Abstract
Tomato breeding has been tremendously efficient in increasing fruit quality and quantity but did not focus on improving herbivore resistance. The biosynthetic pathway for the production of 7-epizingiberene in a wild tomato was introduced into a cultivated greenhouse variety with the aim to obtain herbivore resistance. 7-Epizingiberene is a specific sesquiterpene with toxic and repellent properties that is produced and stored in glandular trichomes. We identified 7-epizingiberene synthase (ShZIS) that belongs to a new class of sesquiterpene synthases, exclusively using Z-Z-farnesyl-diphosphate (zFPP) in plastids, probably arisen through neo-functionalization of a common ancestor. Expression of the ShZIS and zFPP synthases in the glandular trichomes of cultivated tomato resulted in the production of 7-epizingiberene. These tomatoes gained resistance to several herbivores that are pests of tomato. Hence, introduction of this sesquiterpene biosynthetic pathway into cultivated tomatoes resulted in improved herbivore resistance.
Keywords: metabolic engineering, terpenoid, white fly, spider mite, promoter
Herbivorous insects pose serious problems in agricultural production areas. On commercial tomato, whiteflies, spider mites, and aphids are major pests, not only by the feeding damage they cause, but also because they transmit devastating viruses that can cause losses up to 100% (1). Production of tomato, a crop with considerable economic importance, reached 145,751,507 tons worldwide in 2010, representing a value of 53.3 billion dollars (www.fao.org). In general, wild tomatoes are far less attractive to pests than cultivated tomatoes due to an elevated or qualitatively different production of an array of defense compounds such as alkaloids, phenolic compounds, and terpenes.
Toxic and repellent compounds are mostly produced in glandular trichomes, autonomous epidermal protrusions specialized in efficient production, storage, and release of defense compounds (2). These glands have long been regarded as economically important and challenging targets for bioengineering (2). Confining metabolic changes to trichomes ensures that the plant metabolism and crop yield remains unaffected.
Terpenes constitute the largest and most diverse class of plant-produced secondary compounds that maintain a variety of biological functions and applications, including anticancer (taxol) and antimalarial (artemisin) drugs, in flavor and fragrance industry, and for crop improvement through enhanced pest resistance. Wild tomato sesquiterpenes and derivatives have been implicated in defense against herbivores (3–5). Sesquiterpenes are C15-terpenes predominantly derived from precursors of the cytosolic mevalonate pathway where the C5-isoprene units isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are assembled into C15 E-E-farnesyl diphosphate (FPP) by FPP synthase (FPS) and converted by specific sesquiterpene synthases. However, it was recently shown that some wild tomato species contain a sesquiterpene pathway that is confined to the chloroplast (6). In Solanum habrochaites LA1777 an atypical sesquiterpene synthase produces the sesquiterpenes santalene and bergamotene from Z-Z-farnesyl diphosphate (Z,Z-FPP) that, in turn, is produced in the plastids from IPP and DMAPP by a short chain cis-prenyltransferase (zFPS) (6). Recently an additional S. habrochaites sesquiterpene synthase has been identified, along with two new, cis-substrate (neryl diphosphate) using, monoterpene synthases (7). On a sequence level, this sesquiterpene synthase resembles plastidial diterpene synthases more than cytosolic sesquiterpene synthases. In cultivated tomato, the plastidial sesquiterpene synthase pathway appears to be absent, although another cis-prenyltransferase, i.e., neryl-diphosphate synthase, is responsible for producing the precursor of several C10-monoterpenes (8). Cis-prenyltransferases have evolved independently from transprenyltransferases (9). Zingiberene is a sesquiterpene important in plant defense against an array of herbivores due to its toxicity and repellence (10–14) and is found in a variety of plant species including basil, turmeric, cardamom, and sorghum. Recently, we found that tomato contains 7-epizingiberene, a stereoisomer of α-zingiberene (15). Importantly, purified 7-epizingiberene, when applied to the headspace of susceptible cultivated tomato, repelled whiteflies, whereas α-zingiberene isolated from ginger did not (15). Although several zingiberene synthases have been identified (16–18), including one from S. habrochaites (7), we have now unambiguously identified and characterized 7-epizingiberene synthase from S. habrochaites and modified a cultivated tomato to produce 7-epizingiberene. Moreover, plants with improved herbivore resistance were obtained by traditional breeding and these may contribute to reduced pesticide use.
Results
Role for 7-Epizingiberene in Defense Against an Agricultural Pest.
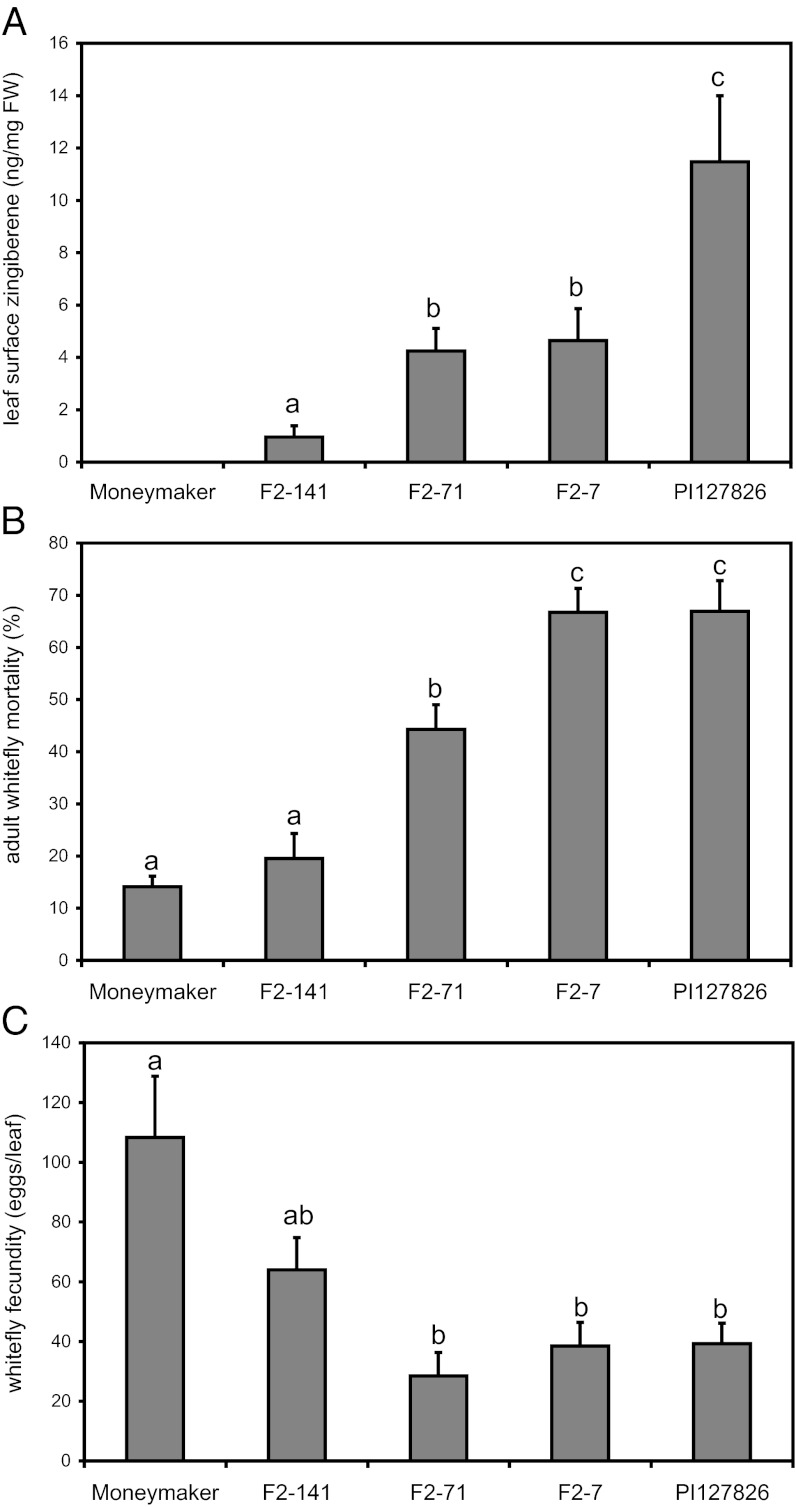
To confirm the biological relevance of 7-epizingiberene in defense against whiteflies, an interspecific cross between Solanum lycopersicum cv Moneymaker and the wild tomato S. habrochaites PI127826 was created. PI127826 contains high levels of 7-epizingiberene (15), which the cultivated tomato lacks. The segregating F2 plants, resulting from F1 selfings, produced 7-epizingiberene in a range of concentrations up to PI127826 levels (Fig. S1A). Six of the 7-epizingiberene containing F2 plants were selected for a terpene-profile analysis. Leaf-surface terpenes were analyzed by GC-MS (Fig. 1A and Fig. S1B). F2-2 contains zingiberene levels equal to PI127826 (10.7 ± 2.0, 14.6 ± 3.4 ng⋅mg−1 FW, respectively). However, it also accumulated monoterpenes and/or other sesquiterpenes at high concentrations (Fig. S1B). F2-7, F2-71, and F2-141 produced medium levels of zingiberene (Fig. 1A) and terpene levels more comparable to the cultivated plant, both in quantity and composition (Fig. S1B). Therefore, these plants were selected for whitefly bioassays. F2-141 contained only 8% 7-epizingiberene compared with the wild tomato and no significant effect on adult mortality was recorded (P = 0.4), although the number of eggs deposited was lower. After 5 d, 67% of the whiteflies were dead on PI127826 and F2-7 (Fig. 1B) whereas mortality on F2-71, containing very few terpenes other than zingiberene, reached 44%. Overall, the data show a correlation between the concentration of 7-epizingiberene on the leaf surface and the performance of whiteflies (Fig. S2). On all zingiberene-producing F2s, a 40–74% reduction in egg deposition was found compared with the cultivated parent (Fig. 1C), showing that tomato zingiberene is toxic to the insect pest Bemisia tabaci in lower doses than produced by the wild tomato plant.
Fig. 1.
Zingiberene levels influence whitefly performance on F2 plants of an interspecific cross between S. lycopersicum cv Moneymaker and S. habrochaites PI127826 and both parents. (A) Leaf surface 7-epizingiberene (ng·mg−1 FW leaf) (n = 5–8). (B) Whitefly mortality (% of total). (C) Whitefly fecundity displayed as the total number of eggs per leaf (n = 5–11). Bars represent means ± SE, different letters signify statistical differences (P < 0.05).
Identification of 7-Epizingiberene Synthase.
To identify the biosynthetic pathway for 7-epizingiberene production in tomato, we determined which terpenes in PI127826 are derived from the cytosolic (MVA; mevalonate) and which from the plastidial (MEP: 2-C-methyl-d-erythritol 4-phosphate) pathway. To this end, we grew plants in hydroponics with the MEP-pathway inhibitor fosmidomycin. In treated plants, the levels of monoterpenes, which are also derived from precursors of the MEP pathway, were reduced by 80% on average (Table 1). Whereas cytosolic sesquiterpenes such as germacrenes were slightly elevated in fosmidomycin-treated plants, 7-epizingiberene and its derivative R-curcumene decreased by 86% and 99.5%, respectively. Also levels of β-sesquiphellandrene were reduced, indicating that these three sesquiterpenes have a plastidial origin.
Table 1.
Terpene concentrations (ng⋅mg−1 leaf FW) in leaf wash of S. habrochaites PI127826 cuttings grown for 5 wk in hydroculture amended with 10 µM fosmidomycin
| Terpene | Control | Treated | P value |
| α-Phellandrene | 0.11 ± 0.01 | 0.04 ± 0.00 | 0.001 |
| α-Terpinene | 0.10 ± 0.01 | nd | 0.002 |
| d-limonene | 0.28 ± 0.03 | 0.09 ± 0.01 | 0.002 |
| γ-Terpinene | 0.04 ± 0.01 | nd | 0.005 |
| Terpinolene | 0.06 ± 0.01 | nd | 0.007 |
| γ-Elemene | 0.02 ± 0.00 | 0.12 ± 0.06 | ns |
| Sesquisabinene | 0.05 ± 0.01 | 0.07 ± 0.02 | ns |
| R-curcumene | 0.30 ± 0.08 | 0.00 ± 0.00 | 0.017 |
| 7-Epizingiberene | 7.64 ± 1.82 | 1.05 ± 0.33 | 0.006 |
| Germacrene A | 0.04 ± 0.01 | 0.08 ± 0.03 | ns |
| β-Sesquiphellandrene | 0.23 ± 0.04 | 0.03 ± 0.03 | 0.015 |
| α-Cadinene | 0.05 ± 0.00 | 0.18 ± 0.11 | ns |
| Selina-3.7 (11)-diene | 0.12 ± 0.02 | 0.25 ± 0.16 | ns |
| Germacrene B | 1.70 ± 0.34 | 4.77 ± 3.18 | ns |
Data are means ± SE, n = 3. nd, not detected; ns, not significant.
Sallaud et al. (6) first provided evidence for a plastid localized sesquiterpene synthase; the santalene-bergamotene synthase (SBS) from LA1777. Based on this sequence we cloned a 778 amino acid terpene synthase from PI127826 with 96% similarity to SBS (Fig. S3A). A 36 amino acid N-terminal transit peptide, similar to that of the chloroplast localized ShSBS protein (6), was predicted (www.cbs.dtu.dk/services/ChloroP). Besides the DDXXD motif necessary for precursor ionization, ShZIS contains the EDXXD motif found in copalyl diphosphate synthases, as well as an N-terminal QW motif found in ent-kaurene synthases. ShZIS belongs to the ent-kaurene Terpene Synthase (TPS)-e/f subfamily (see ref. 19) and is not homologous to other zingiberene synthases, such as sweet basil ObZIS (16), Sorghum SbZIS (SbTPS1) (18), or to other tomato sesquiterpene synthases (19, 20). However, both ShZIS and ShSBS are homologous to SlTPS19-20, which belong to an atypical class of monoterpene synthases in S. lycopersicum and Solanum pennellii (Fig. S3B). In addition, ShZIS was cloned from four other zingiberene-producing S. habrochaites accessions (LA0094, LA1978, LA2167, and LA2650). Comparative analyses revealed 10 SNPs of which 7 led to amino acid changes (Table S1).
Functional Characterization of ShZIS.
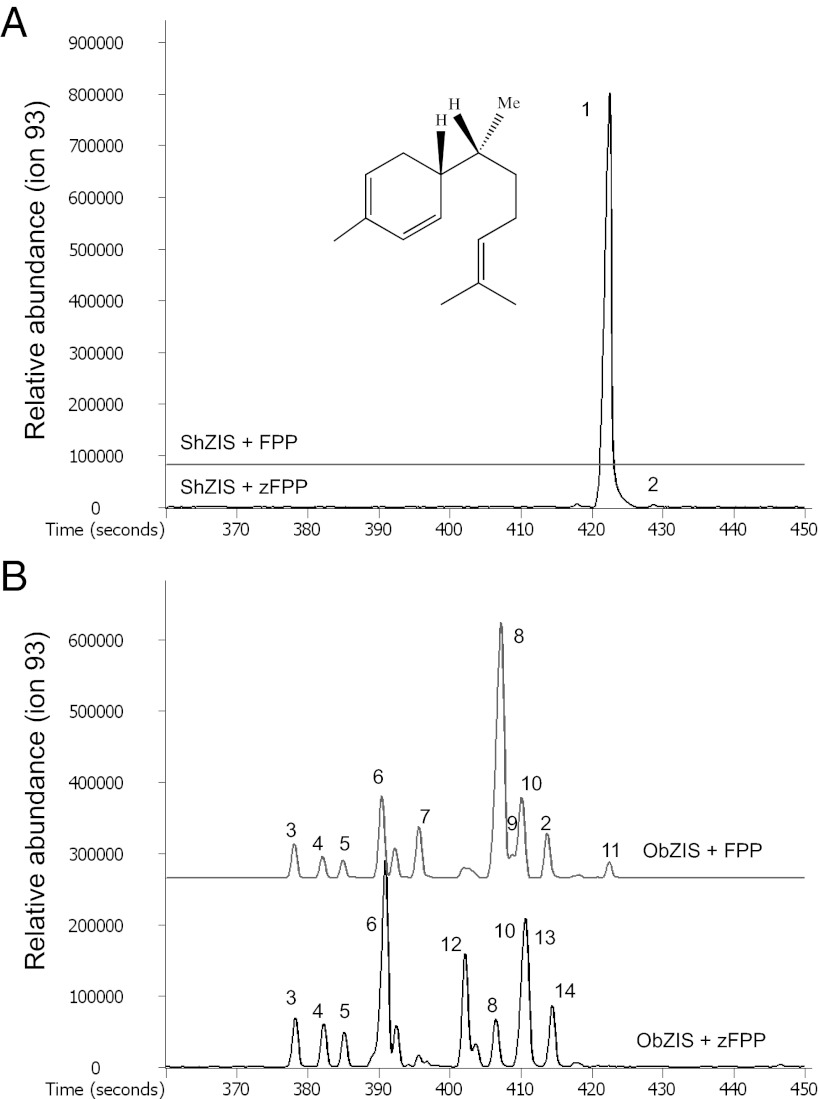
Recombinant ShZIS enzyme exclusively used Z,Z-FPP and was unable to synthesize detectable products from any other terpene precursor offered, including the stereoisomer E,E-FPP (Fig. 2A). Using Z,Z-FPP, the enzyme made zingiberene (98.6%) and trace amounts of β-sesquiphellandrene (0.8%) and curcumene (0.6%), the dehydrogenated derivative of zingiberene. Recombinant zingiberene synthase from lemon basil (ObZIS) (16, 17) was used for comparison with ShZIS (Fig. 2B). ObZIS produced mainly zingiberene from E,E-FPP whereas Z,Z-FPP was converted mostly into α-bergamotene. Moreover, ObZIS was able to convert both C10 precursors GPP and NPP into monoterpenes, although at lower efficiency.
Fig. 2.
Enzymatic activity of recombinant ZIS enzymes. GC-MS chromatograms of ShZIS from S. habrochaites PI127826 (A) and ObZIS from O. basilicum (AY693646) (B) expressed in E. coli, assayed with sesquiterpene precursor E-E-FPP (FPP) or Z,Z-FPP (zFPP) and measured by SPME. Peaks: 1: 7-epizingiberene 2: β-sesquiphellandrene, 3: 7-episesquithujene, 4: sesquithujene, 5: (Z)-α-bergamotene, 6: (E)-α-bergamotene, 7: (E)-β-farnesene, 8: α-zingiberene 9: α-farnesene 10: β-bisabolene 11: (E)-nerolidol, 12: β-acoradiene, 13: (Z)-γ-bisabolene, 14: (E)-γ-bisabolene. The detector response for terpene-specific ion 93 is shown.
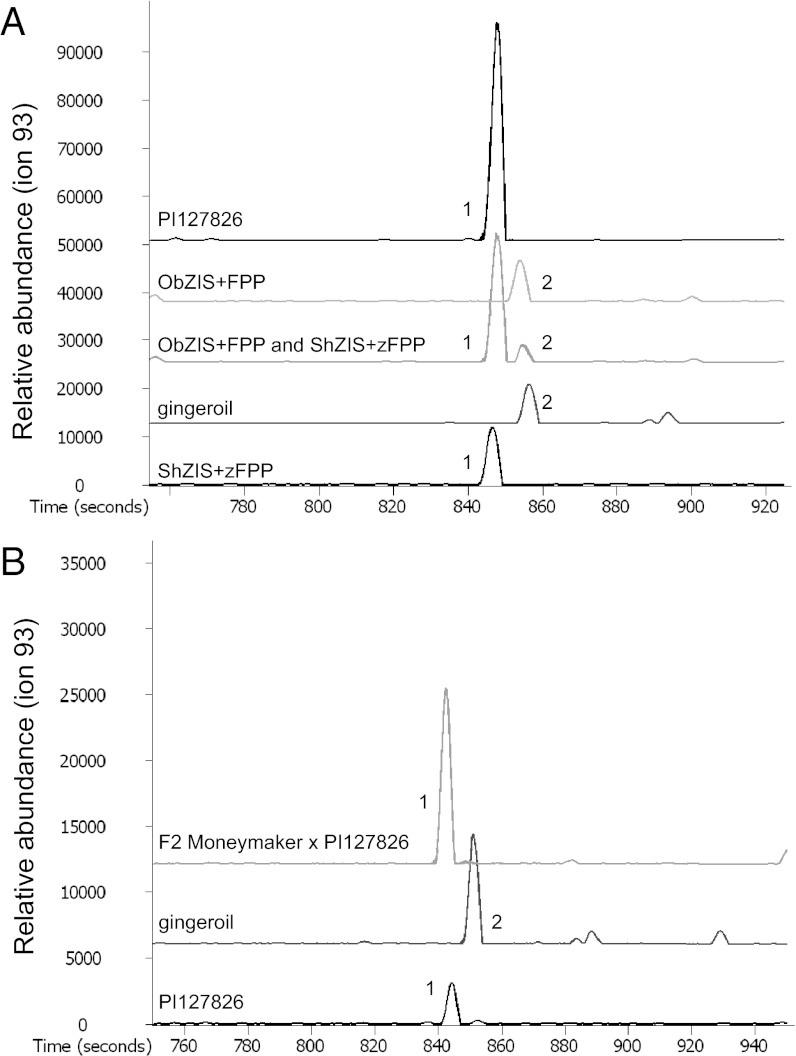
Enantioselective gas chromatography on a β-cyclodextrin coated column allowed identification of the different zingiberene stereoisomers from tomato and ginger (Fig. 3A), previously shown to contain 7-epizingiberene and α-zingiberene, respectively (15). We established that recombinant tomato ShZIS indeed produced 7-epizingiberene from Z,Z-FPP, whereas ObZIS is a bona-fide α-zingiberene synthase that uses E,E-FPP (Fig. 3A). The kinetic constants for recombinant ShZIS (±84 kDa) indicated a substrate affinity and turnover rate comparable to other plant sesquiterpene synthases e.g., the maize (E)-β-caryophyllene synthase and tobacco 5-epiaristolochene synthase (21, 22), with a Km value of 7.12 ± 2.68 µM Z,Z-FPP and Kcat of 0.29 ± 0.10 s−1.
Fig. 3.
Identification of zingiberene stereo-isoforms. (A) GC-MS chromatograms of SPME sampling of S. habrochaites PI127826, ginger oil, recombinant ObZIS with E,E-FPP, and/or ShZIS with Z,Z-FPP. (B) Liquid injection of hexane leaf wash of F2 (plant 2) and parent S. habrochaites PI127826 compared with ginger oil. 1: 7-Epizingiberene and 2: α-zingiberene. The detector response for terpene-ion 93 is shown.
Engineering of zFPS and ShZIS in Cultivated Tomato and Herbivore Responses.
The F2 plants that produced 7-epizingiberene (Fig. 3B) contained both the zFPS and the ShZIS genes that are specifically expressed in trichomes (Fig. S4A). S. habrochaites PI127826 treated with jasmonic acid (JA), a plant hormone involved in response to herbivores, accumulated higher levels of 7-epizingiberene compared with mock-treated plants (Fig. S4B).
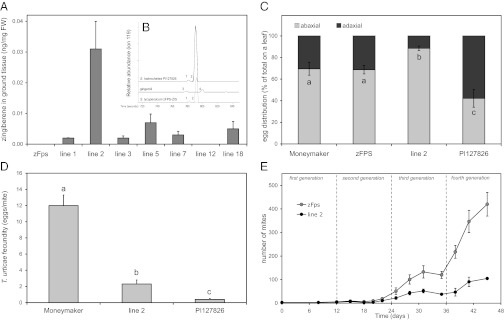
The function of ShZIS was assessed further by stable expression of zFPS and ShZIS genes in S. lycopersicum cv Moneymaker. The zFPS cDNA was expressed under control of the S. habrochaites Methyl Ketone Synthase (MKS1) promoter (23) and ShZIS was driven by the S. lycopersicum Monoterpene Synthase (MTS1) (24) promoter. Transgenic tomato plants containing the uidA reporter (GUS) driven by the MKS1 or the MTS1 promoter revealed that expression under these promoters was specific for type VI glandular trichomes (Fig. S4 C and D). The terpene profiles of leaf material from seven independent primary zFPS/ShZIS-transformants were analyzed by GC-MS and compared with those of plants expressing zFPS alone (Fig. 4A). The transgenic lines containing both genes accumulated 7-epizingiberene (Fig. 4B), proving that 7-epizingiberene synthesis is orchestrated by ShZIS converting Z,Z-FPP in the plastid of tomato trichomes. Coexpression of zFps and ShZis in tomato trichomes did not reduce levels of monoterpenes (P = 0.20).
Fig. 4.
Effect of zFPS and ShZIS engineering in trichomes of cultivated tomato. (A) 7-epizingiberene levels in leaves (ng/mg) of transgenic lines expressing either zFPS alone (zFPS and line 12) or in combination with ShZIS as measured by SPME. Means ± SE (n = 3). (B) (Inset) GC-MS chromatogram of S. habrochaites PI127826, ginger oil and S. lycopersicum transgenic (line 2). 1: R-curcumene, 2: 7-epizingiberene, 3: S-curcumene, 4: α-zingiberene. The detector response for ion 119 is shown. (C) Distribution of whitefly eggs (%). (D) Spider mite (T. urticae) fecundity on Moneymaker, transgenic line 2 and PI127826 displayed as number of eggs per mite per 4 d. Means ± SE (n = 60). (E) Population growth rates of T. urticae on intact tomato plants. Shown are mean number of adult mites per plant (± SE; n = 4) on S. lycopersicum transgenic plants expressing either only zFps (gray symbols) or both zFps and ShZis (black symbols).
Transgenic line 2 contained the highest levels of 7-epizingiberene and was used for bioassays with whiteflies and spider mites (the generalist Tetranychus urticae and a specialist of solanaceous plants, Tetranychus evansi). The leaf washes of line 2 contained only around 1.5% of the 7-epizingiberene in S. habrochaites PI127826. Whitefly mortality and total number of eggs deposited were not significantly affected. However, on transgenic plant 2, significantly more eggs were deposited on the abaxial leaf surface (87.0% ± 2.4) compared with an untransformed plant (P = 0.01) and the lines expressing zFPS alone (P = 0.04) (Fig. 4C). Whereas wild tomato has type IV and VI glandular trichomes on both sides of the leaf, cultivated tomato lacks type IV and has fewer type VI trichomes on the abaxial side (Fig. S5). Therefore, in cultivated tomato 7-epizingiberene will be produced mostly on the adaxial leaf surface in type VI trichomes, which inversely correlates to the distribution of whitefly eggs (Fig. S5E). The production of 7-epizingiberene had no repellent effect on spider mites in a choice assay (P = 0.55), but had a strong effect on their fecundity. Mites placed on leaf disks of transgenic line 2 exhibited a 40% higher mortality and, after 4 d, an 81% reduction in the number of eggs was recorded in the case of T. urticae (Fig. 4D). The fecundity of the invasive JA-response suppressing mite T. evansi was reduced by 54% on line 2 (Fig. S6). Subsequently, we tried to mimic the situation in a production greenhouse by infesting intact tomato plants with three female T. urticae mites and then monitored the population growth. The presence of 7-epizingiberene in the transgenic plants affected the population growth rates leading to significantly lower numbers (P < 0.001) of mites on the transgenic plants compared with control plants after 45 d (Fig. 4E). Moreover, the fecundity of spider mites, which had been allowed to adapt for two generations either on control or transgenic leaves, was still significantly lower on 7-epizingiberene producing transgenic plants independent from the selection regime (Fig. S7).
7-Epizingiberene also influenced the performance of different insect orders. The development of larvae of the tobacco hornworm (Manduca sexta; Lepidoptera) placed on 7-epizingiberene producing F2 plants (F2-100/21; Fig. S1B) was significantly (P < 0.0001) retarded compared with that on F2 plants without 7-epizingiberene (F2-200/2; Fig. S8A). Also, the Colorado Potato Beetle (Leptinotarsa decemlineata; Coleoptera) preferred a diet without 7-epizingiberene in a choice assay (Fig. S8B; P < 0.01).
Discussion
Wild tomato species possess a large genetic diversity and genes for the production of defense-related metabolites (25, 26). Many of these genes were presumably lost during cultivation of tomato leaving them hampered in their defenses against pests such as whiteflies and spider mites. Here we report that a naturally optimized biosynthetic pathway in the wild-tomato germplasm, responsible for the plant’s extremely high production of a specific sesquiterpene, can make susceptible cultivated tomatoes more resistant.
We identified ShZIS, a trichome-specific sesquiterpene synthase, to produce the 7-epi-stereoisoform of zingiberene in wild tomato PI127826. The protein has high sequence homology with the recently discovered ShSBS plastidial sesquiterpene synthase from wild tomato LA1777 (6). In a recent survey several terpene synthases of the TPS-e/f family from S. habrochaites were identified to produce α-pinene, limonene or zingiberene (7). PI127826 ShZIS is identical to ShZIS from LA2167 recently identified (7) (GenBank accession no. JN990661), although we found a few SNPs in that accession (Table S1). We can conclude that a relatively high number of SNPs is present in 7-epizingiberene synthases of different S. habrochaites accessions (see also Table S1). The presence of an N-terminal targeting peptide nearly identical to ShSBS (Fig. S3A), and the fact that ShZIS exclusively accepts plastidial Z,Z-FPP (6) as a substrate (Fig. 2 and Table 1), indicates plastidial localization. Other zingiberene synthases from basil, sorghum, and grape, have promiscuous activity and convert cytosolic E,E-FPP into a variety of sesquiterpenes (17, 18, 27) (Fig. 2B). Tomato appears to possess a set of atypical terpene synthases, closely related to ancestral TPS synthases (Fig. S3B) that have so far not been found in other sequenced species (27).
PI127826 produces 7-epizingiberene in large amounts and appears to invest in a high and constant rate of production. Producing such extreme sesquiterpene levels could, perhaps, be facilitated by a plastidial biosynthetic pathway. Metabolic engineering of a sesquiterpene pathway to the chloroplast resulted in highly elevated sesquiterpene levels in tobacco (28). In S. habrochaites LA1777, the terpenoid profile is dominated by sesquiterpenes of plastidial origin (29) and it is likely that even more plastidial sesquiterpene sythases will be identified.
The presence of zingiberene could be assigned to a single major locus (30). We now have provided evidence that this locus must contain ShZIS. In LA1777, ShSBS and zFPS are located on the top of chromosome 8 in very close proximity to each other (6). We presume that ShZIS is allelic to ShSBS and linked to zFPS as well. Based on geographic distribution and evolutionary divergence, Gonzales-Vigil et al. (7) suggested that ShZIS originated before ShSBS. Because mutagenesis of only three amino acids in the active site of a predominantly zingiberene synthesizing enzyme resulted in an enzyme producing predominantly (E)-α-bergamotene and (E)-β-farnesene (18), mutations could have led to divergence of ZIS and SBS genes in tomato (7). Probably the neo-functionalization of ShZIS and ShSBS enzymes occurred after a duplication event of an ent-kaurene synthase-like gene in the genome of S. habrochaites. Interestingly, SBS and ZIS genes share high homology (88–89%) to the S. pennellii and cultivated tomato monoterpene synthases PHS (TPS20), on top of chromosome 8 (8, 20), which make α- and β-phellandrene, respectively.
The toxic effect of trichome exudates of the wild PI126826 tomato, attributed to the presence of zingiberene, has been associated with resistance against a variety of herbivores (10–13). The tomato stereoisoform, 7-epizingiberene, appears to be a defensive compound in several ways. First, the constitutive presence of high amounts of 7-epizingiberene in the plant’s headspace probably acts as a semiochemical. Even though whitefly antennae detect both zingiberene stereoisomers, repellence is specific for 7-epizingiberene (15). This repellence appears to be innate, but when given no alternative whiteflies will settle on a tomato emitting 7-epizingiberene and die (15). Second, 7-epizingiberene increases tomato-whitefly resistance in a dose-dependent manner (Fig. S2).
We found a severe reduction in fecundity of two major tomato pests, whiteflies and spider mites (Figs. 1C and 4 D and E). Survival rate and reproductive success of whitefly adults on PI127826 and 7-epizingiberene producing F2s was severely compromised (Fig. 1). Even though the levels of 7-epizingiberene on the leaf surface of F2-7 and F2-71 were lower than in the wild tomato, exposure resulted in a comparable reduction of whitefly fitness (Fig. 1). This will most likely also lead to reduced spread of Begomoviruses (26). When 7-epizingiberene levels were below a certain threshold, as was the case for F2-141 and transgenic line 2, a direct toxicity effect was not detected. However, the number of eggs was reduced (Fig. 1C) and with most glandular trichomes on the adaxial surface (Fig. S5), whiteflies appeared to respond by ovipositing away from the source of 7-epizingiberene production (Fig. 4C) as they do for trichome-produced acylsugars (31). Also herbivores belonging to different orders were severely affected by 7-epizingiberene. Neonate M. sexta larvae consume trichomes as a first meal (32), making them particularly sensitive to trichome-produced toxins as reflected in a severe reduction of body mass (Fig. S8A). The Colorado potato beetle was affected in its choice behavior by the presence of 7-epizingiberene (Fig. S8B).
The F2 plants might contain other traits influencing pest resistance, which we cannot completely exclude. For this reason we created transgenic lines identical to cultivated tomato in every sense, but for the production of 7-epizingiberene from their type VI glandular trichomes. In these lines the effect on spider mite fecundity can be attributed to the presence of 7-epizingiberene solely. 7-Epizingiberene production increased even further in response to JA treatment (Fig. S4B), indicating involvement in induced defenses following herbivory. The invasive red spider mite T. evansi, a specialist on tomato, was recently found to suppress JA-activated defense responses (33), and is beginning to be a serious threat in agriculture. T. evansi appeared to perform slightly better than T. urticae on the transgenic line. However, also for this specialist, reproduction was severely compromised on the wild tomato and, more importantly, on the transgenic line (Fig. S6). The effect of 7-epizingiberene on mite survival and fecundity will affect population development on greenhouse tomatoes as exemplified by the multigenerational experiments (Fig. 4E and Fig. S7).
One trait influencing herbivore resistance is the type and density of the glandular trichomes that produce, store and emit 7-epizingiberene. Zingiberene levels had been associated to type IV and VI trichome densities in an F2-population (10). On cultivated tomato type IV trichomes are far outnumbered by type VI (refs. 10, 11, 34, and 35; Fig. S5) and the promoters used for expression of zFPS and ShZIS are active in type VI (Fig. S4), indicating they are the primary source of 7-epizingiberene produced (Fig. 4 A and B). However, we cannot exclude a contribution of other types of glandular trichomes. Sole expression of zFPS and ShZIS is sufficient to allow S. lycopersicum to make repellent and toxic 7-epizingiberene, although concentrations are likely restricted by the number of trichomes (10, 11). Also, the promoters used in this study, chosen for their trichome specificity, are probably not as strong as the endogenous S. habrochaites promoters. We know that SlMTS1 is not highly expressed (24) and the ShMKS1 promoter is expressed out of context in S. lycopersicum, possibly influencing expression levels. However, because expression is restricted to glandular trichomes, transgenic lines developed normally and no effect on flowering, fruit and seed set was observed. Additionally, whereas it would be conceivable that expression of zFps and ShZis in the plastid would lead to precursor competition, we did not find lower levels of monoterpenes in the transgenic line.
In summary, tomato glandular trichomes can be used as biochemical factories for the production of toxic and repellent defense compounds. We exploited the fact that a wild tomato species contains an atypical terpene pathway, confined to the chloroplast, allowing these plants to produce exceptionally high concentrations of 7-epizingiberene. Trichomes emerge early in tomato ontogeny and are found all over the plant, except on ripe fruits, making them ideal for metabolic engineering without affecting fruit flavor and yield. We have shown that, by the addition of two enzymes in the terpenoid-biosynthetic pathway, it is feasible to alter insect-choice behavior and improve cultivated tomato defense, both as proof-of-concept in transgenic lines, as well as via non-GM techniques.
Materials and Methods
Detailed information is described in SI Materials and Methods.
Plant Material.
Solanum habrochaites PI127826 was obtained from Keygene N.V. A cross between S. lycopersicum cv Moneymaker and PI127826 was made and an F2 population was obtained through selfing. Plants were grown in a controlled greenhouse compartment with 16 h light. For the inhibitor study cuttings were placed in a hydroponics system amended with 10 µM fosmidomycin.
Expression Analyses.
Tomato trichomes were shaken from stems frozen in liquid nitrogen. RNA was isolated from trichomes for expression analyses using real-time quantitative PCR.
GC-MS Analyses.
Total tissue terpenes were analyzed by Solid Phase Micro Extraction (SPME) followed by desorption into the injector port of the Gas-Chromatograph Mass Spectrometer (GC-MS). For leaf surface terpenes, leaves were washed for 5 min in hexane. Leaf washes were analyzed by injection into the GC-MS injector port. Compounds were separated on a DB-5 column with helium as a carrier gas. Mass spectra were collected with a Time-of-Flight MS. Zingiberene stereoisoforms were separated on an Astec CHIRALDEXTM B-DM column.
Enzyme Characterization.
ShZIS was cloned into the pGEX-KG expression vector and transformed into Escherichia coli C41. After induction activity assays were performed in the bacterial lysate by addition of various terpene precursors. Products were sampled by SPME and analyzed by GC-MS. For kinetic analyses the recombinant fusion protein was purified using glutathione affinity binding. Purified protein was assayed with different Z,Z-FPP concentrations to determine Km and Kcat values.
In Planta Engineering.
Trichome specific expression of the Methyl Ketone Synthase (MKS1) promoter and the Monoterpene Synthase (MTS1) promoter was confirmed by expression of the uidA reporter (GUS). Next, the MKS1 promoter was used to drive zFPS and the MTS1 promoter was cloned in front of ShZIS. Both constructs were transformed to S. lycopersicum cv Moneymaker. The presence of 7-epizingiberene was analyzed by GC-MS in several independent transformants.
Bioassays.
Performance of the Hemipteran B. tabaci (biotype Q) was assessed on F2 plants and transgenic lines producing 7-epizingiberene. Performance of spider mites (generalist T. urticae and specialist T. evansi; suborder Acari) was assessed in choice or no-choice assays on leaf disks and intact (transgenic) plants. The effect of 7-epizingiberene on the development of the Lepidopteran M. sexta and preference-behavior of the Coleopteran L. decemlineata was ascertained on F2 plants.
Statistics.
Statistical analyses was performed by ANOVA followed by a post hoc test for comparison of individual means (SPSS Inc. 2010). When necessary values were log-transformed before analyses.
Supplementary Material
Acknowledgments
We thank Prof. Eran Pichersky for the basil zingiberene synthase and, with Yoko Iijima, for the MKS1 promoter sequence; SUPELCO (www.sigmaaldrich.com) for help with development of the enantiomer GC-MS program; Ludek Tikovsky, Harold Lemereis, and Thijs Hendrix for plant care; the Max Planck Institute for Chemical Ecology (Jena, Germany) for the M. sexta eggs; and Nicole van Dam (Radboud University Nijmegen) for CPB eggs.
Footnotes
The authors declare no conflict of interest. Description of 7-epizingiberene production and the use of the ShZIS gene and its application for enhanced insect resistance as described in this paper are covered by patents and/or patent applications owned by Keygene N.V. (CT/NL2012/050382).
This article is a PNAS Direct Submission. G.A.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208756109/-/DCSupplemental.
References
- 1.Navas-Castillo J, Fiallo-Olivé E, Sánchez-Campos S. Emerging virus diseases transmitted by whiteflies. Annu Rev Phytopathol. 2011;49:219–248. doi: 10.1146/annurev-phyto-072910-095235. [DOI] [PubMed] [Google Scholar]
- 2.McCaskill D, Croteau R. Strategies for bioengineering the development and metabolism of glandular tissues in plants. Nat Biotechnol. 1999;17(1):31–36. doi: 10.1038/5202. [DOI] [PubMed] [Google Scholar]
- 3.Eigenbrode SD, Trumble JT, Millar JG, White KK. Topical toxicity of tomato sesquiterpenes to the beet armyworm and the role of these compounds in resistance derived from an accession of Lycopersicon hirsutum f. typicum. J Agric Food Chem. 1994;42:807–810. [Google Scholar]
- 4.Frelichowski JE, Jr, Juvik JA. Sesquiterpene carboxylic acids from a wild tomato species affect larval feeding behavior and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera: Noctuidae) J Econ Entomol. 2001;94(5):1249–1259. doi: 10.1603/0022-0493-94.5.1249. [DOI] [PubMed] [Google Scholar]
- 5.Snyder JC, Guo Z, Thacker R, Goodman JP, Pyrek JST. 2,3-Dihydrofarnesoic acid, a unique terpene from trichomes of Lycopersicon hirsutum repels spider mites. J Chem Ecol. 1993;19:2981–2997. doi: 10.1007/BF00980597. [DOI] [PubMed] [Google Scholar]
- 6.Sallaud C, et al. A novel pathway for sesquiterpene biosynthesis from Z,Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell. 2009;21(1):301–317. doi: 10.1105/tpc.107.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzales-Vigil E, Hufnagel DE, Kim J, Last RL, Barry CS. Evolution of TPS20-related terpene synthases influences chemical diversity in the glandular trichomes of the wild tomato relative Solanum habrochaites. Plant J. 2012;71(6):921–935. doi: 10.1111/j.1365-313X.2012.05040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schilmiller AL, et al. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc Natl Acad Sci USA. 2009;106(26):10865–10870. doi: 10.1073/pnas.0904113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama T. Molecular analysis of prenyl chain elongating enzymes. Biosci Biotechnol Biochem. 1999;63(10):1671–1676. doi: 10.1271/bbb.63.1671. [DOI] [PubMed] [Google Scholar]
- 10.Maluf WR, Campos GA, Cardoso MG. Relationships between trichome types and spider mites (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene content. Euphytica. 2001;121:73–80. [Google Scholar]
- 11.Freitas JA, Maluf WR, Graças-Cardoso M, Gomes LAA, Bearzotti E. Inheritance of foliar zingiberene contents and their relationship to trichome densities and whitefly resistance in tomatoes. Euphytica. 2002;127:275–287. [Google Scholar]
- 12.Muigai SG, et al. Mechanisms of resistance in Lycopersicon germplasm to the whitefly Bemisia argentifolii. Phytoparasitica. 2002;30:347–360. [Google Scholar]
- 13.de Azevedo SM, et al. Zingiberene-mediated resistance to the South American tomato pinworm derived from Lycopersicon hirsutum var. hirsutum. Euphytica. 2003;134:347–351. [Google Scholar]
- 14.Zhang W, McAuslane HJ, Schuster DJ. Repellency of ginger oil to Bemisia argentifolii (Homoptera: Aleyrodidae) on tomato. J Econ Entomol. 2004;97(4):1310–1318. doi: 10.1093/jee/97.4.1310. [DOI] [PubMed] [Google Scholar]
- 15.Bleeker PM, et al. Tomato-produced 7-epizingiberene and R-curcumene act as repellents to whiteflies. Phytochemistry. 2011;72(1):68–73. doi: 10.1016/j.phytochem.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Iijima Y, et al. The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol. 2004;136(3):3724–3736. doi: 10.1104/pp.104.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidovich-Rikanati R, et al. Overexpression of the lemon basil α-zingiberene synthase gene increases both mono- and sesquiterpene contents in tomato fruit. Plant J. 2008;56(2):228–238. doi: 10.1111/j.1365-313X.2008.03599.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang X, et al. Dynamic evolution of herbivore-induced sesquiterpene biosynthesis in sorghum and related grass crops. Plant J. 2012;69(1):70–80. doi: 10.1111/j.1365-313X.2011.04771.x. [DOI] [PubMed] [Google Scholar]
- 19.Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66(1):212–229. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- 20.Falara V, et al. The tomato terpene synthase gene family. Plant Physiol. 2011;157(2):770–789. doi: 10.1104/pp.111.179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Köllner TG, et al. A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008;20(2):482–494. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhagen BT, O’Maille PE, Noel JP, Chappell J. Identifying and manipulating structural determinates linking catalytic specificities in terpene synthases. Proc Natl Acad Sci USA. 2006;103(26):9826–9831. doi: 10.1073/pnas.0601605103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu G, et al. Enzymatic functions of wild tomato methylketone synthases 1 and 2. Plant Physiol. 2010;154(1):67–77. doi: 10.1104/pp.110.157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Schie CC, Haring MA, Schuurink RC. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol Biol. 2007;64(3):251–263. doi: 10.1007/s11103-007-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quesada-Ocampo LM, Hausbeck MK. Resistance in tomato and wild relatives to crown and root rot caused by Phytophthora capsici. Phytopathology. 2010;100(6):619–627. doi: 10.1094/PHYTO-100-6-0619. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-López MJ, et al. Whitefly resistance traits derived from the wild tomato Solanum pimpinellifolium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of Tomato yellow leaf curl virus. Phytopathology. 2011;101(10):1191–1201. doi: 10.1094/PHYTO-01-11-0028. [DOI] [PubMed] [Google Scholar]
- 27.Martin DM, et al. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010;10:226–248. doi: 10.1186/1471-2229-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu S, et al. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotechnol. 2006;24(11):1441–1447. doi: 10.1038/nbt1251. [DOI] [PubMed] [Google Scholar]
- 29.Besser K, et al. Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiol. 2009;149(1):499–514. doi: 10.1104/pp.108.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahimi FR, Carter CD. Inheritance of zingiberene in Lycopersicon. Theor Appl Genet. 1993;87:593–597. doi: 10.1007/BF00221883. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-López MJ, et al. Acylsucrose-producing tomato plants forces Bemisia tabaci to shift its preferred settling and feeding site. PLoS ONE. 2012;7(3):e33064. doi: 10.1371/journal.pone.0033064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinhold A, Baldwin IT. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc Natl Acad Sci USA. 2011;108(19):7855–7859. doi: 10.1073/pnas.1101306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarmento RA, et al. A herbivore that manipulates plant defence. Ecol Lett. 2011;14(3):229–236. doi: 10.1111/j.1461-0248.2010.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luckwill LC. The genus Lycopersicon: An historical, biological and taxonomic survey of the wild and cultivated tomatoes. Aberdeen, UK: Aberdeen Univ Press; 1943. [Google Scholar]
- 35.Kang J-H, et al. The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol. 2010;154(1):262–272. doi: 10.1104/pp.110.160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.