lung
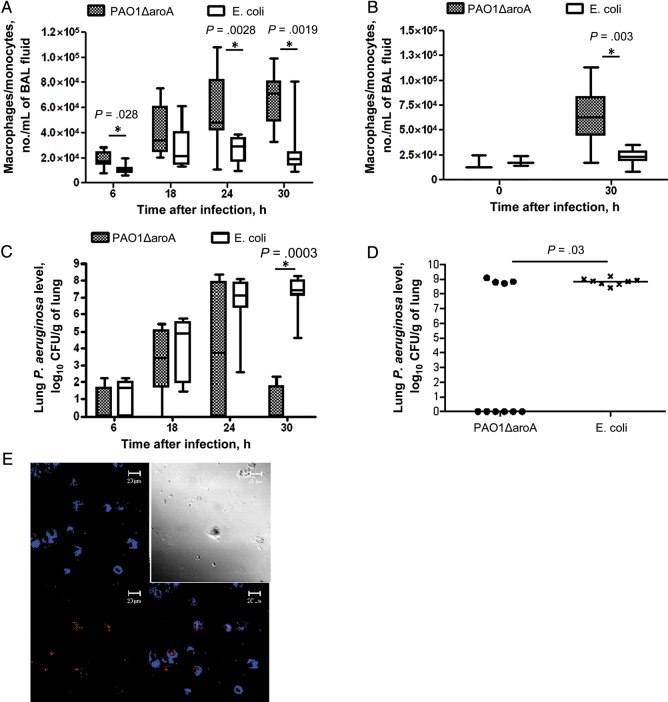
We next compared the numbers of monocytes/macrophages in BAL fluid between vaccinated and control mice at various time points after infection during anti-Gr-1–induced neutropenia. Chronologic accumulation of the monocyte/macrophage lineage cells after infection was found in BAL fluid from both immunized and control mice, but the numbers were higher in the vaccinated group, with significant differences at 6, 24, and 30 hours after infection (Figure 2A). Higher monocyte/macrophage numbers in BAL fluid from immunized mice as compared to control mice was also found 30 hours after infection (the only time point tested) in the setting of CY-induced neutropenia (Figure 2B), where we also observed no difference in monocyte/macrophage numbers before infection. Vaccination was also associated with significantly lower levels of P. aeruginosa in the lung 30 hours after infection in both anti-Gr-1–treated mice (Figure 2C) and CY-treated mice (Figure 2D).
Figure 2.
Vaccine-induced protection is associated with accumulation of monocytes/macrophages in the alveolar space and lower lung Pseudomonas aeruginosa levels. A and C, Numbers of monocytes/macrophages per milliliter of bronchoalveolar lavage (BAL) fluid (A) and lung P. aeruginosa levels (C) at different times after challenge with P. aeruginosa strain IT4 (70–80 colony-forming units [CFU]) in PAO1ΔaroA-immunized or control Escherichia coli–immunized mice made neutropenic with anti-Gr-1 monoclonal antibody. In box plots, horizontal bars show medians, boxes show the 25th and 75th percentiles, and whiskers show the minimum and maximum values. The figures compile the results of 4 independent experiments performed at each time point, with 7–9 mice/group at each time point. P values were determined by Mann–Whitney U tests. B and D, Numbers of monocytes/macrophages per milliliter of BAL fluid (B) and lung P. aeruginosa levels (D) 30 hours after challenge with P. aeruginosa strain IT4 (70 CFU) in PAO1ΔaroA-immunized or control E. coli–immunized mice made neutropenic with cyclophosphamide (CY). Panel B compiles the results of 2 independent experiments performed at each time point, with 3 mice/group at 0 hours and 8–10 mice/group at 30 hours after infection. Each point in panel D represents 1 mouse, and bars indicate medians. P values were determined by the Mann–Whitney U test. E, Expression of cell proliferation marker Ki-67 (red) in monocyte/macrophage marker MOMA-2–positive cells (blue) in collagenase-digested lungs from PAO1ΔaroA-immunized mice with CY-induced neutropenia at 30 hours after challenge with P. aeruginosa strain IT4 (70 CFU). The top left panel is the blue channel only, the bottom left is the red channel only, the top right is phase contrast, and the bottom right is the overlay of the blue and red channels.
Finally, cytospin preparation of total lung leukocytes isolated from collagenase-digested lungs of immunized mice 30 hours after infection during CY-induced neutropenia were stained for Ki-67, a nuclear protein associated with cellular proliferation [20]. Ki-67 was expressed in about 25% of the monocytes/macrophages (ie, MOMA-2–positive cells) isolated from collagen-digested whole lung (Figure 2E), suggesting that proliferation of the cells may possibly contribute in part to the accumulation of monocytes/macrophages in the infected alveolar space.
CD4+ T Cells as Critical Adaptive Effectors of Protection
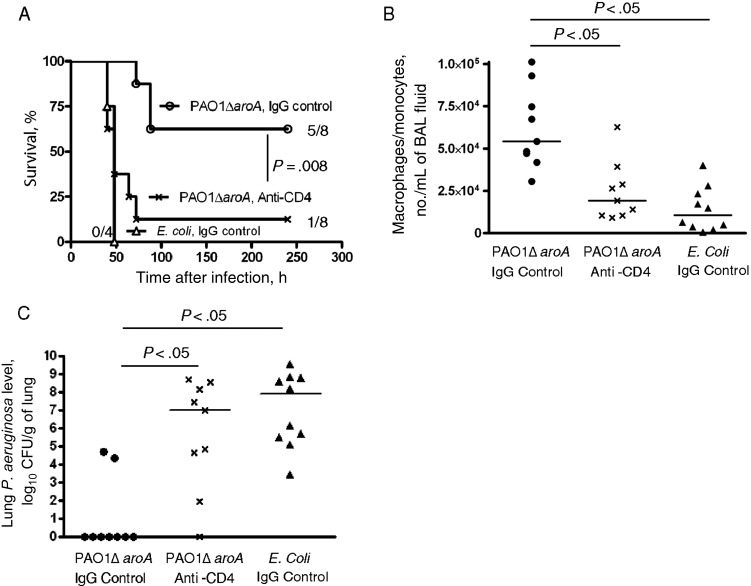
In the nonneutropenic setting, we previously showed that vaccine-induced CD4+ T cells and IL-17 allow rapid recruitment of neutrophils to the infected lung, which is critical for protection against vaccine-heterologous P. aeruginosa strains [6, 7]. We hypothesized that adaptive CD4+ T cells may also play a crucial role in vaccine-induced protection in the setting of neutropenia. In vivo depletion of CD4+ T cells that started 72 hours before challenge removed the protection engendered by PAO1ΔaroA immunization against the vaccine-heterologous strain IT4 (Figure 3A). CD4+ T-cell depletion was associated with loss of the vaccine-induced protective phenotypes described above, including the accumulation of macrophage-lineage cells in the alveolar space (Figure 3B) and the enhanced P. aeruginosa clearance from the lung 30 hours after challenge (Figure 3C). These data highlight the critical collaboration between adaptive effectors (CD4+ T cells) and innate effectors (macrophages) that is induced by this mucosal vaccination strategy.
Figure 3.
Vaccine-induced protective phenotypes are CD4+ T-cell dependent. A, Survival of PAO1ΔaroA-immunized mice with cyclophosphamide (CY)–induced neutropenia after Pseudomonas aeruginosa challenge (strain IT4, 350 colony-forming units [CFU]) with or without in vivo CD4+ T-cell depletion induced by anti-CD4 monoclonal antibody that started 72 hours before infection. Numbers on the right are the number of surviving mice/number of mice challenged. The P value was determined by the log-rank test with Bonferroni correction and compared the survival of CD4+ T-cell depleted and CD4+ T-cell nondepleted PAO1ΔaroA-immunized neutropenic mice. B and C, Numbers of monocytes/macrophages per mL of bronchoalveolar lavage (BAL) fluid (B) and lung P. aeruginosa levels (C) 30 hours after P. aeruginosa challenge (strain IT4, 320 CFU) in PAO1ΔaroA-immunized or control Escherichia coli–immunized mice with CY-induced neutropenia and with or without in vivo CD4+ T-cell depletion. Each point represents 1 mouse. Bars indicate medians. P values were determined by the Kruskal–Wallis test with the Dunn multiple comparison test. Abbreviation: IgG, immunoglobulin G.
GM-CSF as a Member of Effector Cytokines from Vaccine-Induced CD4+ T Cells
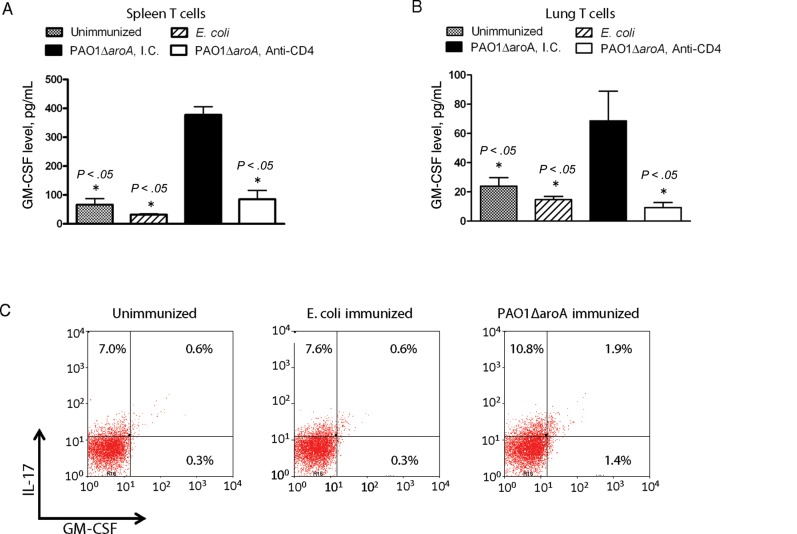
GM-CSF is a multifunctional growth factor for myeloid precursor cells and also a cytokine that can promotes survival, activation, and terminal differentiation of macrophages [21–23]. We hypothesized that our live-attenuated mucosal vaccination strategy can induce CD4+ T cells to produce GM-CSF, which could potentially contribute to vaccine efficacy by stimulating the host innate immune system. We compared GM-CSF production from lymphocytes purified from vaccinated versus control mice during in vitro coculture. GM-CSF levels produced by T cells that were purified from spleens (Figure 4A) or lungs (Figure 4B) of PAO1ΔaroA-immunized mice and then stimulated with gentamicin-killed whole P. aeruginosa cells were higher than those produced by similarly stimulated T cells isolated from unimmunized or E. coli–immunized mice. The production of GM-CSF was inhibited by addition of anti-CD4 mAb (GK1.5) to the culture media, suggesting that CD4+ T cells are the source of GM-CSF under these conditions. After in vitro stimulation of immune splenocytes, intracellular levels of GM-CSF were higher in splenic CD4+ T cells from PAO1ΔaroA-immunized mice, compared with E. coli–immunized mice and unimmunized mice (Figure 4C). More than 50% of the GM-CSF–positive CD4+ T cells were also positive for IL-17, suggesting that GM-CSF is produced primarily, although not exclusively, by Th17 cells (Figure 4C), which is consistent with recent reports implicating Th17-derived GM-CSF as a key mediator of experimental autoimmune encephalitis [8, 9].
Figure 4.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a vaccine-induced CD4+ T-cell effector cytokine. A and B, GM-CSF levels produced by splenic T cells (A) or lung T cells (B) in response to in vitro stimulation with gentamicin-killed Pseudomonas aeruginosa. T cells were isolated from naive mice or from mice 3 weeks after intranasal immunization with either PAO1ΔaroA or Escherichia coli. T cells were stimulated for 7 days with gentamicin-killed whole bacterial cells of P. aeruginosa strain PAO1 in the presence of irradiated splenocytes. Cells were pooled from 3–5 mice per group. Anti-CD4 monoclonal antibody (mAb; clone GK1.5) or isotype control (I.C.) immunoglobulin G2b mAb was added at 1 μg/well. Bars indicate means of triplicate wells, error bars the SEM. *P values were determined by analysis of variance with the Dunnett multiple comparison test, using PAO1ΔaroA/I.C. group as the control comparator group. C, Intracellular staining of CD4+ T cells for interleukin 17 (IL-17) and GM-CSF among splenocytes isolated from vaccinated and unvaccinated mice and then cocultured with gentamicin-killed P. aeruginosa strain PAO1 for 5 days, followed by PMA with ionomycin and brefeldin A for 6 hours before harvest and staining. Cells were pooled from 3 mice per group. The CD4 gate is shown.
Critical Contribution of Local GM-CSF in Vaccine-Induced Lung Immunity
Neutralization of local GM-CSF starting 8 hours before infection significantly diminished the PAO1ΔaroA-induced protection against acute lethal pneumonia caused by strain IT4 during CY-induced neutropenia (Figure 5A). Vaccine-efficacy loss due to GM-CSF neutralization was accompanied by loss of the accumulation of monocytes/macrophages in BAL fluid (Figure 5B) and by diminished P. aeruginosa clearance from the lung (Figure 5C) 30 hours after infection. On the other hand, local delivery of murine recombinant GM-CSF that started 3 hours before infection partially restored the vaccine-efficacy loss induced by in vivo CD4+ T-cell depletion (Figure 5D). These data collectively suggest a critical role of local GM-CSF as a collaborator of the vaccine-induced lung immunity against P. aeruginosa during neutropenia.
Figure 5.
Local granulocyte-macrophage colony-stimulating factor (GM-CSF) is required for vaccine-induced protection. A, Survival of PAO1ΔaroA-immunized mice with cyclophosphamide (CY)–induced neutropenia after Pseudomonas aeruginosa challenge (strain IT4, 130 colony-forming units [CFU]), with or without GM-CSF neutralization in the lung that started 8 hours before infection. Numbers on the right are number of surviving mice/number of mice challenged. The P value was determined by the log-rank test. B and C, Numbers of monocytes/macrophages per milliliter of bronchoalveolar lavage (BAL) fluid (B) and lung P. aeruginosa levels (C) 30 hours after challenge (strain IT4, 160 CFU) in PAO1ΔaroA-immunized or control Escherichia coli–immunized mice with CY-induced neutropenia and with or without in vivo GM-CSF neutralization that started 8 hours before infection. Each point represents 1 mouse. Bars indicate medians. P values were determined by the Kruskal–Wallis test with the Dunn multiple comparison test. D, Survival of PAO1ΔaroA-immunized mice with CY-induced neutropenia after challenge with P. aeruginosa strain IT4 (190 CFU). Before challenge, 2 groups of mice underwent in vivo CD4+ T-cell depletion followed by local supplementation with murine recombinant GM-CSF or phosphate-buffered saline (PBS; given intranasally). Numbers on the right are the number of surviving mice/number of mice challenged. The P value was determined by the log-rank test with the Bonferroni correction and compares the PAO1ΔaroA/anti-CD4/GM-CSF group with the PAO1ΔaroA/anti-CD4/PBS group. Abbreviation: IgG, immunoglobulin G.
DISCUSSION
We conducted this study to investigate the usefulness of a vaccine-based strategy to prevent lethal P. aeruginosa pneumonia during neutropenia and also to define the critical effectors of vaccine-induced lung immunity that can operate independent of neutrophils. We showed that immunity induced by a mucosal immunization with a P. aeruginosa live-attenuated vaccine was protective in the absence of neutrophils against a variety of P. aeruginosa strains that include both vaccine-homologous strains (ie, strains having the same LPS serogroup as the vaccine strain) and vaccine-heterologous strains. The spectrum of protection of the active mucosal vaccination strategy was broader than that of passive immunization, which conferred only vaccine-homologous protection in the neutropenic mice. In vaccine-heterologous protection during neutropenia induced by active immunization, cells in the macrophage-lineage served as crucial innate cellular effectors. The vaccine's efficacy was also CD4+ T-cell dependent: CD4+ T cells were required for the accumulation of monocytes/macrophages in the infected alveolar space and for enhanced P. aeruginosa clearance from the lung in immunized mice. Importantly, protection was observed regardless of the mode of neutrophil induction, either anti-Gr-1 mAb or CY, the latter of which also leads to profound lymphocytopenia of mice [5]. The adaptive CD4+ T cells produced higher levels of GM-CSF in response to P. aeruginosa stimulation in vitro than did CD4+ T cells from naive or E. coli–immunized mice. Local GM-CSF, produced either in the lung and/or outside the lung and delivered to the lung, was also a crucial factor for these vaccine-induced protective phenotypes to be expressed. These findings highlight a form of vaccine-induced immunity in which collaboration between innate and adaptive effectors overcomes neutropenia and confers protection against lethal bacterial infection in the profoundly neutropenic host.
Animal studies have identified both positive and negative roles of GM-CSF in host defense against lung infections. GM-CSF–deficient mice have been shown to have increased susceptibility to P. aeruginosa pulmonary infection that could be restored by targeted expression of GM-CSF in the lung parenchyma [24]. However, in a murine model of P. aeruginosa pneumonia following bone marrow transplantation, genetic ablation of GM-CSF in the donor hematopoietic cells was associated with improved bacterial clearance in recipient mice as compared to that in mice that received a wild-type hematopoietic cell transplant, suggesting a paradoxical role of GM-CSF derived from leukocytes of donor origin in lung immunity in a host who has undergone bone marrow transplantation [25]. Another study showed that culture supernatants of mucoid P. aeruginosa isolates from patients with panbronchiolitis can stimulate human neutrophils to secrete GM-CSF, leading to the enhanced neutrophil survival in an autocrine/paracrine fashion—a process speculated to be one of the contributing factors to chronic airway inflammation in those patients [26]. These previous findings suggest that the role of GM-CSF in host defense is largely dependent on the intersection of a number of factors, including host immunological status, source of GM-CSF, and acute versus chronic infectious disease process.
Here, we describe a critical role of GM-CSF in vaccine-induced immunity against acute P. aeruginosa pneumonia in a host with neutropenia, in which the monocytes/macrophages serve as the primary innate cellular effectors. The relative contributions by CD4+ T cell-derived GM-CSF versus GM-CSF from other cellular sources in the present model were not evaluated in this work. Nevertheless, a vaccine-based strategy that induces adaptive CD4+ T cells having the capacity to produce GM-CSF may serve as an alternative approach to the preventative/therapeutic application of exogenous GM-CSF to the lung of the immunocompromised host [27, 28].
The precise mechanism of protection in the present model remains to be elucidated. The accumulating macrophage-lineage cells in the infected lung in the present model potentially derive from a number of sources, such as monocytes that have acutely emigrated from the bone marrow [29], a subset of endothelium-attached “patrolling” monocytes that have extravasated in response to infection [30], locally proliferating macrophages [31, 32], and/or cells originating from hematopoietic stem and progenitor cells that had circulated in peripheral blood and landed in the lung [33]. Known biological functions of GM-CSF includes effective mobilization of monocytes to the inflamed tissue [34, 35], generation of macrophages from precursors and proliferation of macrophage-lineage cells [36], and enhancement of macrophage resistance against infection-related necrosis and apoptosis [37], any of which could have contributed to the vaccine-induced, GM-CSF–dependent macrophage accumulation in the infected lung. Local GM-CSF may also have contributed to the protection by enhancement of macrophage phagocytic functions, as was shown in a nonneutropenic P. aeruginosa pneumonia model [24]. Finally, it is plausible that IL-17 from vaccine-induced CD4+ T cells [6, 7] may also play a role in protection during neutropenia, which could be mediated by enhanced production of antimicrobial peptides from lung epithelia [38] or by chemokine production from fibroblasts and macrophages, leading to monocyte recruitment and/or activation [39].
In summary, immunity induced by a mucosal vaccination strategy can be protective against acute lethal P. aeruginosa pneumonia even during profound neutropenia. Collaboration between macrophage-lineage cells and vaccine-induced CD4+ T cells as well as local GM-CSF confers protection in the absence of neutrophils. This paradigm of vaccine-based immunity during neutropenia may potentially serve as a framework for new preventive and therapeutic strategies against infectious diseases in the immunocompromised host.
Supplementary Data
Notes
Acknowledgments. We are grateful to J. Golberg, H. Koziel, and E. Tuomanen for insightful discussions and comments.
Financial support. This work was supported by a Pediatric Infectious Diseases Society–St. Jude Children's Research Hospital Fellowship Award (to A. K.) and by the National Institutes of Health (grants AI22535 [to G. B. P.] and HL092515 [to G. P. P.]).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thirumala R, Ramaswamy M, Chawla S. Diagnosis and management of infectious complications in critically ill patients with cancer. Crit Care Clin. 2010;26:59–91. doi: 10.1016/j.ccc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Carratala J, Roson B, Fernandez-Sevilla A, Alcaide F, Gudiol F. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch Intern Med. 1998;158:868–72. doi: 10.1001/archinte.158.8.868. [DOI] [PubMed] [Google Scholar]
- 3.Tam VH, Chang KT, Abdelraouf K, et al. Prevalence, resistance mechanisms, and susceptibility of multidrug-resistant bloodstream isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54:1160–4. doi: 10.1128/AAC.01446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joo EJ, Kang CI, Ha YE, et al. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia: clinical impact of antimicrobial resistance on outcome. Microb Drug Resist. 2011;17:305–12. doi: 10.1089/mdr.2010.0170. [DOI] [PubMed] [Google Scholar]
- 5.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun. 2009;77:5300–10. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priebe GP, Walsh RL, Cederroth TA, et al. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J Immunol. 2008;181:4965–75. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamei A, Coutinho-Sledge YS, Goldberg JB, Priebe GP, Pier GB. Mucosal vaccination with a multivalent, live-attenuated vaccine induces multifactorial immunity against Pseudomonas aeruginosa acute lung infection. Infect Immun. 2011;79:1289–99. doi: 10.1128/IAI.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Codarri L, Gyulveszi G, Tosevski V, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–7. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 9.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–75. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priebe GP, Brinig MM, Hatano K, et al. Construction and characterization of a live, attenuated aroA deletion mutant of Pseudomonas aeruginosa as a candidate intranasal vaccine. Infect Immun. 2002;70:1507–17. doi: 10.1128/IAI.70.3.1507-1517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh AY, Priebe GP, Pier GB. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect. Immun. 2005;73:2262–72. doi: 10.1128/IAI.73.4.2262-2272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allewelt M, Coleman FT, Grout M, Priebe GP, Pier GB. Acquisition of expression of the P. aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 2000;68:3998–4004. doi: 10.1128/iai.68.7.3998-4004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 14.Vlahos R, Bozinovski S, Chan SP, et al. Neutralizing granulocyte/macrophage colony-stimulating factor inhibits cigarette smoke-induced lung inflammation. Am J Respir Crit Care Med. 2010;182:34–40. doi: 10.1164/rccm.200912-1794OC. [DOI] [PubMed] [Google Scholar]
- 15.Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol. 2002;169:3499–506. doi: 10.4049/jimmunol.169.7.3499. [DOI] [PubMed] [Google Scholar]
- 16.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 17.Priebe GP, Meluleni GJ, Coleman FT, Goldberg JB, Pier GB. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect Immun. 2003;71:1453–61. doi: 10.1128/IAI.71.3.1453-1461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraal G, Rep M, Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand J Immunol. 1987;26:653–61. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 20.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–67. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 22.Berclaz PY, Shibata Y, Whitsett JA, Trapnell BC. GM-CSF, via PU.1, regulates alveolar macrophage Fcgamma R-mediated phagocytosis and the IL-18/IFN-gamma -mediated molecular connection between innate and adaptive immunity in the lung. Blood. 2002;100:4193–200. doi: 10.1182/blood-2002-04-1102. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 24.Ballinger MN, Paine R, 3rd, Serezani CH, et al. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:766–74. doi: 10.1165/rcmb.2005-0246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballinger MN, Hubbard LL, McMillan TR, et al. Paradoxical role of alveolar macrophage-derived granulocyte-macrophage colony-stimulating factor in pulmonary host defense post-bone marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2008;295:L114–22. doi: 10.1152/ajplung.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimaki K, Okuyama K, Okada S, Hattori T, Takayanagi M, Ohno I. Prolongation of neutrophil survival by the culture supernatant of Pseudomonas aeruginosa. Respirology. 2007;12:664–9. doi: 10.1111/j.1440-1843.2007.01131.x. [DOI] [PubMed] [Google Scholar]
- 27.Quezada G, Koshkina NV, Zweidler-McKay P, Zhou Z, Kontoyiannis DP, Kleinerman ES. Intranasal granulocyte-macrophage colony-stimulating factor reduces the Aspergillus burden in an immunosuppressed murine model of pulmonary aspergillosis. Antimicrob Agents Chemother. 2008;52:716–8. doi: 10.1128/AAC.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heslet L, Bay C, Nepper-Christensen S. The immunomodulatory effect of inhaled granulocyte-macrophage colony-stimulating factor in cystic fibrosis. A new treatment paradigm. J Inflamm Res. 2012;5:19–27. doi: 10.2147/JIR.S22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maus UA, Janzen S, Wall G, et al. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35:227–35. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- 30.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–70. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 31.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–94. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook AD, Turner AL, Braine EL, Pobjoy J, Lenzo JC, Hamilton JA. Regulation of systemic and local myeloid cell subpopulations by bone marrow cell-derived granulocyte-macrophage colony-stimulating factor in experimental inflammatory arthritis. Arthritis Rheum. 2011;63:2340–51. doi: 10.1002/art.30354. [DOI] [PubMed] [Google Scholar]
- 35.Lenzo JC, Turner AL, Cook AD, et al. Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunol Cell Biol. 2012;90:429–40. doi: 10.1038/icb.2011.58. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton JA, Anderson GP. GM-CSF biology. Growth Factors. 2004;22:225–31. doi: 10.1080/08977190412331279881. [DOI] [PubMed] [Google Scholar]
- 37.Steinwede K, Tempelhof O, Bolte K, et al. Local delivery of GM-CSF protects mice from lethal pneumococcal pneumonia. J Immunol. 2011;187:5346–56. doi: 10.4049/jimmunol.1101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kao CY, Chen Y, Thai P, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173:3482–91. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 39.Shahrara S, Pickens SR, Mandelin AM, 2nd, et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–87. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatano K, Pier GB. Complex serology and immune response of mice to variant high-molecular-weight O polysaccharides isolated from Pseudomonas aeruginosa serogroup O2 strains. Infect. Immun. 1998;66:3719–726. doi: 10.1128/iai.66.8.3719-3726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston MJ, Fleiszig SM, Zaidi TS, et al. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun. 1995;63:3497–501. doi: 10.1128/iai.63.9.3497-3501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleiszig SMJ, Zaidi TS, Preston MJ, et al. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–94. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyer HW, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–72. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.