Abstract
The ability of natural peptides and proteins to influence the formation of inorganic crystalline materials has prompted the design of synthetic compounds for the regulation of crystal growth, including the freezing of water and growth of ice crystals. Despite their versatility and ease of structural modification, peptidomimetic oligomers have not yet been explored extensively as crystallization modulators. This report describes a library of synthetic N-substituted glycine peptoid oligomers that possess “dual-action” antifreeze activity as exemplified by ice crystal growth inhibition concomitant with melting temperature reduction. We investigated the structural features responsible for these phenomena and observed that peptoid antifreeze activities depend both on oligomer backbone structure and side chain chemical composition. These studies reveal the capability of peptoids to act as ice crystallization regulators, enabling the discovery of a unique and diverse family of synthetic oligomers with potential as antifreeze agents in food production and biomedicine.
Keywords: tailored additive, X-ray diffraction
The regulation of crystallization outcomes is an enduring and formidable challenge, with practical implications ranging from the treatment of biomineralization-associated diseases to the manufacture of pharmaceuticals and electronic materials (1, 2). Tailored additives have been reported to regulate crystallization via binding to specific crystal growth sites through molecular recognition, thereby presenting substituents at the crystal surface that perturb the attachment of solute growth units (3–5). For example, the crystallization of amino acids, organic crystals and pharmaceutical compounds, calcium-containing biominerals, and pathological crystals, are influenced by tailored additives (5–10), often resulting in crystal growth inhibition and alterations in crystal morphology (i.e., crystal habit or shape) and sometimes influencing crystal polymorphism. Some of these studies have attempted to unravel the critical structure–property relationships through the use of well-defined small molecule additives thought to resemble residues and segments in naturally occurring proteins that influence biomineralization (8, 11).
Ice formation is arguably the most ubiquitous natural crystallization process, yet in many respects the freezing properties of water are poorly understood (12), particularly with respect to extrinsic factors that can influence the onset of freezing (13). For example, natural antifreeze proteins (AFPs) are known to exert a protective function, permitting fish to survive in subzero marine environments (14, 15). Other protein sequences, however, can promote extracellular freezing in some amphibians during hibernation (16). The underlying mechanisms of these apparently discordant phenomena remain unresolved. Natural AFPs and antifreeze glycoproteins (AFGPs) are thought to influence ice crystal growth through an adsorption–inhibition mechanism in which the apparent freezing point (i.e., the temperature at which the onset of freezing can be observed) is depressed through noncolligative effects (13, 14, 17). The adsorption of these agents on specific crystal faces inhibits growth on these faces, thereby influencing growth rates and ice crystal morphologies (18). This noncolligative effect can be explained by binding of the AFPs and AFGPs to the surfaces of precritical ice nuclei— which cannot be observed readily because of their minute size—thereby frustrating growth of the nuclei beyond their critical size and giving the appearance of freezing point depression. Under these conditions, freezing will be observed only when the temperature is lowered to an extent that the system is driven further from equilibrium, such that the nucleation barrier can be surmounted (19). Moreover, the apparent freezing point depression is greater than anticipated from colligative effects alone (20).
The interactions of proteins with ice crystals have been attributed to a variety of physicochemical characteristics, including hydrophilicity, amphiphilicity, and secondary structure. Elucidating the critical features responsible for molecular recognition at the ice crystal interface, however, is complicated by the wide range of molecular weights, sequences, and structures for AFPs. In addition, the inherent complex structure and heterogeneous chemical composition of proteins make unraveling the role of a particular natural protein in ice crystallization at the molecular level extraordinarily challenging (21). Investigations of ice crystallization in the presence of synthetic small molecules with well-defined features are more likely to reveal the critical structure–property relationships responsible for antifreeze behavior. Such investigations may also reveal pathways to the discovery of chemical additives for preventing ice formation in a variety of applications, ranging from food production to cryopreservation of tissue.
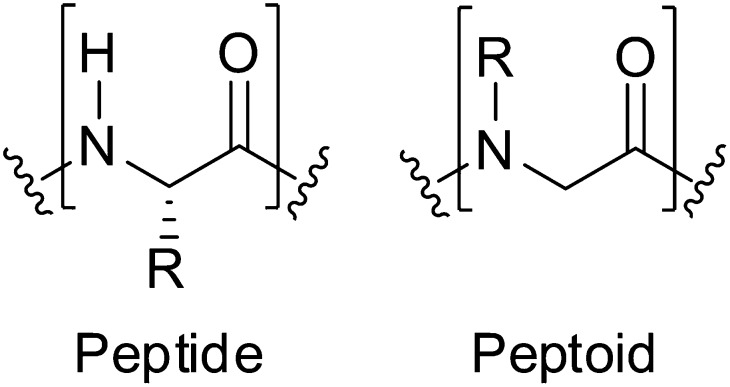
Noncolligative antifreeze activity has been reported for a limited number of synthetic molecules, including oligopeptides (22–25) and bulk polymers (26–28). Synthetic oligomers that can mimic the structures and functions of natural polypeptides (29) present an opportunity for the exploration of antifreeze activity paralleling that observed in natural systems. Among these, N-substituted glycine peptoids stand out as a family of peptidomimetic oligomers (30) with potential as modulators of crystallization processes (Fig. 1). Peptoids can be synthesized with precise control of the sequence of highly diverse side chain functional groups, enabling a robust investigation of structure–property relationships (31). Peptoids can be synthesized efficiently on solid phase using a broad range of primary amines as synthons, thus greatly expanding their chemical diversity compared with natural polypeptides. Moreover, recent advances to induce peptoid conformational order (32–34) have enabled the discovery of biomimetic oligomers for materials (35), catalytic (36), and biomedical applications (37–39). Investigations of sequence-specific synthetic oligomers as modulators of crystallization (40–42), including the crystallization of ice, are sparse (43).
Fig. 1.
Chemical structures of peptides and peptoids. Whereas the side chains of peptides are located on the α-carbon, the side chains of peptoids are located on the amide nitrogen.
Herein we report an investigation of peptoids as regulators of ice crystallization, evaluating their antifreeze activity and mechanism through examination of ice morphologies, crystal growth rates, melting temperature reductions, and ice crystal growth orientations. Through a comparison of the activity of the peptoid oligomers to analogous peptide sequences, these studies illustrate that libraries of well-defined peptidomimetic molecules have the potential to reveal the critical structure–activity relationships responsible for antifreeze activity.
Results and Discussion
Synthesis and Characterization of Peptoids.
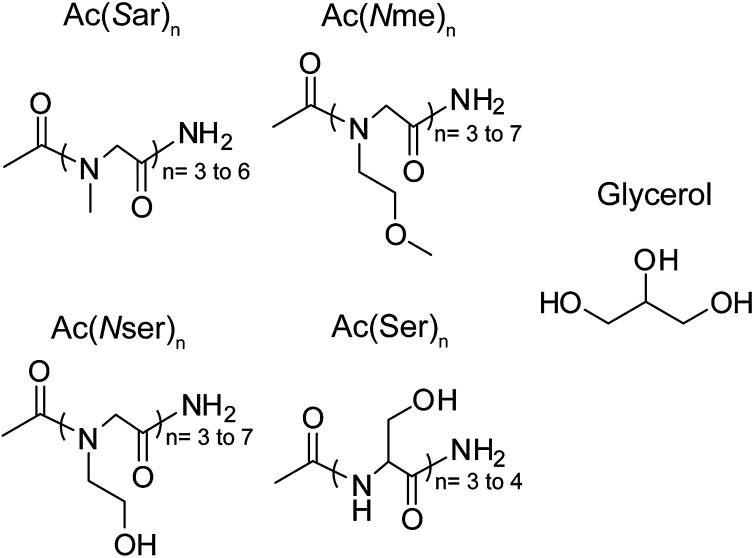
A small library of peptoids (Fig. 2) was synthesized to compare the effects of backbone structure, side chain chemical composition, and sequence length on antifreeze activity. Recognizing the importance of chemical functional groups for noncolligative antifreeze activity (44), peptoids ranging from trimers to heptamers with side chains bearing hydroxyl (Ac(Nser)n), ether (Ac(Nme)n), and methyl (Ac(Sar)n) substituents were synthesized. These functional groups have been used in various antifreeze agents (45). The peptoids were synthesized on Rink amide resin using standard “submonomer” protocols (46). The products were cleaved from the solid support, purified by reverse-phase high-performance liquid chromatography (RP-HPLC), and confirmed by mass spectrometry (SI Appendix). Short oligoserine peptides also were synthesized, and glycerol was used to allow comparison with a nonoligomeric molecule with hydroxyl substituents.
Fig. 2.
Peptoids and peptides with varying sequence lengths (n). Peptoids bearing methyl Sar, ether Nme, and hydroxyl Nser side chains were synthesized. Oligoserine peptides (Ac(Ser)n) and glycerol were used as comparisons for antifreeze activity.
Modulation of Ice Morphology by Peptoid Oligomers.
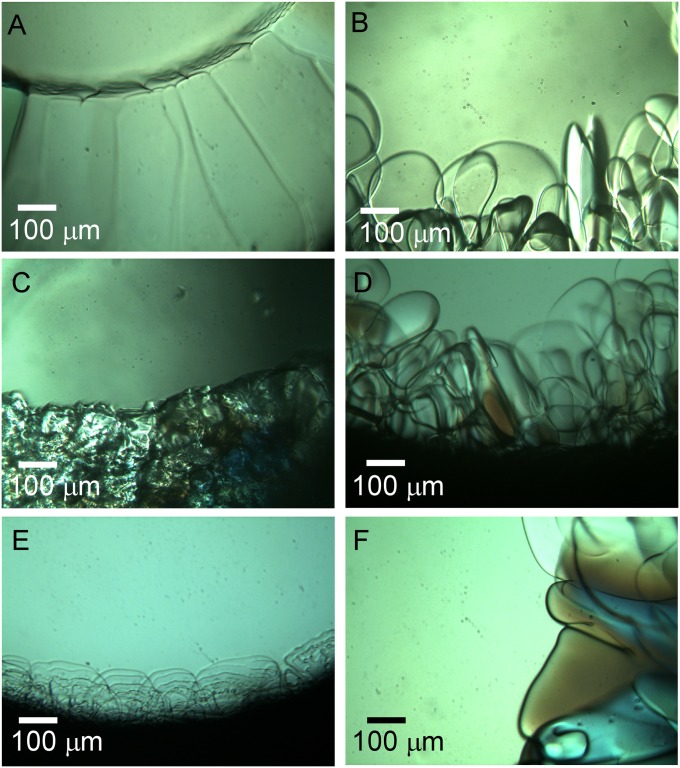
The effect of the aforementioned peptoids on ice morphology was evaluated by variable temperature video microscopy. Each antifreeze peptoid was dissolved in ultrapure water (to a concentration of 10 mg⋅mL−1), and a 20-μL aliquot was added to a circular reservoir constructed by sealing silicone isolators between two glass slides (Methods). This assembly was then mounted on a microscope equipped with a thermal stage that permitted controlled cooling and heating of the samples. To generate seed crystals, the solution was frozen rapidly at a rate of 40 °C⋅min−1 to −30 °C, then melted slowly at 3 °C⋅min−1 to 0.1 °C above the observed melting temperature until ∼90% of ice was melted. The solution was subsequently cooled at 0.05 °C⋅min−1 to −1.0 °C, at which images were obtained. Ice crystals from pure water began growing at −0.6 °C, and the apparent freezing points in the presence of the additives were observed below this temperature, at −1.0 ± 0.1 °C (SI Appendix). Ice crystals grown in pure water appeared as large grains (Fig. 3A), whereas ice crystals grown in the presence of tripeptoids, tripeptides, or glycerol were significantly smaller (Fig. 3 B–F). Similar reductions in ice crystal sizes were observed for peptoid sequences with longer chain lengths (tetramers to heptamers, see SI Appendix, Fig. S6), but the most significant reductions were observed for the tripeptoids Ac(Nser)3, Ac(Nme)3, and Ac(Sar)3. Ice crystals grown in the presence of Ac(Nser)3 (Fig. 3C) were smallest, suggesting that this molecule was the most effective inhibitor of ice grain growth.
Fig. 3.
Ice crystals grown at −1.0 °C in (A) pure water or 10 mg⋅mL−1 (B) glycerol, (C) Ac(Nser)3, (D) Ac(Ser)3, (E) Ac(Nme)3, and (F) Ac(Sar)3 exhibit different morphologies.
In addition to reduced grain sizes, the ice crystal growth fronts in the presence of Ac(Nser)3 (Fig. 3C) and Ac(Nser)6 (SI Appendix, Fig. S6) exhibited a jagged morphology, suggesting a pinning effect during ice growth (47). This effect was not observed for the other oligomers in the library or for glycerol (Fig. 3B). In contrast, ice crystals grown in the presence of Ac(Nme)3 exhibited a lamellar structure of overlapping plate-like ice crystals (Fig. 3E). These observations demonstrate that ice crystal morphology is sensitive to the chemical composition of the additives.
Peptoids as Inhibitors of Ice Crystal Growth.
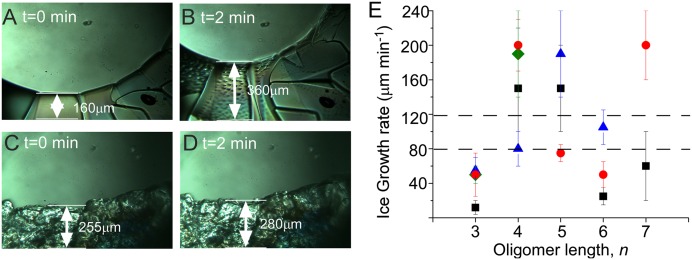
Ice crystal growth rates in the presence of additives were determined by measuring the advance of the ice growth fronts over time at −1.0 ± 0.1 °C (Fig. 4 A–D). In the absence of additives, the ice growth front advanced at a rate of 100 ± 20 μm⋅min−1, but in the presence of some additives the front advanced at significantly slower rates (Fig. 4E; SI Appendix, Table S2). Ice crystals in the presence of Ac(Sar)3 and Ac(Nme)3 grew at a rate of 55 ± 10 μm⋅min−1 and 50 ± 25 μm⋅min−1, respectively, whereas crystals formed in the presence of Ac(Nser)3, which was equipped with hydroxyl substituents, grew at a significantly slower rate of 12 ± 5 μm⋅min−1 (Fig. 4E). These observations suggest an important role for hydrogen-bonding interactions between the peptoid side chain and ice crystal surfaces. Glycerol, however, with three hydroxyl groups like Ac(Nser)3, did not influence the ice growth rate, illustrating that the existence of hydroxyl groups alone is not sufficient for altering ice growth rates. In the presence of the peptide Ac(Ser)3, the growth front advanced at a rate of 50 ± 10 μm⋅min−1, four times faster than in the presence of the peptoid Ac(Nser)3. This comparison suggests that the oligomer backbone structure is also an important feature for the modulation of ice crystal growth.
Fig. 4.
Ice growth rates in aqueous media containing peptoids and peptides (10 mg⋅mL−1) at −1.0 ± 0.1 °C. Sequential microscopy images collected for pure water (A and B) and in the presence of Ac(Nser)3 (C and D) reveal different rates of growth front advancement. (E) The dependence of growth rates on oligomer length, n. Black squares represent Ac(Nser)n sequences, red circles represent Ac(Nme)n sequences, blue triangles represent Ac(Sar)n sequences, and green diamonds represent Ac(Ser)n sequences. Dashed lines indicate the region (within error) of ice growth rates for water and glycerol (100 ± 20 μm⋅min−1). Error bars correspond to SE means (SI Appendix). Note: Ac(Nser)3 and Ac(Ser)3 exhibited growth rates of 12 ± 5 μm⋅min−1 and 50 ± 10 μm⋅min−1, respectively.
The slowest ice growth rates were observed in the presence of peptoid trimers and hexamers (Fig. 4E and SI Appendix, Table S2), regardless of the side chain identity. Ice growth rates in the presence of peptoid tetramers and pentamers were actually faster than that observed in pure water. The ice growth rate also was faster in the presence of the peptoid heptamer Ac(Nme)7 than in the presence of its hexameric counterpart. Although the molecular-level origin of this behavior is not revealed by these measurements, it is reminiscent of the behavior of some additives that have been reported to either promote or prevent crystal growth via adsorption on crystal faces (17–19, 48, 49).
Melting Temperature Analysis.
To evaluate the mechanism of antifreeze action and to ascertain the effect of the additives on the ice-water equilibrium, differential scanning calorimetry (DSC) experiments were conducted. Freezing temperatures, determined from the onset of freezing as observed by microscopy, often are used as a metric of antifreeze activity. The onset of freezing, however, can be an unreliable measure of the freezing point because of the inherent stochastic nature of nucleation and associated kinetic factors. As such, the onset of freezing may not reflect the true equilibrium freezing point. Therefore, the effect of the antifreeze agents was examined by measuring the melting temperatures with DSC, which is not influenced by the kinetic factors that compromise freezing point determinations. Aqueous solutions containing either glycerol or one of the peptoid trimers in Fig. 1, each at a concentration of 10 mg⋅mL−1, were placed in aluminum pans, rapidly cooled to −30 °C at a rate of 1 °C⋅min−1, and equilibrated at −30 °C for 10 min. Heating curves were then collected from −30 °C to 5 °C at a rate of 0.25 °C⋅min−1. These molecules were chosen to compare the effects of side chain chemical composition and backbone structure on the melting temperatures. The melting temperatures were determined from the maxima of the heat curves. Substantial reductions in the melting temperatures compared with water alone (ΔTm(DSC)) were evident (Table 1). The melting temperature depression was identical in the presence of Ac(Nser)3, Ac(Nme)3, and Ac(Ser)3, with ΔTm(DSC) = −0.40 ± 0.15 °C. Solutions of Ac(Sar)3 exhibited ΔTm(DSC) = −0.30 ± 0.15 °C and solutions of glycerol exhibited ΔTm(DSC) = −0.60 ± 0.10 °C. The larger melting temperature depression observed in the glycerol solutions can be attributed to the higher molar concentration compared with the trimer (100 mM vs. ∼30 mM), which would be expected to result in increased adsorption on the ice crystal surfaces.
Table 1.
Reductions in the melting temperatures of ice in the presence of different additives at 10 mg ⋅ mL−1
| Additive | MW (g⋅mol−1)* | ΔTm(DSC) (°C)† | ΔTm(colligative) (°C)‡ | R§ |
| Ac(Nser)3 | 362.38 | −0.40 ± 0.15 | −0.051 | 7.84 |
| Ac(Nme)3 | 404.46 | −0.40 ± 0.15 | −0.046 | 8.70 |
| Ac(Sar)3 | 272.30 | −0.30 ± 0.20 | −0.068 | 4.42 |
| Ac(Ser)3 | 320.30 | −0.40 ± 0.15 | −0.058 | 6.90 |
| Glycerol | 92.13 | −0.60 ± 0.10 | −0.203 | 2.96 |
*Molecular weight.
†Observed reduction in melting temperatures compared with water.
‡Reduction in melting temperatures calculated based on colligative effects (see Melting Temperature Analysis).
§R is the ratio ΔTm(DSC)/ ΔTm(colligative), indicative of the extent of noncolligative effects.
The observed ΔTm(DSC) values were compared with melting point depressions expected from colligative effects alone (ΔTm(colligative)) using a ratio R, determined for each solution according to Eq. 1,
where ΔTm(colligative) = Km · molality and Km is the cryoscopic constant, 1.86 °C⋅kg⋅mol−1 (50). The observed ΔTm(DSC) values will be identical to ΔTm(colligative) if colligative effects alone are operative (R = 1). The data reveal, however, that R > 1 in the presence of the additives (Table 1), indicating melting temperature reductions that exceed those expected from colligative effects. Ac(Nme)3 exerted the largest enhancement (R = 8.70), followed by Ac(Nser)3 (7.84), Ac(Ser)3 (6.90), Ac(Sar)3 (4.42), and glycerol (2.96).
At equilibrium, the ice crystal surface is in contact with liquid water. The observed melting temperature reduction in the presence of the peptoids may be attributed to a shift in solid–liquid equilibrium owing to peptoid adsorption on the ice surface. Another possible contributing factor is the inclusion of the peptoids into the frozen ice crystal. The significant effects exhibited by the peptoid Ac(Nser)3 on the ice crystal morphology and the melting temperature reduction compared with the other additives suggest that hydrogen-bonding interactions between water molecules at the ice crystal surface and oligomer hydroxyl groups is advantageous for this perturbation. The observed variations of the melting temperature reduction with changes in oligomer chemical composition indicate a noncolligative mode of action.
Two-dimensional X-Ray Diffraction Analysis.
Other research groups have investigated the effect of macromolecular additives on ice crystal properties using powder X-ray diffraction (XRD) (26, 27) of frozen macromolecule–ice mixtures. For example, some broadening of the (100) and (101) diffraction peaks for hexagonal ice formed in the presence of a poly(ethylene oxide)-polyethyleneimine block copolymer with polyglycidol side chains was surmised to be evidence of smaller crystals compared with ice crystals formed in the absence of this macromolecule (26). Whereas the intensity of the (002) reflection was comparable to that of the (100) and (101) reflections for pure water, in the presence of this polymer the (002) intensity was negligible. Although this observation was deduced to signal a change in ice crystal morphology, it may also reflect preferred orientations of the ice crystals such that the (002) planes are not parallel to the sample stage. In a related investigation, the (002) peak was absent for ice crystals grown in the presence of a poly(tartar amide) with glycol side chains, and the (101) intensity was substantially reduced (27). This study also revealed that the (002) reflection was negligible when ice crystals were grown in the presence of poly(ethylene glycol), specifically PEG-6 (average Mn = 5,900). This study was attributed to an earlier postulate that macromolecules can block the c-directional growth of ice crystals (51), producing ice nanoplatelets interleaved with polymers such that the diffraction intensity would be diminished (27). Whereas these results may also be attributed to preferred orientation of the ice crystals, ground ice crystals (to ensure a random orientation of crystal planes) from the polymer–ice mixture afforded an identical X-ray diffraction pattern. Moreover, microscopy of bulk ice crystals revealed a substantial influence of the polymers on ice crystal morphology.
In order to examine the influence of additives on ice crystallization in greater detail, we deployed an X-ray microdiffractometer equipped with a 2D area detector that enabled characterization—phase and crystal orientation—of multiple ice crystals located at different regions of a frozen water droplet, rather than sampling the entire frozen droplet (26, 27, 51). The microdiffractometer (0.5-mm incident beam width) enabled acquisition of diffraction patterns from a population of ice crystals formed in situ at the ice–water interface. XRD patterns of ice crystals grown in the presence of the trimers (Fig. 1) were evaluated to investigate the effects of side chain functional groups on ice crystal growth orientations. A 20-μL aqueous droplet, either pure water or a 10 mg⋅mL−1 additive solution, was placed on a microscope slide on top of a cooling stage. The contact angle between the droplet and microscope slide was only ∼15°, such that the air–water interface across the droplet was nearly parallel with the sample stage. Seed crystals were generated by cooling at 40 °C⋅min−1 from room temperature to −30 °C, at which XRD showed diffraction spots corresponding to 2θ values expected for hexagonal ice (SI Appendix, Fig. S8) in the P63/mmc space group (52, 53). The sample was then heated at 3 °C⋅min−1 to 0.1 °C above the melting temperature, as deduced by the disappearance of XRD diffraction spots. The sample was then cooled at 10 °C⋅min−1 to −1 °C, at which new ice crystals were evident from the diffraction spots on the 2D detector. XRD patterns were acquired for individual crystals within a circular annulus ∼1 mm away from the edge of the droplet (Methods).
Typically, the diffraction spots for hexagonal ice were observed at unique azimuthal angles (δ) on the 2D detector. The presence of discrete diffraction spots rather than continuous rings is consistent with the presence of oriented ice crystals at the air–water interface. Ice crystals were sampled by collecting diffraction data at incident X-ray angles of 11°, 12°, and 16.5° to ensure sampling of a reasonable number of crystals with orientations meeting the Bragg condition (Methods). The area of the X-ray beam (incident and exiting) ensures that ∼40% of the droplet is interrogated during each measurement.
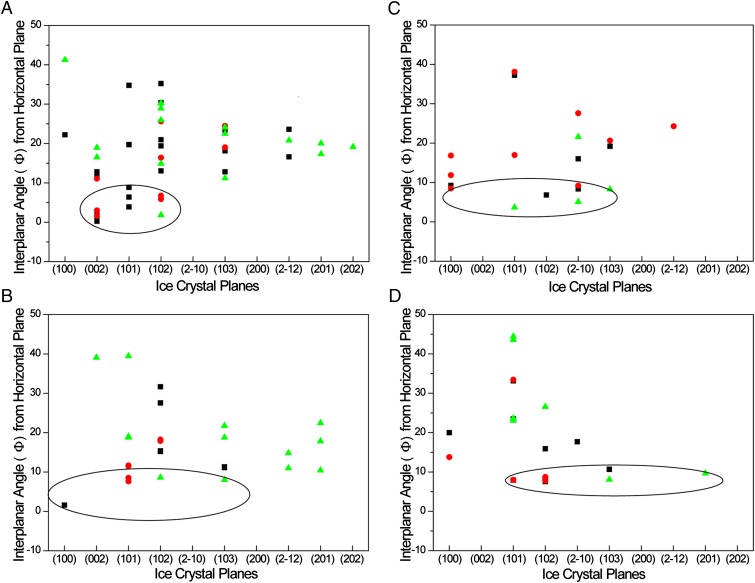
The interplanar angles (Φ) between diffraction planes and the horizontal plane of the microscope slide were calculated for each diffraction spot based on its (2θ, δ) coordinate on the 2D detector. The Φ values represent the orientations of corresponding diffraction planes, and lower Φ values correspond to diffraction planes that are nearly parallel to the microscope slide (Methods). With numerous XRD measurements, a distribution of Φ values from different diffraction planes that reflect the various orientations of crystals with respect to the air–water interface can be generated. The distribution of Φ vs. diffraction plane indicates that ice crystals grown in pure water exhibited the (002), (101), and (102) planes parallel (0° < Φ < 10°) to the horizontal plane of the glass sample stage (Fig. 5A). In contrast, the (002) plane was not observed at 0° < Φ < 10° in the presence of the additives (Fig. 5 B–D) and was only observed at higher Φ angles. In addition to the (101) and (102) orientations, the additive solutions also exhibited the (100), (210), and (103) planes nearly parallel to the microscope slide (0° < Φ < 10°). Among the additives, only Ac(Nme)3 exhibited the (210) orientation nearly parallel to the microscope slide (Fig. 5C).
Fig. 5.
Ice crystals grown from (A) pure water or 10 mg⋅mL−1, (B) Ac(Nser)3, (C) Ac(Nme)3, and (D) Ac(Sar)3 solutions grow as a distribution of different crystal planes corresponding to hexagonal ice. The (001) reflection is not allowed by symmetry in the hexagonal space group P63/mmc (52). Therefore, this orientation is observed through the allowed (002) reflection. The hexagonal ice crystals exhibited a distribution of interplanar angles (Φ) from the horizontal plane of the substrate with different diffraction planes. Each diffraction pattern was taken with unique values of the incident X-ray beam angle (θ1). Data points corresponding to θ1 = 11° are represented as red circles, θ1 = 12° as black squares, and θ1 = 16.5° as green triangles. Circled areas represent diffraction planes corresponding to Φ = 0° to 10°.
Collectively, the data in Fig. 5 demonstrate that ice crystals in the presence of the peptoids display different distributions of ice crystal orientations relative to pure water, indicating a selective adsorption of each peptoid on the various ice crystal planes. It is well established that the most prominent faces of a crystal correspond to those to which an additive binds most strongly, thus retarding growth normal to that crystal face. Therefore, it is reasonable to suggest that additives can influence the orientation at the air–water interface during the early stages of ice crystallization by specific adsorption to the crystal planes of emerging crystal nuclei. These planes would be expected to align parallel with the air–water interface because the source of additives is the aqueous subphase beneath the air–water interface. As such, the fastest growth directions would be parallel to the air–water interface where growth is unconstrained and the temperature would be expected to be less than the temperature of the aqueous bath. As such, crystal nuclei in this orientation would achieve critical size more readily.
Although the microscopy and XRD measurements cannot identify the fast growth (crystallographic) directions unequivocally, these directions will be contained within the planes parallel to the air–water interface, as determined by the XRD data. The data reveal the presence of diffraction peaks from crystal planes that were not observed in the aforementioned investigations of ice crystallization in the presence of macromolecules (26, 27, 51). We found that ice crystals formed in the presence of PEG-8 (average Mn = 400) exhibited a (002) reflection with moderate intensity (SI Appendix, Fig. S17), unlike the previous report of behavior in the presence of PEG-6 (27). Moreover, we observed (102), (210), and (103) reflections in addition to the (100) and (101). This demonstrates that the 2D X-ray microdiffraction method used here is capable of detecting ice crystals in orientations that otherwise may not be observed using conventional 1D powder XRD, which detects Bragg diffraction from only those crystals with planes aligned horizontally, parallel to the sample stage. This capability removes any ambiguity about whether the loss of peak intensity for a particular reflection is due to changes in crystal orientation or crystal size. The data presented here suggests that, in our hands, the peptoids and PEG-8 do not reduce the thickness of ice crystals to the extent that the diffraction intensity from (200) and (101) planes are diminished substantially.
Conclusions
For peptoid aqueous solutions, we observed melting temperature reductions that exceeded those expected from additive concentration effects alone, demonstrating that peptoids can display noncolligative antifreeze activity, most likely through specific binding to ice crystal growth interfaces. The dependence of melting temperature reduction on peptoid structure corroborates the noncolligative behavior. Moreover, the dependence of ice crystal morphology, growth inhibition, and orientation on the peptoid structure illustrates the varying influence of different functional groups in the regulation of ice crystal growth. The peptoid Ac(Nser)3 exhibits dual-action antifreeze effects: enhanced ice growth inhibition and melting temperature reduction. Dual-action antifreeze agents may prove to be particularly useful, as they could impede the formation of ice crystals while also frustrating the further growth of ice crystals once they have formed.
These studies contribute to a foundation for the discovery of synthetic oligomers that regulate crystallization outcomes for a variety of organic and inorganic species. The versatility and modularity of peptoids are well suited for this purpose (40). The short achiral peptoid oligomers described here do not exhibit conformational order (54), but we anticipate that peptoid inhibitors with some degree of secondary structure may further influence ice crystallization based on epitaxial matching with the crystal planes of ice nuclei (11). Given the measurable effects of small perturbations in peptoid sequence length on ice crystal growth—an effect that would easily be masked in polydisperse or random polymer systems—the monodispersity and sequence-specificity of peptoid oligomers hold considerable promise for unraveling the critical structure–activity relationships responsible for the regulation of ice crystallization.
Methods
All solutions of the additives, including glycerol (Alfa Aesar) were prepared in ultrapure water.
Preparation of Peptoid and Peptide Oligomers.
Peptoids were synthesized using solid-phase submonomer protocols (46) and peptides were synthesized using standard Fmoc-based solid-phase peptide synthesis protocols. N-acetylated linear oligomers were synthesized on Rink amide resin solid support and were elongated using iterative bromoacylation with bromoacetic acid and nucleophilic displacement with an amine submonomer. Amine submonomers were generated from 2-methoxyethylamine (Alfa Aesar) for the Nme submonomer and methylamine (Sigma-Aldrich) for the Sar submonomer. The Nser submonomer was generated from O-tert-butyl-dimethylsilyl (TBDMS)-2-ethanolamine, which was prepared using previously published procedures (SI Appendix). The N terminus was acetylated with acetic anhydride (Alfa Aesar) in diisopropylethylamine/N,N-Dimethylformamide, before cleavage from resin (and concomitant deprotection of TBDMS protecting groups for Nser oligomers) with 95% trifluoroacetic acid (TFA) (Sigma-Aldrich) in water at room temperature for 30 min. Preparative HPLC was then performed on a Delta-Pak C18 (Waters, 15 µm, 100 Å, 25 × 100 mm) with a linear gradient of 0–30% acetonitrile/H2O (0.1% TFA) over 40 min with a flow rate of 2.5 mL⋅min−1. Mass spectrometry was performed on an Agilent 1100 Series LC/MSD Trap XCT (Agilent Technologies). Purified HPLC fractions were freeze dried, and samples for antifreeze analysis were prepared with ultrapure water (18.2 MΩ).
Ice Growth Inhibition and Morphology Assays.
Candidate antifreeze agents were dissolved in ultrapure water (to a concentration of 10 mg⋅mL−1), and 20 µL of each sample was added to a circular reservoir constructed by pressing a silicone isolator (Sigma-Aldrich) between two glass slides (SI Appendix, Fig. S3). This assembly was then mounted on a Leitz Ergolux optical microscope equipped with a LTS350 thermal stage (Linkam, UK) and examined in the transmitted light (bright field) mode. The aperture was minimized to prevent light absorption effects. The temperature accuracy and stability of the thermal stage was 0.05 °C. The interior and upper windows of the thermal stage were purged with dry nitrogen to prevent water condensation from ambient air. In each experiment, one of the wells was filled with ultrapure water and used as a reference. Images were collected using a SPOT Insight digital camera. Ice growth rates were calculated from the images using ImageJ software. The ice growth rate was determined from the average of ≥10 different measurements.
DSC.
DSC measurements were performed using a Pyris 1 differential scanning calorimeter (Perkin-Elmer). A 7-µL sample solution was placed in a 40-µL aluminum pan, using an empty aluminum pan as a reference. Nitrogen (20 mL⋅min−1) was used to purge the system to prevent condensation of moisture within the DSC furnace. The melting temperature was determined from the maximum of each heating curve.
Two-Dimensional XRD Analysis.
XRD was conducted by placing 20 μL water or additive solutions on microscope glass slides cleaned by washing with ethanol, acetone, and finally with ultrapure water. The slides were then placed on a cooling stage (Anton Paar; DCS 350) and covered with transparent plastic wrap (without contact to the droplet) to allow visualization of the sample with a digital camera attached to the optical microscope (SI Appendix, Fig. S3). XRD data were obtained with a Bruker D8 Discover GADDS microdiffractometer equipped with a VÅNTEC-2000 area detector and a Cu-Kα source (λ = 1.54178 Å). The X-ray beam was monochromated with a graphite crystal and collimated with a 0.5-mm MONOCAP. The sample-to-detector distance was maintained at 150 mm. The XRD data were analyzed by the XRD2EVAL program of the Bruker PILOT software (version 2009.5-0; Bruker AXS) and DIFFRACplus EVA (version 1.5; Bruker AXS). The interplanar angle generated by the diffraction plane and the horizontal plane of the substrate (Φ) was calculated based on Eq. 2,
in which θ is the Bragg angle for each diffraction plane (hkl), δ is the azimuthal angle of the diffraction spots on the 2D detector, and θ1 is the incident X-ray beam angle (SI Appendix, Fig. S5A). The distribution of Φ vs. diffraction planes for each sample was determined from ∼30 diffraction spots of ≥12 diffraction patterns (SI Appendix, Fig. S5 B and C).
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (NSF) CRIF/CHE-0840277 and CHE-1152317 and by the NSF’s Materials Research Science and Engineering Center Program DMR-0820341. M.L.H. is supported by the New York University Graduate School of Arts and Science Horizon Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212826109/-/DCSupplemental.
References
- 1.Lee AY, Erdemir D, Myerson AS. Crystal polymorphism in chemical process development. Annu Rev Chem Biomol Eng. 2011;2:259–280. doi: 10.1146/annurev-chembioeng-061010-114224. [DOI] [PubMed] [Google Scholar]
- 2.Elliot J, Hancock B. Pharmaceutical materials science: An active new frontier in materials research. MRS Bull. 2006;31:875–879. [Google Scholar]
- 3.Weissbuch I, Addadi L, Leiserowitz L, Leiserowitz L. Molecular recognition at crystal interfaces. Science. 1991;253(5020):637–645. doi: 10.1126/science.253.5020.637. [DOI] [PubMed] [Google Scholar]
- 4.Weissbuch I, Popovitz-Biro R, Lahav M, Leiserowitz L. Understanding and control of nucleation, growth, habit, dissolution and structure of two- and three-dimensional crystals using 'tailor-made' additives. Acta Crystallogr B. 1995;51:115–148. [Google Scholar]
- 5.Rimer JD, et al. Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science. 2010;330(6002):337–341. doi: 10.1126/science.1191968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton BD, Hillmyer MA, Ward MD. Glycine polymorphism in nanoscale crystallization chambers. Cryst Growth Des. 2008;8:3368–3375. [Google Scholar]
- 7.Qiu SR, et al. Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc Natl Acad Sci USA. 2004;101(7):1811–1815. doi: 10.1073/pnas.0307900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sommerdijk NAJM, de With G. Biomimetic CaCO3 mineralization using designer molecules and interfaces. Chem Rev. 2008;108(11):4499–4550. doi: 10.1021/cr078259o. [DOI] [PubMed] [Google Scholar]
- 9.Berkovitch-Yellin Z, et al. Crystal morphology engineering by “tailor-made” inhibitors: A new probe to fine intermolecular interactions. J Am Chem Soc. 1985;107:3111–3122. [Google Scholar]
- 10.Capes J, Cameron RE. The effect of polymer addition on the contact line crystallization of paracetamol. CrystEngCom. 2007;9:84–90. [Google Scholar]
- 11.DeOliveira DB, Laursen RA. Control of calcite morphology by a peptide designed to bind to a specific surface. J Am Chem Soc. 1997;119:10627–10631. [Google Scholar]
- 12.Malkin TL, Murray BJ, Brukhno AV, Anwar J, Salzmann CG. Structure of ice crystallized from supercooled water. Proc Natl Acad Sci USA. 2012;109(4):1041–1045. doi: 10.1073/pnas.1113059109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehre D, Lavert E, Lahav M, Lubomirsky I. Water freezes differently on positively and negatively charged surfaces of pyroelectric materials. Science. 2010;327(5966):672–675. doi: 10.1126/science.1178085. [DOI] [PubMed] [Google Scholar]
- 14.DeVries AL. Glycoproteins as biological antifreeze agents in antarctic fishes. Science. 1971;172(3988):1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 15.Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci USA. 1977;74(6):2589–2593. doi: 10.1073/pnas.74.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storey KB, Storey JM. Natural freeze tolerance in ectothermic vertebrates. Annu Rev Physiol. 1992;54:619–637. doi: 10.1146/annurev.ph.54.030192.003155. [DOI] [PubMed] [Google Scholar]
- 17.Harding MM, Ward LG, Haymet ADJ. Type I ‘antifreeze’ proteins. Structure-activity studies and mechanisms of ice growth inhibition. Eur J Biochem. 1999;264(3):653–665. doi: 10.1046/j.1432-1327.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 18.Gibson MI. Slowing the growth of ice with synthetic macromolecules: Beyond antifreeze(glyco) proteins. Polym Chem. 2010;1:1141–1152. [Google Scholar]
- 19.Tiller WA. The Science of Crystallization: Microscopic and Interfacial Phenomena. New York: Cambridge Univ Press; 1991. pp. 328–330. [Google Scholar]
- 20. Colligative freezing point depression arises only from the number of solute molecules in solution, independent of their characteristics. See Raoult F-M (1882) Loi de congelation des solutions benzeniques des substances neutres. Comptes Rendus, Semestre T. XCV, No. 4, pp. 187–198.
- 21.Doxey AC, Yaish MW, Griffith M, McConkey BJ. Ordered surface carbons distinguish antifreeze proteins and their ice-binding regions. Nat Biotechnol. 2006;24(7):852–855. doi: 10.1038/nbt1224. [DOI] [PubMed] [Google Scholar]
- 22.Hachisu M, et al. One-pot synthesis of cyclic antifreeze glycopeptides. Chem Commun (Camb) 2009;13(13):1641–1643. doi: 10.1039/b815917c. [DOI] [PubMed] [Google Scholar]
- 23.Capicciotti CJ, Trant JF, Leclère M, Ben RN. Synthesis of C-linked triazole-containing AFGP analogues and their ability to inhibit ice recrystallization. Bioconjug Chem. 2011;22(4):605–616. doi: 10.1021/bc100394k. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Damodaran S, Yethiraj A. Retardation of ice crystallization by short peptides. J Phys Chem A. 2009;113(16):4403–4407. doi: 10.1021/jp8110748. [DOI] [PubMed] [Google Scholar]
- 25.Wierzbicki A, et al. Structure-function relationship in the antifreeze activity of synthetic alanine-lysine antifreeze polypeptides. Biomacromolecules. 2000;1(2):268–274. doi: 10.1021/bm000004w. [DOI] [PubMed] [Google Scholar]
- 26.Baruch E, Mastai Y. Antifreeze properties of polyglycidol block copolymers. Macromol Rapid Commun. 2007;28:2256–2261. [Google Scholar]
- 27.Yagci YE, Antonietti M, Bomer HG. Synthesis of poly(tartar amides) as bio-inspired antifreeze additives. Macromol Rapid Commun. 2006;27:1660–1664. [Google Scholar]
- 28.Budke C, Koop T. Ice recrystallization inhibition and molecular recognition of ice faces by poly(vinyl alcohol) ChemPhysChem. 2006;7(12):2601–2606. doi: 10.1002/cphc.200600533. [DOI] [PubMed] [Google Scholar]
- 29.Hill DJ, Mio MJ, Prince RB, Hughes TS, Moore JS. A field guide to foldamers. Chem Rev. 2001;101(12):3893–4012. doi: 10.1021/cr990120t. [DOI] [PubMed] [Google Scholar]
- 30.Kirshenbaum K, et al. Sequence-specific polypeptoids: A diverse family of heteropolymers with stable secondary structure. Proc Natl Acad Sci USA. 1998;95(8):4303–4308. doi: 10.1073/pnas.95.8.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figliozzi GM, Goldsmith R, Ng SC, Banville SC, Zuckermann RN. Synthesis of N-substituted glycine peptoid libraries. Methods Enzymol. 1996;267:437–447. doi: 10.1016/s0076-6879(96)67027-x. [DOI] [PubMed] [Google Scholar]
- 32.Wu CW, Sanborn TJ, Huang K, Zuckermann RN, Barron AE. Peptoid oligomers with alpha-chiral, aromatic side chains: Sequence requirements for the formation of stable peptoid helices. J Am Chem Soc. 2001;123(28):6778–6784. doi: 10.1021/ja003154n. [DOI] [PubMed] [Google Scholar]
- 33.Shin SBY, Yoo B, Todaro LJ, Kirshenbaum K. Cyclic peptoids. J Am Chem Soc. 2007;129(11):3218–3225. doi: 10.1021/ja066960o. [DOI] [PubMed] [Google Scholar]
- 34.Paul B, et al. Peptoid atropisomers. J Am Chem Soc. 2011;133(28):10910–10919. doi: 10.1021/ja2028684. [DOI] [PubMed] [Google Scholar]
- 35.Nam KT, et al. Free-floating ultrathin two-dimensional crystals from sequence-specific peptoid polymers. Nat Mater. 2010;9(5):454–460. doi: 10.1038/nmat2742. [DOI] [PubMed] [Google Scholar]
- 36.Maayan G, Ward MD, Kirshenbaum K. Folded biomimetic oligomers for enantioselective catalysis. Proc Natl Acad Sci USA. 2009;106(33):13679–13684. doi: 10.1073/pnas.0903187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynn KD, et al. GU81, a VEGFR2 antagonist peptoid, enhances the anti-tumor activity of doxorubicin in the murine MMTV-PyMT transgenic model of breast cancer. BMC Cancer. 2010;10:397–311. doi: 10.1186/1471-2407-10-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang ML, Shin SBY, Benson MA, Torres VJ, Kirshenbaum K. A comparison of linear and cyclic peptoid oligomers as potent antimicrobial agents. ChemMedChem. 2012;7(1):114–122. doi: 10.1002/cmdc.201100358. [DOI] [PubMed] [Google Scholar]
- 39.Simon RJ, et al. Peptoids: A modular approach to drug discovery. Proc Natl Acad Sci USA. 1992;89(20):9367–9371. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C-L, Qi J, Zuckermann RN, DeYoreo JJ. Engineered biomimetic polymers as tunable agents for controlling CaCO3 mineralization. J Am Chem Soc. 2011;133(14):5214–5217. doi: 10.1021/ja200595f. [DOI] [PubMed] [Google Scholar]
- 41.Estroff LA, Incarvito CD, Hamilton AD. Design of a synthetic foldamer that modifies the growth of calcite crystals. J Am Chem Soc. 2004;126(1):2–3. doi: 10.1021/ja037614z. [DOI] [PubMed] [Google Scholar]
- 42.Bekele H, Fendler JH, Kelly JW. Self-assembling peptidomimetic monolayer nucleates oriented CdS nanocrystals. J Am Chem Soc. 1999;121:7266–7267. [Google Scholar]
- 43.Norgren AS, et al. On-resin click-glycoconjugation of peptoids. Synthesis-Stuttgart. 2009;3:488–494. [Google Scholar]
- 44.Mehl PM. The effect of the functional groups of organic solutes on the suppression of crystallization in aqueous solutions. Thermochim Acta. 1996;226:325–332. [Google Scholar]
- 45.Fahy GM, Levy DI, Ali SE. Some emerging principles underlying the physical properties, biological actions, and utility of vitrification solutions. Cryobiology. 1987;24(3):196–213. doi: 10.1016/0011-2240(87)90023-x. [DOI] [PubMed] [Google Scholar]
- 46.Zuckermann RN, Kerr JM, Kent SBH, Moos WH. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J Am Chem Soc. 1992;114:10646–10647. [Google Scholar]
- 47.Wilson PW. Explaining thermal hysteresis by the Kelvin effect. Cryo Lett. 1993;14:31–36. [Google Scholar]
- 48.Weissbuch I, Leiserowitz L, Lahav M. Self-poisoning at {011} faces of a-resorcinol crystals may explain its unidirectional growth in the vapor phase: A molecular modeling study. Cryst Growth Des. 2006;6:625–628. [Google Scholar]
- 49.Hussain M, Anwar J. The riddle of resorcinol crystal growth revisited: Molecular dynamics simulations of α-resorcinol crystal-water interface. J Am Chem Soc. 1999;121:8583–8591. [Google Scholar]
- 50.Atkins P, de Paula J. 2002. in Physical Chemistry (Freeman, New York), 7th Ed, p 177.
- 51.Mastai Y, Rudloff J, Cölfen H, Antonietti M. Control over the structure of ice and water by block copolymer additives. ChemPhysChem. 2002;3(1):119–123. doi: 10.1002/1439-7641(20020118)3:1<119::AID-CPHC119>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 52.Goto A, Hondoh T, Mae SJ. The electron-density distribution in ice Ih determined by single-crystal X-ray diffractometry. J Chem Phys. 1990;93:1412–1417. [Google Scholar]
- 53.Fortes AD, et al. No evidence for large-scale proton ordering in Antarctic ice from powder neutron diffraction. J Chem Phys. 2004;120(24):11376–11379. doi: 10.1063/1.1765099. [DOI] [PubMed] [Google Scholar]
- 54.Fafarman AT, Borbat PP, Freed JH, Kirshenbaum K. Characterizing the structure and dynamics of folded oligomers: Pulsed ESR studies of peptoid helices. Chem Commun (Camb) 2007;2(4):377–379. doi: 10.1039/b612198e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.