Abstract
Recently, economists and behavioral scientists have studied the pattern of human well-being over the lifespan. In dozens of countries, and for a large range of well-being measures, including happiness and mental health, well-being is high in youth, falls to a nadir in midlife, and rises again in old age. The reasons for this U-shape are still unclear. Present theories emphasize sociological and economic forces. In this study we show that a similar U-shape exists in 508 great apes (two samples of chimpanzees and one sample of orangutans) whose well-being was assessed by raters familiar with the individual apes. This U-shaped pattern or “midlife crisis” emerges with or without use of parametric methods. Our results imply that human well-being’s curved shape is not uniquely human and that, although it may be partly explained by aspects of human life and society, its origins may lie partly in the biology we share with great apes. These findings have implications across scientific and social-scientific disciplines, and may help to identify ways of enhancing human and ape well-being.
Keywords: aging, primate, satisfaction, evolution, affect
There is accumulating evidence, based on biomarker, spatial, genetic, and brain-science data, for the objective validity of subjective measures of human well-being (1–6). Published results showing a U-shaped relationship between well-being and age, with the lowest point approximately in midlife, can be traced back at least two decades to research on job satisfaction and mental health (7–9). Although some scholars have raised doubts about the existence of the pattern (10–12), a large new literature indicates that human happiness follows a U-shape throughout life (13–17), except in the years right before death (15). There is corroborating evidence. After adjustment for covariates, suicide risk (18) and antidepressant consumption (19) exhibit a midlife peak. U-shaped well-being patterns have been found in over 50 nations (15, 20), including poorer developing nations. Sample sizes vary from a few hundred to millions of participants. One of the most important findings in this literature is that, as shown for example by Stone et al. (14) in their figure 1, the U-shape is virtually unaffected by statistical adjustment for a large range of economic and demographic characteristics. This striking discovery suggests that some of the causes of the U-shape must go beyond standard socioeconomic forces.
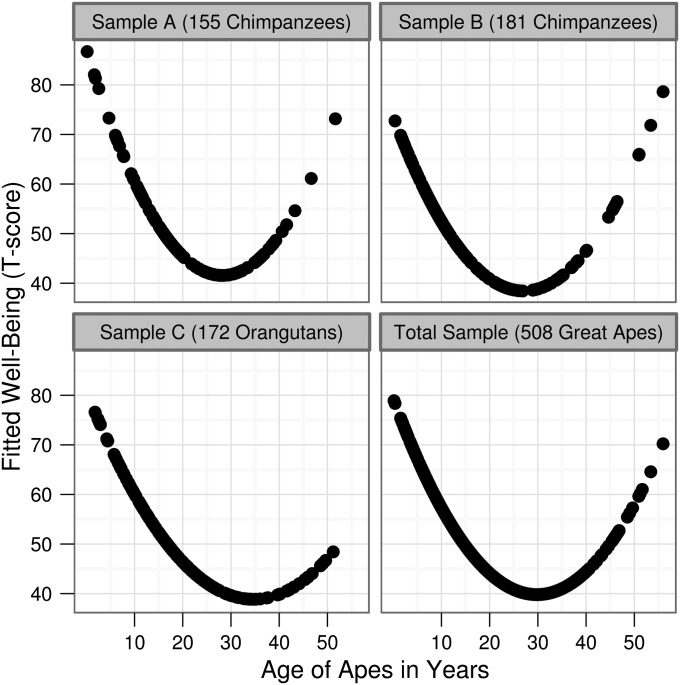
Fig. 1.
The well-being U-shape in three samples of great apes. Well-being scores, collapsed across sex, fitted to a quadratic function for the three samples, both separately (Upper Left, Upper Right, Lower Left), and combined (Lower Right). Fitted scores were rescaled (mean = 50 ± 10 SD).
The midlife dip cannot be explained by the effects of having young children in the household, and it is similar in males and females, so is not likely connected to menopausal changes or to societal sex roles (14, 15). A selection explanation, because of the greater longevity of happy people, is likewise unable to account for the midlife dip (15). One socioeconomic theory (13) is that the U-shape reflects hedonic adaptation in which impossible aspirations are first painfully felt around midlife and then slowly and beneficially given up. Another theory (17) is that the curve is linked to financial hardship and thus likely to be less pronounced in those older individuals with higher resources. A third theory is that human aging may bring with it the ability to experience less regret (21). In short, there is little convergence of explanations about the U-shape’s origins.
We explore an alternative explanation. From a very different research tradition, work on great ape (mostly chimpanzee) development has identified similarities to humans in the development of psychological domains other than well-being (22). Thus, it is worth considering a heretofore untested theory, namely that the U-shape found in human studies of age and well-being evolved in the common ancestors of humans and nonhuman primates, particularly the great apes. If one could establish that the U-shape in well-being exists in nonhuman primates, the implications would be wide-ranging. This finding would also recommend new hypotheses for well-being researchers.
Results
In a sample of 155 chimpanzees from Japanese zoos, research centers, and a sanctuary (sample A), a sample of 181 chimpanzees housed in United States and Australian zoos (sample B), and a sample of 172 orangutans housed in United States, Canadian, Australian, and Singaporean zoos (sample C), multiple regression analyses indicated that linear and quadratic age effects were negative and positive, respectively (Table 1). In other words, all three samples exhibited a U-shape (Fig. 1). The age-related effects were individually significant in sample A, but not samples B or C. The curves’ minima were reached at, respectively, ages 28.3, 27.2, and 35.4, and were thus comparable to human well-being minima, which range from ∼45–50 y. In the fourth regression, for the total sample, the linear and quadratic age effects indicated a U-shape and were significant. Linear and quadratic age effects did not significantly differ across the samples (Table 2). Finally, the linear and quadratic effects again described a U-shaped function (Fig. 1) and were significant after the interaction terms were dropped (Table 2). The curve’s minimum was at age 31.9. Use of 10 banded age variables revealed the same results (SI Text, and Table S2).
Table 1.
Well-being regression equations for samples A (n = 155), B (n = 181), and C (n = 172)
| Sample | b | SE | t | P |
| Sample A | ||||
| Intercept | 56.805 | 2.704 | 21.005 | <0.001 |
| Male | 1.498 | 0.709 | 2.112 | 0.036 |
| Age | −0.735 | 0.253 | −2.910 | 0.004 |
| Age2 | 0.013 | 0.005 | 2.419 | 0.017 |
| Sample B | ||||
| Intercept | 50.557 | 2.094 | 24.147 | <0.001 |
| Male | 0.477 | 0.833 | 0.572 | 0.568 |
| Age | −0.381 | 0.216 | −1.768 | 0.079 |
| Age2 | 0.007 | 0.004 | 1.573 | 0.117 |
| Sample C | ||||
| Intercept | 59.808 | 2.093 | 28.573 | <0.001 |
| Male | 1.992 | 0.606 | 3.287 | 0.001 |
| Age | −0.354 | 0.192 | −1.841 | 0.067 |
| Age2 | 0.005 | 0.004 | 1.348 | 0.179 |
The b coefficient for “Male” indicates the deviation of the mean of well-being in males from the unweighted grand mean of well-being.
Table 2.
Well-being regression equations for the total sample (N = 508) with sample by age interactions and without sample by age interactions
| With or without sample | b | SE | t | P |
| With sample | ||||
| Intercept | 55.764 | 1.400 | 39.835 | <0.001 |
| Sample A | 0.921 | 2.180 | 0.423 | 0.673 |
| Sample B | −5.132 | 1.732 | −2.963 | 0.003 |
| Male | 1.301 | 0.421 | 3.091 | 0.002 |
| Age | −0.491 | 0.132 | −3.714 | <0.001 |
| Age2 | 0.008 | 0.003 | 3.065 | 0.002 |
| Sample A × Age | −0.232 | 0.205 | −1.134 | 0.258 |
| Sample B × Age | 0.118 | 0.169 | 0.696 | 0.487 |
| Sample A × Age2 | 0.004 | 0.004 | 1.015 | 0.311 |
| Sample B × Age2 | −0.001 | 0.004 | −0.395 | 0.693 |
| Without sample | ||||
| Intercept | 55.215 | 1.316 | 41.962 | <0.001 |
| Sample A | −1.502 | 0.598 | −2.510 | 0.012 |
| Sample B | −3.514 | 0.582 | −6.037 | <0.001 |
| Male | 1.260 | 0.418 | 3.017 | 0.003 |
| Age | −0.447 | 0.125 | −3.574 | <0.001 |
| Age2 | 0.007 | 0.003 | 2.920 | 0.004 |
The b coefficients for “Male,” “Sample A,” and “Sample B” indicate the deviation of well-being of these groups from the unweighted grand mean of well-being. The b coefficients for interaction terms indicate the deviation of Age and Age2 effects for sample A and sample B from the unweighted grand mean of the Age and Age2 effects.
Discussion
Although great apes have a close phylogenetic relationship to humans (23) and share many behavioral characteristics, including culture and tool use (24, 25), the research literature on human well-being, dating back to the Second World War (13) and currently used by governments to design economic policy (20), eschewed studies of nonhuman animals. That neglect has encouraged strictly human-centered and socioeconomic explanations for patterns found by demographers, economists, psychologists, and others.
Here we used data on other primates to suggest the value of a cross-species approach in understanding human well-being. It is important to note that our findings do not rule out the possibility that species-specific social, cultural, and psychological forces contribute to the well-being U-shape in humans. However, these results suggest that a persuasive explanation for the human U-shape should also account for the similarity of this trend in our evolutionary cousins, the great apes.
There are several overlapping mechanisms that may explain the well-being U-shape. One possibility is that these age differences reflect the fact that happiness is positively associated with longevity in humans (26) and at least one great ape species (27). Therefore, higher rates of mortality for the least happy apes, especially in later life, could account for part of the higher well-being in the older ape populations. A second possibility is that the U-shape arises in humans, chimpanzee, and orangutans via similar age-related changes in brain structures associated with well-being (2). Finally, older adults in all three species may rely on behavioral mechanisms to regulate their emotions (28). For example, they may seek out situations and group members that elicit more positive emotions or shift to goals that are more attainable in older age. It is also important to consider evolutionary explanations. For example, as well-being is associated with life satisfaction, there may have been selection for individuals who have higher well-being in youth and old adulthood. These individuals, being satisfied at stages of their life where they have fewer resources to improve their lot, would be less likely to encounter situations that could be harmful to them or their kin.
Future focus should be directed toward aspects of human lives and neurodevelopment shared with other great apes. Longitudinal studies of humans and other primates that examine changes in the predictors of well-being across the lifespan could help explain the mechanisms underlying the U-shaped function. Moreover, studies of age and well-being in other species of great apes and studies examining possible fitness costs of high midlife well-being in chimpanzees, orangutans, and humans would lead to a greater understanding of its evolutionary origins. These and other comparative, evolutionary approaches offer applications beyond the midlife nadir in happiness and could affirm Darwin’s (29) view that “He who understands baboon would do more towards metaphysics than Locke.”
Materials and Methods
Subjects.
We used three existing samples of great apes (22, 27, 30), each of which included individuals ranging from infancy to old adulthood. The study was not an experiment and there was no intervention. Data collection complied with regulations and guidelines prescribed by The University of Edinburgh and the institutions that housed the subjects. Sample A comprised 64 male and 91 female chimpanzees (Pan troglodytes) housed in nine zoos, one sanctuary, and two research centers, all located in Japan. Ages ranged from 0.2 to 51.7 y (mean = 22.3 ± 10.6 SD). Sample B comprised 69 male and 112 female chimpanzees housed in 14 United States zoos and 1 Australian zoo. Ages for three subjects were estimated based on the date other subjects in their zoo were rated on well-being. Ages of a further 32 subjects were imputed via regression based on the age at which they were rated on personality (correlated r > 0.99 with age at which well-being was rated). Age ranged from 0.4 to 56.0 y (mean = 17.9 ± 12.5 SD). Sample C comprised 69 male and 103 female orangutans (Pongo spp.), of which 89 were Sumatran (Pongo abelii), 53 were Bornean (Pongo pygmaeus), and 30 were hybrids. These subjects were housed in 35 United States, 2 Canadian, 1 Australian, and 1 Singaporean zoo. Ages for eight subjects were imputed via regression based on the age at which they were rated on personality (correlated r > 0.99 with age at which well-being was rated). Age ranged from 1.8 to 51.2 y (mean = 21.2 ± 11.7 SD). Omitting subjects whose age was imputed did not alter results.
Measure.
Well-being was assessed using a four-item questionnaire based on human subjective well-being measures, but modified for use in nonhuman primates (31, 32). Item one asked raters to assess the degree to which a subject was in a positive versus negative mood. Item two asked raters to assess how much pleasure the subject derives from social situations. Item three asked raters to assess how successful the subject is in achieving its goals. Item four asked raters to indicate how happy they would be if they were the subject for a week. This questionnaire is a well-established method for assessing positive affect in captive nonhuman primates. There is considerable evidence for this measure’s objective validity. Ratings on this questionnaire are consistent across raters and define a single dimension (30, 31, 33). In addition, like human subjective well-being questionnaires (6, 26, 34, 35), scores on the present questionnaire are stable over time (31), associated with analogous personality traits (30, 31, 33), and both heritable and genetically correlated with personality (36, 37). Finally, a study in orangutans (27) indicated that, like human well-being (26), higher scores on this well-being scale were associated with a longer lifespan.
The raters were zoo keepers, volunteers, researchers, and caretakers who knew the subjects, usually for at least 2 y (27, 30, 33). Ratings on the four items were made on 7-point scales. For samples A and C, raters were asked to indicate where on the 7-point scale a particular subject fell on a particular item. Sample B was rated using an older version of the scale that instructed raters to assign a 1 to the chimpanzee at their facility with the lowest score, a 7 to the chimpanzee at their facility with the highest score, and to freely assign values ranging from 2 to 6 to the remaining chimpanzees. In addition, the questionnaires for sample A were written in Japanese and questionnaires for the other two samples were written in English. Well-being in all three samples was computed by taking the mean of each item across raters and then obtaining the mean of these four mean scores. The interrater reliabilities of the individual items for each sample ranged from fair to excellent; the interrater reliability of well-being in all three samples was high (SI Text and Table S1).
Well-being scores were converted into T-scores for all further analyses (mean = 50 ±10 SD). Means and SDs for the samples were: A: 48.1 ± 8.8; B: 47.0 ± 10.9, and C: 54.9 ± 8.1. In a preliminary analysis we tested whether the instructions given to samples A and C on the one hand and sample B on the other influenced the linear or quadratic age effects. The interaction of instruction type and linear age effects was not significant (b = 0.261, t = 1.005, P = 0.315). The interaction of instruction type and quadratic age effect was also not significant (b = −0.003, t = −0.638, P = 0.524). Thus, there was no evidence that the association between the age effects and well-being varied as a function of instruction type.
Analyses.
Researchers on human well-being typically use multiple regression, and age effects are examined after adjusting for several variables, including income, education, marital status, sex, and location. In at least one dataset the U-shape was found not to exist until adjustment for these covariates (15, 38). For our analyses we also examined associations between age and well-being using multiple regressions (39). However, our analysis of ape well-being was more conservative; we only adjusted for sex and the sample used. To avoid the multiple-comparisons problem, we focused on the single hypothesis of a quadratic relationship between well-being and age. Throughout, significance tests were two-tailed and α = 0.05.
In the first three regressions we tested for the U-shape in each sample separately. In each, well-being was predicted by sex (male = 1, female = −1), linear age effects (age in years), and quadratic age effects (age in years squared). In the fourth regression we combined the samples to test whether they described the same linear and quadratic age effects. Predictors in this regression included sex and two effects coded variables that tested for deviations of sample A or B from the well-being grand mean. These effects adjusted for differences arising from sample A being rated in Japanese by Japanese raters or sample C being orangutans instead of chimpanzees. The regression also included variables indicating linear and quadratic age effects, and interaction terms to test whether these age effects differed across samples. The fifth regression was similar to the prior regression except that it did not include interaction terms.
Finally, we conducted a supplementary analysis to assess the robustness of the multiple regression analyses described above. The supplementary analysis examined the appropriateness of fitting a quadratic function to the full ape dataset by checking that we did not overfit these data. To do this, we estimated the effects of age on well-being equation without imposing any parameterized structure or polynomial function. Instead, we estimated the effects of age on well-being using 10 banded age variables.
Supplementary Material
Acknowledgments
We thank the zoological institutions, sanctuaries, and research institutes that agreed to participate in the project and the raters for their assessments of the apes’ well-being; the former director of ChimpanZoo, Virginia Landau, and the head of the Orangutan Species Survival Program, Lori Perkins; and the keepers of the chimpanzee and orangutan studbooks, Megan Elder and Steve Ross, respectively, for granting us access to the studbooks. Collection of chimpanzee data in Japan was funded by Grants 2828 from The University of Edinburgh Development Trust and Small Grant 6515/6818 from The Daiwa Foundation (to A.W.); the Ministry of Education, Culture, Sports, Science and Technology (MEXT) with a Grant-in-aid for Science Research 21310150 (to M.I.-M.); Asia and Africa Science Platform Program under the Japanese Society for the Promotion of Science, Environment Research and Technology Development Fund (D-1007); the Cooperation Research Program of the Primate Research Institute, Kyoto University; Grant 24000001 from MEXT (to T.M.); and Economic and Social Research Council funding of the Centre for Competitive Advantage in the Global Economy at the University of Warwick.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212592109/-/DCSupplemental.
References
- 1.Easterlin RA. Explaining happiness. Proc Natl Acad Sci USA. 2003;100(19):11176–11183. doi: 10.1073/pnas.1633144100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urry HL, et al. Making a life worth living: Neural correlates of well-being. Psychol Sci. 2004;15(6):367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 3.Oswald AJ, Wu S. Objective confirmation of subjective measures of human well-being: Evidence from the U.S.A. Science. 2010;327(5965):576–579. doi: 10.1126/science.1180606. [DOI] [PubMed] [Google Scholar]
- 4.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci USA. 2005;102(18):6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fliessbach K, et al. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318(5854):1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- 6.Bartels M, Boomsma DI. Born to be happy? The etiology of subjective well-being. Behav Genet. 2009;39(6):605–615. doi: 10.1007/s10519-009-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warr P. Age and occupational well-being. Psychol Aging. 1992;7(1):37–45. doi: 10.1037//0882-7974.7.1.37. [DOI] [PubMed] [Google Scholar]
- 8.Clark AE, Oswald AJ. Unhappiness and unemployment. Econ J. 1994;104:648–659. [Google Scholar]
- 9.Clark A, Oswald A, Warr P. Is job satisfaction U-shaped in age? J Occup Organ Psychol. 1996;69:57–81. [Google Scholar]
- 10.Glenn N. Is the apparent U-shape of well-being over the life course a result of inappropriate use of control variables? A commentary on Blanchflower and Oswald (66: 8, 2008, 1733–1749) Soc Sci Med. 2009;69(4):481–485, discussion 486–488. doi: 10.1016/j.socscimed.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 11.Frijters P, Beatton T. The mystery of the U-shaped relationship between happiness and age. J Econ Behav Organ. 2012;82:525–542. [Google Scholar]
- 12.Kassenboehmer SC, Haisken-DeNew JP. Heresy or enlightenment? The well-being age U-shape effect is flat. Econ Lett. 2012;117:235–238. [Google Scholar]
- 13.Frey BS, Stutzer A. Happiness and Economics. Princeton, NJ: Princeton Univ Press; 2002. [Google Scholar]
- 14.Stone AA, Schwartz JE, Broderick JE, Deaton A. A snapshot of the age distribution of psychological well-being in the United States. Proc Natl Acad Sci USA. 2010;107(22):9985–9990. doi: 10.1073/pnas.1003744107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchflower DG, Oswald AJ. Is well-being U-shaped over the life cycle? Soc Sci Med. 2008;66(8):1733–1749. doi: 10.1016/j.socscimed.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Van Landeghem B. A test for the convexity of human well-being over the life cycle: Longitudinal evidence from a 20-year panel. J Econ Behav Organ. 2012;81(2):571–582. [Google Scholar]
- 17.Lang IA, Llewellyn DJ, Hubbard RE, Langa KM, Melzer D. Income and the midlife peak in common mental disorder prevalence. Psychol Med. 2011;41(7):1365–1372. doi: 10.1017/S0033291710002060. [DOI] [PubMed] [Google Scholar]
- 18.Daly M, Wilson DJ. Happiness, unhappiness, and suicide: An empirical assessment. J Eur Econ Assoc. 2009;7:539–549. [Google Scholar]
- 19.Blanchflower DG, Oswald AJ. 2012. Antidepressants and age. IZA Discussion Paper 5785. Available at http://ftp.iza.org/dp5785.pdf. Accessed July 1, 2012.
- 20.Layard R. Measuring subjective well-being. Science. 2010;327(5965):534–535. doi: 10.1126/science.1186315. [DOI] [PubMed] [Google Scholar]
- 21.Brassen S, Gamer M, Peters J, Gluth S, Büchel C. Don’t look back in anger! Responsiveness to missed chances in successful and nonsuccessful aging. Science. 2012;336(6081):612–614. doi: 10.1126/science.1217516. [DOI] [PubMed] [Google Scholar]
- 22.King JE, Weiss A, Sisco MM. Aping humans: Age and sex effects in chimpanzee (Pan troglodytes) and human (Homo sapiens) personality. J Comp Psychol. 2008;122(4):418–427. doi: 10.1037/a0013125. [DOI] [PubMed] [Google Scholar]
- 23.Purvis A. A composite estimate of primate phylogeny. Philos Trans R Soc Lond B Biol Sci. 1995;348(1326):405–421. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]
- 24.Whiten A, et al. Cultures in chimpanzees. Nature. 1999;399(6737):682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 25.van Schaik CP, et al. Orangutan cultures and the evolution of material culture. Science. 2003;299(5603):102–105. doi: 10.1126/science.1078004. [DOI] [PubMed] [Google Scholar]
- 26.Diener E, Chan MY. Happy people live longer: Subjective well-being contributes to health and longevity. Appl Psychol Health Well Being. 2011;3(1):1–43. [Google Scholar]
- 27.Weiss A, Adams MJ, King JE. Happy orang-utans live longer lives. Biol Lett. 2011;7(6):872–874. doi: 10.1098/rsbl.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urry HL, Gross JJ. Emotional regulation in old age. Curr Dir Psychol Sci. 2010;19:352–357. [Google Scholar]
- 29.Darwin C. Notebook M: [Metaphysics on Morals and Speculations on Expression] 1838. Available at http://darwin-online.org.uk/content/frameset?viewtype=side&itemID=CUL-DAR125.-&pageseq=1. Accessed July 1, 2012. [Google Scholar]
- 30.Weiss A, et al. Assessing chimpanzee personality and subjective well-being in Japan. Am J Primatol. 2009;71(4):283–292. doi: 10.1002/ajp.20649. [DOI] [PubMed] [Google Scholar]
- 31.King JE, Landau VI. Can chimpanzee (Pan troglodytes) happiness be estimated by human raters? J Res Pers. 2003;37(1):1–15. [Google Scholar]
- 32.Weiss A, King JE, Murray L. Springer Extras for Personality and Temperament in Nonhuman Primates. New York: Springer; 2011. . Available at http://extras.springer.com/2011/978-1-4614-0175-9. Accessed July 1, 2012. [Google Scholar]
- 33.Weiss A, King JE, Perkins L. Personality and subjective well-being in orangutans (Pongo pygmaeus and Pongo abelii) J Pers Soc Psychol. 2006;90(3):501–511. doi: 10.1037/0022-3514.90.3.501. [DOI] [PubMed] [Google Scholar]
- 34.Steel P, Schmidt J, Shultz J. Refining the relationship between personality and subjective well-being. Psychol Bull. 2008;134(1):138–161. doi: 10.1037/0033-2909.134.1.138. [DOI] [PubMed] [Google Scholar]
- 35.Weiss A, Bates TC, Luciano M. Happiness is a personal(ity) thing: The genetics of personality and well-being in a representative sample. Psychol Sci. 2008;19(3):205–210. doi: 10.1111/j.1467-9280.2008.02068.x. [DOI] [PubMed] [Google Scholar]
- 36.Adams MJ, King JE, Weiss A. The majority of genetic variation in orangutan personality and subjective well-being is nonadditive. Behav Genet. 2012;42(4):675–686. doi: 10.1007/s10519-012-9537-y. [DOI] [PubMed] [Google Scholar]
- 37.Weiss A, King JE, Enns RM. Subjective well-being is heritable and genetically correlated with dominance in chimpanzees (Pan troglodytes) J Pers Soc Psychol. 2002;83(5):1141–1149. [PubMed] [Google Scholar]
- 38.Easterlin R. Life cycle happiness and its sources. Intersections of psychology, economics, and demography. J Econ Psychol. 2006;27(4):463–482. [Google Scholar]
- 39.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.