Abstract
Antibodies hold significant potential for inhibiting toxic protein aggregation associated with conformational disorders such as Alzheimer’s and Huntington’s diseases. However, near-stoichiometric antibody concentrations are typically required to completely inhibit protein aggregation. We posited that the molecular interactions mediating amyloid fibril formation could be harnessed to generate antibodies with potent antiaggregation. Here we report that grafting small amyloidogenic peptides (6–10 residues) into the complementarity-determining regions of a single-domain (VH) antibody yields potent domain antibody inhibitors of amyloid formation. Grafted AMyloid-Motif AntiBODIES (gammabodies) presenting hydrophobic peptides from Aβ (Alzheimer’s disease), α-Synuclein (Parkinson's disease), and islet amyloid polypeptide (type 2 diabetes) inhibit fibril assembly of each corresponding polypeptide at low substoichiometric concentrations (1:10 gammabody:monomer molar ratio). In contrast, sequence- and conformation-specific antibodies that were obtained via immunization are unable to prevent fibrillization at the same substoichiometric concentrations. Gammabodies prevent amyloid formation by converting monomers and/or fibrillar intermediates into small complexes that are unstructured and benign. We expect that our antibody design approach—which eliminates the need for immunization or screening to identify sequence-specific domain antibody inhibitors—can be readily extended to generate potent aggregation inhibitors of other amyloidogenic polypeptides linked to human disease.
Keywords: beta-amyloid, misfolding, protein design, IAPP
The cytotoxicity of protein aggregates (e.g., prefibrillar oligomers and amyloid fibrils) linked to several neurodegenerative diseases has motivated the search for molecules that can inhibit and/or reverse protein aggregation (ref. 1 and references therein). The remarkable specificity of antibodies makes them particularly attractive as inhibitors of protein aggregation (1–3). Sequence-specific antibodies that bind to continuous or discontinuous sequence epitopes within amyloidogenic proteins can sequester monomers and prevent them from oligomerizing. A limitation of this approach is that low substoichiometric antibody concentrations (≤1:10 antibody:monomer molar ratios) are expected to be insufficient to sequester enough monomeric protein to prevent aggregation. In contrast, antibodies that are conformation-specific can selectively bind to and sequester oligomeric nuclei. The strength of this approach is that such antibodies may be inhibitory at low substoichiometric concentrations because they do not bind to monomeric protein. However, a limitation is that aggregated conformers must form before antibody binding, and this binding may be unable to arrest further conformational maturation of oligomeric nuclei into amyloid fibrils.
We recently reported that domain antibodies specific for the Alzheimer’s amyloid β (Aβ) peptide can be designed by grafting hydrophobic Aβ peptide segments into the complementarity-determining regions (CDRs) of a single-domain (VH) antibody (4). These Grafted AMyloid-Motif AntiBODIES (gammabodies) bind to Aβ oligomers and fibrils with nanomolar affinity and recognize Aβ monomers weakly. Gammabodies presenting the central hydrophobic Aβ motif (residues 18-VFFA-21) preferentially bind to Aβ fibrils, whereas gammabodies presenting the C-terminal hydrophobic Aβ motif (residues 34-LMVGGVVIA-42) preferentially bind to Aβ oligomers and fibrils. Moreover, each Aβ gammabody uses homotypic interactions between the grafted Aβ motif and the same motif within Aβ aggregates to mediate binding. Interestingly, gammabodies bind to Aβ oligomers and fibrils noncompetitively with antibodies obtained via immunization that are specific for oligomeric [A11 (5) antibody] and fibrillar [OC (6) and WO1 (7) antibodies] conformers.
The unusual ability of Aβ gammabodies to bind precisely to the hydrophobic peptide segments that mediate Aβ aggregation led us to hypothesize that these domain antibodies would inhibit fibrillization either by interfering with the nucleation of Aβ monomers into prefibrillar oligomers or the conversion of amyloidogenic intermediates into fibrils (Fig. 1). We also posited that gammabodies would be more effective at inhibiting Aβ aggregation than conventional conformation-specific antibodies that do not target hydrophobic linear epitopes recognized by Aβ gammabodies. Finally, we posited that our domain antibody design strategy could be readily extended to other amyloidogenic polypeptides to generate potent sequence-specific inhibitors of amyloid formation. To evaluate these hypotheses, we designed gammabodies that display hydrophobic peptide segments from three polypeptides [Aβ, α-Synuclein, and islet amyloid polypeptide (IAPP)] that form amyloid fibrils and whose aggregation is linked to human disease. Here we report that gammabodies potently inhibit amyloid formation of each polypeptide in a sequence-specific manner at substoichiometric concentrations (1:10 gammabody:monomer molar ratio), whereas sequence- and conformation-specific antibodies obtained via immunization are noninhibitory at the same substoichiometric concentrations.
Fig. 1.
Proposed method for designing gammabody inhibitors of amyloid fibril formation. Small peptide segments (6–10 residues) from amyloidogenic polypeptides (Aβ, IAPP, and α-Synuclein) are grafted into CDR3 of a single-domain (VH) antibody, and the resulting gammabodies are evaluated for their ability to inhibit nucleation of monomers and/or conversion of amyloid intermediates (prefibrillar oligomers or fibrillar intermediates) into fibrils.
Results
Aβ Gammabodies Potently Inhibit Amyloid Formation.
To evaluate our hypotheses related to the design of domain antibody inhibitors of amyloid assembly, we first sought to optimize the biophysical properties of a VH antibody scaffold for CDR grafting (4, 8, 9). We find that gammabodies presenting hydrophobic Aβ peptide segments (e.g., Aβ residues 15–24 and 33–42) within their third CDR (CDR3) readily aggregate when heated, stick to size-exclusion columns, and express at relatively low levels (5–7 mg/L) (4, 9). However, inserting a triad of negatively charged residues (Asp-Glu-Asp) at each edge of the hydrophobic CDR3 loops results in gammabodies that fail to aggregate when heated, elute as monomeric peaks from size-exclusion columns, and express at relatively high levels (15–20 mg/L; Fig. S1) (9). Importantly, the charged Aβ gammabodies bind with much higher affinity to Aβ fibrils (IC50 values of 190–210 nM; Kd value of 320 ± 30 nM for the Aβ33–42 gammabody) than they bind to Aβ monomers (IC50 values >3 μM; Fig. S1), their binding affinity is similar to uncharged Aβ gammabodies (IC50 values of 330–520 nM; Kd values of 330–490 nM) (4, 9), and they fail to bind to soluble or aggregated conformers of other amyloidogenic polypeptides (IAPP and α-Synuclein; Fig. S1).
We next investigated the ability of the charged gammabodies (herein referred to simply as gammabodies) to inhibit Aβ amyloid formation (Fig. 2). We used antibodies specific for prefibrillar oligomers (A11) and fibrillar conformers (OC) to monitor the aggregation of Aβ42 via immunoblotting (Fig. 2A), as we reported previously (4, 10). In the absence of gammabody inhibitors, Aβ forms prefibrillar oligomers (recognized by the A11 antibody) after 1 d; these oligomers convert into fibrillar conformers (recognized by the OC antibody) on the second day and persist for an additional 4 d (longer times not evaluated).
Fig. 2.
Gammabodies inhibit Aβ amyloid formation at substoichiometric concentrations. Aβ42 (25 μM) was incubated in the absence (control) and presence of Aβ gammabodies (2.5 μM; 1:10 gammabody:Aβ molar ratio), and the gammabody–Aβ samples were evaluated via (A) immunoblotting, (B) AFM, (C) ANS fluorescence, and (D) ThT fluorescence. In A, the blots were probed with antibodies specific for prefibrillar oligomers (A11), fibrillar conformers (OC), and the N terminus of Aβ (6E10; loading control). In B, the AFM images are 3 × 3 μm, and the blank images are Aβ samples with heights <1 nm relative to the heights of the Aβ aggregates that are 7–19 nm (average heights of 7 ± 1 nm on day 1, 14 ± 5 nm on day 2, and 19 ± 7 nm on day 3). In C, the wavelength corresponding to the maximum emission fluorescence (λmax) is reported. In C and D, the reported errors are the SDs of three replicates.
We find that Aβ gammabodies inhibit amyloid formation, which we first evaluated at substoichiometric gammabody concentrations (1:10 gammabody:Aβ molar ratio; Fig. 2A). As expected, the Aβ1–10 gammabody (which fails to bind to Aβ) is noninhibitory. The Aβ12–21 and Aβ15–24 gammabodies also fail to inhibit the formation of prefibrillar oligomers (day 1) and fibrillar conformers (day 2) but convert fibrillar Aβ conformers into nonfibrillar ones (days 3–6). In contrast, the Aβ30–39 and Aβ33–42 gammabodies prevent formation of both oligomer and fibrillar Aβ conformers (days 0–6; Fig. 2A). This inhibitory activity is unchanged at higher gammabody concentrations (1:1 gammabody:Aβ molar ratio), whereas each active gammabody is inactive at a molar ratio of 1:100 gammabody:Aβ (Fig. S2). We also find that gammabodies presenting 6mer Aβ peptides (Aβ residues 16–21, 34–39, and 37–42) within CDR3 are as inhibitory as their parent gammabodies presenting 10mer Aβ peptides, whereas gammabodies presenting 4mer Aβ peptides (Aβ residues 36–39 and 39–42) are inactive (Fig. S2). Moreover, scrambling the grafted Aβ peptides (Fig. 2A) or mutating them with single proline substitutions or glycine insertions (Fig. S2) eliminates the inhibitory activity of gammabodies. Finally, Aβ gammabodies that are inhibitory when added before Aβ oligomerization (day 0) are noninhibitory when added after Aβ oligomerization (Fig. S3).
Although the inhibitory Aβ gammabodies eliminated A11- and/or OC-immunoreactivity, we sought additional evidence of their antiaggregation activity. Atomic force microscopy (AFM) imaging and fluorescence analysis using two dyes sensitive to the conformation of Aβ (8-anilino-1-naphthalene sulfonate, ANS; thioflavin T, ThT) confirmed that the Aβ12–21 and Aβ15–24 gammabodies fail to prevent formation of prefibrillar oligomers (day 1) or fibrillar intermediates (day 2), but both prevent fibril formation (days 3–6; Fig. 2 B–D and Fig. S3). AFM and fluorescence analysis also confirmed that the Aβ30–39 and Aβ33–42 gammabodies prevent both Aβ oligomerization and fibrillization (Fig. 2 B–D and Fig. S3). Importantly, the inhibitory activity of gammabodies presenting Aβ peptide segments that overlap (Aβ12–21/Aβ15–24 and Aβ30–39/Aβ33–42) is indistinguishable (Fig. 2 and Fig. S3). Finally, circular dichroism spectroscopy revealed that the Aβ15–24 gammabody converts β-sheet fibrillar intermediates (day 2) into unstructured Aβ conformers (days 3–6), whereas the Aβ33–42 gammabody maintains Aβ monomers (day 0) as unstructured conformers (days 1–6; Fig. S4).
These findings provide further evidence that gammabodies arrest Aβ in soluble conformers that are incompetent for amyloid formation, but they do not provide insight into the local structure of Aβ peptide segments within such conformers. Therefore, we evaluated the impact of the Aβ12–21 and Aβ33–42 gammabodies on the relative solvent accessibility of N-terminal (Aβ residues 3–10), middle (Aβ residues 18–22), and C-terminal (Aβ residues 30–36) Aβ peptide segments during fibrillization using a proteolytic assay that we have reported previously (10). We find that the solvent accessibility of the hydrophilic N terminus of Aβ is unchanged during Aβ fibrillization (days 0–6), and that the Aβ12–21 and Aβ33–42 gammabodies do not alter its solvent accessibility (Fig. S4). In the absence of Aβ gammabodies, the solvent protection of the hydrophobic C terminus of Aβ (residues 30–36) progressively increases upon conversion of Aβ monomers into prefibrillar oligomers (day 1) and fibrillar intermediates (day 2), at which point the Aβ C terminus fails to become more solvent protected upon conversion into fibrils (days 3–6). The Aβ12–21 gammabody converts Aβ fibrillar intermediates (day 2) into Aβ conformers (days 3–6) whose C terminus is as unfolded as within Aβ monomers (Fig. S4). In contrast, the Aβ33–42 gammabody maintains the hydrophobic C terminus of Aβ in an unfolded state without allowing Aβ to initially form solvent-protected aggregated conformers. Both Aβ gammabodies also increase the solvent exposure of the central hydrophobic region of Aβ (residues 18–22) in a similar manner as they do for the Aβ C terminus. Our findings collectively demonstrate that gammabodies inhibit aggregation either by arresting the conformational maturation of Aβ monomers or by converting fibrillar intermediates into unfolded conformers that possess biochemical properties indistinguishable from Aβ monomers.
Gammabodies Inhibit Aβ Amyloid Assembly by Forming Small Gammabody–Aβ Complexes.
We next sought to determine how substoichiometric concentrations of inhibitory gammabodies (1:10 gammabody:Aβ molar ratio) render excess Aβ in a state that is incompetent for amyloid formation. Interestingly, some chaperones, aromatic small molecules, and peptides with antiaggregation activity have also been shown to completely prevent amyloid formation at low substoichiometric concentrations (≤1:10 inhibitor:monomer molar ratios) by converting monomers into unstructured, nonamyloid complexes (11–17). Thus, we posited that gammabodies convert Aβ fibrillar intermediates and monomers into similar complexes that are incompetent for amyloid formation.
To evaluate this hypothesis, we performed size-exclusion chromatography analysis of Aβ amyloid formation in the absence and presence of gammabodies (Fig. 3). In the absence of gammabodies, Aβ sticks to the column (TSKgel G3000SWxl; Tosoh Bioscience) regardless of its conformation and fails to elute in nondenaturing buffers. However, gammabody–Aβ complexes elute as single, symmetric peaks due to the hydrophilicity of gammabodies (Fig. 3). Therefore, we evaluated the increase in size of gammabodies (18–19 kDa) in the presence of Aβ conformers (1:10 gammabody:Aβ molar ratio; Aβ42 molecular weight is 4.5 kDa) to further elucidate the mechanism used by gammabodies to inhibit amyloid formation. The Aβ1–10 gammabody that fails to inhibit amyloid formation (Fig. 2A) does not bind to Aβ (days 0–6; Fig. S5). In contrast, the Aβ15–24 gammabody binds to Aβ monomers (day 0), prefibrillar oligomers (day 1), and fibrillar intermediates (day 2; Fig. 3). Interestingly, on the third day, the Aβ15–24 gammabody converts relatively large gammabody–fibrillar intermediate complexes (>150 kDa) into small complexes (∼75 kDa), and the size of these complexes is invariant for an additional 3 d (longer times not evaluated). The Aβ33–42 gammabody rapidly converts Aβ monomers into small complexes (day 0) that are indistinguishable in size relative to those formed by the Aβ15–24 gammabody (days 3–6), and these complexes fail to change size for an additional 5 d (Fig. 3). We obtained similar sizes of gammabody–Aβ complexes (∼75 kDa) using a different size-exclusion column (Superdex 200; GE Healthcare), as well as via cross-linking and SDS/PAGE analysis (∼75 kDa; Fig. S5). Finally, size-exclusion analysis also revealed that the Aβ15–24 and Aβ33–42 gammabodies bind selectively to Aβ peptide fragments containing their cognate sequences (Fig. S5), suggesting that gammabodies bind to soluble Aβ via homotypic interactions.
Fig. 3.
Gammabodies inhibit amyloid formation by converting Aβ monomers or fibrillar intermediates into small gammabody– Aβ complexes. Size-exclusion chromatography analysis of Aβ42 (25 μM) in the presence of gammabodies (2.5 μM; 1:10 gammabody:Aβ molar ratio). The chromatograms were obtained using an analytical size-exclusion column (TSK Gel G3000SWxl) and monitored at 280 nm.
We next investigated the impact of varying the gammabody:Aβ molar ratio on the size of gammabody–Aβ complexes. We posited that equimolar concentrations of gammabody and Aβ would decrease the size of gammabody–Aβ complexes relative to those formed at substoichiometric gammabody concentrations. However, cross-linking and SDS/PAGE analysis reveals that the size of gammabody–Aβ complexes is unchanged over a wide range of gammabody:Aβ molar ratios (1:1–1:20) for the Aβ33–42 (Fig. 4) and Aβ15–24 (Fig. S6) gammabodies, which we also confirmed via size-exclusion chromatography analysis in the absence of cross-linker (Fig. S6). Strikingly, only the molar ratio of 1:10 gammabody:Aβ resulted in complete complexation of Aβ and gammabody, whereas higher gammabody concentrations (1:9–1:1 gammabody:Aβ molar ratios) resulted in uncomplexed gammabody and lower gammabody concentrations (1:11–1:20 gammabody:Aβ molar ratios) resulted in uncomplexed Aβ (Fig. 4 and Fig. S6). We also measured the stoichiometry of the Aβ33–42 gammabody complexes that elute from size-exclusion columns via fluorescence labeling analysis and find that the stoichiometry of such complexes is 10.9 ± 1.3 Aβ molecules per gammabody. This stoichiometric analysis yields a size of gammabody–Aβ complexes (63–68 kDa) that is similar to their measured size (∼75 kDa; Figs. 3 and 4 and Figs. S5 and S6). Collectively these results suggest that gammabodies form complexes composed of ∼10 Aβ peptides per gammabody, and higher (e.g., stoichiometric) gammabody concentrations do not reduce the size of gammabody–Aβ complexes.
Fig. 4.
Stoichiometric analysis of gammabody–Aβ complexes. Aβ42 (25 μM) was incubated in the presence of the Aβ33–42 gammabody at different stoichiometries (1:1–1:50 gammabody:Aβ molar ratios), the resulting complexes were cross-linked with glutaraldehyde and analyzed via SDS/PAGE and silver staining. As controls, Aβ and gammabodies were separately combined with cross-linker to demonstrate that neither one was cross-linked in a nonspecific manner.
We also evaluated whether gammabody–Aβ complexes are toxic to mammalian cells (Fig. 5 and Fig. S6). In the absence of gammabodies, Aβ prefibrillar oligomers (day 1) are most toxic and Aβ monomers (day 0) are least toxic at high Aβ concentrations (2.5 μM; Fig. 5 and Fig. S6), whereas only prefibrillar oligomers are toxic at lower Aβ concentrations (0.1 μM; Fig. S6). Moreover, the Aβ gammabodies are nontoxic (Fig. 5 and Fig. S6). Addition of the Aβ15–24 gammabody fails to inhibit the toxicity of Aβ monomers (day 0) or prefibrillar oligomers (day 1) but inhibits the toxicity of fibrillar intermediates (day 2). Importantly, the gammabody–Aβ complexes formed by the Aβ15–24 (days 3–6) and Aβ33–42 (days 0–6) gammabodies are nontoxic (Fig. 5. and Fig. S6). We conclude that gammabodies inhibit amyloid formation by converting Aβ into small complexes that are benign.
Fig. 5.
Gammabodies inhibit Aβ-mediated cytotoxicity. Aβ42 (25 μM) was incubated in the absence (control) and presence of gammabodies (2.5 μM; 1:10 gammabody:Aβ molar ratio), and the toxicity of gammabody–Aβ samples to PC12 cells was evaluated via an MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction assay. The final Aβ and gammabody concentrations after dilution into the cell culture media were 2.5 and 0.25 μM, respectively. The reported errors are the SDs of three replicates.
Conventional Sequence- and Conformation-Specific Antibodies Fail to Potently Inhibit Aβ Amyloid Formation.
We suspected that the potent inhibitory activity of Aβ gammabodies is linked to their unusual mode of interaction with the hydrophobic peptide segments that mediate Aβ fibril formation. This led us to posit that conventional Aβ antibodies would be unable to inhibit Aβ fibrillization at similar low substoichiometric concentrations (1:10 antibody:Aβ molar ratio) because such antibodies would either only sequester a small fraction of Aβ monomer (sequence-specific antibodies) or fail to bind to linear hydrophobic Aβ epitopes that mediate aggregation (conformation-specific antibodies).
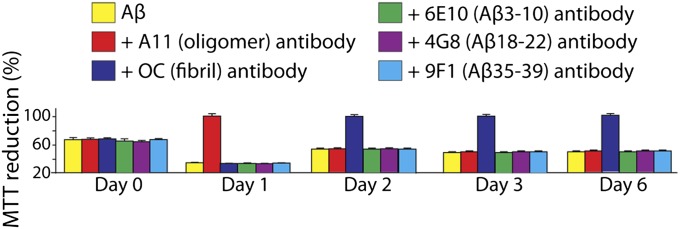
To test these hypotheses, we evaluated the antiaggregation activity of three sequence-specific monoclonal antibodies against the N-terminal (Aβ residues 3–10; 6E10), middle (Aβ residues 18–22; 4G8), and C-terminal (Aβ residues 35–39; 9F1) regions of Aβ, as well as two conformation-specific polyclonal antibodies against prefibrillar oligomers (A11) and fibrillar conformers (OC; Fig. 6 and Fig. S7). Importantly, none of these antibodies inhibit Aβ fibrillization at substoichiometric concentrations (1:10 antibody:Aβ molar ratio). Size-exclusion chromatography analysis confirmed that each noninhibitory antibody bound to Aβ in the expected manner (Fig. S7). Although the conformation-specific polyclonal antibodies A11 and OC fail to arrest amyloid formation, their binding to Aβ oligomers (A11) and fibrillar conformers (OC) inhibits toxicity (Fig. 6). The sequence-specific Aβ antibodies (6E10, 4G8, and 9F1) fail to prevent toxicity at the same substoichiometric concentrations (Fig. 6), as expected on the basis of the small fraction of Aβ sequestered by such antibodies before oligomerization (Fig. S7). Our findings demonstrate that conventional conformation-specific (polyclonal) antibodies bind to aggregated Aβ conformers and prevent their toxicity without inhibiting amyloid formation.
Fig. 6.
Impact of substoichiometric concentrations of conventional sequence- and conformation-specific antibodies on Aβ-mediated cytotoxicity. Aβ42 (25 μM) was incubated in the absence (control) and presence of substoichiometric concentrations (2.5 μM; 1:10 antibody:Aβ molar ratio) of monoclonal sequence-specific (6E10, Aβ residues 3–10; 4G8, Aβ residues 18–22; 9F1, Aβ residues 35–39) and polyclonal conformation-specific (A11, prefibrillar oligomers; OC, fibrillar conformers) antibodies. The antibody–Aβ samples were diluted 10-fold into PC12 cell cultures, and the toxicity was evaluated via an MTT reduction assay. The reported errors are the SDs of three replicates.
IAPP and α-Synuclein Gammabodies Potently Inhibit Amyloid Formation in a Sequence-Specific Manner.
The ability of Aβ gammabodies to potently inhibit amyloid formation led us to investigate whether gammabodies could be designed to inhibit fibrillization of other amyloidogenic polypeptides. Therefore, we selected the peptide hormone IAPP that forms amyloidogenic aggregates associated with type 2 diabetes (18), and the protein α-Synuclein that forms aggregates linked to Parkinson's disease (19). We identified 10-residue amyloidogenic peptide segments in IAPP (residues 22-NFGAILSSTN-31) and α-Synuclein (residues 69-AVVTGVTAVA-78) that are predicted to mediate amyloid formation of each polypeptide by multiple algorithms (20–24). Grafting these peptide segments into CDR3 (along with negatively charged residues at each edge of CDR3; SI Methods) yielded single-domain (VH) gammabodies that are well-expressed (>20 mg/L) and fail to aggregate when heated. Each gammabody binds with higher affinities to its cognate fibrils (IC50 values of 204 ± 7 and 222 ± 10 nM for the IAPP and α-Synuclein gammabodies, respectively; Kd values of 1.37 ± 0.05 and 1.40 ± 0.08 μM for the IAPP and α-Synuclein gammabodies, respectively) than to its monomers (IC50 values >3 μM), and fails to cross-react with soluble or aggregated polypeptides that lack the corresponding peptide segments (Fig. S8).
We next evaluated the ability of the IAPP and α-Synuclein gammabodies to inhibit amyloid formation of each polypeptide at substoichiometric concentrations (Fig. 7 and Figs. S8 and S9). Strikingly, the IAPP and α-Synuclein gammabodies inhibit fibrillization in a sequence-specific manner at both 1:10 (Fig. 7) and 1:1 (Fig. S8) gammabody:monomer molar ratios. Single proline substitution or glycine insertion mutations in the grafted peptide segments eliminate the inhibitory activity of each gammabody (Fig. S8). We also find that the IAPP and α-Synuclein gammabodies inhibit amyloid formation by rapidly converting their respective amyloidogenic polypeptides into small complexes (<100 kDa) that are incompetent for amyloid formation (Fig. S9), as observed for the Aβ33–42 gammabody (Fig. 3). Moreover, the conformation of IAPP and α-Synuclein in these small complexes is indistinguishable from the corresponding monomeric polypeptides, and the complexes are nontoxic (Fig. S9). Importantly, conventional sequence- and conformation-specific antibodies against α-Synuclein and IAPP that were obtained via immunization are noninhibitory at substoichiometric concentrations (1:10 antibody:monomer molar ratio; Fig. 7 and Fig. S9). We conclude that domain antibodies displaying amyloidogenic peptide sequences within their CDRs are more potent inhibitors of amyloid formation than typical conventional antibodies obtained via immunization.
Fig. 7.
IAPP and α-Synuclein gammabodies potently inhibit amyloid formation in a sequence-specific manner. IAPP (32 μM) and α-Synuclein (residues 1–115, 50 μM) were incubated in the absence (control) and presence of gammabodies (1:10 gammabody:monomer molar ratio), and fibrillization was monitored via (A) immunoblotting and (B) AFM. In B, IAPP and α-Synuclein fibrillization was also monitored in the presence of sequence-specific (R10/99, IAPP residues 7–17; 5C2, α-Synuclein residues 61–95) and conformation-specific (A11, prefibrillar oligomers; OC, fibrillar conformers) antibodies (1:10 antibody:monomer molar ratio). In B, the AFM images are 3 × 3 μm, and the blank images are samples with heights <1 nm. The heights of the IAPP and α-Synuclein aggregates are 21 ± 3 and 25 ± 4 nm, respectively.
Discussion
We have demonstrated that potent domain antibody inhibitors of amyloid formation can be designed in a rational manner. Our work is inspired by previous studies that demonstrated the ability of amyloidogenic peptide fragments to inhibit fibrillization of polypeptides containing the cognate peptide sequences (25–31). A common concern when using amyloidogenic peptides as aggregation inhibitors is their poor solubility. Our design strategy overcomes this limitation by inserting charged residues at each edge of the grafted hydrophobic peptides, which yields highly soluble domain antibodies that fail to aggregate even when heated (9). We expect that the simplicity of designing highly soluble gammabodies presenting extremely hydrophobic peptides within their CDR loops will simplify the generation of additional gammabodies for targeting diverse amyloidogenic proteins.
We identified two mechanisms used by gammabodies to inhibit amyloid formation that have not been described previously for antibody inhibitors of protein aggregation. The primary inhibitory mechanism used by the Aβ30–39 and Aβ33–42 gammabodies, as well as by the IAPP and α-Synuclein gammabodies, is to rapidly convert amyloidogenic monomers into small complexes that are unstructured and benign. The formation of these gammabody–polypeptide complexes occurred within the time required to perform size-exclusion chromatography (minutes), revealing that this “nucleation” process is much more rapid than the time required for each polypeptide to nucleate into amyloidogenic aggregates (hours to days). We posit that the grafted peptides are in conformations that are unable to nucleate amyloidogenic conformers, but instead nucleate oligomeric complexes that are incompetent for amyloid formation.
We also identified a second inhibitory mechanism used by two Aβ gammabodies (Aβ12–21 and Aβ15–24) in which the grafted domain antibodies participate in on-pathway oligomerization. These gammabodies disaggregate fibrillar conformers into gammabody–Aβ complexes when Aβ fibrillar intermediates would otherwise mature into amyloid fibrils. Size-exclusion chromatography revealed that the gammabodies only bind to Aβ prefibrillar oligomers if they are added before Aβ oligomerization, suggesting that the Aβ12–21 and Aβ15–24 gammabodies intercalate into on-pathway Aβ oligomers during nucleation. Previous work has established that the central hydrophobic region of Aβ (residues 17–21) undergoes conformational changes when fibrillar intermediates convert into amyloid fibrils (32, 33). Thus, we hypothesize that the conformations of the grafted Aβ12–21 and Aβ15–24 loops are incompatible with the β-sheet conformation formed by the central Aβ motif when fibrillar intermediates convert into amyloid fibrils, which leads to destabilization of Aβ fibrils and formation of small gammabody–Aβ complexes.
Our results also illuminate why conventional antibodies are typically unable to inhibit amyloid formation at low substoichiometric concentrations (≤1:10 antibody:monomer molar ratios). We find that conventional sequence-specific antibodies against three different amyloidogenic polypeptides (Aβ, IAPP, and α-Synuclein) are unable to promote nucleation of oligomeric complexes (as observed for the Aβ30–39, Aβ33–42, IAPP, and α-Synuclein gammabodies) or participate in the aggregation cascade (as observed for the Aβ12–21 and Aβ15–24 gammabodies). Instead, these conventional antibodies sequester a small fraction of amyloidogenic monomer and are unable to prevent uncomplexed monomer from assembling into fibrils. In contrast, we find that conventional conformation-specific (polyclonal) antibodies also fail to use either inhibitory mechanism used by gammabodies. Instead, these polyclonal antibodies bind specifically to prefibrillar oligomers or fibrillar intermediates, but this binding is unable to arrest the conformational maturation of amyloidogenic intermediates into fibrils. We posit that the lack of inhibitory activity of such antibodies is due to their inability to bind to the linear hydrophobic segments that mediate amyloid formation.
Nevertheless, some antibodies that are either sequence- and/or conformation-specific have been reported to inhibit amyloid formation at low substoichiometric antibody concentrations (≤1:10 antibody:monomer molar ratios) (34–38). Interestingly, these antibodies seem to use distinct mechanisms to inhibit amyloid formation relative to those used by gammabodies. For example, a conformation-specific antibody fragment that recognizes Aβ40 fibrillar intermediates (protofibrils) inhibits amyloid formation by preventing protofibrils from converting into fibrils (1:10 antibody:monomer molar ratio) (34). Moreover, some IgG heavy chains inhibit Aβ fibrillization by promoting formation of off-pathway aggregates at even lower substoichiometric concentrations (∼1:20–1:40 antibody:monomer molar ratios) (35). Importantly, these inhibitory antibodies render Aβ in aggregated conformations that are distinct from Aβ monomers. In contrast, our gammabodies render Aβ in complexes that possess structural properties indistinguishable from those of Aβ monomers. We expect that hybrid antibodies that use multiple inhibitory mechanisms will be extremely potent inhibitors of amyloid formation.
The simplicity of our gammabody design strategy deserves further consideration. On the basis of our previous work on designing gammabodies specific for Aβ (4), we used multiple algorithms (20–24) to identify potential amyloidogenic peptide segments within α-Synuclein and IAPP to guide our design of gammabodies against each polypeptide. These algorithms predict amyloidogenic peptides based on properties such as hydrophobicity, charge, and propensity to form β-sheets and/or steric zippers. We find that the most amyloidogenic peptide segments within α-Synuclein and IAPP are capable of potently inhibiting amyloid formation when grafted into antibody loops. However, both α-Synuclein and IAPP contain additional peptide segments that are predicted to be amyloidogenic (e.g., IAPP residues 12–18 and α-Synuclein residues 85–95), which we would also expect to inhibit fibrillization when grafted into similar antibody loops. Moreover, we expect that grafting more than one amyloidogenic peptide into multiple loops within single- and multidomain antibodies will yield gammabodies with even higher potency for inhibiting amyloid formation. The ease of generating gammabodies will allow these and related hypotheses to be rapidly evaluated to further define principles for designing highly potent inhibitors of amyloid formation.
Methods
Αβ42 peptide (American Peptide) was dissolved in 100% hexafluoroisopropanol (HFIP; Fluka), and the HFIP was evaporated overnight. Aβ was then dissolved in 50 mM NaOH (1 mg/mL Aβ), sonicated (30 s), and diluted in PBS (25 μM Aβ). The peptide was incubated at 25 °C for 0–6 d. IAPP was synthesized and purified as described previously (39), and then dissolved in HFIP. After removal of HFIP, IAPP was dissolved in 20 mM Tris·HCl (pH 7.4) at 32 μM and incubated at 25 °C for 0–4 d. α-Synuclein (residues 1–140) and a fragment thereof (residues 1–115) were expressed in bacteria, purified as described previously (40), diluted into buffer [20 mM Hepes, 0.1 M NaCl (pH 7.4)] at 50 μM (α-Synuclein residues 1–115) and 100 μM (α-Synuclein residues 1–140), and agitated (500 rpm) at 37 °C for 0–7 d.
Additional methods are described in SI Methods: cloning, expression and purification of gammabodies, immunoblot analysis, ThT and ANS fluorescence, AFM imaging, cell toxicity analysis, size-exclusion chromatography, cross-linking and SDS/PAGE analysis, circular dichroism spectroscopy, and proteolytic analysis.
Supplementary Material
Acknowledgments
We thank Lila Gierasch for suggesting the cross-linking experiments. This work was supported by American Health Assistance Foundation Grant A2011355 (to P.M.T.), National Science Foundation Grants 954450 and 1159943 (to P.M.T.), the Pew Charitable Trust (Pew Scholar Award in Biomedical Sciences to P.M.T.), and by National Institutes of Health Grants GM078114 (to D.P.R.), 1F32DK089734 (to A.A.), and AG027936 (to R.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208797109/-/DCSupplemental.
References
- 1.Härd T, Lendel C. Inhibition of amyloid formation. J Mol Biol. 2012;421(4–5):441–465. doi: 10.1016/j.jmb.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 2.Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc Natl Acad Sci USA. 1996;93(1):452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emadi S, et al. Inhibiting aggregation of alpha-synuclein with human single chain antibody fragments. Biochemistry. 2004;43(10):2871–2878. doi: 10.1021/bi036281f. [DOI] [PubMed] [Google Scholar]
- 4.Perchiacca JM, Ladiwala AR, Bhattacharya M, Tessier PM. Structure-based design of conformation- and sequence-specific antibodies against amyloid β. Proc Natl Acad Sci USA. 2012;109(1):84–89. doi: 10.1073/pnas.1111232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 6.Kayed R, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Nuallain B, Wetzel R. Conformational Abs recognizing a generic amyloid fibril epitope. Proc Natl Acad Sci USA. 2002;99(3):1485–1490. doi: 10.1073/pnas.022662599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barthelemy PA, et al. Comprehensive analysis of the factors contributing to the stability and solubility of autonomous human VH domains. J Biol Chem. 2008;283(6):3639–3654. doi: 10.1074/jbc.M708536200. [DOI] [PubMed] [Google Scholar]
- 9.Perchiacca JM, Ladiwala AR, Bhattacharya M, Tessier PM. Aggregation-resistant domain antibodies engineered with charged mutations near the edges of the complementarity-determining regions. Protein Eng Des Sel. 2012;25(10):591–602. doi: 10.1093/protein/gzs042. [DOI] [PubMed] [Google Scholar]
- 10.Ladiwala AR, et al. Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J Biol Chem. 2012;287(29):24765–24773. doi: 10.1074/jbc.M111.329763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrnhoefer DE, et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15(6):558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 12.Ladiwala AR, Dordick JS, Tessier PM. Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J Biol Chem. 2011;286(5):3209–3218. doi: 10.1074/jbc.M110.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustafi SM, Garai K, Crick SL, Baban B, Frieden C. Substoichiometric inhibition of Abeta(1-40) aggregation by a tandem Abeta(40-1-Gly8-1-40) peptide. Biochem Biophys Res Commun. 2010;397(3):509–512. doi: 10.1016/j.bbrc.2010.05.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans CG, Wisén S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J Biol Chem. 2006;281(44):33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- 15.Luk KC, Mills IP, Trojanowski JQ, Lee VM. Interactions between Hsp70 and the hydrophobic core of alpha-synuclein inhibit fibril assembly. Biochemistry. 2008;47(47):12614–12625. doi: 10.1021/bi801475r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem. 2005;280(15):14733–14740. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- 17.Mannini B, et al. Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc Natl Acad Sci USA. 2012;109(31):12479–12484. doi: 10.1073/pnas.1117799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KH, O’Brien TD, Betsholtz C, Westermark P. Islet amyloid polypeptide: Mechanisms of amyloidogenesis in the pancreatic islets and potential roles in diabetes mellitus. Lab Invest. 1992;66(5):522–535. [PubMed] [Google Scholar]
- 19.Duda JE, Lee VM, Trojanowski JQ. Neuropathology of synuclein aggregates. J Neurosci Res. 2000;61(2):121–127. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Linding R, Schymkowitz J, Rousseau F, Diella F, Serrano L. A comparative study of the relationship between protein structure and beta-aggregation in globular and intrinsically disordered proteins. J Mol Biol. 2004;342(1):345–353. doi: 10.1016/j.jmb.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 21.Conchillo-Solé O, et al. AGGRESCAN: A server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinformatics. 2007;8:65. doi: 10.1186/1471-2105-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson MJ, et al. The 3D profile method for identifying fibril-forming segments of proteins. Proc Natl Acad Sci USA. 2006;103(11):4074–4078. doi: 10.1073/pnas.0511295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trovato A, Seno F, Tosatto SC. The PASTA server for protein aggregation prediction. Protein Eng Des Sel. 2007;20(10):521–523. doi: 10.1093/protein/gzm042. [DOI] [PubMed] [Google Scholar]
- 24.Maurer-Stroh S, et al. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nat Methods. 2010;7(3):237–242. doi: 10.1038/nmeth.1432. [DOI] [PubMed] [Google Scholar]
- 25.Ghanta J, Shen CL, Kiessling LL, Murphy RM. A strategy for designing inhibitors of beta-amyloid toxicity. J Biol Chem. 1996;271(47):29525–29528. doi: 10.1074/jbc.271.47.29525. [DOI] [PubMed] [Google Scholar]
- 26.Soto C, et al. Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: Implications for Alzheimer’s therapy. Nat Med. 1998;4(7):822–826. doi: 10.1038/nm0798-822. [DOI] [PubMed] [Google Scholar]
- 27.Sievers SA, et al. Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation. Nature. 2011;475(7354):96–100. doi: 10.1038/nature10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tjernberg LO, et al. Arrest of beta-amyloid fibril formation by a pentapeptide ligand. J Biol Chem. 1996;271(15):8545–8548. doi: 10.1074/jbc.271.15.8545. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Mihara H. Peptide and protein mimetics inhibiting amyloid beta-peptide aggregation. Acc Chem Res. 2008;41(10):1309–1318. doi: 10.1021/ar8000475. [DOI] [PubMed] [Google Scholar]
- 30.Scrocchi LA, et al. Design of peptide-based inhibitors of human islet amyloid polypeptide fibrillogenesis. J Mol Biol. 2002;318(3):697–706. doi: 10.1016/S0022-2836(02)00164-X. [DOI] [PubMed] [Google Scholar]
- 31.Kapurniotu A, Schmauder A, Tenidis K. Structure-based design and study of non-amyloidogenic, double N-methylated IAPP amyloid core sequences as inhibitors of IAPP amyloid formation and cytotoxicity. J Mol Biol. 2002;315(3):339–350. doi: 10.1006/jmbi.2001.5244. [DOI] [PubMed] [Google Scholar]
- 32.Scheidt HA, Morgado I, Rothemund S, Huster D, Fändrich M. Solid-state NMR spectroscopic investigation of Aβ protofibrils: Implication of a β-sheet remodeling upon maturation into terminal amyloid fibrils. Angew Chem Int Ed Engl. 2011;50(12):2837–2840. doi: 10.1002/anie.201007265. [DOI] [PubMed] [Google Scholar]
- 33.Kheterpal I, Chen M, Cook KD, Wetzel R. Structural differences in Abeta amyloid protofibrils and fibrils mapped by hydrogen exchange—mass spectrometry with on-line proteolytic fragmentation. J Mol Biol. 2006;361(4):785–795. doi: 10.1016/j.jmb.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 34.Habicht G, et al. Directed selection of a conformational antibody domain that prevents mature amyloid fibril formation by stabilizing Abeta protofibrils. Proc Natl Acad Sci USA. 2007;104(49):19232–19237. doi: 10.1073/pnas.0703793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adekar SP, et al. Inherent anti-amyloidogenic activity of human immunoglobulin gamma heavy chains. J Biol Chem. 2010;285(2):1066–1074. doi: 10.1074/jbc.M109.044321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Nuallain B, Hrncic R, Wall JS, Weiss DT, Solomon A. Diagnostic and therapeutic potential of amyloid-reactive IgG antibodies contained in human sera. J Immunol. 2006;176(11):7071–7078. doi: 10.4049/jimmunol.176.11.7071. [DOI] [PubMed] [Google Scholar]
- 37.Yoshihara T, et al. Immunoreactivity of phage library-derived human single-chain antibodies to amyloid beta conformers in vitro. J Biochem. 2008;143(4):475–486. doi: 10.1093/jb/mvm239. [DOI] [PubMed] [Google Scholar]
- 38.Frenkel D, Balass M, Katchalski-Katzir E, Solomon B. High affinity binding of monoclonal antibodies to the sequential epitope EFRH of beta-amyloid peptide is essential for modulation of fibrillar aggregation. J Neuroimmunol. 1999;95(1–2):136–142. doi: 10.1016/s0165-5728(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 39.Abedini A, Raleigh DP. Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregation-prone polypeptide. Org Lett. 2005;7(4):693–696. doi: 10.1021/ol047480+. [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Margittai M, Chen J, Langen R. Investigation of alpha-synuclein fibril structure by site-directed spin labeling. J Biol Chem. 2007;282(34):24970–24979. doi: 10.1074/jbc.M700368200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.