Abstract
Substantial evidence implicates working memory (WM) as a core deficit in schizophrenia (SCZ), purportedly due to primary deficits in dorsolateral prefrontal cortex functioning. Recent findings suggest that SCZ is also associated with abnormalities in suppression of certain regions during cognitive engagement—namely the default mode system—that may further contribute to WM pathology. However, no study has systematically examined activation and suppression abnormalities across both encoding and maintenance phases of WM in SCZ. Twenty-eight patients and 24 demographically matched healthy subjects underwent functional magnetic resonance imaging at 3T while performing a delayed match-to-sample WM task. Groups were accuracy matched to rule out performance effects. Encoding load was identical across subjects to facilitate comparisons across WM phases. We examined activation differences using an assumed model approach at the whole-brain level and within meta-analytically defined WM areas. Despite matched performance, we found regions showing less recruitment during encoding and maintenance for SCZ subjects. Furthermore, we identified 2 areas closely matching the default system, which SCZ subjects failed to deactivate across WM phases. Lastly, activation in prefrontal regions predicted the degree of deactivation for healthy but not SCZ subjects. Current results replicate and extend prefrontal recruitment abnormalities across WM phases in SCZ. Results also indicate deactivation abnormalities across WM phases, possibly due to inefficient prefrontal recruitment. Such regional deactivation may be critical for suppressing sources of interference during WM trace formation. Thus, deactivation deficits may constitute an additional source of impairments, which needs to be further characterized for a complete understanding of WM pathology in SCZ.
Keywords: schizophrenia, fMRI, working memory, encoding, maintenance, default network
Introduction
Schizophrenia (SCZ) is a disabling neuropsychiatric illness causing substantial cognitive impairment. Working memory (WM) has been postulated as a core cognitive deficit,1 purportedly due to abnormalities in N-methyl-D-aspartate,2 Gamma Amino Butyric Acid (GABA),3 and/or D1 receptor dysfunction.4 Meta-analyses have estimated effects sizes of WM deficits in SCZ as compared with healthy subjects ranging from 0.61 to 1.18 across both verbal and nonverbal WM tasks.5,6 Additionally, functional neuroimaging (functional magnetic resonance imaging [fMRI]) meta-analytic studies have consistently documented abnormalities in dorsolateral prefrontal cortex (DLPFC) activation during WM in SCZ.7,8
One of the critical hurdles in understanding WM dysfunction in this illness is the question of “inefficiency” of DLPFC recruitment. That is, do patients show under or over-recruitment of DLPFC across different levels of WM demands relative to healthy comparison (HC) subjects? To address this, most studies have employed a variety of continuous performance tasks (eg, n-back),7 which “blend” different WM processes. However, WM is not comprised a single operation,9 and both cognitive10 and neurobiological1 models have identified different WM processes: (1) encoding of novel information; (2) maintenance, manipulation and updating; and (3) retrieval of information. Thus, to fully understand WM dysfunction in SCZ, it is critical to employ tasks that assay distinct WM processes as dysfunction of each process may contribute unique sources of deficit in this illness.11
To this end, a number of behavioral studies have documented both encoding and maintenance abnormalities in SCZ, as has been summarized meta-analytically.6 Similarly, neuroimaging studies have specifically examined maintenance12–15 as well as encoding-related16,17 activation abnormalities in SCZ, reporting deficits in prefrontal functioning across both phases. While the work thus far has critically advanced, our understanding of WM-related deficits in this illness, suggesting that there may be abnormalities across multiple WM phases, several issues remain unresolved. First, few studies have explicitly examined both encoding- and maintenance-related deficits in the same sample while ensuring performance matching, which has been established as a critical moderator of WM-related prefrontal recruitment.8 Second, most studies examining encoding deficits16,17 have focused on verbal WM but have not examined both maintenance and encoding deficits in visual WM.18 One recent neuroimaging study that examined spatial WM across both encoding and maintenance12 reported an absence of encoding-related deficits in SCZ. However, Driesen12 and colleagues selected a circumscribed set of right prefrontal areas previously shown to be involved in WM in healthy adults,19 which may overlook encoding deficits in a more distributed network of regions. In fact, most studies examining WM-related abnormalities in SCZ employed either a whole-brain search (which may be statistically too stringent and miss relevant effects) or a selective region-of-interest (ROI) approach (which may be too spatially restrictive, given the distributed networks involved in WM). A compromise may be to comprehensively examine WM-related areas (WMRA) identified by formal meta-analyses20,21—an approach employed in the present study.
Third, most prior work focused on areas where patients may show differences in “activation,” which is a critical question. However, recent work from our laboratory22 indicated that suppressing activity in certain brain areas at encoding, closely corresponding to the default mode network (DMN),23 may be essential for optimal WM operation. Thus, failure to suppress DMN activity may constitute another source of WM-related abnormalities in SCZ. In line with this hypothesis, a number of SCZ studies have reported deactivation deficits in DMN during WM.13,24–27 Therefore, exclusively examining abnormalities in aberrant activation may be insufficient for a complete understanding of WM pathology in SCZ. However, no study has examined suppression abnormalities across both encoding and maintenance of WM in this illness. We hypothesized that patients may show deactivation abnormalities extending across both WM phases. Furthermore, one interesting possibility suggested by Metzak and colleagues26 is that DMN suppression deficits may arise from a breakdown in coordinated and reciprocal modulation via task positive regions. Therefore, we also examined whether there was a relationship between suppressed and activated areas during WM and whether this relationship may be altered in SCZ.
In summary, while there is strong evidence for both encoding- and maintenance-related deficits in SCZ during verbal WM, it is unclear whether such deficits are present across both phases of a “visual” WM, especially when performance is carefully matched and a broad set of WMRA are assayed. Furthermore, no study has systematically examined presence of suppression abnormalities across encoding and maintenance, particularly in regions which when active may interfere with WM. Therefore, the main motivation for the present study was to characterize both activation and deactivation across encoding and maintenance phases of WM in patients suffering from SCZ. To that end, using a well-powered sample, we examined group differences across WM phases with a previously well-validated visual WM task.22,28 Given the aforementioned behavioral meta-analysis findings,6 we hypothesized that (1) SCZ is associated with both encoding and maintenance activation abnormalities when examining meta-analytically defined WMRA, (2) SCZ is associated with abnormalities in deactivation of areas where suppression may be critical for optimal WM operation, and (3) Activation in prefrontal regions may predict deactivation in suppressed regions for HC subjects but not SCZ subjects.
Methods
Subject Recruitment
Twenty-eight subjects meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV) criteria for SCZ and 24 demographically matched HC subjects provided informed consent approved by Washington University. Subjects underwent the Structured Clinical Interview for DSM-IV, symptom ratings using the Scale for Assessment of Positive and Negative Symptoms (SAPS/SANS)29,30 and were administered the Matrix Reasoning and Vocabulary sections of the Wechsler Adult Intelligence Scale—Third Edition. Healthy subjects were recruited from the same community as patients but were excluded for lifetime history of Axis I psychiatric disorder or a first-degree relative with a psychotic disorder. Subjects were excluded if they (1) met DSM-IV criteria for substance abuse/dependence within the past 6 months, anxiety or depression, (2) had any severe medical conditions, (3) suffered head injury (past or present) with neurological symptoms or loss of consciousness, or (4) met DSM-IV criteria for mental retardation. All patients were receiving stable medication for 2 weeks or more (although all but one patient exceeded 6 wk). Groups did not differ for handedness, gender, age, father’s education, mother’s education, and father’s socioeconomic status, but maternal socioeconomic status was higher for patients (table 1). Patients were impaired on standard measures of verbal and nonverbal IQ.
Table 1.
Mean SAPS Global Item Score for Each Subject was the Average of SAPS Global Item Scores for Hallucinations, Delusions, Bizarre Behavior, and Positive Formal Thought Disorder. Mean SANS Global Item Score for Each Subject was the Average of Affective Flattening or Blunting, Alogia, Avolition/Apathy, Anhedonia-Asociality, and Attention. Disorganization Symptoms Were the Sum of Global Scores for Bizarre Behavior, Positive Formal Thought Disorder, and Attention. Poverty Symptoms Were the Sum of Affective Flattening, Alogia, Avolition/Apathy, and Anhedonia-Asociality. Reality Distortion Symptoms Were the Sum of Hallucinations and Delusions
| Clinical and Demographic Characteristics | ||||||
| Characteristic | Controls | Patients | Significance | |||
| M | SD | M | SD | T Value/Chi-Square | P Value (2-tailed) | |
| Age (in years) | 37.18 | 7.59 | 36.39 | 9.54 | 0.31 | .759 |
| Gender (% male) | 0.74 | 0.78 | 0.34 | .737 | ||
| Paternal education (in years) | 12.70 | 1.46 | 13.26 | 2.61 | 0.90 | .370 |
| Maternal education | 12.48 | 1.53 | 13.50 | 3.07 | 1.42 | .162 |
| Paternal SES | 21.59 | 8.92 | 26.59 | 10.73 | 1.67 | .100 |
| Maternal SES | 17.27 | 8.55 | 25.24 | 11.88 | 2.51 | .015 |
| Participant’s education (in years) | 15.26 | 2.12 | 13.04 | 2.14 | 3.50 | .001 |
| Handedness (% right) | 100.00 | 86.96 | 1.45 | .152 | ||
| IQ verbal | 110.23 | 10.85 | 95.23 | 14.18 | 3.88 | .000 |
| IQ performance | 115.45 | 11.64 | 101.82 | 15.24 | 3.30 | .002 |
| Medication (CPZ equivalents) | 584.63 | 563.63 | ||||
| Mean SAPS global item score | 0.02 | 0.11 | 1.91 | 1.21 | ||
| Mean SANS global item score | 0.37 | 0.62 | 2.50 | 0.78 | ||
| Disorganization | 0.78 | 1.17 | 5.48 | 2.71 | ||
| Poverty | 1.13 | 2.39 | 10.43 | 3.53 | ||
| Reality Disotortion | 0.00 | 0.00 | 4.26 | 3.53 | ||
Note: SAPS, scale for assessment of positive symptoms; SANS, scale for the assessment of negative symptoms; CPZ, chlorpromazine; SES, socioeconomic status.
fMRI Acquisition
All structural and blood oxygenation level-dependent (BOLD) data were acquired using a 3T Tim-TRIO scanner at Washington University. Functional images were acquired using an asymmetric spin-echo, echo-planar sequence maximally sensitive to BOLD contrast (T2*) (repetition time [TR] = 2200 ms, echo time [TE] = 27 ms, field of view = 256 mm, flip = 90°, voxel size = 4 mm3). BOLD runs lasted 5.09 minutes and contained 133 sets of oblique axial images (32 slices per volume) acquired parallel to the anterior-posterior commissure. Structural images were acquired using a sagittal magnetization-prepared radio-frequency rapid gradient-echo 3D T1-weighted sequence (TR = 2400 ms, TE = 3.16 ms, flip = 8°; voxel size = 1 mm3).
fMRI Preprocessing
Preprocessing followed our previously published work.22,28,31–33 Preprocessing included: (1) Slice-time correction, (2) Removal of first 5 images from each run to reach steady state, (3) Elimination of odd/even slice intensity differences given interpolated acquisition, (4) Rigid body motion correction, (5) Intensity normalization to a whole-brain mode value of 1000 without bias or gain field correction, (6) Registration using a 12-parameter affine transform of the structural image to a template image in the Talairach coordinate system, (7) Coregistration of fMRI volumes to the structural image with 3 mm3 resampling, (8) Smoothing using a 6 mm full-width at half-maximum Gaussian kernel.
To ensure comparable across-group signal-to-noise ratios (SNR), subjects were excluded if a BOLD run had SNR < 150 (5 SCZ and 1 HC were rejected based on these criteria). After removal, there were no significant between-group SNR differences across BOLD runs (mean-SCZ = 313.85; mean-HC = 333.72) (t 44 = 0.72, P = .47, NS). SNR was calculated following preprocessing but prior to atlas transformation (ie, in each subject’s native space).31 Briefly, SNR estimates were computed by obtaining the mean signal and SD for a given slice across the BOLD run, while excluding all nonbrain voxels across all frames. The overall SNR estimate was expressed as mean/SD for a given slice and averaged across all slices.
Task Design and Stimuli
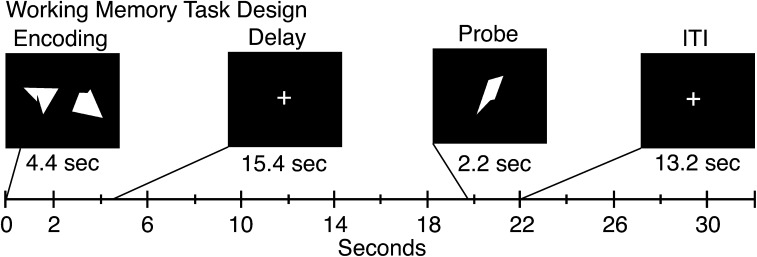
While in the scanner subjects completed a modified Sternberg-type delayed match-to-sample WM task (24 trials) (figure 1) employed in our prior work.22,28 First, subjects were given instructions and a brief (8 trial) practice session. The task was difficulty matched such that all subjects performed at ∼80% correct, given previous studies indicating that prefrontal activation differences vary as a function of task performance.8 Subject-specific difficulty matching was accomplished by manipulating the nontarget probe similarity but holding the encoding load constant across subjects (see Supplement in online supplementary material). Details describing the difficulty manipulation procedure and pilot work validating this approach are reported elsewhere.32 Briefly, during the diagnostic visit, subjects completed an out-of-scanner WM task with 3 difficulty levels (low, medium, and high) to extrapolate at which difficulty each subject performs at ∼80% (results from this task session are reported in figure 2 along with fMRI behavioral results). This approach was critical to ensure equal encoding and maintenance load levels across groups while maintaining comparable performance levels.
Fig. 1.
Task design. Task design is displayed with different components and their onsets marked along the timeline. Each box represents a trial component with the duration marked below. The memory sets were presented centrally subtending a visual angle of 15.75 for a duration of 4.4 s, followed by a 15.4 s delay, and a probe presented for 2.2 s. Each trial was followed by a 13.2 s fixation period to allow the hemodynamic response to return to baseline, as employed in our prior work.
Fig. 2.
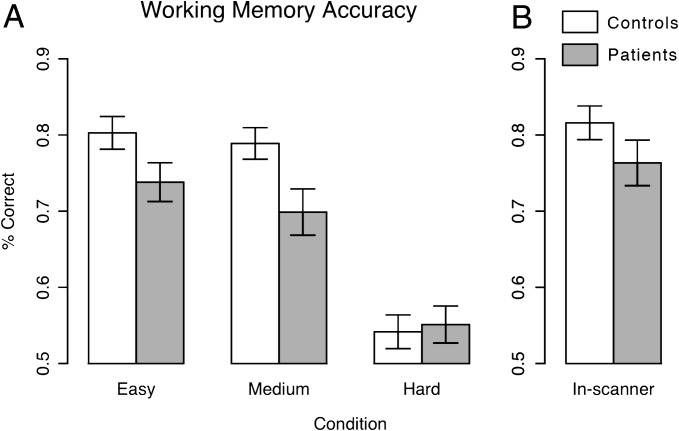
Behavioral results. Behavioral results are shown for (A) working memory (WM) performance on the task used to calibrate performance outside of the scanner and (B) WM performance on the task performed inside the scanner following performance matching. Prior to performance-matching schizophrenia (SCZ) subject performed worse than healthy comparison subjects across 2 conditions (ie, easy and medium) that were above chance for both groups. In-scanner WM performance was closely matched between groups (t 50 = 1.20, P = .23, NS).
An alternative to the present approach is to focus exclusively on accurate trials. Our a priori reasoning for including all trials in the analyses (instead of accurate trials only) is based on the motivation to remain maximally powered for within-subject analyses. That is, there were only 24 trials per subject and dropping ∼20% of trials per subjects (and more in some cases) could have ultimately reduced our within-subject estimates (although post hoc analyses confirmed the same findings for accurate trials).
All stimuli were complex geometric shapes that were difficult to verbally encode and were generated using a MATLAB algorithm described in detail previously.22 Stimuli were presented through a projector to a screen behind the scanner. Of note, while in the scanner, subjects completed 2 additional tasks: an additional modified Sternberg WM task with distraction and a simple visual detection task reported elsewhere.31,32
fMRI Analyses
As a first step, a general linear model (GLM) approach was used to estimate voxel-wise magnitudes of task-related activity. We employed an assumed hemodynamic response function (HRF) GLM approach to specifically isolate encoding- and maintenance-related activation—validated previously using the same paradigm.22 Briefly, we obtained an estimate of encoding- and maintenance-related activity by convolving a block function reflecting the neuronal response with an assumed BOLD response function. The resulting beta estimates were entered into a second-level analysis treating subjects as a random factor. To verify all assumed HRF analyses and to visualize activation time courses, we computed a GLM without assuming an HRF by modeling 15 frames following trial onset (details of this approach are reported elsewhere28). At the second level, we computed independent samples t tests at both encoding and maintenance using the assumed GLM magnitudes for each trial component.
All analyses were computed at the whole-brain level (ie, voxel wise) with the appropriate P < .05 type I error correction (Z > 3 and k = 13 contiguous voxels). We also constrained the analyses to WMRA showing meta-analytic evidence of involvement in WM, by computing the above t tests within a constrained mask,20,21 which allowed for a less stringent type I correction given the constrained search space (Z > 2 and k = 33 contiguous voxels) (see Supplement and Figure S1 in online supplementary material). We opted for a constrained search since hypothesized smaller effects may be especially identifiable in regions with a priori theoretical precedence and given that such an approach yields less stringent false-positive protection. Furthermore, there is a theoretical advantage when using a mask based on previous meta-analysis articles in that it examines regions with known involvement in the neural mechanisms of WM in healthy individuals.
Results
Behavioral Results
To replicate work showing WM deficits in SCZ, we compared group performance on the preliminary WM task completed outside the scanner (ie, used for performance matching, see Supplement for details in online supplementary material). These results are a critical validity check in another investigation reported elsewhere.34 Briefly, we computed a 2-way repeated-measures ANOVA with “Difficulty” (low, medium, and high) as a within-subject factor and “Diagnosis” (SCZ vs CON) as a between-subject factor. Results revealed a trend level main effect of Diagnosis (F 1,50 = 2.98, P < .095), a significant main effect of Difficulty (F 2,100 = 86.6, P < .0001), and a significant Diagnosis × Difficulty interaction (F 2,100 = 3.97, P < .025) (figure 2A). SCZ subjects performed worse than HC subjects at the 2 “easier” levels but not at the most difficult level. The highest level of difficulty produced chance performance and did not provide across-group discrimination. We computed a post hoc comparison to verify that SCZ subjects performed worse across easier levels providing performance discrimination. Results revealed a significant main effect of Diagnosis (F 1,50 = 5.45, P = .02), main effect of Difficulty (F 1,50 = 3.81, P = .05) but no Diagnosis × Difficulty interaction (F 1,50 = 0.80, P = .37). Critically, no significant group differences in WM performance emerged for the in-scanner task (t 50 = 1.20, P = .23, NS), indicating close performance matching (figure 2B).
Encoding-Related Deficits
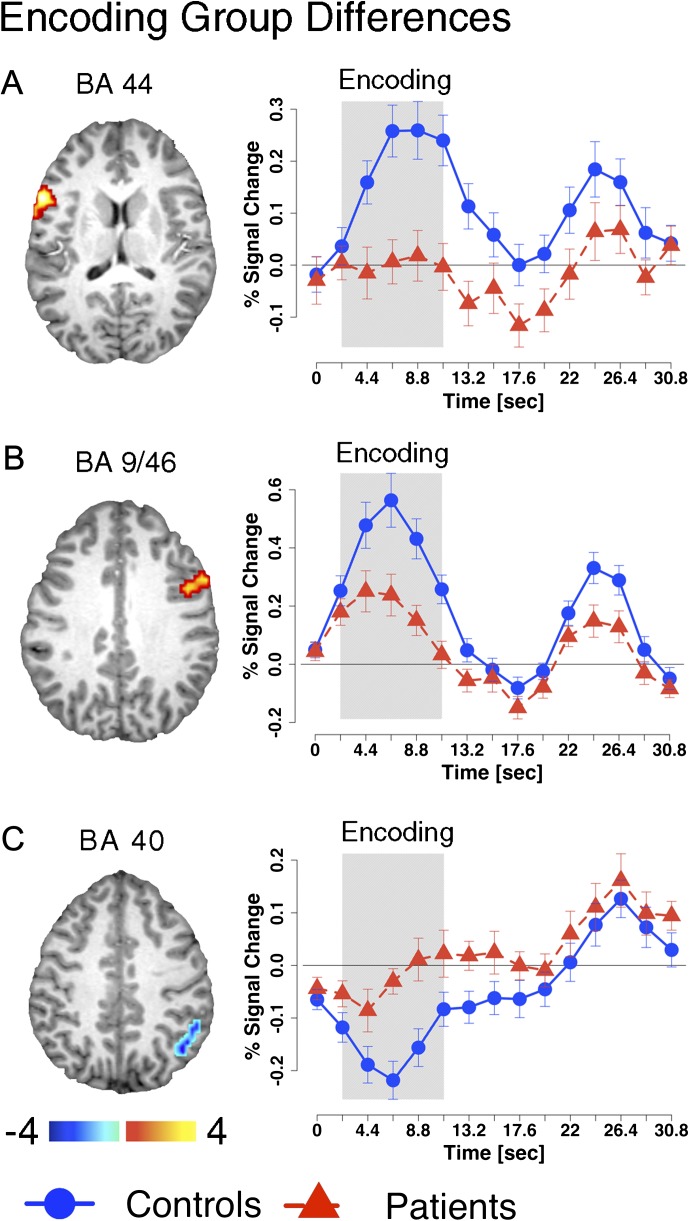
To test hypotheses regarding encoding deficits, we examined group differences by computing an independent samples t test (HC vs SCZ) for the encoding-related magnitude specifically (both at the whole-brain level and within WMRA). Whole-brain analysis did not reveal significant group differences. However, constrained WMRA analysis revealed 3 areas: left inferior frontal gyrus (Brodmann’s Area [BA] 44), right middle frontal gyrus (BA 9/46), and right inferior parietal lobe (BA 40) (figure 3A–C and table 2). The 2 prefrontal areas showed encoding-related activation in HC subjects, whereas SCZ subjects exhibited substantially lower encoding-related signal but little evidence for maintenance-related activation differences. The parietal region matched a posterior component of the DMN35 and showed task-related deactivation in HC subjects. Consistent with predictions, SCZ subjects exhibited significantly less deactivation during encoding relative to HC subjects.
Fig. 3.
Encoding-related deficits. Regions showing group differences during the encoding phase of working memory (WM). Precise coordinates are listed in the top panel of table 2. All foci shown were identified within the WM-related areas constrained space using the assumed hemodynamic response function (HRF) analysis (see Methods). Time courses were extracted for each region using an unassumed HRF approach to facilitate visual inspection of group differences across trial epochs. The approximate relevant encoding period of the time course is marked using the gray box. Time courses for control subjects are shown using circles whereas time courses for patients are shown using triangles.
Table 2.
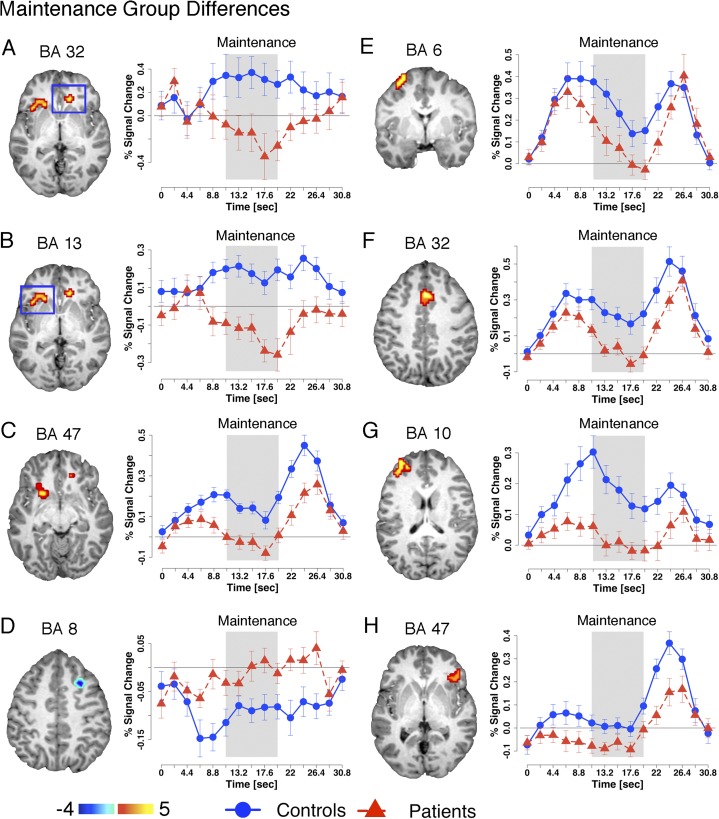
Significant Foci Surviving Appropriate Type I Error Correction Either at the Whole-Brain Level (Z > 3, k = 13 Contiguous Voxels, P < .05) or Within WMRA Shown to be Involved in WM Based on Meta-Analytic Work20,21(Z > 2, k = 33, P < .05)
| Regions Showing Encoding and Maintenance Group Differences | ||||
| X | Y | Z | Hemisphere | Anatomical Landmark |
| Encoding—WMRA effects | ||||
| SCZ > CON | ||||
| 43 | −54 | 39 | Right | Inferior parietal louble (BA 40) |
| SCZ < CON | ||||
| −56 | 9 | 12 | Left | Inferior frontal gyrus (BA 44) |
| 44 | 5 | 28 | Right | Precentral gyrus (BA 9/6) |
| Maintenance—voxel-wise effects | ||||
| SCZ > CON | ||||
| 21 | 15 | 45 | Right | Superior frontal gyrus (BA 8) |
| SCZ < CON | ||||
| 9 | 27 | −6 | Right | Anterior cingulate (BA 32) |
| −26 | 10 | −12 | Left | Inferior frontal gyrus (BA 47) |
| −32 | 18 | −2 | Left | Insular cortex (BA 13) |
| Maintenance—WMRA effects | ||||
| SCZ < CON | ||||
| 36 | 25 | 0 | Right | Inferior frontal gyrus (BA 47) |
| −35 | 43 | 18 | Left | Middle frontal gyrus (BA 10) |
| −3 | 11 | 40 | Left | Cingulate gyrus (BA 32) |
| −33 | −7 | 59 | Left | Precentral gyrus (BA 6) |
Note: WMRA, working memory-related areas; WM, working memory; SCZ, schizophrenia; BA, Brodmann’s Area.
Maintenance-Related Deficits
Next, we tested maintenance-related hypotheses by computing an independent samples t test (HC vs SCZ) specifically for magnitudes representing the period following encoding but prior to the probe phase (both at the whole-brain level and within WMRA). The whole-brain analysis revealed 4 regions (figure 4A–D and table 2) showing group differences. WMRA analysis revealed 4 additional areas (figure 4E–H and table 2) showing group differences. Interestingly, all identified foci were located prefrontally and for all foci except one SCZ subjects exhibited substantial signal reductions across the entire maintenance phase, closely replicating and extending prior work.12 The single exception was a superior frontal gyrus region (figure 4D) that SCZ failed to deactivate relative to HC subjects—a region which closely matched a superior frontal component of the DMN.35 Taken together, maintenance-related findings revealed a set of prefrontal areas for which SCZ relative to HC subjects failed to sustain delay signals. Furthermore, consistent with predictions, we indentified a region previously shown to deactivate during cognitive engagement23 that SCZ subjects failed to suppress during WM maintenance.
Fig. 4.
Maintenance-related deficits. Regions showing group differences during the maintenance phase of working memory (WM). Foci shown in panels A–D were identified using voxel-wise analyses (shown in table 2 middle panel), and foci shown in panels E–H were identified within the WM-related areas constrained space (shown in table 2 bottom panel). As in figure 3, all foci were identified using the assumed hemodynamic response function (HRF) analysis (see Methods), whereas time courses were extracted for each region using an unassumed HRF approach to facilitate visual inspection of group differences. The approximate relevant maintenance period of the time course is marked using the gray box. Time courses for control subjects are shown using circles whereas time courses for patients are shown using triangles.
Relationship Between Suppression and Activation
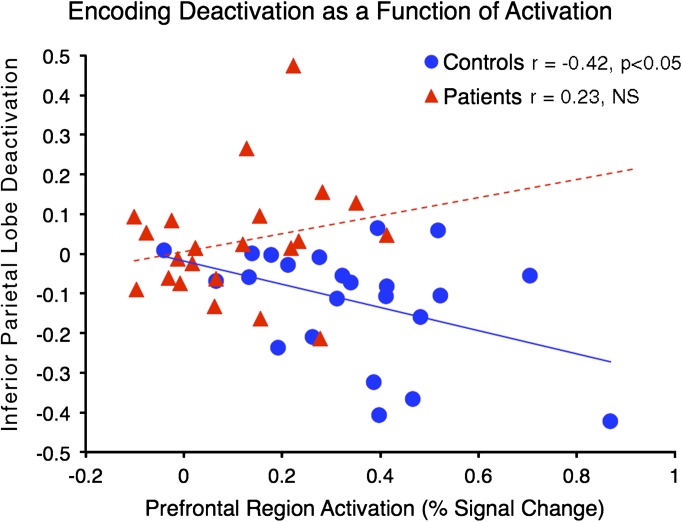
Given that we identified regions showing group differences in suppression at both encoding and maintenance, we investigated whether the degree of activation in prefrontal areas predicted the degree of suppression in the deactivated regions. We extracted the average percent signal change across all voxels for the identified regions (table 2) across relevant epochs (encoding vs maintenance). We averaged signal across all encoding activation regions and then across maintenance activation regions given no a priori motivation to focus on any one area (but also to avoid stringent multiple-comparison protection that arises from multiple tests). Next, we computed 2 across-subject correlations examining the degree of suppression as a function of activation for both encoding and maintenance phases. No significant results emerged for maintenance. However, the amount of prefrontal activation significantly predicted suppression for the parietal region during encoding for HC (r =−.42, P < .045, 2-tailed) but not SCZ subjects (r = .23, NS) (figure 5), which constituted a significant difference between the 2 correlation coefficients (Z = −2.16, P < .035, 2-tailed). We also investigated whether the degree of suppression predicted symptom severity but no significant correlations emerged. Of note, when excluding one HC outlier, the correlation was attenuated (r =−.21).
Fig. 5.
Relationship between suppression and activation at encoding. We examined the relationship between the degree of encoding-related suppression and encoding-related activation for regions identified as showing group differences during encoding (figure 3) (as noted in the main text, no significant findings emerged for maintenance-related analyses). Healthy comparison subjects (blue circles) showed a significant inverse relationship between suppression and activation (r = −.42, P < .045, 2-tailed), whereas patients (red triangles) did not (r = .23, NS). Of note, we averaged across both prefrontal areas showing activation given no a priori motivation to examine them separately.
Discussion
The present investigation replicates prior work showing WM maintenance deficits in SCZ and extends the existing literature in several important ways: (1) SCZ subjects exhibited encoding-related activation deficits during visual WM; (2) there were 2 regions for which SCZ subjects showed a lack of encoding- and maintenance-related suppression; (3) CON but not SCZ subjects showed a relationship between the degree of prefrontal recruitment and suppression at encoding; and (4) these findings were true even when subjects were matched for performance and when examining correct trials, helping to rule out the possibility that these deficits are solely the result of inaccurate performance.
Behavioral Performance
In the present study, we attempted to performance-match groups during the fMRI task. Preliminary testing, which was conducted to ascertain the optimal WM difficulty, indicated that SCZ relative to HC subjects showed WM deficits (especially at the 2 difficulty levels that were above chance performance for both groups), replicating a large body of prior work.6 Although there were no statistical differences in performance on the fMRI portion of the task, SCZ subjects were still numerically less accurate. Although unlikely (as results remained unchanged when focusing on correct trials only), this small group performance difference (5.25% less accurate performance for SCZ) may have accounted for some of the brain activation differences, which should be ruled out in further studies with more precise performance matching.
Encoding-Related Deficits
Behavioral meta-analytic work suggested that WM deficits observed in SCZ subjects may be primarily due to encoding-related abnormalities and/or deficits present during the early maintenance phase.6 Studies examining verbal WM identified prefrontal regions for which SCZ exhibited less activation at encoding relative to HC subjects.16,17 However, prior work examining spatial WM did not reveal encoding-related abnormalities when examining selected right prefrontal cortex (PFC) ROIs.12 We broadened the search for encoding deficits, both at the whole-brain level (which was afforded by our more powered sample) and within a larger set of areas shown to be involved in WM operations.20,21 With these analytic approaches, we identified 2 prefrontal ROIs that clearly displayed encoding deficits during visual WM in SCZ subjects. Thus, even when matched for performance, SCZ subjects failed to recruit PFC regions during the encoding period to the same extent as HC subjects, which may lead to less stable memory trace formation.
Of note, performance was matched by manipulating the similarity of the nontarget probe to the memory set. This, by definition, creates a constant load level for all subjects across WM epochs. One possibility is that memoranda in the present study (2 complex geometric shapes) were more challenging to encode for SCZ relative to HC subjects. If so, the encoding activation differences could, in some part, still reflect effort or task difficulty rather than an underlying neural deficit in prefrontal recruitment. However, we would argue that “effort” is an unlikely explanation as patients performed at a high accuracy level (close to 80% correct), suggesting adequate task engagement. Alternatively, one could accomplish performance matching by producing different information load requirements.16 One problem with this approach is that encoding and maintenance comparisons are complicated given a mismatch in information load. One venue for future work is to systematically compare prefrontal recruitment across different performance-matching strategies within subjects, to help understand the source of encoding-related impairments in SCZ.
Another potential explanation for encoding (and maintenance) abnormalities may be in part related to SCZ subjects’ inability to recruit attentional resources when novel information is presented and to suppress activity in areas that may disrupt ongoing memory trace formation. A meta-analysis by Shulman and colleagues23 identified a network of regions consistently suppressed during cognitive engagement, which was later more carefully characterized using resting-state techniques35 and termed the DMN. Regions belonging to this network have been consistently implicated in so-called spontaneous cognition. Our prior work has revealed that within-subject trial-by-trial deactivation of the DMN network during encoding is associated with better WM performance22 (of note, present data revealed a nonsignificant trend in the same direction). Also, prior work has shown that SCZ is associated with less DMN suppression during cognitive engagement.36 We found regions that are part of the DMN, which SCZ subjects failed to deactivate during encoding and maintenance. One possibility is that suppression of regions, which when active may disrupt novel memory trace formation,22 is compromised in SCZ. Such an abnormality in regional suppression may introduce additional “noise” during WM encoding in this illness, which may arise in part from aberrant PFC recruitment that may be critical for coordinated DMN network deactivation.26
Consistent with the hypothesis that PFC recruitment may be critical for DMN-region suppression, we showed that HC subjects with greatest prefrontal activation at encoding also exhibited greatest suppression of a parietal region overlapping the DMN. However, SCZ subjects failed to show such a relationship, which suggests that there may be a deficit in coordinated regional suppression as a function of abnormal prefrontal recruitment. It is unclear if present results extend to all components of the DMN—a question to be examined in prospective work. One possibility, supported by present results, is that prefrontal recruitment and regional DMN suppression may be part of the same deficit (or at least a function of aberrant PFC engagement). As such, it will be critical to determine whether deactivation deficits in SCZ are a consequence of PFC dysfunction or a separate abnormality (ie, failure to suppress spontaneous cognition) that independently contributes to WM deficits and other domains of cognitive dysfunction in SCZ.
Maintenance-Related Deficits
We also found a number of prefrontal foci exhibiting a more prominent signal drop during maintenance phase for patients. This pattern closely replicated prior work showing that SCZ subjects fail to maintain the same level of activation throughout maintenance of WM, even when encoding-related signals are similar in PFC regions.12 Unlike prior work showing abnormalities during visual WM maintenance, we used an assumed HRF approach that allowed us to isolate activity associated with specific WM processes.22 Despite the methodological differences, present data provide a strong replication of prior work. As argued before, the loss of signals during the maintenance phase may reflect an underlying disturbance in the ability to form stable attractor networks in PFC, which results in diminished stability of WM trace maintenance.37
One difference between present and prior results is that we identified regions exhibiting activity deficits across both phases of spatial WM in SCZ. It is likely that by focusing on very circumscribed prefrontal regions, known to be involved primarily in WM trace maintenance, prior work could not detect areas that show deficits at both stages. Thus, present findings suggests that there may be 2 types of visual WM disturbances in SCZ: (1) deficits at encoding reflected as lower activation, which implies less robust WM trace formation and (2) subsequent failure to sustain stable delay period activity, which further contributes to WM deficits. Importantly, encoding and maintenance deficits may constitute unique sets of abnormalities in different regions. That is, failure to encode novel information in SCZ may be related to both an inability to “focus” attention when novel information is presented and possibly suppress regions that are active in the task-free state (as articulated above),22 whereas maintenance-related abnormalities may arise from neuropathology in attractor network formation and propagation.37
Prior work examining verbal WM failed to find prominent group activation differences during WM maintenance—Schlösser and colleagues17 demonstrated more prominent group differences when examining conditions requiring manipulation of verbal information (ie, alphabetizing the remembered letters). However, this reflected mainly “over-recruitment” of executive regions in the patient group relative to HC subjects. One possibility is that this activation difference reflected task demands—that is Schlösser and colleagues17 employed a task that was substantially more challenging for patients and performance has been shown to be a critical moderating variable of group activation differences.8 Another possibility may be that there are critical neural activation differences between maintenance of verbal vs spatial WM representations in SCZ—a hypothesis that awaits direct and systematic prospective testing.
Lastly, prior studies examined the relationship between encoding and maintenance deficits in SCZ.12 We did not attempt a direct replication of these effects. Instead, we focused on both activation and deactivation deficits—which is the main substantive advance over prior work. However, an intriguing question is whether there is a relationship between deactivation and activation deficits across stages of WM.
Implications for Neural Abnormalities of WM Function in SCZ
An important point is that we searched for foci showing encoding- and maintenance-related abnormalities at the whole-brain level and within a set of a priori ROIs implicated in WM operations. Both approaches identified PFC regions (apart from a posterior region showing a lack of suppression during WM encoding). While the whole-brain approach may have been statistically conservative to detect small effects in regions other than PFC, the less stringent WMRA analysis still revealed abnormalities mainly in PFC regions and not elsewhere, despite equal likelihood of detecting group differences in other foci (eg, thalamus, basal ganglia, visual cortex, cerebellum, and parietal cortex). This suggests that PFC activation abnormalities, possibly due to underlying dopamine, glutamate, and/or GABA pathology2,37,38 are central to WM-related deficits observed in SCZ. This is not to say that when using other measures of systems-level interactions (eg, functional connectivity approaches), there are no abnormalities that span a more distributed set of circuits (eg, reverberations between fronto-parietal regions), but the core deficit contributing to WM pathology in SCZ may be stemming from abnormalities in recruitment of prefrontal cortical regions.1
One finding not predicted by prior work39 was less recruitment of PFC regions when groups were performance matched. However, Johnston and colleagues16 reported a similar effect to ours when using load-based difficulty manipulation to accomplish performance matching. Furthermore, the pattern of “hypoactivation” in the present study may be in part related to the way in which difficulty was manipulated—we did not adjust load levels but rather the requirement of maintaining a given level of WM trace precision by adjusting the similarity of nontarget probes. One interesting possibility is that patients, in the context of the present paradigm, are able to accomplish WM operations when provided lower difficulty, but their mnemonic traces, due to noisy attractor network formation, may be substantially less precise and degrade rapidly.40 In turn, less robust neural attractor formation in distributed cortical networks may result in a lower signal across encoding and maintenance.
Conclusions
Present results replicated prior work and advanced the basic understanding of WM encoding- and maintenance-related neural abnormalities in SCZ by showing that: (1) When examining a broad set of regions implicated in WM,20,21 there are not only maintenance but also encoding-related PFC signal decreases in SCZ subjects and (2) There are deficits in deactivating DMN regions across both WM encoding and maintenance. This suggests that SCZ subjects may show abnormalities in both precise WM formation as well as robust maintenance of ongoing traces. Further, by examining the relationship between activation and deactivation at encoding, we show that SCZ subjects may exhibit a breakdown in coordinated suppression of regional activity. Therefore, present results suggest that characterizing both activation and deactivation deficits is critical for a complete understanding of WM dysfunction in this illness.
Funding
National Institute of Mental Health (R01 MH 066031 to D.M.B.).
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
We would like to thank the McDonnell Center for Systems Neuroscience for providing invaluable financial support. We would also like to thank Dr Hedy Kober for her invaluable input and comments regarding figure preparation. Lastly, we would like to thank 2 anonymous Referees for their thoughtful feedback and review. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 2.Krystal JH, D'Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 4.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 7.Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- 9.Jonides J, Lewis RL, Nee DE, et al. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baddeley AD. The episodic buffer: a new component of working memory? Trends in Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 11.Neufeld RWJ. On the centrality and significance of stimulus-encoding deficit in schizophrenia. Schizophr Bull. 2007;33:982–993. doi: 10.1093/schbul/sbm056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driesen NR, Leung HC, Calhoun VD, et al. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64:1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Manoach D, Mathalon D, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009;30:3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luck D, Danion J-M, Marrer C, et al. Abnormal medial temporal activity for bound information during working memory maintenance in patients with schizophrenia. Hippocampus. 2010;20:936–948. doi: 10.1002/hipo.20689. [DOI] [PubMed] [Google Scholar]
- 15.Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MR, Morris NA, Astur RS, et al. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry. 2006;60:11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Schlösser RGM, Koch K, Wagner G, et al. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: an fMRI study. Neuropsychologia. 2008;46:336–347. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 19.Leung H-C, Gore JC, Goldman-Rakic PS. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 2002;14:659–671. doi: 10.1162/08989290260045882. [DOI] [PubMed] [Google Scholar]
- 20.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 22.Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulman GL, Corbetta M, Buckner RL, et al. Common blood flow changes across visual tasks .1. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J Cogn Neurosci. 1997;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008;29:1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrity AG, Pearlson GD, McKiernan K, et al. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 26.Metzak PD, Riley JD, Wang L, et al. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbq154. [published online ahead of print January 11, 2011] doi: 10.1093/schbul/sbq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomarol-Clotet E, Salvador R, Sarró S, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 28.Anticevic A, Repovs G, Barch DM. Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn Affect Behav Neurosci. 2010;10:159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: University of Iowa; 1983. [Google Scholar]
- 30.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1983. [PubMed] [Google Scholar]
- 31.Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbq168. [published online ahead of print March 17, 2011] doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anticevic A, Repovs G, Corlett PR, Barch DM. Negative and non-emotional interference with visual working memory in schizophrenia. Biol Psychiatry. doi: 10.1016/j.biopsych.2011.07.010. In press. [DOI] [PubMed] [Google Scholar]
- 33.Anticevic A, Repovs G, Van Snellenberg JX, Csernansky JG, Barch DM. Subcortical alignment precision in patients with schizophrenia. Schizophr Res. 2010;120:76–83. doi: 10.1016/j.schres.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cole MW, Anticevic A, Repovs G, Barch DM. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield-Gabrieli S, Thermenos H, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald AW, Chafee MV. Translational and developmental perspective on N-methyl-D-aspartate synaptic deficits in schizophrenia. Dev Psychopathol. 2006;18:853–876. [PubMed] [Google Scholar]
- 39.Callicott JH, Mattay VS, Verchinski BA, et al. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 40.Badcock JC, Badcock DR, Read C, Jablensky A. Examining encoding imprecision in spatial working memory in schizophrenia. Schizophr Res. 2008;100:144–152. doi: 10.1016/j.schres.2007.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.