Abstract
Schizophrenia is a severe chronic psychiatric illness, characterized by hallucinations and delusions. Decreased brain volumes have been observed in the disease, although the origin of these changes is unknown. Changes in the n-methyl-d-aspartate (NMDA)-receptor mediated glutamatergic neurotransmission are implicated, since it is hypothesized that NMDA-receptor dysfunction in schizophrenia leads to increased glutamate release, which can have excitotoxic effects. However, the magnitude and extent of changes in glutamatergic metabolites in schizophrenia are not clear. With 1H magnetic resonance spectroscopy (1H-MRS), in vivo information about glutamate and glutamine concentrations can be obtained in the brain. A systematic search through the MEDLINE database was conducted to identify relevant 1H-MRS studies that examined differences in glutamate and glutamine concentrations between patients with schizophrenia and healthy control subjects. Twenty-eight studies were identified and included a total of 647 patients with schizophrenia and 608 healthy-control subjects. For each study, Cohen’s d was calculated and main effects for group analyses were performed using the random-effects model. Medial frontal region glutamate was decreased and glutamine was increased in patients with schizophrenia as compared with healthy individuals. Group-by-age associations revealed that in patients with schizophrenia, glutamate and glutamine concentrations decreased at a faster rate with age as compared with healthy controls. This could reflect aberrant processes in schizophrenia, such as altered synaptic activity, changed glutamate receptor functioning, abnormal glutamine-glutamate cycling, or dysfunctional glutamate transport.
Keywords: magnetic resonance spectroscopy, glutamatergic system, aging
Introduction
Schizophrenia is a severe chronic psychiatric disease, characterized by hallucinations and delusions, starting in late adolescence or early adulthood. Structural magnetic resonance imaging (sMRI) studies have established that schizophrenia is a brain disease with approximately a 3% tissue loss.1 Moreover, this loss of brain tissue appears to be progressive as suggested by numerous longitudinal MRI studies.2–7 It may be explained by reduced neuropil rather than by neuronal loss, implying changes in synaptic, dendritic, and axonal organization in schizophrenia. Reduced neuropil has been observed mostly in the prefrontal region, hippocampus, and thalamus.8 This observation, together with the progressive brain tissue loss found in patients suggests that synaptic plasticity and cortical microcircuitry may be abnormal in schizophrenia. Interestingly, progressive brain changes are also present in discordant cotwins of patients with schizophrenia, suggesting that these changes are familial, and possibly genetic, and can therefore not solely be explained by antipsychotic medication intake.9
The progressive loss of brain tissue in schizophrenia may represent an ongoing pathophysiological process, which could be an important target for therapeutic intervention. One of the possible mechanisms that may be involved is dysfunction of the glutamatergic system (figure 1), which might affect synaptic plasticity and cortical microcircuitry, in particular (n-methyl-d-aspartate) NMDA-receptor signaling.10 NMDA-receptors are glutamate-gated ion channels, which play an important role in excitatory neurotransmission, plasticity, and excitotoxicity.11,12 Indeed, NMDA-receptor antagonists, such as ketamine and phencyclidine (PCP), produce symptoms that mimic psychosis as seen in schizophrenia.13–16 Depending on the severity and duration of the NMDA-receptor hypofunction state, postsynaptic neurons can develop morphological changes and may cause chronic psychosis and structural brain changes.17–19 However, in vivo measurement of NMDA-receptor function has been challenging. Although its agonist glutamate can be measured in vivo, evidence of aberrant glutamate levels in schizophrenia is inconsistent. It has been suggested that glutamate levels decrease with age in healthy individuals,20 but it is not known whether glutamate levels change with longer illness duration. Glutamate can be measured using 1H magnetic resonance spectroscopy (1H-MRS). 1H-MRS allows in vivo assessment of the chemical composition of tissues in a noninvasive manner, by using the magnetic resonance signal of hydrogen to determine the concentrations of metabolites. With 1H-MRS, both physiologically active and inactive glutamate are measured. The majority of physiologically active glutamate is derived from glutamine; therefore, high levels of glutamine may suggest high glutamatergic activity. The glutamatergic transmission and metabolism are strongly coupled, and findings on glutamate and glutamine levels in schizophrenia could give an insight in possible changes in neuronal activity during the course of the disease.21 The aims of this structured review and meta-analysis were therefore to determine the extent to which glutamatergic changes occur in patients with schizophrenia compared with those of healthy controls and whether, if present, these changes become more pronounced with increasing age. For this purpose, we evaluated 1H-MRS studies investigating glutamatergic metabolites in patients with schizophrenia.
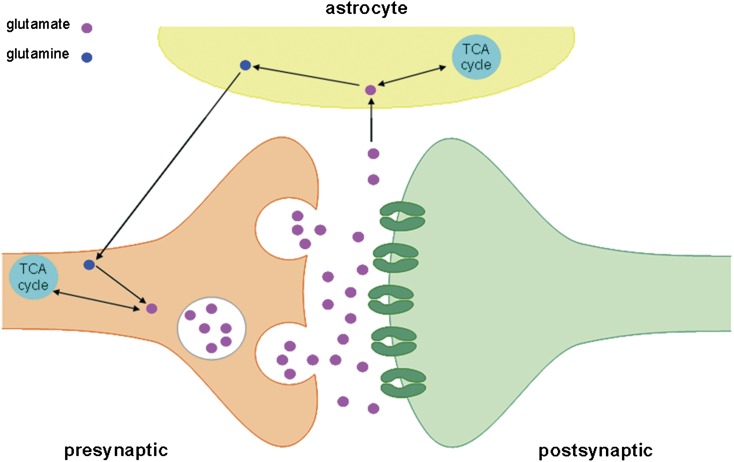
Fig. 1.
The glutamatergic synapse. Glutamate is an amino acid, a building block for proteins, therefore, it is abundant in all cells of the body. It is also the most important excitatory neurotransmitter in the central nervous system. Glutamate is synthesized in axon terminals of glutamatergic neurons. It can be produced from α-ketoglutarate -a tricarboxylic acid (TCA)-cycle intermediate- or from glutamine. For glutamate synthesis from glutamine, the enzyme glutamine synthase is transported to the axon terminal. In the cytosol, it converts glutamine into glutamate. Transporters then concentrate glutamate in vesicles. Release of glutamate is triggered by influx of calcium (Ca2+) into the presynaptic neuron. The synaptic vesicles fuse with the cell membrane and release glutamate into the synaptic cleft. Glutamate is taken up by the postsynaptic neuron, by glia, or it is recycled in the presynaptic neuron.
Methods
Data Sources
1H-MRS studies that examined differences in glutamate levels between patients with schizophrenia and healthy control subjects were obtained through a MEDLINE search, using the keywords “glutamate,” “spectroscopy,” and “schizophrenia.” Titles and abstracts of the articles were examined to see whether they fulfilled the inclusion criteria.
Study Selection
Studies were included if they (1) used 1H-MRS to examine glutamate concentrations in schizophrenia, (2) compared patients with a healthy control group, (3) did not use any interventions (ie, were not subjected to certain actions or imposed to certain behaviors at the time of the study; patients taking antipsychotic medication were included), and (4) were published in the English language. Twenty-eight articles, published between 1994 and 2011, met these criteria. The magnetic field of the MRI-scanners used varied between 1.5 and 4.0 Tesla. The numbers of patients varied between 9 and 40 and the number of healthy controls varied between 11 and 46. Studies were divided based on the stage of illness of their patient populations into high risk, first-episode (FE), and chronic patient studies.
Three studies reported on individuals with a high genetic risk of schizophrenia.22–24 One study reported on individuals with prodromal symptoms of psychosis.25 Twelve studies reported on FE patients.26–37 Eight of these studies reported on a patient group of which the majority was antipsychotic-naive.26,27,30–35 In 3 studies on antipsychotic-naive FE patients, (part of) the patient group was rescanned after receiving antipsychotic treatment.32,34,35 In 4 studies on FE patients, the majority of patients had received antipsychotic medication.28,29,36,37 Four studies report on FE patients and chronically ill patients.30 – 32 , 37
Thirteen studies reported on chronically ill patients,30–32,37–44 of which 1 study only included antipsychotic-naive patients,39 1 study also included unaffected cotwins of patients,40 and 1 study also assessed gender differences.42 One study reported on a sample consisting of both FE and chronically ill patients.45
For 2 studies, the stage of illness of the patient population could not be determined46 , 47 (table 1). These 2 studies did provide all the information that was necessary for the meta-analysis and were thus included.
Table 1.
Study Overview
| Source | Field Strength | Controls | Patients | Area | Disease Stage | Medication |
| Bartha et al26 | 1.5T | 10 | 14 | Frontal | first-episode (FE) | none |
| Bartha et al27 | 1.5T | 11 | 11 | Temporal | FE | none |
| Bustillo et al28 | 4.0T | 10 | 14 | Frontal | FE | atypical |
| Bustillo et al37 | 4.0T | 28 | 30 | Whole brain (1 slice) | FE + chronic | ? |
| Chang et al38 | 4.0T | 22 | 23 | Frontal, temporal, occipital | Chronic | atypical |
| Choe et al39 | 1.5T | 22 | 23 | Frontal | Chronic | none |
| Galińska et al29 | 1.5T | 19 | 30 | Frontal, temporal, thalamus | FE | atypical |
| Keshavan et al22 | 1.5T | 46 | 40 | Frontal, temporal, parietal, occipital, basal ganglia | High risk | none |
| Lutkenhoff et al40 | 3.0T | 21 | 9 | Frontal, hippocampus | Chronic | ? |
| 12 | Cotwins | none | ||||
| Ohrmann et al30 | 1.5T | 20 | 18 | Frontal | FE | none |
| 21 | Chronic | atypical | ||||
| Ohrmann et al31 | 1.5T | 20 | 15 | Frontal | FE | none |
| 20 | Chronic | atypical | ||||
| Olbrich et al36 | 2.0T | 32 | 9 | Frontal, hippocampus, amygdala | FE | atypical |
| Öngür et al41 | 4.0T | 21 | 17 | Frontal, occipital | Chronic | atypical |
| Reid et al48 | 3.0T | 23 | 26 | Frontal | Chronic | atypical |
| Rowland et al49 | 3.0T | 11 | 20 | Frontal, parietal | Chronic | atypical |
| Rüsch et al45 | 2.0T | 31 | 29 | Frontal, hippocampus | FE + chronic | atypical |
| Shirayama et al46 | 3.0T | 18 | 19 | Frontal | ? | atypical |
| Stanley et al32 | 1.5T | 24 | 11 | Frontal | FE drug-naive | none |
| 10 | FE medicated | ? | ||||
| 11 | Chronic | ? | ||||
| Stone et al25 | 3.0T | 27 | 27 | Frontal, hippocampus, thalamus | Prodromal | none |
| Tayoshi et al42 | 3.0T | 25 | 30 | Frontal, basal ganglia | Chronic | ? |
| Tebartz van Elst et al43 | 2.0T | 32 | 21 | Frontal, hippocampus | Chronic | atypical |
| Théberge et al33 | 4.0T | 21 | 21 | Frontal, thalamus | FE | none |
| Théberge et al44 | 4.0T | 21 | 21 | Frontal, thalamus | Chronic | atypical/typical |
| Théberge et al34 | 4.0T | 16 | 16 | Frontal, thalamus | FE drug-naive | none |
| 16 | FE 10M medicated | atypical | ||||
| 16 | FE 30M medicated | atypical | ||||
| Tibbo et al23 | 3.0T | 22 | 20 | Frontal | High risk | none |
| Wood et al47 | 3.0T | 14 | 15 | Frontal | ? | atypical |
| Wood et al35 | 3.0T | 19 | 15 | Temporal | FE drug-naive | none |
| 19 | FE medicated | atypical | ||||
| Yoo et al24 | 1.5T | 22 | 22 | Frontal, thalamus | High risk | none |
Two studies reported insufficient information to calculate Cohen’s d, and these studies were excluded from the meta-analysis.22,32
In total, 19 studies report on medial (pre) frontal brain areas (including the anterior cingulate cortex),22,24–29,33,34,38–42,44,46–49 7 studies report on the dorsolateral prefrontal cortex,24,30–32,36,43,45 5 studies report on temporal brain areas,22,27,29,35,38 5 studies report on the hippocampus,25,36,40,43,45 8 studies report on the thalamus,22,24,25,28,29,33,34,44 3 studies report on occipital brain areas,22,38,41 2 studies report on basal ganglia,22,42 2 studies report on parietal brain areas,22,49 and 1 study reports results from a whole-brain slice.37 Glutamatergic metabolites that entered the meta-analysis were glutamate, the major excitatory neurotransmitter in the central nervous system, glutamine, a glutamate precursor, and glx, the sum of glutamate and glutamine. Meta-analysis was performed if 3 or more studies on glutamate, glutamine, or glx were available in a particular brain region. Thus, a meta-analysis was performed on glutamate, glutamine, and glx levels in the medial (pre)frontal region and only on glutamate levels in the thalamus and hippocampus. Two studies reported on the same group of subjects33 , 34 and to prevent potential bias of overlap, we decided to include only 1 study33 in the meta-analysis.
Data Extraction
Within a meta-analysis, one defines an effect size statistic, representing the quantitative findings of a set of research studies in a standardized form that permits meaningful comparison and analyses across the studies.50 For each study in this meta-analysis, the effects size statistic Cohen’s d was calculated.51 The Cohen’s d is the difference between the mean of the experimental group and the mean of the control group divided by the pooled SD. The Cohen’s d was calculated as follows. The mean concentration of glutamate, glutamine, or glx for patients were subtracted from mean concentration for comparison subjects and divided by the pooled SD of both. When means and SDs were not available, d-values were calculated from exact P values, t values, or F values. After computing individual effect sizes for each study, meta-analytic methods were applied to obtain a combined effect size, which indicated the magnitude of the association across all studies.52 Meta-analyses and meta-regressions were performed with a random-effects model using the statistical package Comprehensive Meta-Analysis V2.53 A random-effects model assumes that the effect size estimated by different studies varies among studies not only because of coincidence but also because of differences in samples or paradigms and that these effect sizes have a normal distribution.54 A t test was subsequently performed on the null hypothesis that the d value is 0.00, i.e., indicating no significant difference between the patient and control populations. According to Cohen, absolute d-values of 0.2 represent small effects, d-values between 0.4 and 0.6 moderate effects, and d values of 0.8 or higher large effects.51In addition, heterogeneity was tested using Cochran’s Q and I 2.55,56 When significant heterogeneity was found, meta-regressions of experimental variables were performed to investigate potential sources of heterogeneity and to identify possible outliers.
When data were available, we also performed a meta-analysis and meta-regression on N-acetyl aspartate (NAA), a potential marker of neuronal viability, to see if changes in glutamate, glutamine, or glx could be a consequence of a decrease in functioning of neurons. A meta-analysis and meta-regression, when data were available, were performed on the gln/glu ratio to explore the changes of glutamate and glutamine relative to each other.
Results
Meta-Analysis of the Medial Frontal Region
Glutamate.
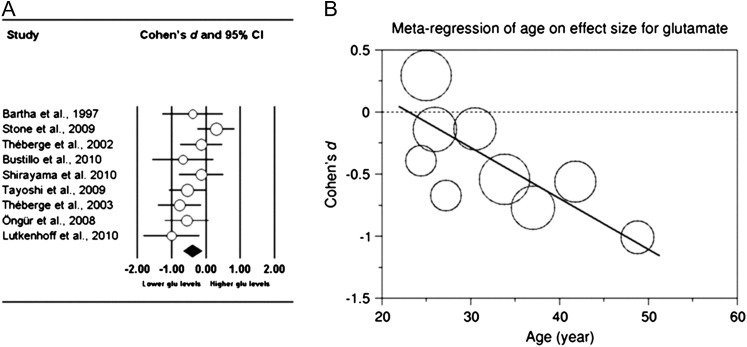
The meta-analysis on glutamate (glu) included 9 studies with a total of 166 patients and 171 healthy controls.25,26,28,33,40–42,44–46 The glutamate level in the frontal region was lower in patients than in controls (combined effect Cohen’s d = −0.391, P = .006) (figure 2A).
Fig. 2.
(A). Meta-analysis on frontal region glutamate. Displayed are the Cohen's d and 95% CI per study. The size of the circles is proportional to the study’s weight in the meta-analysis. Studies are ordered according to mean age of the patient group, from young to old. Data were recorded from the following anatomical regions: anterior cingulate cortex (Stone et al25, Théberge et al33, Bustillo et al28, Öngür et al41), medial prefrontal cortex (Bartha et al26, Lutkenhoff et al40, Shirayama et al46). The combined Cohen's d is −0.391 (P = .006). (B). Meta-regression of age of patients on effect size in frontal region glutamate. Each circle represents one study; the size of the circles is proportional to the study’s weight in the meta-regression. (Slope, −0.04; SE of the slope, 0.015; P = .008).
Glutamine.
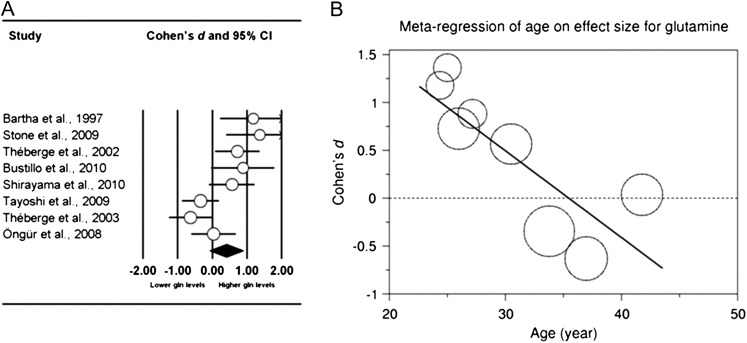
The meta-analysis on glutamine (gln) included 8 studies with a total of 140 patients and 135 healthy controls.25,26,28,33,41,42,44–46 The glutamine level in the frontal region was higher in patients than in controls (combined-effect Cohen’s d = 0.403, P = .045) (figure 3A).
Fig. 3.
(A) Meta-analysis of frontal region glutamine. Displayed are the Cohen's d and 95% CI per study. The size of the circles is proportional to the study’s weight in the meta-analysis. Studies are ordered according to mean age of the patient group, from young to old. Data were recorded from the following anatomical regions: anterior cingulate cortex (Stone et al25; Théberge et al33; Bustillo et al28; Öngür et al41), medial prefrontal cortex (Bartha et al26; Shirayama et al46). The combined Cohen’s d is 0.403 (P = .045). B. Meta-regression of age of patients on effect size in frontal region glutamine. Each circle represents one study; the size of the circles is proportional to the study’s weight in the meta-regression. (Slope, –0.1; SE of the slope, 0.02; P = .0005).
Glx (Glutamate+Glutamine).
The meta-analysis on glx included 8 studies with a total of 186 patients and 144 healthy controls.24,25,29,38,39,47–49 The glx level in the frontal region was lower in patients than in controls but this finding was not significant (combined-effect Cohen’s d = 0.122, P = .393) (see online supplementary figure 1).
Gln/glu Ratio.
The meta-analysis on the gln/glu ratio included 6 studies with a total of 112 patients and 116 healthy controls.28,33,41,42,44,46 The gln/glu ratio in the frontal region was higher in patients than in controls but this was not significant (combined-effect Cohen’s d = 0.308, P = .062) (see online supplementary figure 2A)
N-acetyl aspartate.
The meta-analysis on NAA based on the studies included in this meta-analysis, included 19 studies with a total of 401 patients and 378 healthy controls.24–26,28–31,33,38–42,44–49 NAA in the frontal region was lower in patients than in controls (combined-effect Cohen’s d = −0.320, P = .019) (see online supplementary figure 3A). The meta-analysis on the glu/NAA ratio included 7 studies.26,28,33,40–42,44 The glu/NAA ratio in the frontal region was lower in patients than in controls (combined-effect Cohen’s d = −0.357, P = .038).
Meta-Regression of Age on Effect Size in the Medial Frontal Region
Glutamate.
The meta-regression of age on effect size across 9 studies with a total of 166 patients and 171 healthy controls showed a progressive decrease with age of glutamate in the frontal region in patients as compared with healthy controls25,26,28,33,41,42,44–46 (P = .008) (figure 2B).
Glutamine.
The meta-regression of age on effect size across 8 studies with a total of 140 patients and 135 healthy controls showed a progressive decrease with age of glutamine in the frontal region in patients as compared with healthy controls25,26,28,33,41,42,44–46 (P = .0005) (figure 3B).
Gln/glu Ratio.
The meta-regression of age on effect size across 6 studies with a total of 112 patients and 116 healthy controls showed a progressive decrease with age of the gln/glu ratio in the frontal region in patients as compared with controls28,33,41,42,44,46 (P = .02) (see online supplementary figure 2B).
N-acetyl aspartate.
The meta-regression of age on effect size across 19 studies with a total of 401 patients and 378 healthy controls showed a progressive decrease with age of NAA in the frontal region in patients as compared to controls24 – 26 , 28 – 31 , 33 , 38 – 42 , 44 – 49 (P = .04) (see online supplementary figure 3B). The meta-regression of age on effect size across 7 studies with a total of 121 patients and 126 healthy controls showed a progressive decrease with age of the glu/NAA ratio in the frontal region in patients as compared with controls26 , 28 , 33 , 40 – 42 , 44 (P = .049).
Meta-Analysis of the Hippocampus
Glutamate.
The meta-analysis of glutamate in the hippocampus included 3 studies, with a total of 47 patients and 60 healthy controls.25,40,45 No differences in glutamate between patients and controls were observed (combined Cohen’s d = 0.031, P = .92) (see online supplementary figure 4). There were not enough data available to perform a meta-analysis of glutamine in the hippocampus.
Meta-Analysis of the Thalamus
Glutamate.
The meta-analysis of glutamate in the thalamus included 3 studies, with a total of 64 patients and 64 controls.25 , 33 , 44 No significant difference was observed for glutamate between patients and controls (combined Cohen’s d = −0.286, P = .20) (see online supplementary figure 5). There were not enough data available to perform a meta-analysis of glutamine in the thalamus.
Outlier and Sensitivity Analysis
We performed an outlier and sensitivity analysis and found 3 potential confounding variables. First, lower magnetic field strengths account for more variation in Cohen’s d. Second, the spectroscopic acquisition method STimulated Echo Acquisition Mode (STEAM) shows less variation in Cohen’s d then the acquisition method Point-RESolved Spectroscopy (PRESS) does. Third, shorter echo times account for more pronounced Cohen’s d effects. For the meta-analyses on the medial frontal cortex, we did not find any outliers.
Discussion
In this meta-analysis of 1H-MRS studies in schizophrenia, 24 studies were analyzed for changes in glutamate and glutamine. We found that frontal region glutamate is lower, and glutamine is higher in patients as compared with healthy controls. Interestingly, both glutamate and glutamine levels in the frontal region decrease progressively with age in patients with schizophrenia, which could suggest a progressive loss of synaptic activity. This is supported by the gln/glu ratio, which is increased in patients and declines with age. Also, we found decreasing NAA-levels in patients, which may be associated with the progressive brain volume reductions observed in patients with schizophrenia.2–7 However, the altered frontal glutamate concentration in patients as compared with controls could not be solely explained by the changing NAA levels (with age). Thus, the findings from this meta-analysis suggests a decrease glutamate and an increased glutamine contentration in the brain of patients with schizophrenia, particularly in older patients compared with older controls.
Several limitations have to be taken into account while interpreting the results of this meta-analysis. The most important limitation is that there is only a small number of studies that reported on glutamatergic changes in schizophrenia, as measured with in vivo 1H-MRS of which most focused on frontal brain areas. It is of course possible that glutamatergic alterations only take place in the frontal region and only in older patients, but the hippocampus and thalamus have been examined in only a few studies, as are the basal ganglia and temporal, parietal, and occipital lobes. Differences in paradigms, such as magnetic field strength, acquisition mode, quantification method, and frontal brain region, also have to be taken into account because this could have an impact on the between-study variability.57 Moreover, the progressive decline of glutamatergic levels observed has not been corrected for brain volume changes in this meta-analysis. Several, but not all, of the included studies did take into account partial voluming effects. Therefore, we cannot completely exclude that some of the age-related decline in glutamate concentration in schizophrenia may have been due to brain volume changes. A longitudinal study which assessed patients when medication-naive and after treatment found reductions in precuneal gray matter after 10 months and in frontal, temporal, parietal, and limbic lobes after 30 months of treatment, which was correlated with thalamic glutamine reductions.34 It is therefore too early to make definitive conclusions about the involvement of glutamate in schizophrenia.
Secondly, this meta-analysis only includes cross-sectional data. The conclusion that glutamatergic concentrations progressively decrease with age in schizophrenia is therefore preliminary. Also, the effect size statistic Cohen’s d displays the deviation of patients as compared with healthy controls, which means that possible age-related changes in healthy controls are not taken into account in the meta-analysis. There is evidence though that glutamate changes with age in the healthy brain.20 Unfortunately, medication effects could not be taken into account in this analysis because a major part of the studies did not report sufficient information on antipsychotic intake. The effects of antipsychotic medication cannot be discounted because it has been suggested that haloperidol, clozapine, and olanzapine cause reductions in glutamatergic levels in the rat brain.58 However, long-lasting effects of antipsychotic medication have not been found in human studies so far.28,34
Thirdly, in this meta-analysis, we focused on glutamatergic neurotransmission, however, of course, the measurement of glutamatergic levels alone is not sufficient to draw conclusions about possible neurochemical alterations in patients with schizophrenia. Also, changes in glutamatergic levels may not be detectable with 1H-MRS. Glutamate and glutamine are difficult to distinguish at lower magnetic field strengths, which makes the quantification of glutamate and glutamine problematic.59 Also, 1H-MRS only provides information about the concentration of metabolites. Other metabolite levels, such as compounds involved in energy metabolism and cell proliferation, should also be taken into account. γ-aminobutyric acid (GABA) is also likely to play an important role. An earlier meta-analysis made clear that N-acetyl aspartate levels are reduced in patients with schizophrenia in frontal lobe gray and white matter.60
In conclusion, glutamatergic levels appear to decrease progressively with age in patients with schizophrenia as compared with healthy controls. It is possible though that these progressive reductions are, at least partly, caused by the decrease of brain volume or accumulative intake of antipsychotic medication. In addition, we find an overall increased glutamine level in patients with schizophrenia also in the early (medication-naïve) phase of the disease. Indeed, from the meta-regression, it appears that glutamine levels drop below the healthy control level after patients have reached the age of 35 years, when the majority has reached the chronic phase of the illness. Thus, while we can only draw conclusions with great caution, increased glutamine levels could possibly represent an early marker for glutamate changes in schizophrenia. In fact, the gln/glu ratio is most prominently increased in patients with schizophrenia as compared with the controls at a young age and seems to normalize in older patients This may reflect a deficiency in glutaminase, the enzyme that converts glutamine into glutamate, which results in reduced glutamate levels and increased glutamine levels in the frontal region.61 In line with this, the amount of glutamate that is released into the synaptic cleft and taken up by astrocytes would decrease. In astrocytes, diminishing levels of glutamate are converted back into glutamine, which in the long term could result in reduced glutamine levels. However, we should be aware that while glutamine represents the major precursor for neuronal glutamate (and GABA), glutamate can also be synthesized from the tricarboxylic acid cycle62 and changes in glutamate may also present changes in this second pathway.
There are several possible causes that may explain the altered glutamate levels in schizophrenia. In fact, diminished glutamate receptor activation, in particular of the NMDA-type of glutamate receptor, may be a possible underlying mechanism of reduced synaptic activity because NMDA-receptor antagonists produce the same symptoms as those seen in schizophrenia.17,18,25 The NMDA-receptor hypofunction hypothesis was evolved from studies with NMDA-receptor antagonists, such as PCP and ketamine.17 Injection of these NMDA-receptor antagonists leads to increased glutamine levels and decreased glutamate levels15,63,64 suggesting that NMDA-receptor blockade results in a shift in the glutamine-glutamate cycle.63 Animal studies show that the absence of NMDA-receptor subunits can cause alterations at the molecular and behavioral level and produce schizophrenia-like symptoms.65–78 This might suggest that changes in NMDA-receptor functioning play an important role in schizophrenia, supporting our current meta-results of age-related decreased glutamatergic levels in patients.
Diminished activation of NMDA-receptors results in insufficient excitatory activity, disrupting the monitoring function of GABAergic inhibitory neurons. In response, these inhibitory neurons may downregulate their own activity.79,80 This results in disinhibition and hyperactivity of excitatory pathways, which will eventually cause neuronal damage.17 GABA could thus be an important target for future human in vivo 1H-MRS research.
Knockout models suggest that other types of glutamate receptors may be involved in the pathophysiology of schizophrenia. There is evidence that knockout of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor subunits is implicated in psychosis-like behavior.81 Deletion of subunits of metabotropic glutamate receptors induce glutamatergic function deficits82 , 83 and schizophrenia-like symptoms.84,85
Interestingly, mouse models have shown that a number of proteins associated with glutamate receptors, some of which are directly linked with schizophrenia susceptibility genes, have been suggested to show modifications in patients, which in turn cause schizophrenia-like phenotypes.86,87 Another cause of NMDA-receptor hypofunction could be altered expression of intracellular and extracellular glutamate transporters.88–91 Dysfunction of glutamate transport in the synaptic cleft, and also glutamate transport inside presynaptic neurons, can play a role in the altered glutamatergic levels. Changes in the membrane metabolism in schizophrenia may also be implicated in the changed concentrations of glutamate in schizophrenia. 31Phosphorus magnetic resonance spectroscopy has revealed alterations in membrane metabolism in schizophrenia. An increase in membrane breakdown products has been measured in FE schizophrenia, while a reduction of membrane breakdown products has been measured in chronic schizophrenia.92–94 Together with increased glutamatergic metabolites in early schizophrenia followed by decreased glutamatergic metabolites in chronic schizophrenia, this is consistent with an excitotoxic process.94
More information is essential to make solid conclusions on glutamatergic changes over time in schizophrenia. Up until now, studies were mostly cross-sectional and investigated different brain areas. Also, studies did not control adequately for medication intake, duration of illness, and severity of symptoms. It is thus important that future studies take into account the abovementioned limitations and are carried out in a more uniform manner in order to obtain accurate information about alterations in the glutamatergic system in schizophrenia.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Netherlands Organization for Scientific Research (NWO) VIDI Grant 917-46-370 (to H.H.); Utrecht University High Potential Grant (to H.H.).
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Van Haren NEM, Hulshoff Pol HE, Schnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Cahn W, Hulshoff Pol HE, Lems EBTE, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 4.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 5.DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34:312–321. doi: 10.1093/schbul/sbm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantelis C, Yücel M, Wood SJ, McGorry PD, Velakoulis D. Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. Aust N Z J Psychiatry. 2003;37:399–406. doi: 10.1046/j.1440-1614.2003.01193.x. [DOI] [PubMed] [Google Scholar]
- 7.Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 9.Brans RGH, Van Haren NEM, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008;65:1259–1268. doi: 10.1001/archpsyc.65.11.1259. [DOI] [PubMed] [Google Scholar]
- 10.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 11.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 12.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 14.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 15.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 18.Kondziella D, Brenner E, Eyjolfsson EM, Sonnewald U. How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem Int. 2007;50:291–301. doi: 10.1016/j.neuint.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Stone JM, Morrison PD, Pilowsky LS. Review: Glutamate and dopamine dysregulation in schizophrenia—a synthesis and selective review. J Psychopharmacol. 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman DL, Behar KL, Hyder F, Shulman RG. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu Rev Physiol. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- 22.Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a 1H spectroscopy study. Schizophr Res. 2009;115:88–93. doi: 10.1016/j.schres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Tibbo P, Hanstock C, Valiakalayil A, Allen P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry. 2004;161:1116–1118. doi: 10.1176/appi.ajp.161.6.1116. [DOI] [PubMed] [Google Scholar]
- 24.Yoo SY, Yeon S, Choi CH, et al. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: Investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res. 2009;111:86–93. doi: 10.1016/j.schres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Stone JM, Day F, Tsagaraki H, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- 27.Bartha R, Al-Semaan YM, Williamson PC, et al. A short echo proton magnetic resonance spectroscopy study of the left mesial-temporal lobe in first-onset schizophrenic patients. Biol Psychiatry. 1999;45:1403–1411. doi: 10.1016/s0006-3223(99)00007-4. [DOI] [PubMed] [Google Scholar]
- 28.Bustillo JR, Rowland LM, Mullins P, et al. 1H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galinska B, Szulc A, Tarasów E, et al. Duration of untreated psychosis and proton magnetic resonance spectroscopy (1H-MRS) findings in first-episode schizophrenia. Med Sci Monit. 2009;15:CR82–CR88. [PubMed] [Google Scholar]
- 30.Ohrmann P, Siegmund A, Suslow T, et al. Evidence for glutamatergic neuronal dysfunction in the prefrontal cortex in chronic but not in first-episode patients with schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr Res. 2005;73:153–157. doi: 10.1016/j.schres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Ohrmann P, Siegmund A, Suslow T, et al. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J Psychiatr Res. 2007;41:625–634. doi: 10.1016/j.jpsychires.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Stanley JA, Williamson PC, Drost DJ, et al. An in vivo proton magnetic resonance spectroscopy study of schizophrenia patients. Schizophr Bull. 1996;22:597–609. doi: 10.1093/schbul/22.4.597. [DOI] [PubMed] [Google Scholar]
- 33.Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 34.Théberge J, Williamson KE, Aoyama N, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- 35.Wood SJ, Berger GE, Wellard RM, et al. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naïve and early-treated first episode psychosis. Schizophr Res. 2008;102:163–170. doi: 10.1016/j.schres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Olbrich HM, Valerius G, Rusch N, et al. Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry. 2008;9:59–63. doi: 10.1080/15622970701227811. [DOI] [PubMed] [Google Scholar]
- 37.Bustillo JR, Chen H, Gasparovic C, et al. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biol Psychiatry. 2011;69:19–27. doi: 10.1016/j.biopsych.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62:1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choe BY, Kim KT, Suh TS, et al. 1H magnetic resonance spectroscopy characterization of neuronal dysfunction in drug-naive, chronic schizophrenia. Acad Radiol. 1994;1:211–216. doi: 10.1016/s1076-6332(05)80716-0. [DOI] [PubMed] [Google Scholar]
- 40.Lutkenhoff ES, van Erp TG, Thomas MA, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- 41.Öngür D, Jensen JE, Prescot AP, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tayoshi S, Sumitani S, Taniguchi K, et al. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophr Res. 2009;108:69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Tebartz van Elst L, Valerius G, Büchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 44.Théberge J, Al-Semaan Y, Williamson PC, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 45.Rüsch N, Tebartz van Elst L, Valerius G, et al. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr Res. 2008;99:155–163. doi: 10.1016/j.schres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 46.Shirayama Y, Obata T, Mathalon DH, et al. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage. 2010;49:2783–2790. doi: 10.1016/j.neuroimage.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Wood SJ, Yücel M, Wellard RM, et al. Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3áT. Schizophr Res. 2007;94:328–331. doi: 10.1016/j.schres.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Reid MA, Stoeckel LE, White DM, et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. doi: 10.1016/j.biopsych.2010.04.013. 2010;68:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White matter alterations in deficit schizophrenia. Neuropsychopharmacology. doi: 10.1038/npp.2008.207. 2008;34:1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipsey MW, Wilson DB. The way in which intervention studies have “personality” and why it is important to meta-analysis. Eval Health Prof. 2001;24:236–54. doi: 10.1177/016327870102400302. [DOI] [PubMed] [Google Scholar]
- 51.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 52.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York, NJ: Academic Press; 1985. [Google Scholar]
- 53.Comprehensive Meta-analysis. A Computer Program for Research Synthesis [Computer Program]. Englewood, NY; : BioStat, Inc.; 1999. [Google Scholar]
- 54.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986:7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 55.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 57.Olson DP, Hirashima F, Yurgelun-Todd DA, Renshaw PF. Relaxation effects in spectroscopic studies of schizophrenia. In: Ng V, Barker GJ, Hendler T, editors. Psychiatric Neuroimaging. Amsterdam, The Netherlands: IOS Press; 2003. pp. 79–185. [Google Scholar]
- 58.McLoughlin GA, Ma D, Tsang TM, et al. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. J Proteome Res. 2009;8:1943–1952. doi: 10.1021/pr800892u. [DOI] [PubMed] [Google Scholar]
- 59.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7T. Magn Reson Med. 2001;46:451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 60.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 61.Gaisler-Salomon I, Miller GM, Chuhma N, et al. Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia. Neuropsychopharmacology. 2009;34:2305–2322. doi: 10.1038/npp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 63.Iltis I, Koski DM, Eberly LE, et al. Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized 1H MRS study. NMR Biomed. doi: 10.1002/nbm.1385. 2009;22:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 65.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 66.Duncan GE, Moy SS, Perez A, et al. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 67.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 68.Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 69.Niewoehner B, Single FN, Hvalby Ø, et al. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eu J Neurosci. 2007;25:837–846. doi: 10.1111/j.1460-9568.2007.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheli V, Adrover M, Blanco C, et al. Knocking-down the NMDAR1 subunit in a limited amount of neurons in the rat hippocampus impairs learning. J Neurochem. 2006;97(suppl 1):68–73. doi: 10.1111/j.1471-4159.2005.03592.x. [DOI] [PubMed] [Google Scholar]
- 71.Inada K, Ishigooka J, Anzai T, Suzuki E, Miyaoka H, Saji M. Antisense hippocampal knockdown of NMDA-NR1 by HVJ-liposome vector induces deficit of prepulse inhibition but not of spatial memory. Neurosci Res. 2003;45:473–481. doi: 10.1016/s0168-0102(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 72.Inta D, Monyer H, Sprengel R, Meyer-Lindenberg A, Gass P. Mice with genetically altered glutamate receptors as models of schizophrenia: a comprehensive review. Neurosci Biobehav Rev. 2010;34:285–294. doi: 10.1016/j.neubiorev.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Bannerman DM, Niewoehner B, Lyon L, et al. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakimura K, Kutsuwada T, Ito I, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor [epsi]1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 75.Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- 77.Spooren W, Mombereau C, Maco M, et al. Pharmacological and genetic evidence indicates that combined inhibition of NR2A and NR2B subunit containing NMDA receptors is required to disrupt prepulse inhibition. Psychopharmacology. 2004;175:99–105. doi: 10.1007/s00213-004-1785-y. [DOI] [PubMed] [Google Scholar]
- 78.von Engelhardt J, Doganci B, Jensen V, et al. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors to performance on spatial learning tasks. Neuron. 2008;60:846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 79.Lisman JE, Coyle JT, Green RW, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 81.Fuchs EC, Zivkovic AR, Cunningham MO, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 82.Gray L, Van den Buuse M, Scarr E, Dean B, Hannan AJ. Clozapine reverses schizophrenia-related behaviours in the metabotropic glutamate receptor 5 knockout mouse: association with N-methyl-d-aspartic acid receptor up-regulation. Int J Neuropsychopharmacol. 2009;12:45–60. doi: 10.1017/S1461145708009085. [DOI] [PubMed] [Google Scholar]
- 83.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 84.Patil ST, Zhang L, Martenyi F, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 85.Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (–)-(1R,4S,5S,6S)-4-Amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic Acid (LY404039) J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- 86.Szumlinski KK, Lominac KD, Kleschen MJ, et al. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- 87.Miyakawa T, Leiter LM, Gerber DJ, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci USA. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered vesicular glutamate transporter expression in the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2008;63:766–775. doi: 10.1016/j.biopsych.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Vesicular glutamate transporter transcript expression in the thalamus in schizophrenia. Neuroreport. doi: 10.1097/00001756-200109170-00026. 2001;12:2885–2887. [DOI] [PubMed] [Google Scholar]
- 90.Uezato A, Meador-Woodruff JH, McCullumsmith RE. Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord. 2009;11:711–25. doi: 10.1111/j.1399-5618.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 91.Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117:92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen JE, Al-Semaan YM, Williamson PC, et al. Region-specific changes in phospholipid metabolism in chronic, medicated schizophrenia: (31)P-MRS study at 4.0 Tesla. Br J Psychiatry. 2002;180:39–44. doi: 10.1192/bjp.180.1.39. [DOI] [PubMed] [Google Scholar]
- 93.Jensen JE, Miller J, Williamson PC, et al. Focal changes in brain energy and phospholipid metabolism in first-episode schizophrenia: 31P-MRS chemical shift imaging study at 4 Tesla. Br J Psychiatry. 2004;184:409–415. doi: 10.1192/bjp.184.5.409. [DOI] [PubMed] [Google Scholar]
- 94.Miller J, Williamson P, Jensen JE, et al. Longitudinal 4.0 Tesla 31P magnetic resonance spectroscopy changes in the anterior cingulate and left thalamus in first episode schizophrenia. Psychiat Res Neuroim. doi: 10.1016/j.pscychresns.2008.10.001. 2009;173:155–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.