Abstract
Innate-like B-1a cells contribute significantly to circulating natural antibodies and mucosal immunity as well as to immunoregulation. Here we show that these classic functions of B-1a cells segregate between two unique subsets defined by expression of plasma cell alloantigen 1 (PC1), also known as ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1). These subsets, designated B-1a.PC1lo and B-1a.PC1hi, differ significantly in IgH chain utilization. Adoptively transferred PC1lo cells secreted significantly more circulating natural IgM and intestinal IgA than PC1hi cells. In contrast, PC1hi cells produced more IL-10 than PC1lo cells when stimulated with LPS and phorbol 12-myristate 13-acetate (PMA). PC1hi cells were also more efficient than PC1lo cells in regulating Th1 cell differentiation, even though both B-1a subsets were comparably active in stimulating T-cell proliferation. Furthermore, PC1lo cells generated antigen-specific IgM responses to pneumococcal polysaccharide antigens, whereas PC1hi cells do not. We found that PC1lo cells develop from an early wave of B-1a progenitors in fetal life, whereas PC1hi cells are generated from a later wave after birth. We conclude that identification of B-1a.PC1lo and B-1a.PC1hi cells extends the concept of a layered immune system with important implications for developing effective vaccines and promoting the generation of immunoregulatory B cells.
Keywords: B-1 cells, natural antibody, innate immunity, B regulatory cells
A subset of mouse B cells expressing CD5 was first described almost 30 y ago (1). These cells, later termed B-1a cells in mice (2), are localized primarily to the peritoneum and pleural cavities with low frequencies (<5%) in the spleen. B-1a cells differ from conventional CD5− B-2 cells, which are derived from bone marrow (BM) precursors, in that they develop from fetal liver progenitors and are maintained by self-replenishment throughout life. The recent identification of B-1a progenitors in BM and spleen supports a multiorigin model of B-1a cell development (3, 4). Indeed, analyses of Ig use have revealed heterogeneous B-1a clones with some expressing Ig sequences with essentially no nontemplated (N) nucleotide additions, evidence of a fetal origin, and some with N nucleotide insertions, evidence of an adult BM origin (5, 6). Whereas B-1a progenitors (4, 7–9) and transitional B-1a cells wane dramatically within the first few weeks after birth (10), there are reports showing that adult BM progenitors can generate B-1a cells with substantial N nucleotide additions (11, 12). The facts that conventional B-2 cells can be induced to acquire a CD5+ B-1a phenotype and that the B-1a cell fate is determined by B-cell antigen receptor (BCR) signaling strength and antigen specificity (13–16) are well in line with multiorigin mechanisms for shaping the adult B-1a repertoire.
Classic B-1a cell functions include production of serum IgM “natural antibodies,” secretion of intestinal IgA, and immunoregulation. B-1a cells are a major source of natural antibodies that bind self-antigens, bacterial cell wall components, and viruses (17–19). These antibodies are encoded by “stereotyped” germ-line sequences and can be of IgM, IgG, or IgA classes. The lymphoid compartments in which B-1a cells secrete natural antibodies have been shown to include the spleen and BM (20–22). Recently, Cole et al. showed that spleens of mice immunized with the atypical LPS of Francisella tularensis contain antigen-specific plasma cells of B-1a origin (23). In the gut, B-1a cells are able to differentiate and switch to IgA-producing plasma cells in a T-cell–independent fashion (24, 25) and contribute to most intestinal IgA (26, 27). The hypersensitivity of B-1a cells to microbial products leads to rapid IgM secretion to limit the spread of pathogens before the development of germinal center-dependent adaptive immune responses. In addition to secreting natural antibodies, B-1a cells are also a major source of IL-10 (28), an anti-inflammatory cytokine, and have been shown to play regulatory roles in certain pathological conditions (29, 30).
There are inconsistencies in the literature regarding the phenotype of B-1a cells, their gene expression profile, and the functional attributes of B-1a cells isolated from different anatomical locations including the spleen and peritoneum (31–33). Although microenvironmental influences could affect behavior of resident B-1a cells (34), the nature of these factors has not been defined. Given that the various functional attributes described above are often presented as reflecting the features of all B-1a cells, understanding of how these functions are manifested at the clonal level is rather limited. Here we show that B-1a cells can be subdivided into two distinct, stable subsets based on differing expression of the plasma cell alloantigen 1 (PC1), also known as ectonucleotide pyrophosphatase phosphodiesterase 1 (ENPP1), an enzyme involved primarily in hydrolysis of ATP at the cell surface. Based on their differing levels of PC1 expression, we have termed one subset B-1a.PC1lo and the second subset B-1a.PC1hi. These subsets are distinguishable by additional surface markers, gene expression profiles, VH gene utilization, and time of development. Importantly, the classic B-1a functions of spontaneous production of natural IgM and gut IgA, responses to stimulation with microbial antigens, and IL-10 secretion segregate quite cleanly between the two subsets.
Results
Levels of PC1 Expression Distinguish Peritoneal B-Cell Subpopulations.
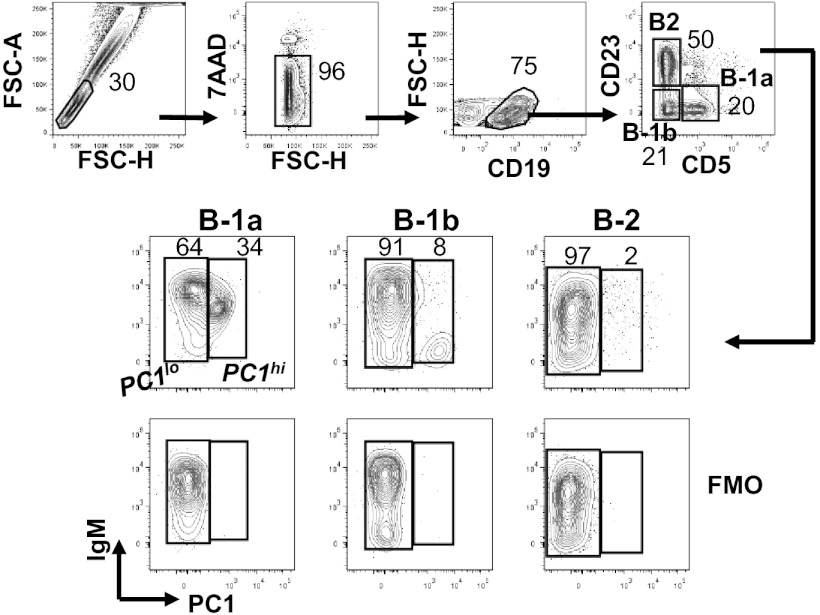
Peritoneal B cells of adult B6 mice are composed of three well-characterized subpopulations, B-2 (CD19+CD5−CD23+), B-1a (CD19+CD5+CD23−), and B-1b (CD19+CD23−CD5−) (Fig. 1A). These cells expressed different levels of PC1 on the cell surface as assessed by a mAb against PC1 (35). Most B-2 and B-1b cells expressed low levels, whereas B-1a cells consisted of two distinct subsets defined by differing levels of PC1 expression. We designated the subsets B-1a.PC1lo and B-1a.PC1hi and will hereafter refer to them as PC1lo and PC1hi (Fig. 1). Comparisons of the B-1a subsets showed that PC1hi cells expressed lower levels of IgM and B220 (Fig. S1A). Both B-1a subsets expressed two other markers used for distinguishing B-2 and B-1a cells, CD11b and CD43, at comparable levels (Fig. S1A).
Fig. 1.
Expression of PC1 distinguishes peritoneal B-cell subsets. Peritoneal cells were stained with indicated antibodies and were analyzed by FACS. The numbers are percentages of cells falling in each gate. FMO, fluorescence minus one control.
PC1hi cells comprised 35% of peritoneal B-1a cells of B6 mice 3–5 mo of age (n = 8) and 29% in 16-mo-old mice (n = 3, P > 0.1). Parallel studies of splenic B-1a cells showed that PC1hi cells made up ≤10% of the B-1a population but that their total numbers were nearly equal to their peritoneal counterparts (Fig. S1B). It should be noted that PC1hi cells were also present in the B-2 and B-1b subsets but at much lower levels than in the B-1a compartment (Fig. 1); these populations were not further examined in this study. Cells with levels of PC1 expression characteristic of the peritoneal PC1lo and PC1hi subsets of B6 mice were present among peritoneal B-1a cells from 2- to 3-mo-old BALB/c, C3H, NZB, FVB, and CB17 mice, but with strain-dependent differences in their relative proportions (Fig. S1C). Unless otherwise indicated, B6 mice were used throughout this study.
Differential Developmental Kinetics and Origins of PC1lo and PC1hi Cells.
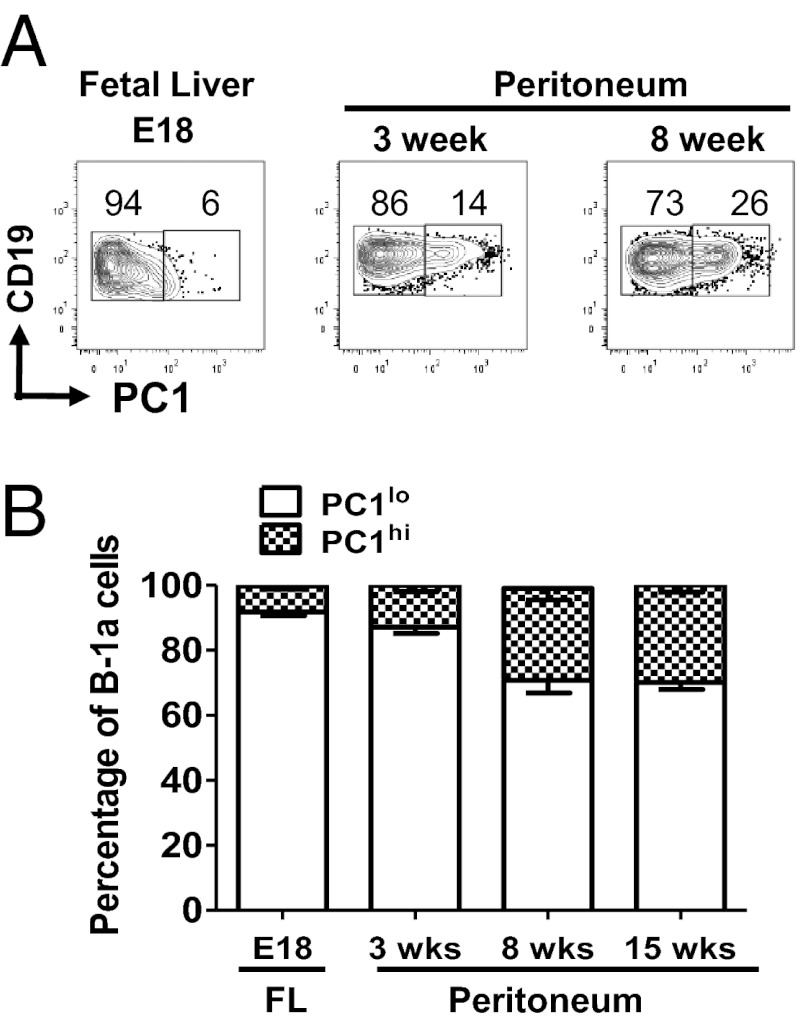
Phenotypic differences between PC1lo and PC1hi cells suggest that they might derive from different lineages. To examine this possibility, we examined the frequencies of the PC1lo and PC1hi subsets among B-1a cells of day-18 fetal liver and peritoneal cells of 3-, 8-, and 15-wk old mice. PC1hi cells comprised <7% of B-1a cells in fetal liver. The frequency of PC1hi cells was increased twofold among the peritoneal B-1a population of 3-wk-old mice and reached adult proportions in 8-wk-old mice (Fig. 2 A and B). These data identified PC1lo cells as the dominant B-1a subset in late fetal and early neonatal life and suggested two waves of development that successively shape the adult B-1a repertoire.
Fig. 2.
B-1a subset ontogeny in fetal and adult life. (A) Fetal liver (day 18) and peritoneal cells of 3- and 8-wk-old CB17 mice were analyzed by FACS. Cells were gated on CD19+CD5+ B-1a cells. The numbers are percentages of cells falling in each gate. (B) Combined data for six to eight mice per group.
PC1lo and PC1hi Cells Exhibit Different Migration Patterns and Turnover Rates in Vivo.
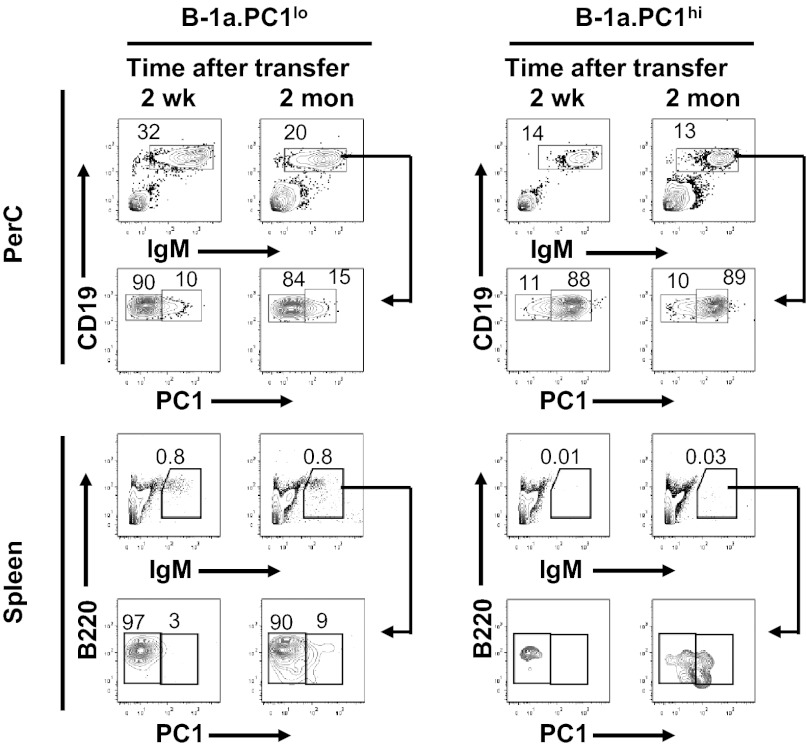
Previous studies showed that peritoneal B-1a cells can migrate to the spleen (36, 37) and that those present in the pleural cavity can migrate to mediastinal lymph nodes (38). To determine whether the peritoneal B-1a subsets differ in their migratory potential, we adoptively transferred equal numbers of sort-purified subsets i.p. to Rag1-deficient mice. The stains and gating strategy used to sort the PC1-defined subsets and analyses of postsort purity are shown in Fig. S2A. Studies performed at 1 wk to 2 mo following transfer showed that comparable numbers of both PC1lo and PC1hi donor cells were recovered from the peritoneal cavities of recipient mice. In addition, the PC1 profiles of the transferred cells were remarkably stable (Fig. 3). This indicated that one subset did not acquire the PC1 phenotype of the other to any significant extent, and that the differences in levels of PC1 expression were subset-intrinsic. Studies of spleen cells (Fig. 3 Lower) and mesenteric lymph nodes from the same recipients (Fig. S2B) showed that PC1lo cells migrated much more efficiently to these compartments than did PC1hi cells. Furthermore, the sessile feature of PC1hi cells was further confirmed by an in vivo carboxyl fluorescent succinimidyl ester (CFSE) labeling assay (Fig. S3). Comparisons of the CFSE dilution patterns during the first 3 wk after injection with CFSE showed that the proportions of dividing cells were lower for PC1hi than for PC1lo cells (Fig. S3).
Fig. 3.
Persistence and migration of B-1a subsets in vivo. Equal numbers (3 × 105) of sort-purified B-1a subsets were injected i.p. to Rag1−/− mice (8 wk old). Peritoneal cells and spleens of recipients were analyzed by FACS at 2 wk and 2 mo following transfer. Cells were gated on lymphocytes. Data represent multiple mice from at least three independent experiments. PerC, peritoneal cavity.
The possibility that the low representation of PC1hi cells in the spleens of mice reconstituted i.p. was due to an inability of migrating cells to establish residence in this site prompted us to compare the peritoneal and spleen cell populations of Rag1-deficient mice that had been reconstituted with the subsets either i.v. or i.p. As expected, the splenic migratory activity of PC1hi cells inoculated i.p. was much lower than that of PC1lo cells given by the same route; however, comparable proportions of both subsets were found in spleens of mice that had been reconstituted i.v. (Fig. S2C). This demonstrated that PC1hi cells could establish residence and survive in the spleen but did so very poorly as migrants from the peritoneum.
PC1lo and PC1hi Subsets Express Distinct IgH Repertoires.
The Ig repertoire of B-1a cells differs from that of other B-cell subsets in being enriched for self-reactive BCRs and utilization of a restricted set of heavy chains encoded by genes lacking N nucleotide additions at the V-D and D-J joining regions. This is exemplified by the high proportion of B-1a cells with BCRs reactive with the phosphatidylcholine (PtC) determinant expressed on senescent red blood cells that use primarily VH genes of the VH11 and VH12 families. These biases are considered by some to result from strong antigen-dependent selection of the B-1a cell repertoire (39, 40).
To determine whether these predispositions are shared by the B-1a subsets, we first compared peritoneal B-1a and B-2 cells for their binding of PtC-tagged fluorescent liposomes; essentially all PtC-binding cells belonged to the PC1hi subset of B-1a cells (Fig. S4A). Previous studies showed that a very high proportion of B cells in mice expressing a VH12 transgene were CD5+ and bound PtC (41). Studies of these cells for PC1 expression showed that the great majority had the PC1hi phenotype (Fig. S4B). Analysis of VH11 B cells yielded similar results (Fig. S4C). These data indicated that the “canonical B-1a” VH11 and VH12 family genes were overrepresented in the repertoire of PC1hi cells.
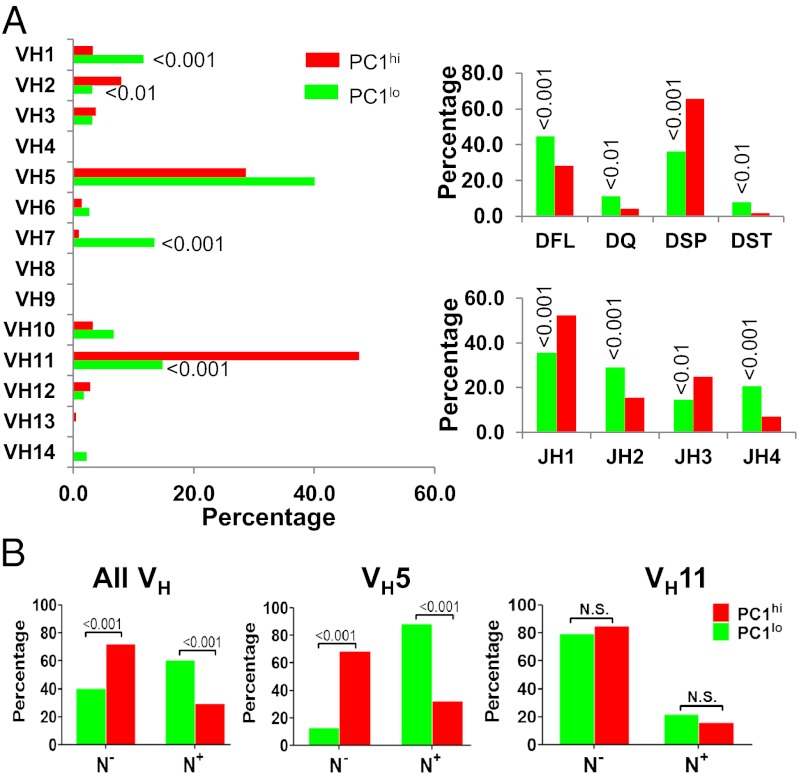
To obtain a more detailed picture of the BCR repertoires used by these subsets, we cloned and sequenced expressed IgH genes from sort-purified peritoneal B cells of adult B6 mice. Analyses of VH family utilization revealed significantly increased representation of the VH1 and VH7 families within the PC1lo subset and of the VH2 and VH11 families within the PC1hi subset (Fig. 4A Left). These data indicated that the PC1hi repertoire aligned more closely than that of PC1lo cells, similar to that reported previously for total peritoneal B-1a cells of BALB/c mice (5). Analyses of D-region genes showed a biased representation of DSP2 sequences among the PC1hi subset and DFL, DQ, and DST sequences among the PC1lo population (Fig.4A Right). Again, data from the PC1hi subset aligned more closely to published studies of total B-1a cells (5). Moreover, the pattern of J-gene utilization by PC1hi cells was skewed to JH1 and JH3 families, whereas that of PC1lo cells was biased to the JH2 and JH4 members (Fig. 4A Right). Finally, the overall frequency of N region additions at the V-D and D-J joins was significantly higher for PC1lo than for PC1hi sequences (P < 0.001) (Fig. 4B). It is noteworthy, however, that this generalization did not apply uniformly to all VH families. Thus, N-region additions were comparably low for VH11 sequences from either B-1a subset but were significantly higher for VH5 sequences of PC1lo than of PC1hi cells (P < 0.001) (Fig. 4B). Finally, VH7 sequences, found almost exclusively in the PC1lo subset, were markedly biased toward those lacking N additions. These observations preclude an assignment of either subset to origins from “fetal” or “bone marrow” cells. Instead, they appear to reflect mixed origins of the adult B-1a repertoire, including components of individual VH families in both B-1a subsets, from precursors arising during a transient fetal/neonatal wave of B-1a development (10) and other precursors identified in adult bone marrow and spleen (3, 8).
Fig. 4.
B-1a subsets express different IgH chain repertoires. Sort-purified B-1a subsets from three mice were cloned and sequenced for IgH VDJ regions as indicated. Data were pooled from two independent experiments. Totals of 222 and 213 sequences were analyzed for PC1lo and PC1hi cells, respectively. Frequencies of VH, D, and JH sequences (A) and N nucleotide additions (B) are depicted for PC1lo and PC1hi subsets. N−, no N nucleotide; N+, with more than one N nucleotide.
Contributions of B-1a B-Cell Subsets to Serum and Mucosal Natural Antibodies and to Antigen-Specific Responses to T-Independent Antigens.
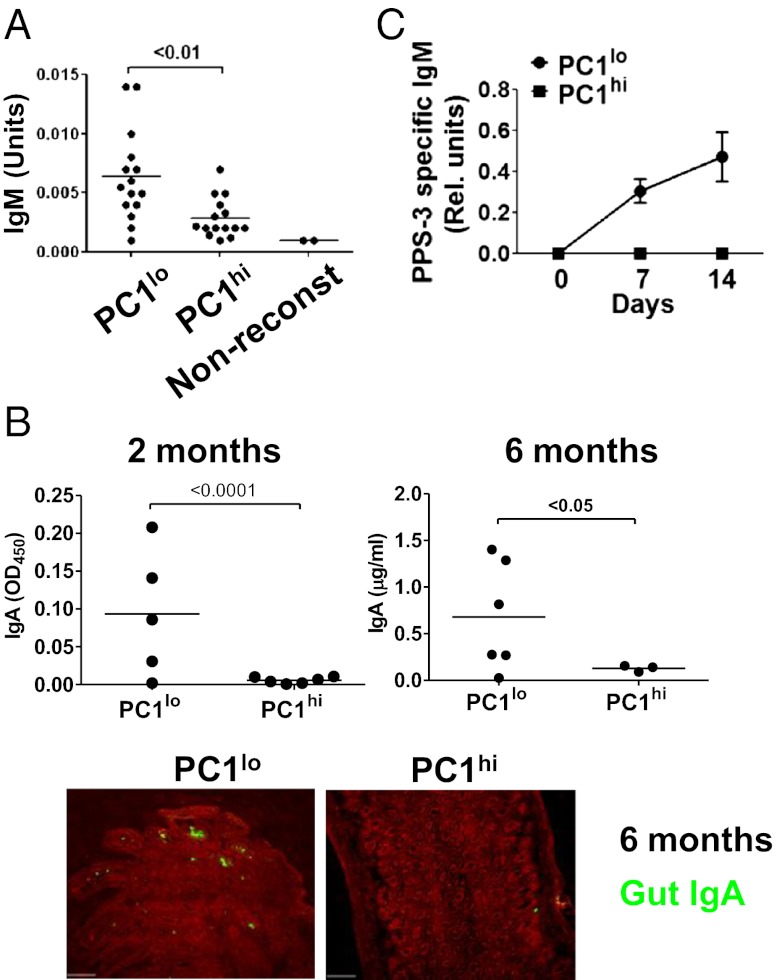
The fact that B-1a cells are considered responsible for the production of most serum natural IgM (17, 19) and to contribute significantly to mucosal IgA responses in the gut (42) prompted us to evaluate the contributions of the B-1a subsets to these aspects of innate-like immunity. To do this, we quantified levels of serum IgM in Rag1−/− mice 2 wk following the i.p. transfer of equal numbers of sort-purified cells. We found the levels of serum IgM to be significantly higher in recipients of PC1lo cells (Fig. 5A). The facts that PC1lo cells migrate more readily to the spleen than PC1hi cells and that peritoneal B-1 cells become secretory only after migration to the spleen (20) suggested that PC1hi cells homing to the spleen after i.v. transfer might also acquire secretory activity. In support of this notion, we found that Rag1−/− mice reconstituted i.v. with either B-1a subset exhibited comparable splenic localization (Fig. S2C) and had comparable levels of serum IgM (Fig. S5).
Fig. 5.
B-1a subsets show different capacities to secret IgM and IgA. (A) Rag1−/− mice were reconstituted i.p. with equal numbers of sort-purified B-1a subsets for 2 wk. Serum IgM levels were measured by ELISA. Data are pooled from three independent experiments. Each symbol represents a mouse. Nonreconst, Rag1−/− mouse serum control. (B) Rag1−/− mice were reconstituted i.p. with B-1a subsets for 2 or 6 mo. The IgA levels in intestinal lavage were measured by ELISA. Each symbol represents a mouse. Small-intestinal tissue was examined by immunofluorescence microscopy (Lower). IgA-secreting cells in lamina propria appear as green. Data represent two independent experiments with similar results. (C) Rag1−/− mice were reconstituted i.p. with equal numbers of sort-purified B-1a subsets for 2 wk before they were immunized i.p. with PPS-3. The serum levels of total IgM before immunization were measured by ELISA to confirm efficient reconstitution. Antigen-specific IgM following immunization was quantified by ELISA. Data are means ± SEM (n = 7–8 mice per group) and represent two independent experiments with similar results.
The relative contributions of B-1a subsets to gut IgA expression were examined in studies of Rag1−/− mice inoculated i.p. 2 or 6 mo previously with purified cell populations. At both time points, intestinal IgA levels determined by ELISA were significantly higher in recipients of PC1lo cells (Fig. 5B). In keeping with this observation, lamina propria IgA+ plasma cells were much more common in mice reconstituted with PC1lo cells (Fig. 5B). It is worth noting that the lack of IgA secretion in mice receiving PC1hi cells was not due to poor reconstitution, because the numbers of PC1lo and PC1hi cells recovered from peritoneum of recipient mice at 2 mo following transfer were comparable [5.4 ± 0.6 × 104 for PC1hi recipients (n = 6) vs. 7.2 ± 1.8 ×104 for PC1lo recipients (n = 5, P > 0.1)]. We therefore conclude that B-1a.PC1lo cells are the predominant B-1a contributors to the steady-state production of serum IgM and intestinal IgA.
Previous studies showed that B-1a and marginal-zone B cells are uniquely poised to respond rapidly to challenges with T-independent antigens, such as pneumococcal polysaccharides, by generating large numbers of plasma cells secreting high levels of IgM (43). Studies of sorted B-1a subsets treated with LPS in vitro showed that they responded comparably for plasma cell formation and for secretion of IgM (Fig. S6 A and B).
To examine the antigen-specific responsiveness of these populations in vivo, Rag1−/− mice reconstituted with sort-purified PC1lo or PC1hi cells were challenged with type-3 pneumococcal polysaccharide (PPS-3), a protective immunogen during immune responses to infection with Streptococcus pneumoniae. Analyses of sera obtained at 7 and 14 d after challenge showed that only PC1lo cells generated antigen-specific IgM antibodies (Fig. 5C). These results indicated that the contributions of the PC1lo subset to natural antibodies in the serum and gut and to challenge with T-independent antigens far outweighed those of PC1hi cells.
PC1lo and PC1hi Cells Are Comparably Active in Stimulating Antigen-Specific T-Cell Proliferation but Differ in Elicitation of IFNγ Expression.
B cells are capable of presenting antigens to T cells, and it has been suggested that B-1a and marginal-zone B cells are particularly potent in this regard (44, 45). To determine whether the PC1lo and PC1hi subsets differ in their ability to present antigen, we used an antigen-specific T-cell proliferation assay. Sort-purified populations of all peritoneal B-cell subsets were primed with ovalbumin peptide OVA323–339 and then cocultured with naïve CD4+ T cells isolated from OVA-specific OT-II TCR transgenic mice. The results showed that all four subsets stimulated antigen-specific T-cell proliferation to comparable levels (Fig. S7A).
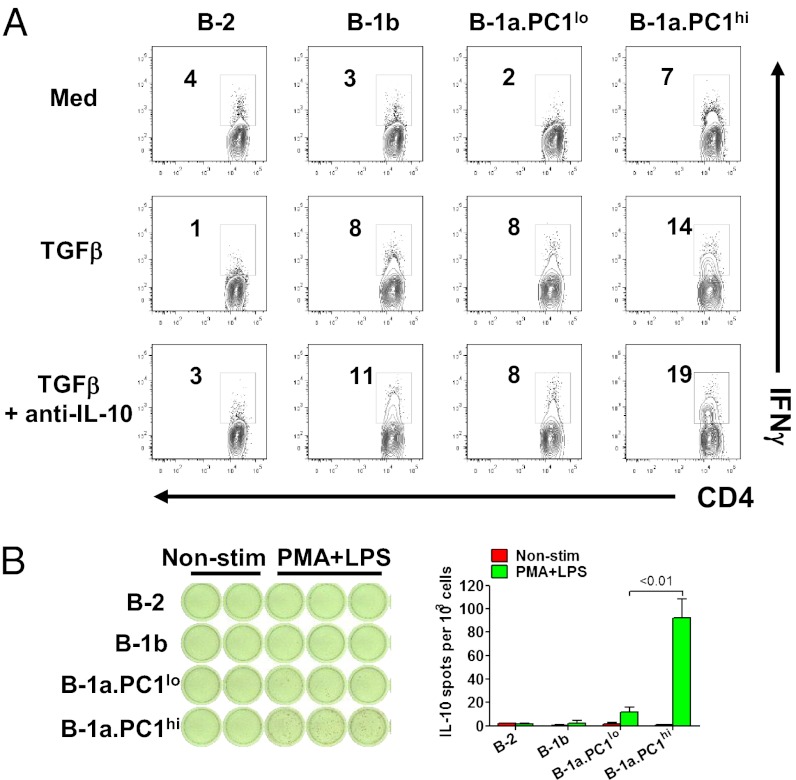
Interestingly, about 7% of OVA-specific T cells cocultured with PC1hi cells spontaneously produced IFN-γ as assessed by intracellular staining, whereas T cells cocultured with B-1a.PC1lo, B-2, or B-1b cells had only background levels of intracellular IFN-γ (Fig. 6A). Stimulation with TGF-β significantly increased the frequency of cells expressing IFN-γ in cultures containing PC1hi, PC1lo, and B-1b cells, but not B-2 cell cells. Addition of neutralizing antibody to IL-10 slightly increased the number of IFN-γ+ T cells in PC1hi and B-1b cultures but not PC1lo and B-2 cultures. These data suggested that PC1hi cells are more efficient than PC1lo cells in regulating Th1 cell differentiation, even though both B-1a subsets were comparably active in stimulating CD4+ T-cell proliferation.
Fig. 6.
Antigen presentation and differential IL-10 secretion by B-1a subsets. (A) Sort-purified peritoneal B-cell subsets were cultured for 3 d with OVA323–396 peptide, CD4+ T cells purified from OT-II transgenic mice and TGFβ ± anti-IL-10 Ab. The cells were stained for CD4 and intracellular IFN-γ and analyzed by FACS. Representative data of three independent experiments is shown. (B) Sort-purified peritoneal B-cell subsets were cultured with and without PMA plus LPS overnight. IL-10–secreting cells were visualized by ELISPOT. Data represent two independent experiments with similar results.
PC1lo and PC1hi Cells Differ in Their Ability to Produce IL-10.
An increasing body of evidence attests to the importance of B cells as potent regulators of immunity independent of their ability to produce antibodies (46, 47). These activities include suppression of a variety of autoimmune and inflammatory responses by IL-10–producing regulatory B cells (termed “Bregs” by some), including those characterized phenotypically as CD5− transitional 2/MZ precursor cells (48) or CD5+ “B10” cells (49). In other studies, about 30% of activated peritoneal B-1a cells were found to express IL-10 (50). Here we analyzed unstimulated peritoneal B-cell subsets of mice for expression of an IL-10 GFP reporter gene. Flow cytometric studies clearly showed more GFP signals in PC1hi than in PC1lo cells; B-2 and B-1b cells were almost silent (Fig. S7B). We next used an enzyme-linked immunosorbent spot (ELISPOT) assay to assess IL-10 production by peritoneal B-2, B-1b, PC1lo, and PC1hi cells stimulated with LPS and phorbol 12-myristate 13-acetate (PMA). B-2 and B-1b cells were essentially nonresponsive to these stimuli, and the numbers of IL-10–secreting cells were nearly 10-fold higher for PC1hi than for PC1lo cells (Fig. 6B). We conclude that the immunoregulatory potential of B-1a cells associated with expression of IL-10 is almost exclusively an attribute of the PC1hi subset.
Discussion
In this report, we identified two subsets of mouse B-1a B cells defined by differential expression of ENPP1 that we designated as PC1lo and PC1hi. The subsets differ not only in phenotype, BCR specificity, and migration, but also in immunological functions, including production of natural IgM, secretion of intestinal IgA, and production of IL-10 (Fig. S8). They appear to develop from two successive waves of progenitors; the PC1lo subset arises during fetal life and persists into adulthood, and the PC1hi subset appears in adult proportions only after 8 wk of life. When immunized with pneumococcal polysaccharides, PC1lo cells generated antigen-specific IgM-responses, whereas PC1hi cells did not. These results revealed features of the peritoneal B-1a repertoire that contains layered populations with distinct functions.
Early studies of total B-1a cells indicated an origin from fetal progenitors that waned soon after birth and the mature population was maintained by self-renewal (51). In addition, it was thought that the B-1a IgH repertoire was heavily biased toward sequences lacking N additions because TdT is not expressed during fetal life. More recent work, however, has shown that B-1a precursors do exist in adult bone marrow to some extent and that their progeny can exhibit Ig sequences with high levels of N additions. In view of our findings indicating an earlier developmental origin for PC1lo than for PC1hi cells, it might have been expected that the PC1lo Ig repertoire would have a “fetal signature,” whereas PC1hi sequences would indicate more frequent origins from bone marrow cells. Instead, the Ig sequences of the B-1a subsets from young adult mice demonstrated that they both comprise diverse repertoires that include substantial contributions of genes with N additions. This suggests that in adults cells included in both subsets have origins from both fetal and bone marrow progenitors. Interestingly, despite these complexities, it is noteworthy that the great majority of sequences from the “canonical B-1a” VH11 family lack N additions regardless of their origins from either subset. This is compatible with the finding that the VH11 repertoire was greatly underrepresented among PtC-binding B-1a cells uniquely of bone marrow origin, thereby suggesting that N additions in CDR3 are incompatible with selection for this specificity (11).
It is noteworthy that PtC-binding B-1a cells were greatly enriched in the PC1hi subset, which we characterized as having less secretory potential than PC1lo cells, whereas PtC antibodies are well represented in circulating natural antibodies. It may be that the small numbers of PC1hi cells that migrate to the spleen are the precursors to splenic IgM-secreting cells, similar to the migratory cells found to respond to stimulation with the atypical LPS of F. tularensis (52).
The subdivision of labor between the two subsets defined by our studies suggests a predominant immunoregulatory role for the PC1hi subset, with PC1lo cells responsible for mediating antigen-specific responses and producing natural antibodies. Previously identified populations of regulatory B cells were associated with important roles in controlling T-cell–mediated autoimmunity and inflammation, often through the production of IL-10 (53). High-level expression of PC1, which promotes the conversion of immunostimulatory extracellular ATP to immunosuppresive adenosine (54), could represent a second means for PC1hi cells to repress autoimmune and inflammatory responses. Because natural antibody-producing cells are enriched for self-reactive B-cell receptors, PC1hi cells may play an important role in limiting this activity of the PC1lo subset as well as autoreactive B2 cells. Studies of antibody responses by immunized Rag1−/− mice reconstituted with PC1lo cells alone or with both B-1a subsets would be informative in this regard.
Since the discovery of the B-1a subset of B cells (1), phenotypic characterization of this population determined by flow cytometry has led to the suggested existence of CD5+ B-1a cells with noncanonical features. For example, Clarke and Arnold reported CD23+ B-1a cells in mouse spleen, which they designated B-0 cells (55). The Rothstein and Herzenberg laboratories identified a CD11b− B-1a subset in the peritoneum (9, 56), questioning the reliability of CD11b as a pan B-1 cell marker that has been widely used for positive gating on B-1a and B-1b cells. Recently, Rothstein and coworkers identified PD-L2+ (57) and CD25+ B-1a subsets (58). By using a decoy receptor for many CC chemokines, D6, as a marker, Hansell et al. described the division of B-1a cells into four subpopulations (59). None of these subsets, however, has been well characterized in terms of their developmental origins, molecular features, and cellular functions. It will be essential to directly compare the features of each subset to gain better understanding of the heterogeneity of the B-1a compartment.
The identification of B-1a B-cell subsets has important implications for developing vaccines that effectively stimulate the dominant B-cell population of infants. Infections of infants with encapsulated bacteria such as Haemophilus influenza, Neisseria meningitides, and Streptococcus pneumoniae are a leading cause of death in children worldwide (60–62). Strategies for protection from infection have been focused on immunization with vaccines, mainly produced from polysaccharides purified from bacterial capsules. These polysaccharide antigens induce mostly T-independent responses through innate-like marginal-zone and B-1 cells. Unlike adults, however, young children develop poor antibody responses to the vaccines, a finding that has been attributed to the underdeveloped marginal-zone B-cell compartment of children under 2 y of age (63). Because B-1 cells are abundant in children (64), it is puzzling why these cells fail to respond to polysaccharide antigens. With the discovery of PC1-defined B-1a subsets with distinct capacities to respond to PPS-3, our data raise an important question as to whether humans have similar functionally segregated B-1a–like cell populations. Better understanding of the B-1a cell repertoire in young children would certainly benefit development of more effective vaccines.
Materials and Methods
The methods for FACS phenotyping, adoptive transfer, Ig repertoire analyses, ELISA, antigen presentation assay, and immunohistology are described in SI Text. For animals and methods of immunization, see SI Materials and Methods. All animal studies were performed under protocols of LIG-14 and -16E approved by the Institutional Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases (NIAID).
Supplementary Material
Acknowledgments
We thank Dr. Fumio Takei (University of British Columbia) for providing us with the anti-PC1 monoclonal antibody, Larry Lantz (NIAID Research Technologies Branch) for antibody purification and labeling, Mehrnoosh Abshari for cell sorting, and Alfonso Macias for technical assistance. We thank Dr. Stephen H. Clarke (University of North Carolina) for providing us VH12 Tg mice and FITC-liposomes, and Drs. Kyoko Hayakawa and Richard R. Hardy (Fox Chase Cancer Center) for valuable advice and analysis of VH11 cells. We also thank Drs. Leonore A. Herzenberg, Yang Yang, and Eliver Eid Bou Ghosn (Stanford University) for valuable technical input.This study was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212428109/-/DCSupplemental.
References
- 1.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stall AM, Adams S, Herzenberg LA, Kantor AB. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann N Y Acad Sci. 1992;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- 3.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci USA. 2011;108(7):2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7(3):293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 5.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158(3):1175–1186. [PubMed] [Google Scholar]
- 6.Vale AM, et al. The peritoneal cavity B-2 antibody repertoire appears to reflect many of the same selective pressures that shape the B-1a and B-1b repertoires. J Immunol. 2010;185(10):6085–6095. doi: 10.4049/jimmunol.1001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci USA. 2011;108(33):13700–13704. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106(14):5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung JW, Mrazek MD, Yang Y, Herzenberg LA, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci USA. 2006;103(16):6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montecino-Rodriguez E, Dorshkind K. Formation of B-1 B cells from neonatal B-1 transitional cells exhibits NF-κB redundancy. J Immunol. 2011;187(11):5712–5719. doi: 10.4049/jimmunol.1102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Düber S, et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114(24):4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 12.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39(9):2383–2394. doi: 10.1002/eji.200838920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 14.Casola S, et al. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5(3):317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 15.Lam KP, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J Exp Med. 1999;190(4):471–477. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Clarke SH. Positive selection focuses the VH12 B-cell repertoire towards a single B1 specificity with survival function. Immunol Rev. 2004;197:51–59. doi: 10.1111/j.0105-2896.2004.0098.x. [DOI] [PubMed] [Google Scholar]
- 17.Förster I, Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987;17(4):521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986;16(4):450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa K, et al. Ly-1 B cells: Functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol. 2003;171(10):5406–5414. doi: 10.4049/jimmunol.171.10.5406. [DOI] [PubMed] [Google Scholar]
- 21.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol. 2012;42(1):120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Oudenaren A, Haaijman JJ, Benner R. Frequencies of background cytoplasmic Ig-containing cells in various lymphoid organs of athymic and euthymic mice as a function of age and immune status. Immunology. 1984;51(4):735–742. [PMC free article] [PubMed] [Google Scholar]
- 23.Cole LE, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci USA. 2009;106(11):4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagarasan S, Honjo T. T-Independent immune response: New aspects of B cell biology. Science. 2000;290(5489):89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 25.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413(6856):639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 26.Kroese FG, et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1(1):75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- 27.Macpherson AJ, et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288(5474):2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 28.O’Garra A, et al. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22(3):711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 29.Klinker MW, Lundy SK. Multiple mechanisms of immune suppression by B lymphocytes. Mol Med. 2012;18(1):123–137. doi: 10.2119/molmed.2011.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 31.Dasu T, Sindhava V, Clarke SH, Bondada S. CD19 signaling is impaired in murine peritoneal and splenic B-1 B lymphocytes. Mol Immunol. 2009;46(13):2655–2665. doi: 10.1016/j.molimm.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer GM, et al. Splenic and peritoneal B-1 cells differ in terms of transcriptional and proliferative features that separate peritoneal B-1 from splenic B-2 cells. Cell Immunol. 2001;213(1):62–71. doi: 10.1006/cimm.2001.1860. [DOI] [PubMed] [Google Scholar]
- 33.Tumang JR, Hastings WD, Bai C, Rothstein TL. Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur J Immunol. 2004;34(8):2158–2167. doi: 10.1002/eji.200424819. [DOI] [PubMed] [Google Scholar]
- 34.Stoermann B, Kretschmer K, Düber S, Weiss S. B-1a cells are imprinted by the microenvironment in spleen and peritoneum. Eur J Immunol. 2007;37(6):1613–1620. doi: 10.1002/eji.200636640. [DOI] [PubMed] [Google Scholar]
- 35.Abbasi S, et al. Characterization of monoclonal antibodies to the plasma cell alloantigen ENPP1. Hybridoma (Larchmt) 2011;30(1):11–17. doi: 10.1089/hyb.2010.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatu C, Ye J, Arnold LW, Clarke SH. Selection at multiple checkpoints focuses V(H)12 B cell differentiation toward a single B-1 cell specificity. J Exp Med. 1999;190(7):903–914. doi: 10.1084/jem.190.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen L, Shinton SA, Hardy RR, Hayakawa K. Association of B-1 B cells with follicular dendritic cells in spleen. J Immunol. 2005;174(11):6918–6926. doi: 10.4049/jimmunol.174.11.6918. [DOI] [PubMed] [Google Scholar]
- 38.Waffarn EE, Baumgarth N. Protective B cell responses to flu—No fluke! J Immunol. 2011;186(7):3823–3829. doi: 10.4049/jimmunol.1002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haughton G, Arnold LW, Whitmore AC, Clarke SH. B-1 cells are made, not born. Immunol Today. 1993;14(2):84–87. doi: 10.1016/0167-5699(93)90064-R. discussion 87–91. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa K, et al. Positive selection of anti-thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J Exp Med. 2003;197(1):87–99. doi: 10.1084/jem.20021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnold LW, Pennell CA, McCray SK, Clarke SH. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J Exp Med. 1994;179(5):1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fagarasan S, Honjo T. Intestinal IgA synthesis: Regulation of front-line body defences. Nat Rev Immunol. 2003;3(1):63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 43.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 44.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2(5):323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 45.Zhong X, et al. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009;60(12):3734–3743. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20(3):332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 2010;22(6):761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Evans JG, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 49.Yanaba K, et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 51.Herzenberg LA. B-1 cells: The lineage question revisited. Immunol Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 52.Yang Y, et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci USA. 2012;109(14):5388–5393. doi: 10.1073/pnas.1121627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 54.Goding JW, Grobben B, Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta. 2003;1638(1):1–19. doi: 10.1016/s0925-4439(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 55.Clarke SH, Arnold LW. B-1 cell development: Evidence for an uncommitted immunoglobulin (Ig)M+ B cell precursor in B-1 cell differentiation. J Exp Med. 1998;187(8):1325–1334. doi: 10.1084/jem.187.8.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hastings WD, Gurdak SM, Tumang JR, Rothstein TL. CD5+/Mac-1- peritoneal B cells: A novel B cell subset that exhibits characteristics of B-1 cells. Immunol Lett. 2006;105(1):90–96. doi: 10.1016/j.imlet.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37(9):2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 58.Tumang JR, et al. A CD25− positive population of activated B1 cells expresses LIFR and responds to LIF. Frontiers in Immunology. 2011;2(6):8. doi: 10.3389/fimmu.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansell CA, et al. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117(20):5413–5424. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Brien KL, et al. Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 61.Pollard AJ, Frasch C. Development of natural immunity to Neisseria meningitidis. Vaccine. 2001;19(11-12):1327–1346. doi: 10.1016/s0264-410x(00)00333-9. [DOI] [PubMed] [Google Scholar]
- 62.Watt JP, et al. Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: Global estimates. Lancet. 2009;374(9693):903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 63.Kruschinski C, Zidan M, Debertin AS, von Hörsten S, Pabst R. Age-dependent development of the splenic marginal zone in human infants is associated with different causes of death. Hum Pathol. 2004;35(1):113–121. doi: 10.1016/s0046-8177(03)00422-2. [DOI] [PubMed] [Google Scholar]
- 64.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70- J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.