Abstract
The combination of irradiated fibroblast feeder cells and Rho kinase inhibitor, Y-27632, conditionally induces an indefinite proliferative state in primary mammalian epithelial cells. These conditionally reprogrammed cells (CRCs) are karyotype-stable and nontumorigenic. Because self-renewal is a recognized property of stem cells, we investigated whether Y-27632 and feeder cells induced a stem-like phenotype. We found that CRCs share characteristics of adult stem cells and exhibit up-regulated expression of α6 and β1 integrins, ΔNp63α, CD44, and telomerase reverse transcriptase, as well as decreased Notch signaling and an increased level of nuclear β-catenin. The induction of CRCs is rapid (occurs within 2 d) and results from reprogramming of the entire cell population rather than the selection of a minor subpopulation. CRCs do not overexpress the transcription factor sets characteristic of embryonic or induced pluripotent stem cells (e.g., Sox2, Oct4, Nanog, or Klf4). The induction of CRCs is also reversible, and removal of Y-27632 and feeders allows the cells to differentiate normally. Thus, when CRCs from ectocervical epithelium or tracheal epithelium are placed in an air–liquid interface culture system, the cervical cells form a well differentiated stratified squamous epithelium, whereas the tracheal cells form a ciliated airway epithelium. We discuss the diagnostic and therapeutic opportunities afforded by a method that can generate adult stem-like cells in vitro without genetic manipulation.
We recently have shown that human keratinocytes (1) and nonkeratinocyte epithelial cells (e.g., prostate, breast, liver, and lung) (2) can be propagated indefinitely in vitro when cocultured with irradiated fibroblast feeder cells and a Rho kinase (ROCK) inhibitor, Y-27632 (3). These cultures are referred to as conditionally reprogrammed cells (CRCs) due to the conditional induction of cell proliferation, and offer many possibilities for personalized and regenerative medicine. Moreover, both normal and tumor cells can be cultured using the technique, affording opportunities for genetic and phenotypic characterization, including chemosensitivity testing. Being able to rapidly establish karyotype-stable cell cultures from normal human epithelium enables cell biology studies without the pitfalls of cell cultures established from induced pluripotent stem cells (iPSCs) (e.g., genetic instability, tumorigenicity, and altered antigenicity) (4–6).
Adult stem cells enable the regeneration and repair of damaged epithelial tissues. These cells reside in the basal layer of the epithelium, and have both the capacity for continuous self-renewal and the ability to produce transit amplifying (TA) cells, which terminally differentiate after a brief period of rapid proliferation (7, 8). The capacity of CRCs for self-renewal suggested that they might resemble adult stem cells. Here, we show that the transfer of primary human ectocervical cells (HECs) to irradiated feeders in the presence of Y-27632 rapidly induces numerous markers of epithelial stem cells, and that the induction results from generalized cell reprogramming, rather than selection. CRC markers revert completely upon removal of the reprogramming conditions, and the cells differentiate in a tissue-specific manner. We further show that CRCs do not express high levels of proteins characteristic of iPSCs or embryonic stem cells (ESCs).
Results
ROCK Inhibitor and Feeder Cells Induce Adult Epithelial Stem Cell Markers in Primary HECs.
The demonstration that ROCK inhibitor Y-27632, in combination with irradiated fibroblasts, enables epithelial cells to proliferate indefinitely in vitro suggests that the cells might be acquiring a stem-like phenotype. To address this question, lysates were prepared from early-passage primary HECs in keratinocyte growth medium (KGM), which does not induce immortalization, and from the same HECs after plating on irradiated fibroblast feeder cells in the presence of Y-27632. Lysates also were prepared from HECs in the presence of Y-27632, but without feeder cells, to determine the contribution of feeders to the phenotype.
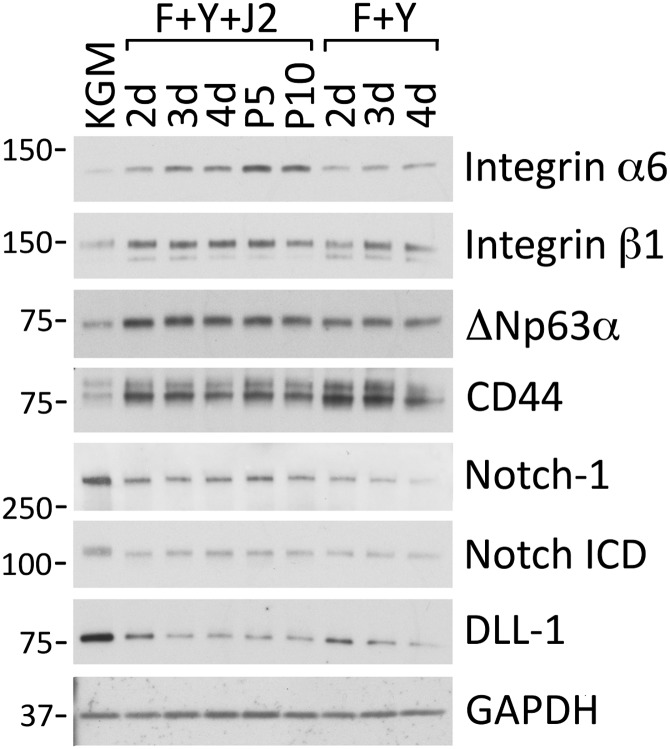
In vitro studies with human keratinocytes have identified integrins α6 and β1 as markers of epithelial stem cells, and keratinocytes expressing high levels of integrin α6 (9) or integrin β1 (10) exhibit increased colony-forming efficiency and rapid attachment to components of the extracellular matrix. As shown in Fig. 1, the expression of integrin α6 in HECs increased after 2 d in the presence of Y-27632 and feeder cells, and reached a maximum level by passage 5 (10-fold increase). Integrin β1 was also increased at 2 d and reached maximum expression after 3 d (2.2-fold increase). For both integrins, expression was greater in the presence of feeder cells (Fig. 1).
Fig. 1.
Rapid induction of epithelial stem cell markers by ROCK inhibitor and feeders. Irradiated 3T3 J2 fibroblasts were plated on 10-cm tissue culture dishes in complete DMEM at 75% confluence. Two hours later, primary HECs (growing in KGM) were plated on 10-cm dishes with (F+Y+J2) or without (F+Y) J2 cells, in F-medium containing 10 µM Y-27632. Whole-cell lysates were prepared after 2–4 d of growth, and after 5 or 10 passages (P5, P10) for analysis by Western blotting. Control cells (KGM) were harvested from KGM. Lanes contain equal amounts of protein. Molecular mass markers (in kDa) are shown on the left.
The p63 transcription factor is required for the maintenance of stem cells in mouse epidermis (7). It is specifically expressed by stem cells in human epidermis and limbal epithelium, and not by adjacent TA cells (11). An anti-pan p63 Western blot indicated that ΔNp63α was the only detectable p63 isoform in HECs (Fig. S1). Both the combination of Y-27632 and feeder cells, or Y-27632 alone, induced a rapid (2 d) 3.1-fold increase in ΔNp63α in the HECs (Fig. 1). Consistent with published values for the apparent molecular weight of recombinant p63 isoforms on SDS polyacrylamide gels, ΔNp63α migrated at 72 kDa (12). The expression of CD44, another established marker of epithelial stem cells (13), similarly increased 3.4-fold in 2 d in the presence of Y-27632, independently of feeder cells (Fig. 1).
The Notch 1 receptor and its ligand, Delta 1 (human homolog Delta-like ligand 1; DLL-1), are transmembrane proteins expressed on the surface of basal and suprabasal cells of stratified epithelia. Binding of DLL-1 to Notch 1 on adjacent cells induces proteolytic processing of Notch, which releases the Notch intracellular domain (ICD). Notch ICD then translocates to the nucleus and initiates a brief period of rapid proliferation, followed by terminal differentiation (14). Therefore, it is critical for stem cells to maintain a low level of Notch signaling. In the epithelium, Notch signaling in stem cells appears to be repressed in two ways: First, expression of the Notch 1 receptor is lower in basal-layer cells than in suprabasal cells (15, 16), and second, high expression of the DLL-1 ligand in stem cells reduces Notch signaling while stimulating adjacent TA cells to proliferate and differentiate (14). In our system, Y-27632 repressed Notch 1 signaling in HECs (independently of feeder cells), as evidenced by decreased levels of the Notch ICD (2.3-fold decrease; Fig. 1). Reduced signaling was correlated with decreased expression of both the Notch 1 receptor (4.0-fold) and DLL-1 (6.0-fold). A glyceraldehyde 3-phosphate dehydrogenase (GAPDH) immunoblot demonstrated that all samples contained equal amounts of cell protein (Fig. 1). Thus, Y-27632 was sufficient to increase expression of the epithelial stem cell markers ΔNp63α and CD-44 while decreasing Notch-1 signaling. In combination with fibroblast feeder cells, Y-27632 increased expression of the α6 and β1 integrins.
Primary HECs Are Reprogrammed to a Stem Cell Phenotype.
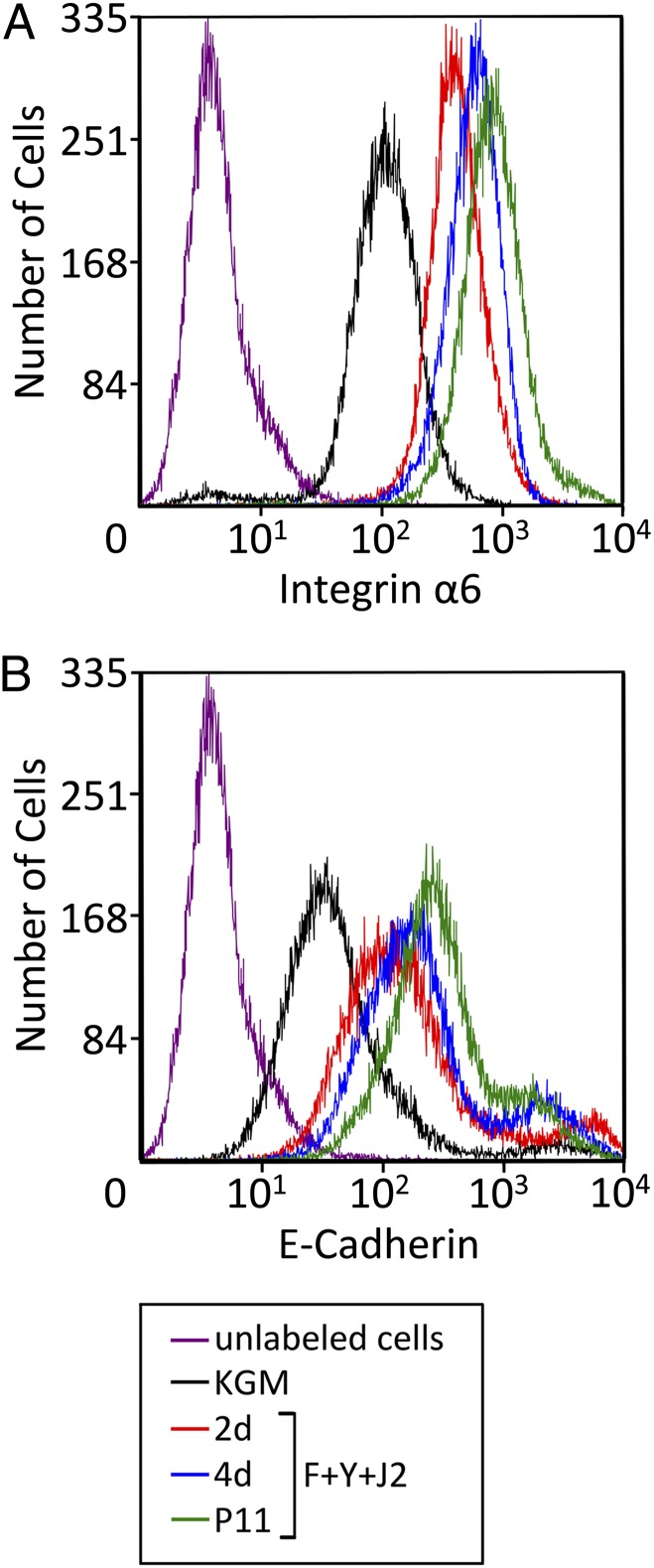
Although Western blots measure the average level of stem cell markers in a population of cells, the finding that expression generally was fully up-regulated (or down-regulated) after only 2 d in the presence of Y-27632 and feeders (Fig. 1) suggested that these conditions were reprogramming HECs to a stem cell phenotype, rather than selecting for cells that already displayed the phenotype. To distinguish between these possibilities, flow cytometry was used to determine the level of integrin α6 per cell for HECs growing in KGM, and after plating on feeder cells in the presence of Y-27632. Integrin α6 was chosen for this analysis because it exhibited the most gradual increase of stem cell markers we tested. In KGM, the level of integrin α6 on the surface of HECs was distributed as a single peak (Fig. 2A), signifying a single population of cells. Consistent with our immunoblot results, the mean value of integrin α6 expression in the HECs progressively increased after 2 d (4.3-fold), 4 d (5.8-fold), and 11 passages (8.8-fold) of coculture with feeders and Y-27632; however, expression levels continued to be distributed as a single peak (Fig. 2A). These results are indicative of a single population of cells undergoing gradual reprogramming. A similar result was obtained for E-cadherin on the cell surface (7.6-fold increase; Fig. 2B). The small “shoulder” at the upper end of the major E-cadherin peak did not indicate selection, because it remained constant at ∼10% of the population (Fig. 2B).
Fig. 2.
ROCK inhibitor and feeders reprogram primary epithelial cells. Flow cytometric analysis of integrin α6 (A) and E-cadherin (B) on the surface of primary HECs in KGM, or growing on irradiated J2 cells in F-medium containing 10 µM Y-27632 (F+Y+J2) for 2 d, 4 d, or 11 passages. Unlabeled cells were not incubated with the primary antibodies.
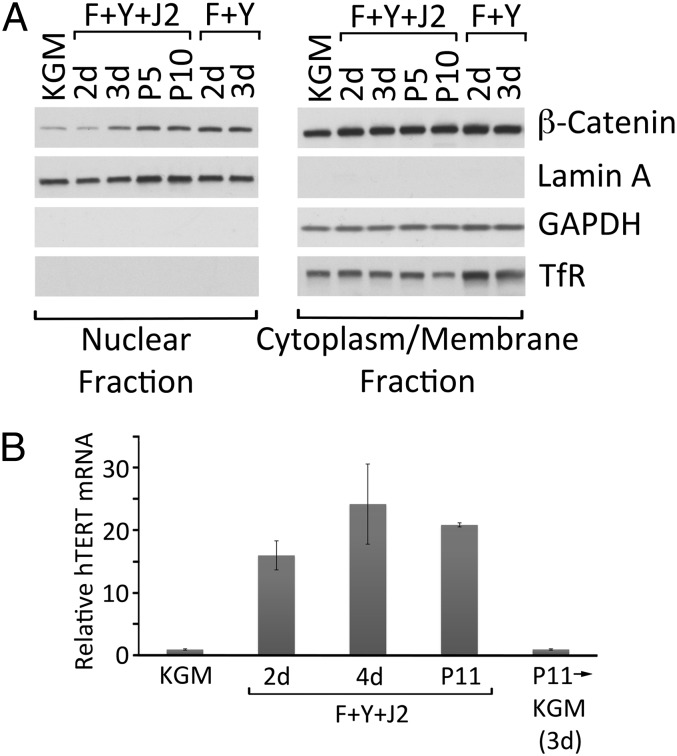
The stabilization and subsequent nuclear translocation of β-catenin is required for epithelial stem cell maintenance, as well as for promoting proliferation in TA cells (17). In primary HECs, Y-27632 induced a 3.3-fold increase in nuclear β-catenin, even in the absence of feeder cells (Fig. 3A). Under the same conditions, the level of nonnuclear β-catenin was much higher but remained constant (Fig. 3A). The efficiency of cell fractionation into nuclear and nonnuclear (i.e., cytoplasm and membrane) compartments was demonstrated by the presence (or absence) of fraction-specific proteins. Lamin A, an integral component of the nuclear envelope, was exclusively present in the nuclear fraction, whereas cytoplasmic GAPDH and the membrane-associated transferrin receptor were exclusively present in the nonnuclear fraction (Fig. 3A).
Fig. 3.
Induction of nuclear β-catenin and telomerase during conditional reprogramming. (A) Nuclear and nonnuclear fractions were prepared from primary HECs growing in KGM, or in F-medium containing 10 µM Y-27632 in the presence (F+Y+J2) absence (F+Y) of irradiated J2 cells for 2 d, 3 d, 5 passages (P5) or 10 passages (P10). The fractions were solubilized for SDS/PAGE and Western blot analysis (equal cell equivalents per lane). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TfR, transferrin receptor. (B) Quantitative real-time RT-PCR measurement of hTERT mRNA levels in primary HECs growing in KGM, or on irradiated J2 cells in F-medium containing 10 µM Y-27632 for 2 d, 4 d or 11 passages (P11). The level of hTERT in KGM was set to 1.0. Some P11 reprogrammed HECs were harvested and replated in KGM for 3 d (P11→KGM) before RNA isolation and PCR analysis. Error bars indicate SEM (n = 3).
In contrast to differentiating cells of the epithelium, stem cells and TA cells exhibit elevated telomerase activity, which undoubtedly contributes to the capacity of stem cells for self-renewal (18). To determine whether the reprogramming of HECs by Y-27632 and feeders is associated with increased telomerase activity, quantitative real-time RT-PCR was used to measure levels of mRNA encoding the catalytic subunit of human telomerase, telomerase reverse transcriptase (hTERT). As shown in Fig. 3B, hTERT mRNA was almost undetectable in HECs growing in KGM, but increased 16-fold after 2 d of reprogramming and remained highly elevated even after 11 passages under reprogramming conditions.
CRC Stem Cell Phenotype Is Rapidly Reversible.
The demonstration that primary human keratinocytes conditionally immortalized by Y-27632 and feeder cells can differentiate into a stratified epithelium following removal of the ROCK inhibitor (1) implies that reprogramming to an adult stem cell phenotype is conditional and reversible. This view is supported by the observation that transferring cervical CRCs to KGM after 11 passages under reprogramming conditions (Y-27632 and feeder cells) caused hTERT mRNA to return to a preprogramming level within 3 d (>20-fold decrease; Fig. 3B). More importantly, the adult epithelial stem cell markers that were induced in the CRCs reverted within 3 d after transfer to KGM. Integrin α6, which increased 10-fold during reprogramming, decreased 6-fold in KGM. ΔNp63α and CD44, which increased 3.1-fold and 3.4-fold during reprogramming, decreased 3.1-fold and 3.4-fold, respectively (Fig. 4). Components of the Notch signaling pathway, which decreased in conditionally reprogrammed HECs, rapidly increased when the cells were shifted into KGM (3.3-fold increase in Notch 1 and 10-fold increase in DLL-1; Fig. 4).
Fig. 4.
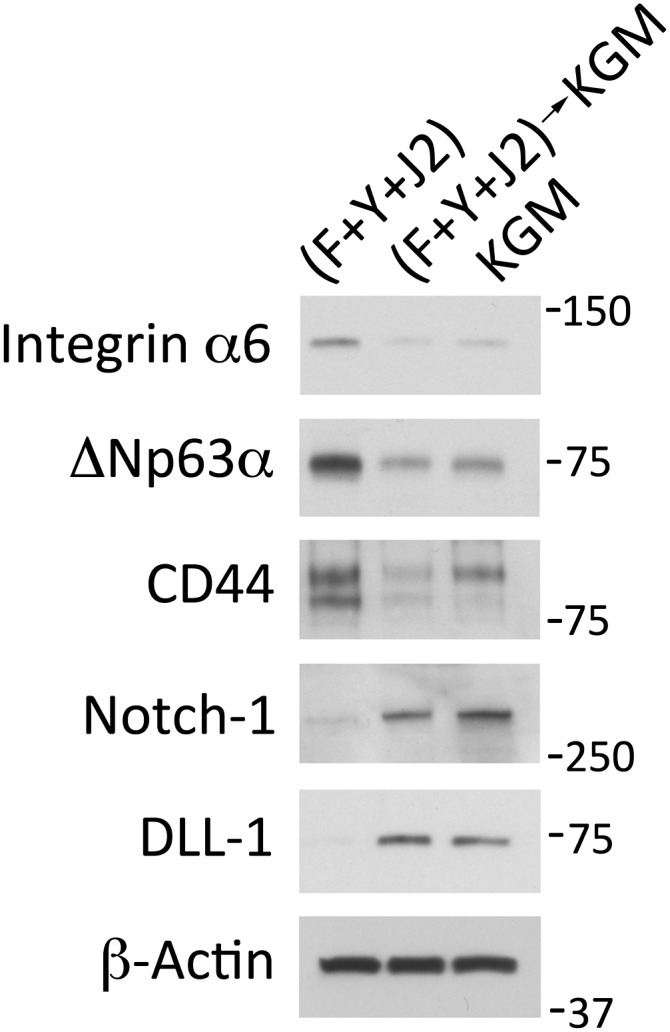
Rapid loss of the stem cell phenotype after removing reprogramming conditions. Western blots of whole cell lysates that were prepared from primary HECs growing in KGM, in F-medium with irradiated J2 cells and 10 µM Y-27632 for 11 passages (F+Y+J2), or for 3 d in KGM after 11 passages in F-medium with irradiated J2 cells and Y-27632 ((F+Y+J2)→KGM). Lanes contain equal amounts of protein. Molecular mass markers (in kDa) are shown on the right.
Conditionally Reprogrammed Cells Maintain Tissue-Specific Differentiation Potential.
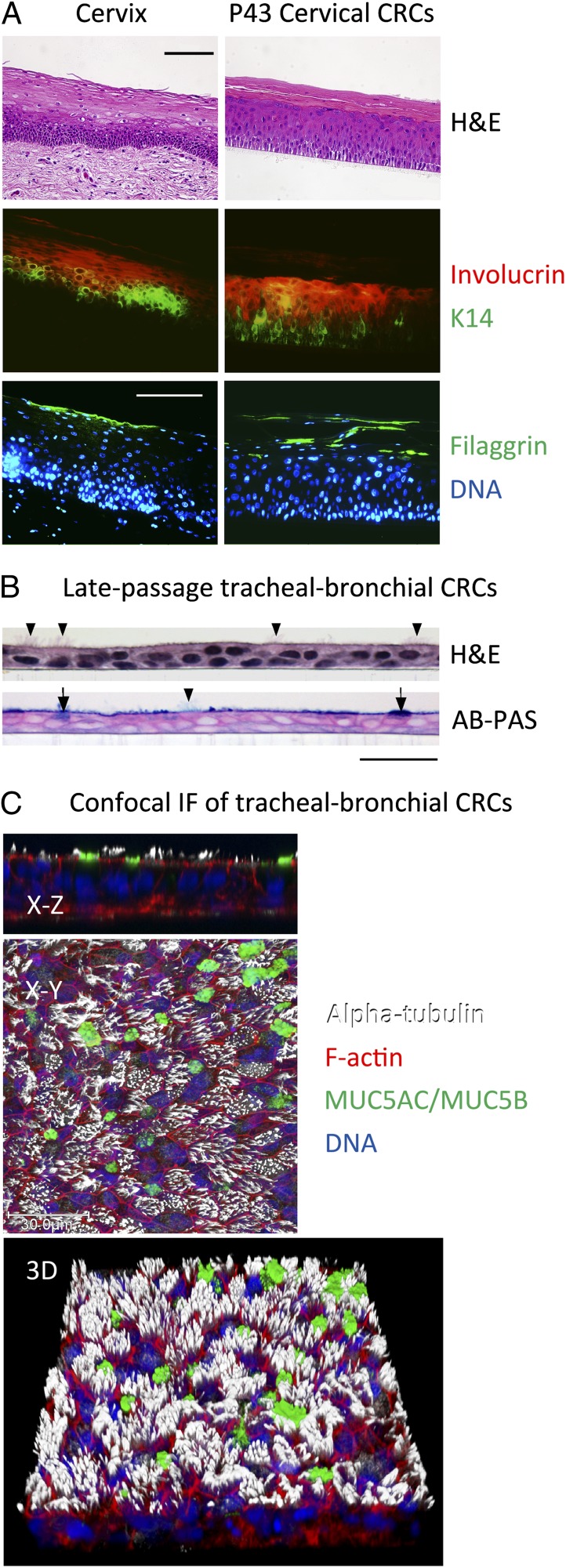
An intrinsic property of epithelial stem cells in vivo is the ability to generate TA cells that eventually differentiate to maintain the epithelium. The stratified squamous epithelium of the cervix is shown in Fig. 5A. Immunostaining of deparaffinized sections of cervix shows that basal layer cells express keratin 14, spinous layer cells express the early differentiation marker, involucrin, and cells of the granular and cornified layers express the late differentiation marker, filaggrin (Fig. 5A, cervix). Despite 43 passages (>200 doublings) under reprogramming conditions, cervical CRCs formed a morphologically similar stratified epithelium in vitro when cultured on an air–liquid interface following the removal of Y-27632 and feeder cells (Fig. 5A, H&E of cervical CRCs). Moreover, this epithelium exhibited the correct pattern of differentiation-specific protein expression: keratin 14 in the basal layer, involucrin in the spinous layer and filaggrin in the granular and cornified layers (Fig. 5A, cervical CRCs). Late-passage human foreskin keratinocyte CRCs also differentiated into a stratified squamous epithelium in vitro under these conditions (Fig. S2). In contrast to the stratified epithelium formed by cervical and foreskin cells, when CRCs derived from tracheal epithelium were cultured on the same air–liquid interface, the cells formed a mucociliary airway epithelium (Fig. 5B and Fig. S2). Although both the ROCK inhibitor and J2 cells were removed in these differentiation experiments, epithelial cell differentiation has been induced in the presence of J2 feeders simply by removing Y-27632 (1). Thus, CRCs retain lineage commitment and, when removed from Y-27632 and placed into an appropriate 3D culture system (with or without feeders), differentiate into the epithelium from which they were derived.
Fig. 5.
Tissue-specific developmental potential is maintained during conditional reprogramming. (A) H&E-stained histological sections of normal cervix, and of primary HECs that were differentiated in air–liquid interface culture after 43 passages on irradiated J2 cells in F-medium containing 10 µM Y-27632. Serial sections of the samples were deparaffinized, rehydrated, and stained with primary antibodies specific for cytokeratin 14 (K14), involucrin and filaggrin, followed by labeling with fluorescent secondary antibodies and Hoechst dye 33258 (DNA). (Scale bars: 100 µm.) (B) Histological sections of primary tracheal-bronchial cells that were cultured to late passage as above, followed by differentiation in vitro. Sections were stained with H&E or a combination of alcian blue and periodic acid-Schiff reaction (AB-PAS). Note the presence of ciliated cells (arrowheads) and mucus-producing cells (arrows). (Scale bar: 30 μm.) (C) Confocal microscopy of tracheal-bronchial CRCs from a second donor that were differentiated in air–liquid interface culture for 47 d, fixed and fluorescently labeled with phalloidin (F-actin), Hoechst dye 33342 (DNA), or antibodies demonstrating the presence of cilia (alpha-tubulin) and mucins 5AC and 5B (MUC5AC/MUC5B). An X–Z cross-section, extended focus X–Y view, and corresponding three-dimensional (3D) view are shown.
Conditional Reprogramming Does Not Induce a Pluripotent Stem Cell Phenotype.
The finding that CRCs differentiated exclusively into the tissue from which they were derived distinguishes them from iPSCs, which exhibit an embryonic stem cell phenotype and can differentiate into tissues of ectodermal, mesodermal and endodermal origin (19, 20). To further characterize the differences between CRCs and iPSCs/ESCs, Western blots were performed on whole-cell lysates of cervical CRCs to detect proteins commonly expressed in ESCs and used to generate iPSCs (20) (Fig. 6). The typical expression of Sox2, Oct4, and Nanog in mouse ESCs was included as a reference. As shown, the levels of these proteins were almost undetectable in cervical CRCs. Klf4 increased 5-fold in cervical CRCs, but was 2.2-fold more abundant in the ESCs. The level of c-Myc increased less than 2-fold during reprogramming, and was comparable to ESCs.
Fig. 6.
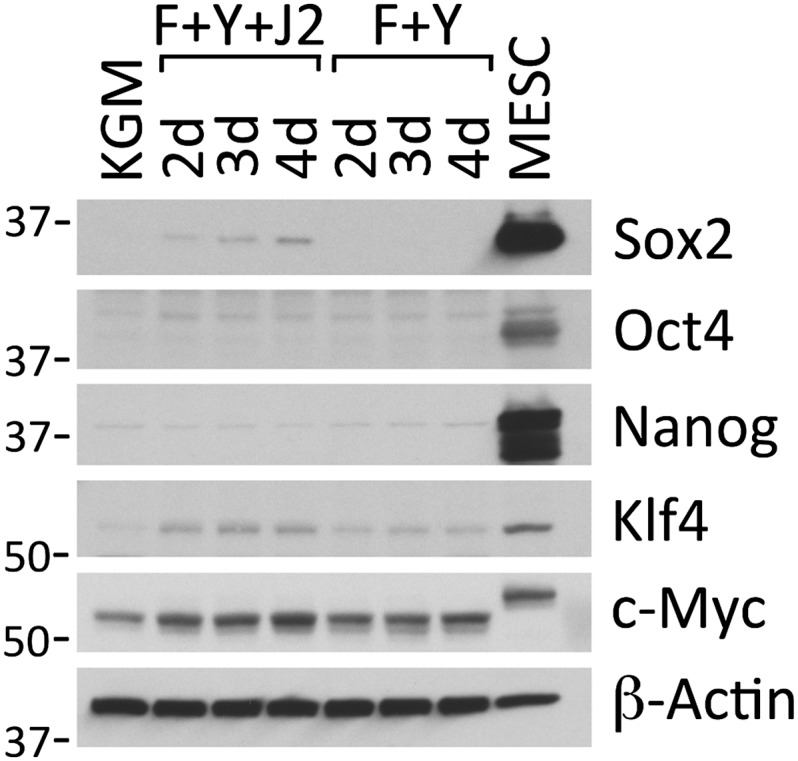
Conditional reprogramming does not up-regulate markers of iPSCs. Western blots of whole cell lysates prepared from primary HECs growing in KGM, or in F-medium containing 10 µM Y-27632 in the presence (F+Y+J2) or absence (F+Y) of irradiated J2 cells for 2 d, 3 d, or 4 d. As a positive control, an identical lysate was prepared from mouse ESCs (MESC). All antibodies were generated against human antigens (and demonstrated to cross-react with the mouse homologs), or were polyclonal antibodies raised against full-length mouse proteins (and demonstrated to cross-react with the human homologs). Lanes contain equal amounts of protein. Molecular mass markers (in kDa) are shown on the left.
Discussion
Previous studies have shown that the combination of fibroblast feeder cells and ROCK inhibitor Y-27632 conditionally immortalize epithelial cells from many tissues (2). Our investigation provides a biochemical characterization of the CRC phenotype. We show that cervical CRCs exhibit increased expression of many proteins that are up-regulated in adult epithelial stem cells, such as integrin α6, integrin β1, ΔNp63α, CD44, hTERT, and nuclear β-catenin (9–11, 13, 17, 18). In contrast, the cervical CRCs express low levels of Sox2, Oct4, and Nanog, which are abundant in ESCs and iPSCs (20, 21). These results are consistent with the different developmental potential of CRCs compared with ESCs and iPSCs. Whereas ESCs and iPSCs can differentiate into tissues of all lineages in culture and form teratomas in mice (5, 19), CRCs are nontumorigenic (2) and specifically differentiate into the tissue from which they are derived (cervical and foreskin CRCs form a stratified squamous epithelium and tracheal CRCs form a ciliated tracheobronchial epithelium). The CRC method rapidly and efficiently generates large numbers of cells (2), which further distinguishes it from relatively inefficient iPSC technologies (20). The rapid induction of highly proliferative cell cultures is consistent with our finding that CRCs are undergoing reprogramming rather than selection as determined by flow cytometry studies of integrin α6 and E-cadherin induction. Finally, unlike ESCs and iPSCs, we show that the CRC phenotype is rapidly (≤3 d) and completely reversible. The differences between CRCs and ESCs/iPSCs are summarized in Table S1 and validate that CRCs are indeed a unique category of stem-like cells, with many properties similar to adult stem cells.
Epithelial stem cells divide slowly in vivo, and can be identified by their ability to retain radioactive thymidine longer than adjacent, rapidly dividing TA cells in pulse–chase experiments (22). In this respect, highly proliferative CRCs more closely resemble TA cells; however, unlike TA cells (but like stem cells), CRCs remain in an undifferentiated state (provided that they are cocultured with feeders and Y-27632). These characteristics may be explained by the combined actions of β-catenin and Notch. Cervical CRCs exhibit higher levels of nuclear β-catenin, which is known to promote the transition of stem cells to TA cells in the epidermis of transgenic mice (17), thus providing a mitogenic stimulus to the CRCs. At the same time, Notch signaling (that is requisite for differentiation) is at low levels in CRCs due to decreased expression of the Notch 1 receptor and DLL-1 ligand. Thus, increased nuclear β-catenin and decreased Notch signaling may be responsible for maintaining CRCs in a highly proliferative, undifferentiated state.
Materials and Methods
Cell Culture.
Primary HECs were isolated from cervical tissue after hysterectomy for benign uterine disease and passaged one to two times in KGM (Invitrogen) without feeder cells before freezing (23). CRC cultures were maintained on irradiated 3T3 J2 murine fibroblasts in F-medium (Invitrogen) containing 10 μM Y-27632 (Enzo Life Sciences), and were passaged every 3–4 d due to rapid proliferation induced by the ROCK inhibitor. After removal of the feeder cells, confluent HECs were detached by trypsin treatment and passaged 1:10 (4.2 × 105 HECs per 75-cm2 tissue culture flask) for 3 d or 1:20 (2.1 × 105 HECs per 75-cm2 flask) for 4 d onto freshly irradiated feeders (1). To induce differentiation, 2 × 105 HECs were plated on 12-mm Millipore PCF polycarbonate inserts (0.4-μm pore size) in CELLnTEC CnT57 medium (containing 10 μM Y-27632 to inhibit deprogramming). Two days later, the medium was replaced with CELLnTEC CnT-02–3D differentiation medium (without Y-27632) according to the manufacturer’s instructions, and the cells were cultured at an air–liquid interface for 14 d. Cultures of human foreskin keratinocyte CRCs were maintained and differentiated in the same manner. An institutional review board exemption was granted by Georgetown University for procurement of human cervical tissue.
Primary human tracheobronchial epithelial cells were isolated by protease dissociation from excess lung donor tissue under the auspices of the Office of Human Research Ethics using protocols approved by the University of North Carolina Biomedical Institutional Review Board. Procedures for the isolation, growth and differentiation of these cells have been described (24). For extended passage, tracheobronchial epithelial cells were cocultured with irradiated feeders and Y-27632 as described for HECs.
Flow Cytometry.
A total of 5 × 105 nonpermeabilized cells were labeled with phycoerythrin-conjugated primary antibodies (Table S2) as described (23).
Western Blotting.
J2 feeder cells were removed from 10-cm tissue culture dishes by treatment with 0.02% EDTA (in PBS) for 5 min at 37 °C. HECs were then scraped into 0.5 mL of RIPA buffer containing protease inhibitors (25) at 4 °C, mixed with 0.5 mL of 2× SDS/PAGE sample buffer [4% (wt/vol) SDS/20% (vol/vol) glycerol/0.01% bromophenol blue/125 mM Tris⋅HCl, pH 6.8] and subjected to ultrasonic disruption at room temperature. After heating at 110 °C for 10 min, aliquots were removed for determination of protein concentrations (Bio-Rad DC protein assay). Mercaptoethanol (5% vol/vol) was added to the remaining portion of the samples, which were incubated for 15 min at 37 °C and frozen. SDS polyacrylamide gels (Novex) were transferred to Immobilon PVDF membranes (Millipore) and labeled (25), using antibodies listed in Table S2. Immunoblots were digitally imaged using a Canon CanoScan 8800F scanner. Bands were quantified using Kodak MI software.
Cell Fractionation.
Nuclear and nonnuclear fractions were prepared from HECs (after removing J2 cells with EDTA) using the Thermo/Pierce NE-PER extraction kit according to the manufacturer’s instructions.
Immunohistology.
Differentiated cell cultures were fixed in formalin, paraffin embedded, sectioned, and stained with H&E using standard procedures. Sections were deparaffinized and rehydrated for fluorescent immunolabeling as described (26), using antibodies listed in Table S2.
Real-Time Quantitative RT-PCR.
Real-time quantitative RT-PCR for detection of hTERT mRNA (normalized to endogenous levels of GAPDH mRNA) was performed as described (27).
Supplementary Material
Acknowledgments
We thank Dr. G. Ian Gallicano (Georgetown University Medical Center) for providing mouse ESCs. We thank the Histopathology and Tissue Shared Resource and the Flow Cytometry Shared Resource at the Georgetown University Medical Center for expert assistance. Human cervical tissue was kindly provided by the Mid-Atlantic Cooperative Human Tissue Network, which is funded by the National Cancer Institute, and the National Disease Research Interchange, with support from National Institutes of Health Grant 5 U42 RR006042. This work was supported by National Institutes of Health Grant R01-OD11168 (to R.S.). S.C.K. and J.D.H. were supported by a grant to Georgetown University from the Howard Hughes Medical Institute through the Precollege and Undergraduate Science Education Program.
Footnotes
Conflict of interest statement: Georgetown University has submitted a PCT patent application on this cell culture technology entitled “Immortalization of epithelial cells and methods of use” (PCT/US2011/060378).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213241109/-/DCSupplemental.
References
- 1.Chapman S, Liu X, Meyers C, Schlegel R, McBride AA. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120(7):2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180(2):599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narumiya S, Ishizaki T, Uehata M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000;325:273–284. doi: 10.1016/s0076-6879(00)25449-9. [DOI] [PubMed] [Google Scholar]
- 4.Tenzen T, Zembowicz F, Cowan CA. Genome modification in human embryonic stem cells. J Cell Physiol. 2010;222(2):278–281. doi: 10.1002/jcp.21948. [DOI] [PubMed] [Google Scholar]
- 5.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 6.Dressel R, et al. Multipotent adult germ-line stem cells, like other pluripotent stem cells, can be killed by cytotoxic T lymphocytes despite low expression of major histocompatibility complex class I molecules. Biol Direct. 2009;4:31. doi: 10.1186/1745-6150-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12(3):170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- 9.Kaur P, Li A. Adhesive properties of human basal epidermal cells: An analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol. 2000;114(3):413–420. doi: 10.1046/j.1523-1747.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 10.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73(4):713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrini G, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98(6):3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petitjean A, et al. Properties of the six isoforms of p63: p53-like regulation in response to genotoxic stress and cross talk with DeltaNp73. Carcinogenesis. 2008;29(2):273–281. doi: 10.1093/carcin/bgm258. [DOI] [PubMed] [Google Scholar]
- 13.Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4(3):193–201. doi: 10.1007/s12015-008-9033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowell S, Jones P, Le Roux I, Dunne J, Watt FM. Stimulation of human epidermal differentiation by delta-notch signalling at the boundaries of stem-cell clusters. Curr Biol. 2000;10(9):491–500. doi: 10.1016/s0960-9822(00)00451-6. [DOI] [PubMed] [Google Scholar]
- 15.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27(38):5115–5123. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: Differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20(2):171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry WE, et al. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19(13):1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin H. The disparity between human cell senescence in vitro and lifelong replication in vivo. Nat Biotechnol. 2002;20(7):675–681. doi: 10.1038/nbt0702-675. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Gourronc FA, Klingelhutz AJ. Therapeutic opportunities: Telomere maintenance in inducible pluripotent stem cells. Mutat Res. 2012;730(1-2):98–105. doi: 10.1016/j.mrfmmm.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallanna SK, Rizzino A. Systems biology provides new insights into the molecular mechanisms that control the fate of embryonic stem cells. J Cell Physiol. 2012;227(1):27–34. doi: 10.1002/jcp.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci Suppl. 1988;10:45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- 23.Suprynowicz FA, et al. The human papillomavirus type 16 E5 oncoprotein inhibits epidermal growth factor trafficking independently of endosome acidification. J Virol. 2010;84(20):10619–10629. doi: 10.1128/JVI.00831-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Randell SH, Fulcher ML, O’Neal W, Olsen JC. Primary epithelial cell models for cystic fibrosis research. Methods Mol Biol. 2011;742:285–310. doi: 10.1007/978-1-61779-120-8_18. [DOI] [PubMed] [Google Scholar]
- 25.Suprynowicz FA, Disbrow GL, Simic V, Schlegel R. Are transforming properties of the bovine papillomavirus E5 protein shared by E5 from high-risk human papillomavirus type 16? Virology. 2005;332(1):102–113. doi: 10.1016/j.virol.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Krawczyk E, Suprynowicz FA, Sudarshan SR, Schlegel R. Membrane orientation of the human papillomavirus type 16 E5 oncoprotein. J Virol. 2010;84(4):1696–1703. doi: 10.1128/JVI.01968-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Roberts J, Dakic A, Zhang Y, Schlegel R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology. 2008;375(2):611–623. doi: 10.1016/j.virol.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.