Abstract
G-protein–activated inward-rectifying K+ (GIRK) channels hyperpolarize neurons to inhibit synaptic transmission throughout the nervous system. By accelerating G-protein deactivation kinetics, the regulator of G-protein signaling (RGS) protein family modulates the timing of GIRK activity. Despite many investigations, whether RGS proteins modulate GIRK activity in neurons by mechanisms involving kinetic coupling, collision coupling, or macromolecular complex formation has remained unknown. Here we show that GIRK modulation occurs by channel assembly with R7-RGS/Gβ5 complexes under allosteric control of R7 RGS-binding protein (R7BP). Elimination of R7BP occludes the Gβ5 subunit that interacts with GIRK channels. R7BP-bound R7-RGS/Gβ5 complexes and Gβγ dimers interact noncompetitively with the intracellular domain of GIRK channels to facilitate rapid activation and deactivation of GIRK currents. By disrupting this allosterically regulated assembly mechanism, R7BP ablation augments GIRK activity. This enhanced GIRK activity increases the drug effects of agonists acting at G-protein–coupled receptors that signal via GIRK channels, as indicated by greater antinociceptive effects of GABA(B) or μ-opioid receptor agonists. These findings show that GIRK current modulation in vivo requires channel assembly with allosterically regulated RGS protein complexes, which provide a target for modulating GIRK activity in neurological disorders in which these channels have crucial roles, including pain, epilepsy, Parkinson’s disease and Down syndrome.
Many neurotransmitters, therapeutic agents, and drugs of abuse activate metabotropic receptors coupled to the Gi/o family of heterotrimeric G proteins. These agonists modulate neuronal excitability and synaptic transmission in part by activating G-protein–activated inward-rectifying K+ channels (GIRKs or Kir3s) to hyperpolarize neurons (1). The importance of this mechanism is illustrated by the diverse phenotypes exhibited by mice lacking GIRK channel subtypes, including reduced anxiety (GIRK1) (2), hyperactivity (GIRK2) (3), propensity for seizures (GIRK2) (4), and decreased morphine-mediated analgesia (GIRK2/3) (5, 6). Conversely, augmentation of GIRK activity caused by trisomic expression of the GIRK2 gene (Kcnj6) causes cognitive impairment and other phenotypes in mouse models of Down syndrome (7–9).
GIRK activity is controlled in vivo by the regulator of G protein signaling (RGS) family (10–13). Regulator of G-protein signaling (RGS) proteins accelerate rates of G-protein deactivation by acting as GTPase-activating proteins (GAPs) for G-protein α-subunits (14–16). Thus, rapid deactivation of GIRK currents requires RGS proteins because GAP activity accelerates the rate that Gi/oα subunits hydrolyze GTP and reform inactive GDP-bound Gαβγ heterotrimers.
RGS proteins have been suggested to regulate GIRK gating kinetics by several mechanisms, including kinetic coupling, collisional coupling, and macromolecular complex formation with GIRK channels. Complex formation between GIRK channels and RGS proteins has been suggested by several lines of evidence (17). RGS protein overexpression can accelerate receptor-evoked GIRK gating kinetics without suppressing steady-state current amplitude (18, 19). RGS8 overexpressed in sympathetic neurons can regulate GIRK currents by a GAP-independent mechanism (20). Overexpressed RGS4 coimmunoprecipitates with complexes of GIRK1/2 heteromers and various receptors (21), and endogenously expressed members of the RGS7 (R7) class of RGS proteins (RGS6, -7, -9, and -11) bound to their obligate partner Gβ5 coimmunoprecipitate with GIRK channels (13). Despite such evidence, whether GIRK channels function in neurons as macromolecular assemblies with regulatory RGS proteins remains unknown.
Here we have addressed this question by analyzing R7-RGS/Gβ5 complexes because they are expressed preferentially and widely in neurons throughout the nervous system (22), possess Gi/oα-specific GAP activity (23), and regulate receptor-evoked GIRK current gating kinetics in neurons (13). We focus on elucidating the function of R7 RGS binding protein (R7BP), a palmitoylated SNARE-like protein that has been suggested to function as a membrane anchor, scaffold, or allosteric regulator for R7-RGS/Gβ5 complexes (24–28). Our studies support a model in which GIRK modulation occurs by channel assembly with R7-RGS/Gβ5 complexes that are allosterically regulated by R7BP.
Results
R7BP Controls Modulation of GABA(B) Receptor-Evoked GIRK Currents.
To explore the function of R7BP in neurons, we determined whether GABA(B) receptor-evoked GIRK currents are dysregulated in hippocampal pyramidal neurons cultured from R7BP−/− mice (Fig. S1) relative to wild-type controls. Whereas we found that baclofen-evoked GIRK current density was similar in wild-type and R7BP−/− neurons (−5.6 ± 1.1 pA/pF and −7.6 ± 1.3 pA/pF, respectively), R7BP ablation increased the deactivation time and magnitude of baclofen-evoked GIRK currents (Fig. 1). GIRK current deactivation kinetics (offset) following baclofen washout were approximately twofold slower in R7BP−/−neurons (Fig. 1 A and B), similar to neurons void of all R7-RGS/Gβ5 complexes (13). R7BP ablation augmented steady-state GIRK current amplitude elicited by a submaximal dose of baclofen (Fig. 1 C and D) relative to that evoked by a maximal dose of this agonist. Importantly, transient expression of red fluorescent protein (RFP)-tagged R7BP in R7BP−/− neurons restored GIRK current deactivation kinetics (Fig. 1 E and F). Together these findings demonstrate that R7BP controls modulation of GABA(B) receptor-evoked GIRK currents in hippocampal neurons.
Fig. 1.
R7BP ablation augments GIRK activity. (A) GABA(B) receptor-evoked GIRK currents in wild-type and R7BP−/− hippocampal pyramidal neurons. Boxed inset highlights GIRK offset kinetics. (B) Quantification of GIRK current onset and offset kinetics in wild-type and R7BP−/− neurons. (C) R7BP ablation augments GIRK activation by a submaximal concentration of baclofen. (D) Quantification of GIRK currents elicited by a submaximal concentration of baclofen. (E) Rescue of GIRK current regulation by expression of R7BP in R7BP−/− neurons. Representative baclofen-evoked GIRK currents from R7BP−/− hippocampal pyramidal neurons that were untransfected or transfected with RFP-R7BP or RFP are shown. Arrows indicate current offset. (F) Quantification of GIRK current offset kinetics in transfected (RFP-R7BP or RFP) and untransfected R7BP−/− neurons. *P < 0.05, **P < 0.01, ***P < 0.0001.
R7BP Allosterically Regulates Assembly of R7-RGS/Gβ5 Complexes with GIRK Channels.
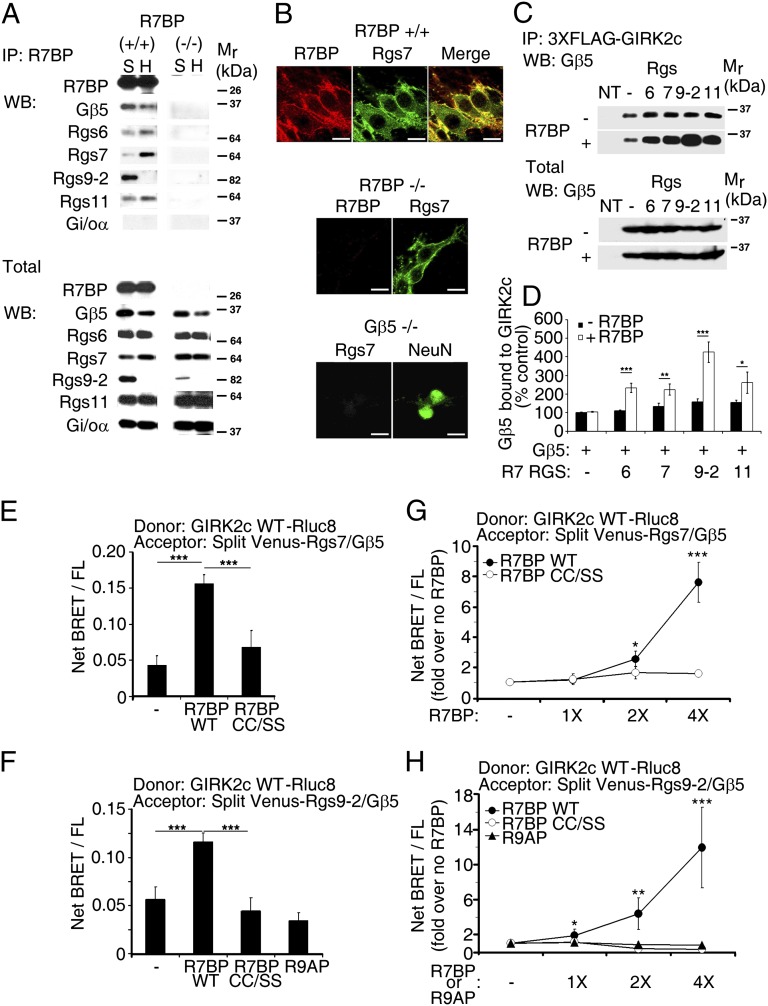
When palmitoylated, R7BP can facilitate targeting of R7-RGS/Gβ5 heterodimers to the plasma membrane; when unpalmitoylated, R7BP can transport them into the nucleus (24, 25). Therefore, R7BP has the potential to determine the subcellular localization of R7-RGS/Gβ5 complexes. Although we found that R7BP coimmunoprecipitated with R7-RGS/Gβ5 complexes in the hippocampus and striatum (Fig. 2A), R7BP ablation did not affect expression or plasma membrane localization of RGS7 in hippocampal neurons (Fig. 2 A and B), consistent with prior evidence that the absence of R7BP has an insignificant effect on membrane localization of RGS7 (29). R7BP therefore is likely to regulate GIRK currents by mechanisms other than anchoring R7-RGS/Gβ5 complexes to the plasma membrane.
Fig. 2.
R7BP facilitates assembly of R7-RGS/Gβ5 complexes with GIRK2c in living cells. (A) Coimmunoprecipitation of R7-RGS/Gβ5 complexes with R7BP from hippocampal (H) and striatal (S) extracts. (B) R7BP is dispensable for plasma membrane localization of RGS7 in cultured hippocampal neurons. (Scale bar: 10 μm.) (C) R7BP augments coimmunoprecipitation between GIRK2c and R7-RGS/Gβ5 complexes. (D) Quantification of coimmunoprecipitation between GIRK2c and R7-RGS/Gβ5 complexes in the absence and presence of R7BP. (E and F) R7BP augments BRET between GIRK2c-Rluc8 and split Venus-tagged R7-RGS/Gβ5 heterodimers containing RGS7 (E) or RGS9 (F), but a nonpalmitoylated R7BP mutant (CC/SS) or the R7BP-related protein R9AP does not. Data are expressed as BRET values normalized to the level of net BRET/FL. (G and H) Quantification of BRET between GIRK2c-Rluc8 and split Venus-tagged R7-RGS/Gβ5 heterodimers containing RGS7 (G) or RGS9 (H) with increasing expression of wild-type R7BP, nonpalmitoylated R7BP (CC/SS), or R9AP. ***P < 0.0005, **P < 0.005, *P < 0.05.
Because R7-RGS/Gβ5 heterodimers have been shown to coimmunoprecipitate with GIRK channels (13), we determined whether R7BP affects this interaction, potentially as a scaffold or allosteric regulator. We found that R7BP enhanced coimmunoprecipitation of overexpressed GIRK2c and R7-RGS/Gβ5 heterodimers two- to fourfold (Fig. 2 C and D and Fig. S2). This effect required R7BP palmitoylation as a plasma membrane targeting signal (Fig. S2).
To determine whether R7BP regulates complex formation in real time at normal expression levels, we performed bioluminescence resonance energy transfer (BRET) experiments with living cells expressing luciferase-tagged GIRK2 as donor and split Venus-tagged forms of R7-RGS proteins (RGS7 or RGS9) and Gβ5 as acceptors. Importantly, experiments used the lowest detectable level of donor to minimize the contribution of BRET signals arising from simple collisional interaction between overexpressed proteins in the membrane. Furthermore, acceptors and R7BP were expressed at native levels (Fig. S3), and BRET was normalized to the level of fluorescent acceptor protein expression (net BRET/FL) to eliminate any potentially confounding effects of R7BP on acceptor expression.
Under these conditions, in cells expressing varying levels of wild-type R7BP we found that BRET was increased up to ∼10-fold relative to cells lacking R7BP (Fig. 2 E–H). An unpalmitoylated R7BP mutant (CC/SS) had an insignificant effect on BRET between GIRK2 and complexes containing Gβ5 and RGS7 (Fig. 2 E and G) or RGS9 (Fig. 2 F and H) because R7BP must, presumably, associate with the plasma membrane where the GIRK2 donor localizes. However, simple plasma membrane targeting of R7-RGS/Gβ5 heterodimers and collisional interaction with GIRK channels do not fully account for the effects of R7BP on BRET because expression of R9AP, an R7BP-like transmembrane protein that interacts with Gβ5 complexes containing RGS9 or RGS11 in the retina (30, 31), had no impact on BRET between GIRK2 and RGS9/Gβ5 heterodimers (Fig. 2 F and H). Furthermore, insignificant BRET occurred between the GIRK2 donor and acceptor-tagged R7BP in the absence of the R7-RGS/Gβ5 heterodimers (Fig. S4), indicating that minimal collisional interaction occurs and suggesting that R7BP does not function as a scaffold that bridges interaction between R7-RGS/Gβ5 complexes and GIRK channels.
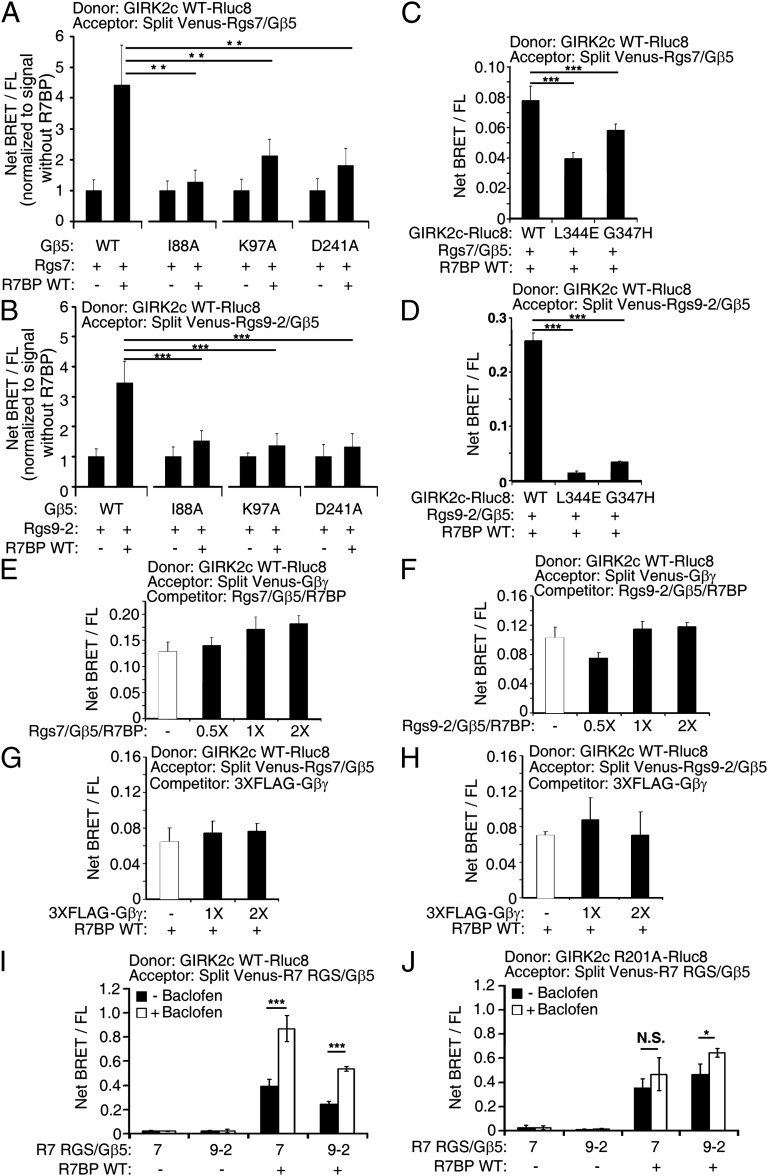
In lieu of serving as a scaffold, R7BP may function allosterically to change the conformation of R7-RGS/Gβ5 complexes and facilitate interaction of the Gβ5 subunit with GIRK channels. This hypothesis is supported by evidence indicating that Gβ5 expressed without an R7-RGS partner coimmunoprecipitates with GIRK channels (13) and that R7BP alters the fate or functional properties of R7-RGS/Gβ5 heterodimers (26–28, 32). We tested this hypothesis by identifying Gβ5 mutants that impair the ability of R7BP to facilitate complex formation with GIRK2c. Because Gβ5 and other Gβ isoforms potentially interact similarly with GIRK channels, we analyzed Gβ5 mutants (I88A, K97A, and D241A) corresponding to Gβ1 mutants defective in GIRK activation (33). In BRET experiments, we found that R7-RGS complexes containing these Gβ5 mutants impaired the ability of R7BP to promote interaction with GIRK2c (Fig. 3 A and B). In contrast, these Gβ5 mutants were normal with respect to complex formation with R7-RGS subunits (Fig. S5A) and R7BP (Fig. S5 B and C), indicating that they specifically impair GIRK2 interaction. Thus, these results support the hypothesis that R7BP allosterically regulates R7-RGS/Gβ5 complexes to facilitate interaction of Gβ5 with GIRK channels.
Fig. 3.
Mechanism of R7BP-faciliated assembly of R7-RGS/Gβ5 complexes with GIRK2. (A and B) Gβ5 mutants that impair R7BP-facilitated assembly of R7-RGS/Gβ5 complexes containing RGS7 (A) or RGS9 (B) with GIRK2c detected by BRET. (C and D) GIRK2c mutants that impair R7BP-facilitated assembly with R7-RGS/Gβ5 complexes containing RGS7 (C) or RGS9 (D) detected by BRET. (E and F) R7-RGS/Gβ5/R7BP complexes containing RGS7 (E) or RGS9 (F) do not compete with Gβγ for binding to GIRK2c as detected by BRET. (G and H) Gβγ does not compete for binding of R7-RGS/Gβ5/R7BP complexes containing RGS7 (G) or RGS9 (H) to GIRK2c as detected by BRET. (I and J) R7BP facilitates assembly of R7-RGS/Gβ5 heterodimers containing RGS7 or RGS9 with wild type (I) or constitutively active R201A (J) GIRK2c activated by baclofen-stimulated GABA(B) receptors as detected by BRET. ***P < 0.0005, **P < 0.005, *P < 0.05.
Because the preceding results suggested that homologous regions of Gβ5 and Gβ1 mediate interaction with GIRK channels, we hypothesized that R7-RGS/Gβ5/R7BP heterotrimers might compete with Gβγ dimers for binding to GIRK2. Indeed, GIRK2c mutations (L344E or G347H) that impaired Gβγ binding (Fig. S5D) (34, 35) also blunted the ability of R7BP to promote interaction with R7-RGS/Gβ5 complexes (Fig. 3 C and D). However, other results indicated that Gβ5 and Gβ1 interact noncompetitively with this surface of GIRK2c. We found that BRET was not diminished significantly in heterotypic competition experiments using donor-tagged GIRK2c, split Venus-tagged Gβγ as acceptor, and untagged overexpressed R7-RGS/Gβ5/R7BP heterotrimers as competitor, or when split Venus-tagged R7-RGS/Gβ5/R7BP complexes were used as acceptor and untagged overexpressed Gβγ as competitor (Fig. 3 E–H). In contrast, homologous competition for donor-tagged GIRK2c by acceptor-tagged and untagged forms of Gβγ was detectable readily by BRET (Fig. S5E). These results suggested that GIRK2c can interact simultaneously with Gβγ and R7-RGS/Gβ5/R7BP heterotrimers. Consistent with this hypothesis, BRET between GIRK2c and R7-RGS/Gβ5 complexes containing R7BP was increased when coexpressed GABA(B) receptors were activated with baclofen (Fig. 3I), whereas this agonist had no effect on BRET between GIRK2c and R7-RGS/Gβ5 complexes in the absence of R7BP (Fig. 3I). Furthermore, R7BP facilitated interaction between R7-RGS/Gβ5 complexes and a constitutively active GIRK2c mutant (R201A) irrespective of whether coexpressed GABA(B) receptors were activated with baclofen (Fig. 3J and Fig. S6). Thus, by promoting assembly of GIRK channels with R7-RGS/Gβ5 complexes possessing Gi/o-specific GAP activity, R7BP should permit GIRK channels to be activated efficiently by Gβγ subunits while facilitating G-protein deactivation and consequent channel closure. Conversely, our findings indicate that the absence of R7BP results in occlusion of the Gβ5 subunit, thereby impairing recruitment of R7-RGS/Gβ5 GAP complexes to GIRK channels and prolonging channel opening.
R7BP Ablation Augments GIRK-Dependent Antinociception.
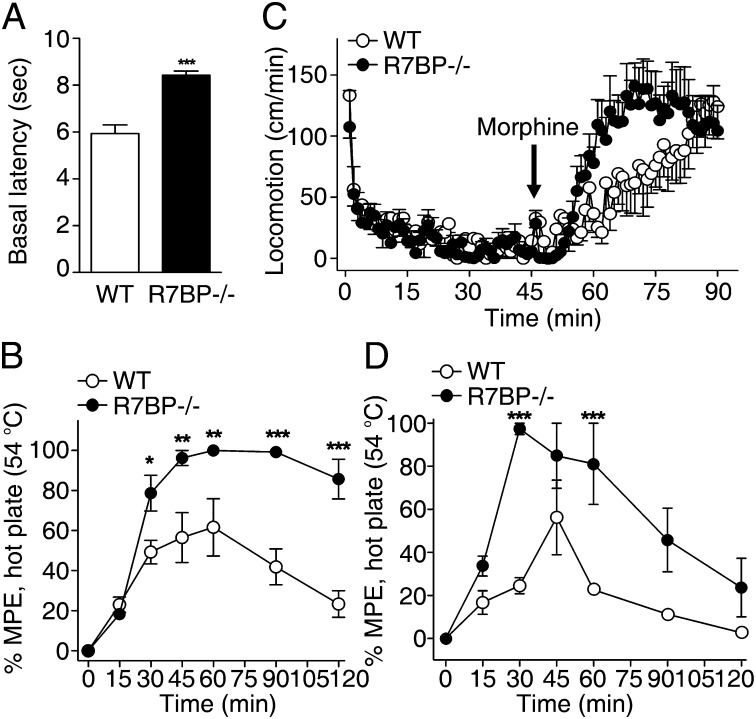
The preceding results coupled with prior evidence indicating that GIRK channels, R7BP, and R7-RGS/Gβ5 complexes are coexpressed widely in the nervous system (1, 36) led us to determine whether R7BP ablation augments GIRK function in vivo, as indicated by phenotypes dependent upon GIRK activity (6, 37–39). For example, agonists of the μ-opioid receptor, such as enkephalins and morphine, promote Gβγ-dependent activation of GIRK channels in GABAergic neurons. The resulting disinhibition of these neurons has been shown to underlie some of the effects associated with opioid actions in vivo, including antinociception and locomotor activity (40, 41). We found that R7BP−/− mice exhibited enhanced basal latency to a thermal stimulus (Fig. 4A) and augmented morphine-elicited antinociception (Fig. 4B). Similarly, morphine-evoked locomotor activity was enhanced in R7BP−/− mice (Fig. 4C). Furthermore, activation of GABA(B) receptors, which also act via GIRK channels to disinhibit GABAergic neuron firing in nociceptive pathways (41), also elicited enhanced antinociception in R7BP−/− mice (Fig. 4D). These results support a model in which R7BP-faciliated assembly of R7-RGS/Gβ5 complexes with GIRK channels modulates inhibitory neurotransmission in vivo.
Fig. 4.
R7BP ablation augments behavioral responses elicited by GABA(B) or μ-opioid receptor agonists. (A) R7BP−/− mice displayed enhanced basal thermal latencies (n = 5/genotype). (B) A μ-opioid receptor agonist (morphine, 10 mg/kg, s.c.) augments antinociceptive responses in R7BP−/− mice compared with wild-type mice (interaction of time × genotype: F(6-56) = 6.46; P < 0.0001; *P < 0.05, **P < 0.01, ***P < 0.001 Bonferroni post hoc analysis). (C) Morphine enhances ambulatory behaviors in R7BP−/− mice more than in wild-type mice (interaction of time × genotype: (F(44-360) = 1.61, P = 0.011). Analysis of the habituation phase (1–45 min) reveals that the R7BP−/− mice are slightly, but significantly less active before drug treatment (for genotype: F(1-720) = 5.59, P = 0.018). (D) GABA(B) receptor agonist (R-baclofen, 2 mg/kg, i.p.) augments antinociceptive responses in R7BP−/− mice compared with wild-type mice (interaction of time × genotype: F(6-42) = 3.2; P = 0.011; ***P < 0.001 Bonferroni post hoc analysis).
Discussion
Our findings indicate that GIRK channels assemble with R7BP-bound R7-RGS/Gβ5 heterodimers to control neuronal excitability and antinociception regulated by Gi/o-coupled GABA(B) and mu-opioid receptors. As discussed below, these findings reveal a function for allosteric regulation of RGS proteins, indicate that GIRK channels function in vivo as macromolecular assemblies with regulatory R7-class RGS proteins, and suggest a strategy for modulating GIRK channel activity in neurological disorders.
In conjunction with prior investigations, our findings indicate that allosteric regulation of R7-RGS/Gβ5 complexes by R7BP has several functions. First, this mechanism protects RGS9 from proteolytic degradation in vivo (32, 42, 43). Second, in heterologous expression systems, it attenuates the ability of Gβ5 complexes containing RGS7 or RGS9 to blunt interaction with and signaling by, respectively, Gq-coupled m3 muscarinic receptors and Gi-coupled D2-like dopamine receptors (26–28). Third, our findings indicate that R7BP facilitates interaction between the Gβ5 subunit of R7-RGS/Gβ5 heterodimers and GIRK channels to limit the duration of GIRK activity evoked by GABA(B) receptors in hippocampal neurons and restrain antinociception elicited by GABA(B) and mu-opioid receptor agonists, which is GIRK-dependent.
Results of our investigation strongly support the emerging concept that GIRK channels function as macromolecular signaling complexes including receptors, G proteins, and regulatory RGS proteins (1). A principal challenge has been to establish the extent to which such complexes form and execute specific functions in vivo relative to alternative mechanisms such as kinetic coupling, collisional coupling, or segregation of signaling proteins within membrane microdomains. Our identification of GIRK2 and Gβ5 point mutants that impair R7BP-faciliated interaction between these channels and R7-RGS/Gβ5 heterodimers supports the existence of macromolecular complexes rather than the occurrence of simple collisional interaction. Such macromolecular complexes appear to be critical for regulating GIRK channel function because R7BP ablation, like elimination of all R7-RGS/Gβ5 complexes (13), delays GIRK current deactivation kinetics in hippocampal neurons, yet does not disrupt plasma membrane targeting of RGS7, the principal R7-RGS isoform in these neurons.
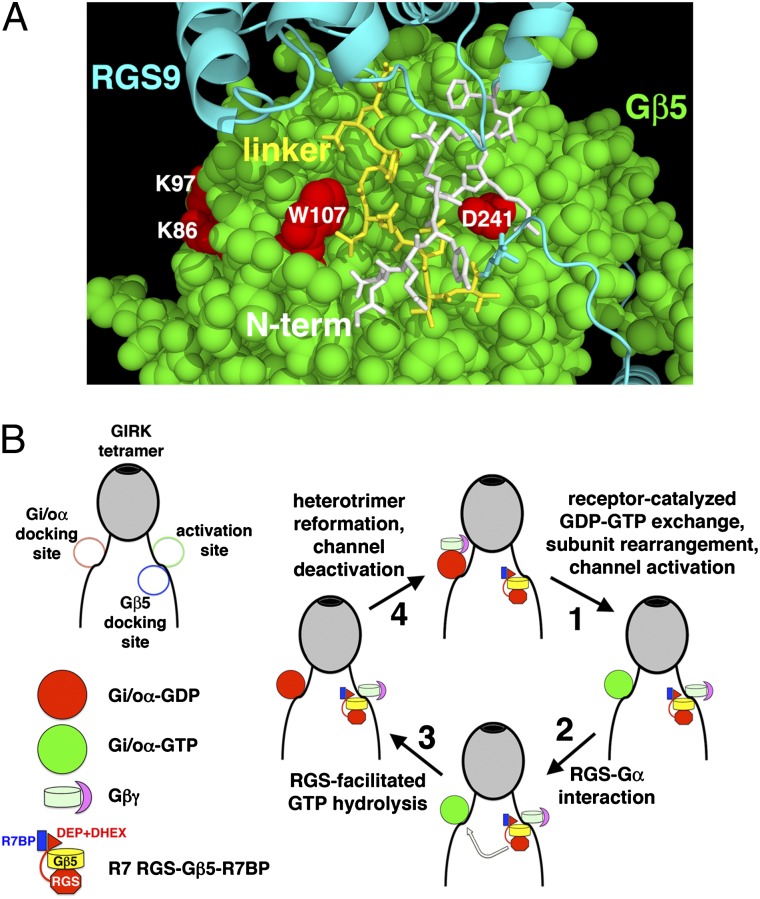
A model for GIRK channel regulation by G proteins and R7BP-bound R7-RGS/Gβ5 complexes (Fig. 5) is suggested by our findings, the structure of RGS9/Gβ5 heterodimers (44), features of RGS9 that mediate R7BP binding (32), and evidence of distinct binding sites on GIRK channels for Gi/oα and Gβγ dimers (45). R7BP binds a linker in RGS9 that occludes one of two surfaces of Gβ5 required for GIRK interaction (Fig. 5A). By displacing this linker, R7BP is proposed to facilitate docking of the Gβ5 subunit near the Gβγ-binding site responsible for GIRK channel activation (Fig. 5B). Once activated by receptors, the Gi/oα subunit bound at a distinct docking site releases the Gβγ dimer to interact with the activation site of the channel. GAP activity of channel-bound R7-RGS/Gβ5/R7BP complexes accelerates deactivation of Gi/oα subunits, which capture Gβγ subunits to close GIRK channels. This model does not preclude the involvement of other RGS proteins that have been implicated in GIRK channel regulation (1).
Fig. 5.
Model of GIRK channel regulation by G proteins and R7BP-bound R7-RGS/Gβ5 complexes. (A) Structure of RGS9/Gβ5 heterodimers in the absence of R7BP suggests that part of the apparent GIRK-binding site of Gβ5 is occluded. Amino acid residues of Gβ5 (spacefill) corresponding to those in Gβ1 that are required for GIRK channel regulation are indicated (red). The linker (yellow sticks) and N-terminal (white sticks) regions of RGS9 occlude part of the GIRK-binding site of Gβ5. R7BP binding to the linker region is proposed to displace these regions, facilitating Gβ5 binding to GIRK channels. (B) Model of GIRK channel regulation by G proteins and R7BP-bound R7-RGS/Gβ5 complexes. R7BP binds the R7-RGS subunit, facilitating docking of the Gβ5 subunit near the activation site of GIRK channels where Gβγ dimers bind. The GAP activity of R7-class RGS proteins tethered by Gβ5 to GIRK channels facilitates reformation of inactive Gαβγ heterotrimers and consequent channel closure.
Intriguingly, R7-RGS/Gβ5 complexes regulate parasympathetic activation and GIRK channel deactivation kinetics in atrial cardiomyocytes (11, 12), which do not detectably express R7BP (36). Thus, it would be interesting to determine whether R7-RGS/Gβ5 heterodimers regulate GIRK channels in cardiomyocytes or other cells normally lacking R7BP by mechanisms involving macromolecular complex formation by R7BP-independent mechanisms, kinetic coupling, collisional coupling, or segregation within membrane microdomains.
Finally, because GIRK channel dysregulation underlies several neurological disorders (1), including pain, epilepsy, Parkinson’s disease, and Down syndrome, pharmacological targeting of allosterically regulated R7-RGS/Gβ5 complexes may provide a means of modulating GIRK function in these disorders. Whereas inhibiting complex formation by targeting protein–protein interfaces could be difficult, inhibiting enzymes responsible for R7BP palmitoylation or depalmitoylation should alter the subcellular localization of R7BP (46) and potentially modulate receptor-evoked GIRK current activity.
Materials and Methods
Detailed methods are described in SI Materials and Methods. Exon 2 of the R7BP gene was deleted to produce R7BP−/− mice, which failed to express detectable levels of R7BP protein (Fig. S1). Methods used for electrophysiological analysis of mouse hippocampal pyramidal neurons, BRET assays, and mouse behavioral pharmacology have been described previously (47–51).
Supplementary Material
Acknowledgments
We thank Nevin Lambert (Georgia Health Sciences University) for providing reagents and advice regarding BRET experiments. We acknowledge support from National Institutes of Health Grants R01GM44592 and R01HL075632 (to K.J.B.), R01MH78823 (to S.J.M.), R01DA018860 (to L.M.B.), and P30NS057105 (to Washington University in St. Louis) and an International Promotion of Young Researchers “Montalcini Program” grant from the Italian Ministry of Education, University and Research (to M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214337109/-/DCSupplemental.
References
- 1.Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11(5):301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pravetoni M, Wickman K. Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav. 2008;7(5):523–531. doi: 10.1111/j.1601-183X.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- 3.Blednov YA, Stoffel M, Chang SR, Harris RA. GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav. 2001;74(1–2):109–117. doi: 10.1016/s0031-9384(01)00555-8. [DOI] [PubMed] [Google Scholar]
- 4.Signorini S, Liao YJ, Duncan SA, Jan LY, Stoffel M. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci USA. 1997;94(3):923–927. doi: 10.1073/pnas.94.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torrecilla M, et al. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci. 2002;22(11):4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitrovic I, et al. Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences. Proc Natl Acad Sci USA. 2003;100(1):271–276. doi: 10.1073/pnas.0136822100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Best TK, Siarey RJ, Galdzicki Z. Ts65Dn, a mouse model of Down syndrome, exhibits increased GABAB-induced potassium current. J Neurophysiol. 2007;97(1):892–900. doi: 10.1152/jn.00626.2006. [DOI] [PubMed] [Google Scholar]
- 8.Best TK, Cramer NP, Chakrabarti L, Haydar TF, Galdzicki Z. Dysfunctional hippocampal inhibition in the Ts65Dn mouse model of Down syndrome. Exp Neurol. 2012;233(2):749–757. doi: 10.1016/j.expneurol.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper A, et al. Trisomy of the G protein-coupled K+ channel gene, Kcnj6, affects reward mechanisms, cognitive functions, and synaptic plasticity in mice. Proc Natl Acad Sci USA. 2012;109(7):2642–2647. doi: 10.1073/pnas.1109099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cifelli C, et al. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103(5):527–535. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, et al. RGS6, a modulator of parasympathetic activation in heart. Circ Res. 2010;107(11):1345–1349. doi: 10.1161/CIRCRESAHA.110.224220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posokhova E, Wydeven N, Allen KL, Wickman K, Martemyanov KA. RGS6/Gβ5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ Res. 2010;107(11):1350–1354. doi: 10.1161/CIRCRESAHA.110.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie K, et al. Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat Neurosci. 2010;13(6):661–663. doi: 10.1038/nn.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996;86(3):445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 15.Hunt TW, Fields TA, Casey PJ, Peralta EG. RGS10 is a selective activator of G alpha i GTPase activity. Nature. 1996;383(6596):175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- 16.Watson N, Linder ME, Druey KM, Kehrl JH, Blumer KJ. RGS family members: GTPase-activating proteins for heterotrimeric G-protein alpha-subunits. Nature. 1996;383(6596):172–175. doi: 10.1038/383172a0. [DOI] [PubMed] [Google Scholar]
- 17.Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: Details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39(1):9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- 18.Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of gbetagamma-activated inwardly rectifying K+ channels. Proc Natl Acad Sci USA. 1997;94(19):10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang HH, Yu M, Jan YN, Jan LY. Evidence that the nucleotide exchange and hydrolysis cycle of G proteins causes acute desensitization of G-protein gated inward rectifier K+ channels. Proc Natl Acad Sci USA. 1998;95(20):11727–11732. doi: 10.1073/pnas.95.20.11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong SW, Ikeda SR. Differential regulation of G protein-gated inwardly rectifying K(+) channel kinetics by distinct domains of RGS8. J Physiol. 2001;535(Pt 2):335–347. doi: 10.1111/j.1469-7793.2001.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaén C, Doupnik CA. RGS3 and RGS4 differentially associate with G protein-coupled receptor-Kir3 channel signaling complexes revealing two modes of RGS modulation. Precoupling and collision coupling. J Biol Chem. 2006;281(45):34549–34560. doi: 10.1074/jbc.M603177200. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman M, Zhou H, Jia L, Cain MD, Blumer KJ. R9AP and R7BP: Traffic cops for the RGS7 family in phototransduction and neuronal GPCR signaling. Trends Pharmacol Sci. 2009;30(1):17–24. doi: 10.1016/j.tips.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooks SB, et al. RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J Biol Chem. 2003;278(12):10087–10093. doi: 10.1074/jbc.M211382200. [DOI] [PubMed] [Google Scholar]
- 24.Drenan RM, et al. Palmitoylation regulates plasma membrane-nuclear shuttling of R7BP, a novel membrane anchor for the RGS7 family. J Cell Biol. 2005;169(4):623–633. doi: 10.1083/jcb.200502007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song JH, Waataja JJ, Martemyanov KA. Subcellular targeting of RGS9-2 is controlled by multiple molecular determinants on its membrane anchor, R7BP. J Biol Chem. 2006;281(22):15361–15369. doi: 10.1074/jbc.M600749200. [DOI] [PubMed] [Google Scholar]
- 26.Narayanan V, et al. Intramolecular interaction between the DEP domain of RGS7 and the Gbeta5 subunit. Biochemistry. 2007;46(23):6859–6870. doi: 10.1021/bi700524w. [DOI] [PubMed] [Google Scholar]
- 27.Sandiford SL, Slepak VZ. The Gbeta5-RGS7 complex selectively inhibits muscarinic M3 receptor signaling via the interaction between the third intracellular loop of the receptor and the DEP domain of RGS7. Biochemistry. 2009;48(10):2282–2289. doi: 10.1021/bi801989c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M, et al. β-Arrestin2 plays permissive roles in the inhibitory activities of RGS9-2 on G protein-coupled receptors by maintaining RGS9-2 in the open conformation. Mol Cell Biol. 2011;31(24):4887–4901. doi: 10.1128/MCB.05690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Y, et al. Targeting of RGS7/Gbeta5 to the dendritic tips of ON-bipolar cells is independent of its association with membrane anchor R7BP. J Neurosci. 2008;28(41):10443–10449. doi: 10.1523/JNEUROSCI.3282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu G, Wensel TG. R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9-1. Proc Natl Acad Sci USA. 2002;99(15):9755–9760. doi: 10.1073/pnas.152094799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, et al. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29(29):9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuho I, Wakasugi-Masuho H, Posokhova EN, Patton JR, Martemyanov KA. Type 5 G protein beta subunit (Gbeta5) controls the interaction of regulator of G protein signaling 9 (RGS9) with membrane anchors. J Biol Chem. 2011;286(24):21806–21813. doi: 10.1074/jbc.M111.241513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford CE, et al. Molecular basis for interactions of G protein betagamma subunits with effectors. Science. 1998;280(5367):1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 34.He C, Zhang H, Mirshahi T, Logothetis DE. Identification of a potassium channel site that interacts with G protein betagamma subunits to mediate agonist-induced signaling. J Biol Chem. 1999;274(18):12517–12524. doi: 10.1074/jbc.274.18.12517. [DOI] [PubMed] [Google Scholar]
- 35.He C, et al. Identification of critical residues controlling G protein-gated inwardly rectifying K(+) channel activity through interactions with the beta gamma subunits of G proteins. J Biol Chem. 2002;277(8):6088–6096. doi: 10.1074/jbc.M104851200. [DOI] [PubMed] [Google Scholar]
- 36.Grabowska D, et al. Postnatal induction and localization of R7BP, a membrane-anchoring protein for regulator of G protein signaling 7 family-Gbeta5 complexes in brain. Neuroscience. 2008;151(4):969–982. doi: 10.1016/j.neuroscience.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: Activation of GIRK2 channels. Proc Natl Acad Sci USA. 2003;100(1):277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda K, Kobayashi T, Kumanishi T, Niki H, Yano R. Involvement of G-protein-activated inwardly rectifying K (GIRK) channels in opioid-induced analgesia. Neurosci Res. 2000;38(1):113–116. doi: 10.1016/s0168-0102(00)00144-9. [DOI] [PubMed] [Google Scholar]
- 39.Marker CL, Stoffel M, Wickman K. Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia. J Neurosci. 2004;24(11):2806–2812. doi: 10.1523/JNEUROSCI.5251-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomazzi M, Slesinger PA, Lüscher C. Addictive drugs modulate GIRK-channel signaling by regulating RGS proteins. Trends Pharmacol Sci. 2008;29(11):544–549. doi: 10.1016/j.tips.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson GR, et al. Expression and localization of RGS9-2/G 5/R7BP complex in vivo is set by dynamic control of its constitutive degradation by cellular cysteine proteases. J Neurosci. 2007;27(51):14117–14127. doi: 10.1523/JNEUROSCI.3884-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson GR, Semenov A, Song JH, Martemyanov KA. The membrane anchor R7BP controls the proteolytic stability of the striatal specific RGS protein, RGS9-2. J Biol Chem. 2007;282(7):4772–4781. doi: 10.1074/jbc.M610518200. [DOI] [PubMed] [Google Scholar]
- 44.Cheever ML, et al. Crystal structure of the multifunctional Gbeta5-RGS9 complex. Nat Struct Mol Biol. 2008;15(2):155–162. doi: 10.1038/nsmb.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berlin S, et al. G alpha(i) and G betagamma jointly regulate the conformations of a G betagamma effector, the neuronal G protein-activated K+ channel (GIRK) J Biol Chem. 2010;285(9):6179–6185. doi: 10.1074/jbc.M109.085944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia L, Linder ME, Blumer KJ. Gi/o signaling and the palmitoyltransferase DHHC2 regulate palmitate cycling and shuttling of RGS7 family-binding protein. J Biol Chem. 2011;286(15):13695–13703. doi: 10.1074/jbc.M110.193763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chisari M, et al. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: Evidence for a low-affinity interaction. J Neurophysiol. 2009;102(2):1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eisenman LN, et al. Anticonvulsant and anesthetic effects of a fluorescent neurosteroid analog activated by visible light. Nat Neurosci. 2007;10(4):523–530. doi: 10.1038/nn1862. [DOI] [PubMed] [Google Scholar]
- 49.Hollins B, Kuravi S, Digby GJ, Lambert NA. The C-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell Signal. 2009;21(6):1015–1021. doi: 10.1016/j.cellsig.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raehal KM, et al. Morphine-induced physiological and behavioral responses in mice lacking G protein-coupled receptor kinase 6. Drug Alcohol Depend. 2009;104(3):187–196. doi: 10.1016/j.drugalcdep.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60(1):58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.