Abstract
Background: Alterations in striatal dopamine neurotransmission are central to the emergence of psychotic symptoms and to the mechanism of action of antipsychotics. Although the functional integrity of the presynaptic system can be assessed by measuring striatal dopamine synthesis capacity (DSC), no quantitative meta-analysis is available. Methods: Eleven striatal (caudate and putamen) [11C/18F]-DOPA positron emission tomography studies comparing 113 patients with schizophrenia and 131 healthy controls were included in a quantitative meta-analysis of DSC. Demographic, clinical, and methodological variables were extracted from each study or obtained from the authors and tested as covariates. Hedges’ g was used as a measure of effect size in Comprehensive Meta-Analysis. Publication bias was assessed with funnel plots and Egger’s intercept. Heterogeneity was addressed with the Q statistic and I 2 index. Results: Patients and controls were well matched in sociodemographic variables (P > .05). Quantitative evaluation of publication bias was nonsignificant (P = .276). Heterogeneity across study was modest in magnitude and statistically nonsignificant (Q = 19.19; P = .078; I 2 = 39.17). Patients with schizophrenia showed increased striatal DSC as compared with controls (Hedges’ g = 0.867, CI 95% from 0.594 to 1.140, Z = 6.222, P < .001). The DSC schizophrenia/control ratio showed a relatively homogenous elevation of around 14% in schizophrenic patients as compared with controls. DSC elevation was regionally confirmed in both caudate and putamen. Controlling for potential confounders such as age, illness duration, gender, psychotic symptoms, and exposure to antipsychotics had no impact on the results. Sensitivity analysis confirmed robustness of meta-analytic findings. Conclusions: The present meta-analysis showed consistently increased striatal DSC in schizophrenia, with a 14% elevation in patients as compared with healthy controls.
Keywords: neuroimaging, PET, striatum, dopamine, schizophrenia, psychosis, antipsychotic
Introduction
While it is now generally accepted that schizophrenia is a neurodevelopmental disorder whose initial pathophysiology begins long before the initial clinical manifestation,1 consensus is building that the emergence of the psychotic symptoms reflect a final common pathway that remains of central clinical relevance.2 Subcortical and striatal hyperdopaminergia are fundamental to the emergence of these psychotic symptoms and to the mechanism of action of antipsychotics.2 A number of striatal structural and functional alterations in established schizophrenia3 and in subjects4 at risk for the disease supports this finding. Over the past 15 years, neurochemical imaging techniques have enabled to characterize the striatal dopaminergic alterations separately at the presynaptic and postsynaptic levels.5 It is also possible to investigate both the functional and the structural integrity of the presynaptic dopamine (DA) neurotransmission in schizophrenia, by adopting different molecular imaging approaches. In the companion study6 published in this issue, we showed no structural abnormalities in the number of dopaminergic neurons or the density of the synaptic connections they make in striatum of patients with schizophrenia. We complete here the previous analysis by assessing the compound functional state of presynaptic dopaminergic neurotransmission, investigating functional alterations in amount or regulation of DA released, bound and taken up into the synapse, or both (dopamine synthesis capacity [DSC] see below).
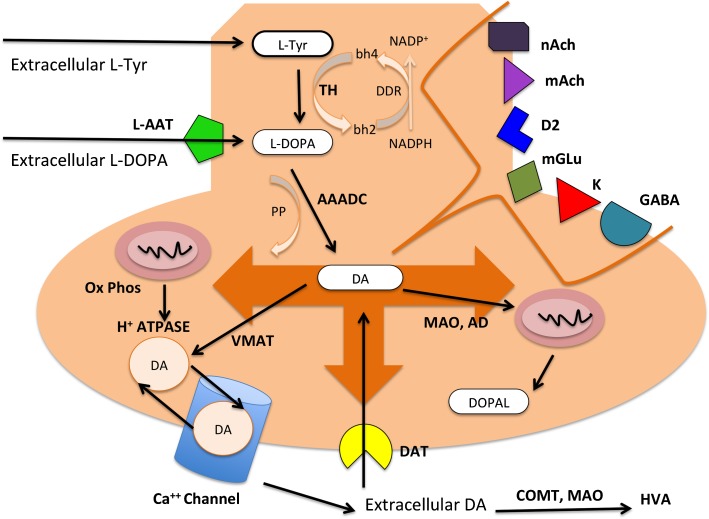
Early studies addressing the functional integrity of striatal presynaptic neurotransmission have employed radiotracers whose receptor binding is sensitive to endogenous DA levels. They found that the baseline levels of striatal synaptic DA and the DA release in response to amphetamine are increased in patients with schizophrenia.7 Moreover, the magnitude of that increase was related to the severity of amphetamine-induced psychotic symptoms and the response to subsequent antipsychotic treatment. Availability of new radiotracers such as carbon [11C] and [18F]-DOPA has allowed researchers to use another functional index of presynaptic DA neurotransmission, the DSC. The molecular regulation of DSC is detailed in figure 1. DA cannot enter the brain to an appreciable degree because it is a polar molecule and the blood-brain barrier does not contain carriers for DA. The blood-brain barrier and brain cells do contain carrier systems for amino acids, and one of these is able to transport the L-DOPA. Because the amino acid transporter and aromatic amino acid decarboxylase (AAADC) also recognize radiolabeled analogs of l-DOPA, a 11C label can be introduced in the β-carbon atom as in the case of [β-11C]-l-DOPA and a 18F label can be introduced as in the case of [18F]-l-DOPA. Thus, in the case of [18F]-DOPA positron emission tomography (PET), once entering striatum, [18F]-DOPA is decarboxylated by AAADC, yielding [18F]-fluorodopamine, which is retained for a time within DA vesicles and ultimately decomposed (figure 1). DSC can be computed with a number of different kinetic approaches reflecting all of the above pathways.
Fig. 1.
Molecular basis of presynaptic dopamine (DA) regulation. The majority of circulating L-tyrosine (Tyr) originates from dietary sources, but small amounts are derived from hydroxylation of phenylalanine by the liver. Blood-borne Tyr is taken up into the brain by a low-affinity amino acid transport system and subsequently from brain extracellular fluid into dopaminergic neurons by high- and low-affinity amino acid transporters. In the presynaptic dopaminergic neuron, Tyr is converted to L-3,4-dihydroxyphenylalanine (l-DOPA) by the enzyme tyrosine hydroxylase (TH). TH does so using tetrahydrobiopterin (bh4) and dihydrobiopterin (bh2) as coenzymes and dihydrobiopterin reductase (DDR) with NADP+/NADH. TH is a rate-limiting enzyme in DA synthesis and is inhibited by its own substrate. DA cannot enter the brain to an appreciable degree but the blood-brain barrier contains the large (L)-type amino acid transporter (l-AAT), which is able to transport the DA precursor, l-DOPA, and its radiolabelled analogs. Subsequently, DOPA decarboxylase or aromatic amino acid decarboxylase (AAADC) converts l-DOPA to DA (3) using pyridoxal phosphate (PP). DA is then transported and concentrated from the cytoplasm to specialized storage vesicles by the vesicular monoamine transporter (VMAT). Most DA is packaged in vesicles from which it is released on the arrival of action potentials. This process relies on the activity of an ATP-dependent vesicular proton pump (H+-ATPase) using ATP formed during oxidative phosphorylation (Ox Phos) at local mitochondria. Synaptic DA release is regulated by tonic activity and bursts by a large number of receptors and second messengers at the level of the dendrites. The secretory response at the neuronal terminal is regulated by the complex interplay of DA autoreceptors (D2) and heterosynaptic receptors (metabotropic glutamate, mGlu; nicotinic and muscarinic acetylcholine, nACh and mAChR; GABA and opiate, K). Their second messengers modulate different pathways and ultimately the voltage-gated Ca++ channels, affecting the targeting of the vescicles to the active zone of the presynaptic membrane, docking, fusion, release of the vescicular content, retrieval by endocytosis, and refilling with the neurotransmitter. After release, DA is rapidly taken up by dopamine transporters (DAT) on the terminal regulating extracellular dopamine homeostasis. Cytosolic DA is catabolized by monoamine oxidase (MAO) and aldehyde dehydrogenase (AD) to 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is exported from the neuron and methylated by catecholamine methyl transferase (COMT) to homovanillic acid (HVA). Extracellular DA catabolism is regulated by COMT, which with extraneuronal MAO and AD produce again HVA. The overall dopamine synthesis capacity (DSC) reflects the complex interplay of the above synthesis, storage, release, and reuptake processes.
Several [11C] and [18F]-DOPA studies in schizophrenia have been published to date8; however, the results are contrasting including both significant7, 9–15 and inconclusive16–18 outcomes. Potential confounding factors that could contribute to these conflicting results could be different imaging methods across centers, sociodemographic and clinical characteristics of the samples and exposure to medications. In addition, the magnitude of striatal DSC in schizophrenia has yet to be consistently measured across different studies. Although some reviews have addressed presynaptic DA functioning in schizophrenia,8,19,20 no quantitative meta-analysis has ever measured the [11C/18F]-DOPA striatal abnormalities in schizophrenia controlling for the modulating effect of potential confounders. In the present article, we sought to address these issues, focusing on [11C/18F]-DOPA PET investigations of striatum in schizophrenia. Our first aim was to examine the evidence for a consistent functional alteration of striatal presynaptic DA neurotransmission in schizophrenia. We then estimated the magnitude of putative differences in synthesis capacity between patients with schizophrenia and matched controls. Finally, we assessed the potential confounding role played by a number of moderators such as sociodemographic characteristics of the sample, illness duration, severity of psychotic symptoms, and exposure to antipsychotics.
Methods
Selection Procedures
Search Strategies.
A systematic search strategy was used to identify relevant studies. Two independent researchers conducted a 2-step literature search. First, we carried out a PubMed, Science Direct, and Scopus search to identify putative [11C/18F]-DOPA studies in subjects affected with schizophrenia. The search was conducted in March 2011, and no time span was specified for date of publication. We combined the following search terms: “fluoro-dopa,” “carbon-dopa,” “psychosis,” “schizophrenia,” “PET.” In a second step, the reference lists of the articles included in the review were manually checked for relevant studies not identified by computerized literature searching. Next, the corresponding authors were contacted by e-mail requesting any detail not included in the original manuscripts. There was no language restriction, though all included articles were in English.
Selection Criteria.
Studies were included according to the following criteria: (a) being an original article in a peer-reviewed journal, (b) having enrolled a group of subjects affected with Diagnostic and Statistical Manual of Mental Disorders (DSM)/or International Classification of Diseases schizophrenia and a matched control group (c) having analyzed the 2 groups with [18F] or [11C]-DOPA PET, and (d) having reported the mean (SD) for striatal DSC in both groups. In cases of 2 or more studies from the same center, we have carefully checked for overlapping samples by contacting the authors to verify there was not a significant overlap in the samples.
Recorded Variables.
The recorded variables for each article included in the meta-analysis were disease stage (first episode and chronic), illness duration, gender (proportion of females), mean age of participants, striatal areas analyzed, exposure to antipsychotics (proportion of drug naive subjects), type of radiotracer, severity of psychotic symptoms, and modeling approach. Results are reported in tables to assist the reader in forming an independent view on the core findings (see online supplementary diagram 1).
Quality Assessment.
We used a simple objective rating system21 that coded studies on a scale of 0–10, assigning 2 points each for sampling method, presence of clearly stated inclusion criteria, sociodemographic diversity, and response assessment. Studies that did not report these methodological issues received lower scores. To achieve a high standard of reporting, we adopted the MOOSE22 approach of broadly including studies and using sensitivity analysis to determine incremental effects of lower quality studies.
Molecular Imaging of DSC
A molecular imaging summary of DSC detailing the different kinetic approaches employed across each study is available as online supplementary material. The molecular regulation of DSC is detailed in figure 1.
Statistical Analysis.
Data were then entered in an electronic database and analyzed with a meta-analytical approach by using Comprehensive Meta-Analysis Software version 2 (Biostat, Inc.).23 This package employs the same computational algorithms used by the Cochrane Collaborators to weight studies. The primary outcome was the striatal DSC in the patient and in the control group. As a measure of effect size, the Hedges’ g was adopted, in order to correct for bias from small sample sizes.24 This metric is normally computed by using the square root of the mean square error from the ANOVA testing for differences between the 2 groups, as indicated by the formula:
where
and
where X is the raw score, M is the mean, and N is the number of cases.24
In a secondary step, we conducted additional meta-analyses to regionally address DSC in the caudate and putamen. Finally, we tested the potential confounding effect of moderators on the meta-analytical estimates: year of publication, age of participants, severity of psychotic symptoms (as measured with the Positive and Negative Syndrome Scale [PANSS]), gender (proportion of females), duration of illness (months), type of radiotracer (18F/11C), modeling approach (see online supplementary materials), and exposure to antipsychotics (proportion of drug naive subjects). The influence of continuous moderators was tested using meta-regression analyses. The slope of meta-regression (β-coefficient: direct [+] or inverse [−]) of the regression line indicated the strength of a relationship between moderator and outcome. To limit risk of false positive (type I) errors arising from multiple comparisons, we adjusted P < .05 by dividing α with the number of meta-regressions.
Heterogeneity among study point estimates was assessed with the Q statistic with magnitude of heterogeneity being evaluated with the I 2 index. In general, random-effects models are more conservative than fixed-effect models and argued to better address heterogeneity (even at trend level as in the present study) between studies and study populations, allowing for greater flexibility in parsing effect size variability. Moreover, they are less influenced by extreme variations in sample size. The possibility of publication bias was examined by visually inspecting funnel plots and applying the regression intercept of Egger et al.25 In this way, we assessed whether there was a tendency for selective publication of studies based on the nature and direction of their results. In addition, we used the fail-safe procedure,26 to generate a number of unpublished studies that would be needed to move estimates to a nonsignificant threshold. To assess the robustness of the results, we performed sensitivity analyses by sequentially removing each study and rerunning the analysis. We also conducted a separate analysis excluding studies with quality ratings in the lowest third to determine if potential methodological weaknesses influenced meta-analytic estimates.
Results
Studies Found and Sample Characteristics
Eleven studies published between 1994 and 2011 met inclusion criteria (see online supplementary diagram 1). The overall database contained 113 subjects with schizophrenia (mean age 35.1 y, 24% females) and 131 controls (mean age 33.6 y, 25% females). Duration of illness ranged from few months to several years. Patients and controls were well matched with respect to age, gender, and IQ (all P > .05). Most studies have assessed psychotic positive and negative symptoms by using the PANSS.7,10,11,14,16,18,27 Although some of them have assessed potential correlations between DSC and psychopathology, only 2 of them uncovered significant positive correlations between striatal DSC and psychotic symptoms.7,27 One study employed the Comprehensive Assessment of Symptoms and history12 uncovering no significant correlation with symptoms. The remaining studies did not report any psychopathological assessment.13,15,17 The sociodemographic details of the whole sample are presented in table 1.
Table 1.
Striatal [18F]-/[11C]-DOPA Studies Included in the Meta-Analysis
| Author | Year | Radiotracer | Illness Stage | Illness Duration (mo) | Schizophrenia | Controls | Treatment | DSC SCZ/C | ||||
| N | Females | Age | N | Females | Age | |||||||
| Reith et al11 | 1994 | [18F]-DOPA | C | 168 | 5 | 0 | 38 | 13 | 4 | 36 | 4DN,1DF | 1.20 (sig) |
| Dao-Castellana et al16 | 1997 | [18F]-DOPA | C | 72 | 6 | 0 | 26 | 7 | 0 | 25 | 2DN, 4DF | 1.05 (ns) |
| Hietala et al27 | 1999 | [18F]-DOPA | FEP | 49 | 10 | 6 | 30 | 13 | 5 | 30 | DN | 1.18 (sig) |
| Lindstrom et al13 | 1999 | [11C]-DOPA | FEP, C | 252 | 12 | 2 | 31 | 10 | 2 | ? | 10DN, 2DF | 1.16 (sig) |
| Elkashef et al17 | 2000 | [18F]-DOPA | C | 204 | 19 | 4 | 36 | 13 | 5 | 35 | 9DF, 10T | 1.00 (ns) |
| Meyer-Lindenberg et al15 | 2002 | [18F]-DOPA | C | ? | 5 | 1 | 35 | 6 | 1 | 34 | DF | 1.19 (sig) |
| McGowan et al12 | 2004 | [18F]-DOPA | C | 132 | 16 | 0 | 38 | 12 | 0 | 40 | T | 1.15 (sig) |
| Kumakura et al10 | 2007 | [18F]-DOPA | C | ? | 8 | 0 | 37 | 15 | 0 | 37 | 3DN,5DF | 1.40 (sig)a |
| Nozaki et al14 | 2009 | [11C]-DOPA | FEP, C | 26 | 18 | 8 | 36 | 20 | 10 | 36 | 14DN, 4DF | 1.14 (sig) |
| Howes et al7 | 2009 | [18F]-DOPA | C | ? | 7 | 3 | 36 | 12 | 4 | 24 | 3DN, 4DF | 1.11 (sig) |
| Shotbolt et al18 | 2011 | [18F]-DOPA | Cb | ? | 7 | 3 | 43 | 10 | 5 | 39 | T | 1.02 (ns) |
Note: C, chronic schizophrenia; FEP, first-episode schizophrenia; DN, drug naive; DF, drug free; T, treated with antipsychotics; DSC SCZ/C, striatal dopamine synthesis capacity ratio (schizophrenia/controls); sig, significant difference between patients and controls; ns, nonsignificant result.
A post hoc reanalysis employing a different kinetic model is provided as online supplementary materials.
Twin study.
Tests for Heterogeneity
According to the criteria set by Higgins and Thompson, the heterogeneity in published studies was modest in magnitude and statistically nonsignificant (Q = 19.19; P = .078; I 2 = 39.17).
Tests for Publication Bias
Visual inspection of funnel plots revealed no evidence of publication bias. Additionally, quantitative evaluation of publication bias, as measured by the Egger intercept, was nonsignificant (P = .276). Finally, the fail-safe procedure determined that 115 unpublished studies would be needed to bring the overall meta-analytic estimate to a nonsignificant threshold.
DSC in Striatum
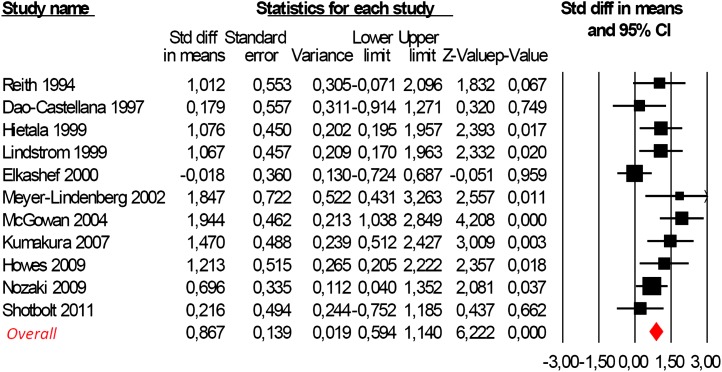
Three of 11 studies reported no significant differences between DSC of the schizophrenic group and controls (P > .05, see table 1). However, the meta-analysis of the whole database provided strong statistical evidence for a significant difference between the 2 groups. Striatal DSC was higher in subjects with schizophrenia as compared with controls (Hedges’ g = 0.867, CI 95% from 0.594 to 1.140, Z = 6.222, P < .001, figure 2). The DSC schizophrenia/control ratio ranged from 1 to 1.40, with an average increase of 14% in schizophrenic patients as compared with controls.
Fig. 2.
Meta-analysis of striatal dopamine synthesis capacity (DSC) in schizophrenia employing random effect models (test for heterogeneity Q = 19.19; P = .078; I 2 = 39.17). Positive values of Hedges’ g indicate greater DSC in patients as compared with controls.
DSC in Caudate and Putamen
Meta-analysis of studies investigating the caudate confirmed an increased DSC in the patient group (Hedges’ g = 0.569, CI 95% from 0.176 to 0.961, Z = 2.839, P = .005). Similarly, DSC was increased in the putamen of schizophrenics as compared with controls (Hedges’ g = 0.643, CI 95% from 0.098 to 1.189, Z = 2.311, P = .021).
Effect of Moderators
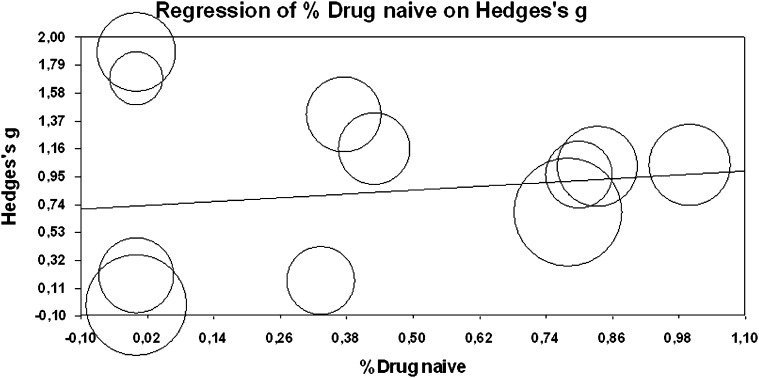
The type of radiotracer (18F/11C) did not influence the meta-analytical results (Q = 3.8100, P = .701). The meta-regressions revealed no significant effects for the examined moderators (year of publication β = .008, CI 95% from −0.041 to 0.059, Z = 0.349, P = .727; age of participants β = .006, CI 95% from −0.059 to 0.071, Z = 0.181, P = .856; duration of illness β = −.001, CI 95% from −0.004 to 0.003, Z = −0.337, P = .736; psychotic symptoms β = .004 CI 95% from −0.031 to 0.023, Z = −.274, P = .783; exposure to antipsychotics β = .239, CI 95% from −0.457 to 0.934, Z = 0.674, P = .501, figure 3; and gender of patients β = −.813, CI 95% from −2.345 to 0.719, Z = −1.039, P = .299) on the findings reported above here. No effect for modeling approach was detected (see online supplementary material).
Fig. 3.
Meta-regression of antipsychotic exposure (proportion % of drug naive subjects) on striatal dopamine synthesis capacity point estimates (Hedges’ g). Circle size reflects the weight a study obtained in the meta-regression. Note that excluding the potential outliers did not affect statistical significance (P > .05).
Sensitivity Analyses
Robustness of the meta-analytic findings was examined by sequentially removing each study and reanalyzing the remaining data set (producing a new analysis for each study removed). No study affected the overall meta-analytic estimate more than 5%. Removing studies with quality ratings in the lowest 30% influenced the meta-analytic estimate by 9% (Hedges’ g = 0.787, P < .05). The pattern of differences across the subanalyses remained essentially unchanged in direction and magnitude.
Discussion
Our meta-analysis for striatal [11C/18F]-DOPA PET studies in schizophrenia found strong evidence supporting an overall increase (on average 14%) in striatal DSC in schizophrenia as compared with controls. This result was regionally evident both in the putamen and in the caudate. Age of subjects, gender, year of publication, duration of illness, psychotic symptoms, or exposure to medication did not influence DSC, and there was no evidence for publication bias.
The present meta-analysis tested the hypothesis that striatal hyperdopaminergia in schizophrenia is accompanied by functional alterations of presynaptic integrity. We identified 13 [11C/18F]-DOPA PET studies of striatal DSC comparing patients with schizophrenia and controls. We found statistical evidence that elevation in DSC represents a reliable and consistent neurobiological marker of manifest schizophrenia. On average, schizophrenia was associated with an increase of 14% in DSC as compared with controls. As the Q statistic was modest in magnitude and not statistically significant, there was sufficient homogeneity between the results of the different studies. We therefore present formal meta-analytic support for increased striatal presynaptic DSC as one of the most widely replicated brain dopaminergic abnormalities in schizophrenia. Our result is consistent with the studies of amphetamine-induced displacement of DA D2 receptor radioligands28 indicating that there are increased levels of striatal DSC in schizophrenia. The results of the present study are of particular interest when they are interpreted in the light of the negative findings of our companion meta-analysis, which suggested no structural alterations of DA neurotransmission.6 A change in the integrity of presynaptic DS terminals is thus unlikely to contribute to the pathogenesis, course, or treatment of schizophrenia. With the present meta-analysis available, we conclude that altered presynaptic DA neurotransmission in schizophrenic patients likely reflects a true difference in functional status rather than an epiphenomenon of structural- or treatment-related changes. Our result is also in line with studies employing other indexes of striatal functional integrity such as DA release, which can be indirectly measured by assessing changes in binding of D2 radiotracers after pharmacological manipulation with DA releasing or depleting agents. Most of these studies confirm altered DA neurotransmission in schizophrenia.8
Striatal presynaptic hyperdopaminergia is of crucial relevance for understanding the symptoms of psychosis in the light of the crucial role played by the striatum in cognition3 and salience.29 Because the striatum and the prefrontal cortex are anatomically interconnected and prefrontal dysfunction is linked to cognitive symptoms of schizophrenia, corticostriatal pathways are critical in the generation of cognitive symptoms in schizophrenia, which may be mediated by DA. In fact, there is evidence supporting a strong correlation between DSC in striatum of healthy volunteers and performance of cognitive tasks linked to the prefrontal cortex.30 Other studies have confirmed a significant relationship between striatal DSC and prefrontal cortex activation in subjects with established schizophrenia15 or in subjects experiencing prodromal symptoms for psychosis.31 In addition to executive cognition, the signaling of salience and reward is also dependent on midbrain DA neurons projecting to the ventral striatum and dorsolateral prefrontal cortex. Functional neuroimaging studies show that the abnormal engagement of striatum is associated with altered reward response in schizophrenia.32 Despite this convergent evidence for a strong link between prefrontal cortex alterations and striatal DA abnormalities, the direction of this relationship is still unknown and may operate in both directions. The prefrontal cortex projects directly to the striatum and to the cell bodies of the midbrain DA neurons that project to the striatum.33 Prefrontal cortical lesions in experimental animals result in elevated striatal DA function, and prefrontal activity is correlated with midbrain DA function in human volunteers.15 Activity in DA terminals within the striatum may thus be controlled by prefrontal cortex, and it has been proposed that these serve as a “brake” on the striatal DA system.34 A primary dysfunction of prefrontal cortex, or of it’s glutamatergic efferents, could thus lead to increased striatal dopaminergic function15 and the development of the positive and cognitive symptoms of manifest schizophrenia. This account agrees with the concept of schizophrenia as a neurodevelopmental disorder and the observation that both candidate35 and genome-wide significant36 risk genes for the disorder have been observed to impact on prefrontal cortex connectivity. On the other hand, there are also indirect projections to prefrontal cortex from the striatum via the thalamus.37 Animal studies indicate that experimentally elevated striatal dopaminergic function leads to impaired behavioral flexibility and cognitive functioning and reduced DA turnover and DA receptor levels in prefrontal cortex.38 This suggests that striatal hyperdopaminergia can have downstream effects in prefrontal cortex and impair neurocognitive function in schizoprenia.3 In summary, therefore, pathways to DA abnormality exist in which striatal hyperdopaminergia is primary or secondary, for example as a consequence of prefrontal dysfunction, and further work is necessary to define which of these routes are common in the prepsychotic stage of the illness.
Since antipsychotics are effective and used clinically in nonschizophrenic psychosis, for example in bipolar disorder, major depression, or delirium, progress in defining a specific role of striatal DA for schizophrenia can be made by comparing PET findings across these nosological categories. Striatal presynaptic hyperdopaminergia seems indeed to be somewhat specific to schizophrenia as DSC is not elevated in patients with affective illnesses although this distinction merits further study, in particular in patients without schizophrenia, which are and are not psychotic. A [18F]-DOPA PET study in subjects with acute mania found no significant differences in the striatal DSC, which was numerically lower for the patients than that for the comparison subjects.39 Another study of change in D2 receptor availability after amphetamine challenge in euthymic bipolar disorder patients failed to demonstrate enhanced DA release.40 Similarly, DSC was lower in unipolar depressed patients as compared with controls.41 In addition, no significant correlations between striatal DSC and acute symptoms were observed in subjects experiencing affective psychoses.42 Conversely, in schizophrenia, greater elevation in striatal DSC has been found to be associated with greater symptom severity.27 We uncovered no significant association between DSC and severity of psychotic symptoms, although this may be the consequence of limited statistical power of the meta-regression analysis. However, all the studies included in the present meta-analysis where patients were acutely psychotic at the time of the scanning, there was a significant increased level of striatal DSC, in agreement with the hypothesis linking striatal hyperdopaminergia to positive symptoms. Conversely, studies in patients who were not acutely psychotic have reported no difference from controls or even a reduction in synthesis capacity.16,17 Interestingly, the few catatonic subjects tested in some of the included studies showed DSC that were by far the lowest of any of the schizophrenic subjects and were also lower than controls, with values comparable in magnitude to those measured in Parkinson disease. Although this is consistent with a number of parallel features in the catatonic syndrome and neurological extrapyramidal disorders, suggesting that the catatonic subtype of schizophrenia might be associated with abnormally low levels of DA, more patients need to be studied to substantiate this finding.
The finding of increased striatal synthesis capacity is also of potential practical interest to preventive interventions in psychosis. In fact, one study has demonstrated that DA synthesis is already increased in patients with prodromal symptoms of schizophrenia, prior to the onset of frank psychosis.7 Identification of early neurobiological markers of an impending psychosis (see reviews of structural43,44 and functional45,46 markers of psychosis) may increase the low predictive value of available diagnostic instruments. These are currently based on psychopathological assessment of “attenuated” psychotic symptoms that are present below the threshold of full psychosis, brief and self-limiting psychotic symptoms, or a significant decrease in functioning in the context of a genetic risk for schizophrenia as well as early subjective disturbances of cognitive processes and the perception of the self and the world.1 However, despite the growing interest in the field—which has lead to the proposal to include the psychosis risk syndrome as a new diagnosis in the coming DSM-547— the predictive validity of current prodromal criteria is low.1 The finding of increased DSC in subjects at clinical risk for psychosis could therefore be used as a neurobiological marker of an impending risk of psychosis. In support of this idea, the striatal DSC was positively related to the severity of psychotic symptoms,27 irrespective of whether the rating was of symptoms associated with the risk or schizophrenia.7 However, before such a proposal could enter clinical practice, it is imperative to clarify the position of striatal hyperdopaminergia in the natural course of the illness. Currently, it is not clear whether the increased striatal DSC is specific to true prodromal subjects (who will later develop psychosis) as opposite to subjects who are at high risk but will not become psychotic. Some studies have begun to address the question of where in the risk architecture of schizophrenia striatal hyperdopaminergia comes into play, suggesting progressive DA increases with the onset of overt psychosis.48 However, a study using [18F]-DOPA PET in nonpsychotic first-degree relatives of patients with schizophrenia4 found an elevation of striatal DSC as compared with healthy controls. Because the majority of the nonpsychotic relatives had passed the highest risk age of schizophrenia, and the overall risk in this population is around 10%, a later conversion to psychosis was unlikely in a relevant proportion of this sample. Thus, this study indicates a link to genetically mediated psychosis vulnerability independent of current psychotic symptoms. However, a more recent study in cotwins of patients affected with schizophrenia showed contrasting findings indicating striatal DSC is not elevated in symptom-free individuals at genetic risk of schizophrenia.18 Secondly, 2 studies have suggested a role for environmental risk factors for the illness because striatal DA release in individuals with low maternal care49 and in subjects with schizophrenia-associated personality characteristics50 was stronger than in controls. Here again, the majority of these subjects are not clinically expected to develop overt psychosis, suggesting that altogether increased striatal dopaminergic neurotransmission is better seen as a neural mediator of psychosis vulnerability than as an indicator of a true prodromal state.
Of course, it still remains possible that elevated striatal DA synthesis may represent both a trait and state marker of schizophrenia. In other words, vulnerability to psychosis, through genetic or environmental factors or their interaction, could be associated with increased striatal synthesis capacity, while transition to psychosis may be associated with a further increase of presynaptic DA. There is in fact evidence that magnitude of DA increase in subjects at clinical of psychosis is less severe than that observed during a first episode of illness9 and that transition to psychosis is associated with progressive increase of DSC.48 Future longitudinal studies in large cohorts of subjects at clinical risk for psychosis, and studies reaching back farther into early adolescence in at-risk populations, are needed to definitively clarify the state or trait-like characteristics of such alterations. If quantitative thresholds for striatal DSC need to be established for the development of intervention strategies, our finding of a consistent and relatively homogenous elevation of around 14% in manifest disease should serve as a useful baseline. Additionally, longitudinal and well-powered studies are also required around relapses of psychosis to better characterize the state-related changes in striatal DSC.
We uncovered no significant moderator factors. Year of publication did not affect the sensitivity of the PET analyses, and this was further confirmed by the lack of significant publication bias in current literature. There was no effect of participant’s age, in line with previous results of studies using [18F]-DOPA measuring age-related changes in DSC and reporting no age effect.51 Finally, there was no effect of exposure to antipsychotics on DSC. This result seems surprising as it is usually thought that antipsychotic medications modulate DA neurotransmission. Recent structural imaging studies also found significant modulation of subcortical gray matter volumes by antipsychotic treatment.52,53 The effect of antipsychotic on the DSC has been investigated in different studies with evidence indicating a biphasic effect of medications. One study found a significant decrease in DSC after 5 weeks administration of haloperidol.54 Two studies found no change in DSC after a single dose of risperidone.55,56 Another study uncovered a significant increase in putamen DSC following 3 days of haloperidol.57 Unluckily, we did not have enough statistical power to contrast subgroups of studies with acute and chronic antipsychotic exposure. However, as elevation of striatal DSC was observed in different groups of subjects antipsychotic-naive (untreated first-episode subjects,9,27 individual at clinical risk for psychosis,9 relatives of patients affected with schizophrenia4), it does not seem to be primarily due to antipsychotic treatment. However, the lack of significant moderators may be also the consequence of limited statistical power in the meta-regression analyses. Additional limitations of the study relates to the heterogeneous radiotracers employed with variable sensitivity, kinetic, and imaging parameters which may account for the unexplained heterogeneity across studies. Furthermore, a more complex network of interactions implicating neurotransmitters other than DA may significantly affect the kinetic of the radioligands.
Conclusions
The present meta-analysis showed consistently increased striatal DSC in schizophrenia, with a 14% elevation in patients as compared with healthy controls. Taken together with the companion study,6 we suggest striatal hyperdopaminergia in schizophrenia is accompanied by alterations of presynaptic functional rather than structural integrity. Further clarification of the role of striatal DA in the risk architecture of the illness, in particular in its prepsychotic stage, may have practical relevance for intervention strategies and treatment development.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We thank the referees for their valuable comments on the 2 manuscripts. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012 doi: 10.1001/archgenpsychiatry.2011.1472. In press. [DOI] [PubMed] [Google Scholar]
- 2.Howes O, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson E, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huttunen J, Heinimaa M, Svirskis T, et al. Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry. 2008;63:114–117. doi: 10.1016/j.biopsych.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 5.McGuire P, Howes OD, Stone J, Fusar-Poli P. Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci. 2008;29:91–98. doi: 10.1016/j.tips.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part I: meta-analysis of dopamine active transporter (DAT) density. Schizophr Bull. 2012 doi: 10.1093/schbul/sbr111. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howes O, Montgomery A, Asselin M, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 8.Miyake N, Thompson J, Skinbjerg M, Abi-Dargham A. Presynaptic dopamine in schizophrenia. CNS Neurosci Ther. 2011;17:104–109. doi: 10.1111/j.1755-5949.2010.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hietala J, Syvalahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 10.Kumakura Y, Cumming P, Vernaleken I, et al. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–8087. doi: 10.1523/JNEUROSCI.0805-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reith J, Benkelfat C, Sherwin A, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci U S A. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Arch Gen Psychiatry. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- 13.Lindstrom LH, Gefvert O, Hagberg G, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry. 1999;46:681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 14.Nozaki S, Kato M, Takano H, et al. Regional dopamine synthesis in patients with schizophrenia using L-[beta-11C]DOPA PET. Schizophr Res. 2009;108:78–84. doi: 10.1016/j.schres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- 16.Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, et al. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res. 1997;23:167–174. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 17.Elkashef AM, Doudet D, Bryant T, Cohen RM, Li SH, Wyatt RJ. 6-(18)F-DOPA PET study in patients with schizophrenia. Positron emission tomography. Psychiatry Res. 2000;100:1–11. doi: 10.1016/s0925-4927(00)00064-0. [DOI] [PubMed] [Google Scholar]
- 18.Shotbolt P, Stokes PR, Owens SF, et al. Striatal dopamine synthesis capacity in twins discordant for schizophrenia. Psychol Med. 2011;41:2331–2338. doi: 10.1017/S0033291711000341. [DOI] [PubMed] [Google Scholar]
- 19.Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–117. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howes OD, Montgomery AJ, Asselin M, Murray R, Grasby P, McGuire P. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–s18. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. 2010;303:1961–1969. doi: 10.1001/jama.2010.605. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Borenstein MHL, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 24.Hedges L, Holkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic Press; 1985. [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Stat. 1983;8:157–159. [Google Scholar]
- 27.Hietala J, Syvalahti E, Vilkman H, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- 28.Abi-Dargham A, Gil R, Krystal J, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 29.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 30.Vernaleken I, Buchholz HG, Kumakura Y, et al. 'Prefrontal' cognitive performance of healthy subjects positively correlates with cerebral FDOPA influx: an exploratory [18F]-fluoro-L-DOPA-PET investigation. Hum Brain Mapp. 2007;28:931–939. doi: 10.1002/hbm.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fusar-Poli P, Howes OD, Allen P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67:683–691. doi: 10.1001/archgenpsychiatry.2010.77. [DOI] [PubMed] [Google Scholar]
- 32.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 34.Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Lindenberg A, Straub RE, Lipska BK, et al. Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition. J Clin Invest. 2007;117:672–682. doi: 10.1172/JCI30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esslinger C, Walter H, Kirsch P, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- 37.Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247(suppl 5):V1–15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- 38.Kellendonk C, Simpson EH, Polan HJ, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Yatham LN, Liddle PF, Shiah IS, et al. PET study of [(18)F]6-fluoro-L-dopa uptake in neuroleptic- and mood-stabilizer-naive first-episode nonpsychotic mania: effects of treatment with divalproex sodium. Am J Psychiatry. 2002;159:768–774. doi: 10.1176/appi.ajp.159.5.768. [DOI] [PubMed] [Google Scholar]
- 40.Anand A, Verhoeff P, Seneca N, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. Am J Psychiatry. 2000;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- 41.Martinot M, Bragulat V, Artiges E, et al. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158:314–316. doi: 10.1176/appi.ajp.158.2.314. [DOI] [PubMed] [Google Scholar]
- 42.Abbott C, Bustillo J. What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr Opin Psychiatry. 2006;19:135–139. doi: 10.1097/01.yco.0000214337.29378.cd. [DOI] [PubMed] [Google Scholar]
- 43.Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies [published online ahead of print November 17, 2011] Schizophr Bull. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis—a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Fusar-Poli P, Perez J, Broome M, et al. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Fusar-Poli P, Yung A. Should attenuated psychosis syndrome be included in the DSM5? Lancet. 2012 doi: 10.1016/S0140-6736(11)61507-9. In press. [DOI] [PubMed] [Google Scholar]
- 48.Howes OD, Bose SK, Turkheimer F, et al. Dopamine Synthesis Capacity Before Onset of Psychosis: a Prospective [18F]-DOPA PET Imaging Study [published online ahead of print July 18, 2011] Am J Psychiatry. doi: 10.1176/appi.ajp.2011.11010160. doi:10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soliman A, O'Driscoll GA, Pruessner J, et al. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology. 2008;33:2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- 51.Braskie MN, Wilcox CE, Landau SM, et al. Relationship of striatal dopamine synthesis capacity to age and cognition. J Neurosci. 2008;28:14320–14328. doi: 10.1523/JNEUROSCI.3729-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borgwardt SJ, Smieskova R, Fusar-Poli P, Bendfeldt K, Riecher-Rossler A. The effects of antipsychotics on brain structure: what have we learnt from structural imaging of schizophrenia? Psychol Med. 2009;39:1781–1782. doi: 10.1017/S0033291709006060. [DOI] [PubMed] [Google Scholar]
- 53.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grunder G, Vernaleken I, Muller MJ, et al. Subchronic haloperidol downregulates dopamine synthesis capacity in the brain of schizophrenic patients in vivo. Neuropsychopharmacology. 2003;28:787–794. doi: 10.1038/sj.npp.1300103. [DOI] [PubMed] [Google Scholar]
- 55.Mamo D, Remington G, Nobrega J, et al. Effect of acute antipsychotic administration on dopamine synthesis in rodents and human subjects using 6-[18F]-L-m-tyrosine. Synapse. 2004;52:153–162. doi: 10.1002/syn.20016. [DOI] [PubMed] [Google Scholar]
- 56.Ito H, Takano H, Takahashi H, et al. Effects of the antipsychotic risperidone on dopamine synthesis in human brain measured by positron emission tomography with L-[beta-11C]DOPA: a stabilizing effect for dopaminergic neurotransmission? J Neurosci. 2009;29:13730–13734. doi: 10.1523/JNEUROSCI.4172-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vernaleken I, Kumakura Y, Cumming P, et al. Modulation of [18F]fluorodopa (FDOPA) kinetics in the brain of healthy volunteers after acute haloperidol challenge. Neuroimage. 2006;30:1332–1339. doi: 10.1016/j.neuroimage.2005.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.